Abstract
To control the sensory–motor system, internal models mimic the transformations between motor commands and sensory signals. The present study proposed to assess the effects of physiological adult ageing on the proprioceptive control of movement and the related internal models. To this aim, one group of young adults and one group of older adults performed an ankle contralateral concurrent matching task in two speed conditions (self-selected and fast). Error, temporal and kinematic variables were used to assess the matching performance. The results demonstrated that older adults used a different mode of control as compared to the young adults and suggested that the internal models of proprioceptive control were altered with ageing. Behavioural expressions of these alterations were dependent upon the considered condition of speed. In the self-selected speed condition, this alteration was expressed through an increased number of corrective sub-movements in older adults as compared to their young peers. This strategy enabled them to reach a level of end-point performance comparable to the young adults' performance. In the fast speed condition, older adults were no more able to compensate for their impaired internal models through additional corrective sub-movements and therefore decreased their proprioceptive control performance. These results provided the basis for a model of proprioceptive control of movement integrating the internal models theory and the continuous and intermittent modes of control. This study also suggested that motor control was affected by the frailty syndrome, i.e. a decreased resistance to stressors, which characterises older adults.
Keywords: Motor control, Internal models, Proprioception, Frailty, Physiological ageing
Introduction
Control of the sensory–motor system lies in transformations between sensory signals and motor commands. The internal model theory proposes that the central nervous system models these transformations on the basis of information from previous experience. Convincing data supporting the existence of internal models have been provided by computational, behavioural, neurophysiological and imagery studies (Dean et al. 2010; Flanagan and Wing 1997; Miall and King 2008; Scarchilli and Vercher 1999; Wolpert et al. 1995, 1998). Two types of internal models (inverse and forward) have been discriminated depending on the direction of the mimicked transformation.
Mathematical approaches showed that an inverse kinematic transformation could solve for the set of joint angles required to move the limb along a given spatial trajectory (Saltzman 1979; Whitney 1972). The fact that humans can view a target in space and imagine their hand moving to this target suggests they have an internal model of this inverse kinematic transformation (Jeannerod 2001). The fact that they can actually move to this target with eyes closed suggests they also have an internal model of the inverse dynamic transformation from the desired pattern of motion to the muscle activation (Atkeson 1989). These so-called inverse models implement the desired sensory consequences of actions into a motor command as a function of the current sensory–motor state, context and task (Kawato 1999).
The efference copy (von Holst 1954) is some information derived from the motor commands of cortical areas involved in planning and executing a motor action. On the basis of this information, the so-called forward models mimic the causal relationship between a motor command and its sensory consequences as a function of a current state, context and task (Jordan and Rumelhart 1992; Wolpert et al. 1995). The efference copy is first implemented by a so-called forward dynamic model into a predicted dynamic state (Miall and Wolpert 1996; Wolpert and Ghahramani 2000). Then, the forward sensory model implements this dynamic state into its corresponding sensory information, cancelling out sensory signal induced by self-motion, taking the noisy sensory measurement apparatus into account and eliminating the effect of the delay related to the sensory control loop (Kleinman 1969). To better account for the human ability to adapt to different and uncertain environmental conditions, Wolpert and Kawato (1998) proposed a theory of multiple internal models. In this theory, the transformation between sensory signals and motor commands is not modelled by a single internal model but by multiple ones. In addition, the authors proposed to pair each inverse model with a corresponding forward model. Finally, responsibility signals are distributed across the multiple pairs of internal models to determine how much a particular pair contributes to the sensori-motor transformation.
From this internal model theory, the control of movement can be divided into several stages (Wolpert and Ghahramani 2000). The central nervous system first selects inverse models that implement the initial state, context and task into the desired states at each point along the movement trajectory. These desired states are then implemented into appropriate motor commands (Kawato et al. 1987). Previous studies showed that movement could be corrected with a shorter latency (30–45 ms) than the minimal latency required for processing the sensory afferences (80–100 ms) (Cooke and Diggles 1984; van Sonderen et al. 1989). These results suggested that the desired state issued from the inverse models and the predicted dynamic state issued from the forward dynamic models could be compared to control the movement before availability of the sensory afferences. Finally, these sensory afferences become available to adjust the ongoing trajectory onto the sensory prediction of the forward sensory models, when necessary (Bard et al. 1999; Branch Coslett et al. 2008; Desmurget et al. 1999; Farrer et al. 2003; Hansen and Elliott 2009; Miall and Wolpert 1996; Wolpert and Ghahramani 2000).
A commentary inspired from the cybernetics proposed that physiological ageing is associated with a decline in the variety of available system states and responses (Thaler 2002). Such a decline would result in a decreased variety of internal models and thus a reduced ability to finely adjust movements in older adults. The alteration of internal models in adult ageing has been experimentally assessed using different methods. One of these methods is motor imagery (Personnier et al. 2008, 2010; Skoura et al. 2005). In this method, a subject internally simulates a movement without moving his limbs or activating the muscles involved in the execution of this movement. On a temporal basis, these studies showed weaker correlations and greater absolute differences between executed and imagined movements in older adults as compared to young ones. Conclusions from these results suggested that internal models become imprecise with advance in age. However, if comparisons between temporal features of executed and imagined movements are consistent with an age-related alteration of the internal models, this method does not assess the spatial features of these models. In addition, time is not explicitly modelled in the internal models theory. Therefore, it could not be valid to infer age-related differences in the quality of internal models based on differences in the correlation of actual and imagined movement time. Indeed, such differences might only reflect an age-related slowing of a general timing mechanism instead of deficits in the accuracy of sensory–motor transformations represented in the internal models.
Conversely, previous studies assessing the spatial adaptation to visuo-motor distortions suggested that inverse models in older adults were comparable to the ones in young adults. Indeed, both age groups showed similar after-effects which are thought to account for the space recalibration mediated by the internal models and which are presumably not confounded by strategic corrections (Bock 2005; Buch et al. 2003; Hegele and Heuer 2010). Nevertheless, results from these visuo-motor adaptation studies were not that univocal. Bock (2005) hypothesised that the absence of age-related alteration of the after-effects reflected deficits of recalibration which were balanced out by increased perseveration. To test this hypothesis, Bock intended to transfer the visuo-motor adaptation from a pointing task to a tracking task. Adaptation in the tracking task would therefore not involve the replication of previously learned behaviour and would thus not be biased by perseveration. If the study concluded to an absence of statistical difference of adaptation in the tracking task between the two age groups, a closer data analysis revealed that older adults' adaptation still tended to be degraded as compared to the adaptation of the young ones.
Analyses of movement kinematics in young and older adults have been performed to investigate the underlying mechanisms of motor control. Most of these studies used goal-directed aiming movements and divided these movements into different phases for analysis. Two main methods have been used to determine these phases. The first one divides movements into acceleration and deceleration phases, with the instant of peak velocity being the division point (Cooke et al. 1989; Darling et al. 1989; Goggin and Meeuwsen 1992; Goggin and Stelmach 1990; Lyons et al. 1996; Murrell and Entwisle 1960; Rey-Robert et al. 2012; Welford et al. 1969; Yan et al. 2000). Results of these studies showed age-related differences in the duration of the acceleration and/or deceleration phases, as well as in the positional, velocity and acceleration profiles. These kinematic alterations could originate from changes in movement strategies and/or from an alteration of the internal models in adult ageing. However, the ability for older adults to reach end-point performances comparable to those obtained in young adults suggested that internal models were as efficient in young and older adults.
The second method divides the movement into initial and secondary sub-movements (Ketcham et al. 2002; Lyons et al. 1996; Pratt et al. 1994; Seidler-Dobrin and Stelmach 1998; Welsh et al. 2007; Yan et al. 2000). In this method, a sub-movement is considered to have ended when the acceleration profile changes from negative to positive. One of these studies showed that older adults exhibited a greater number of corrective sub-movements than young adults near the end of the movement (Lyons et al. 1996). These corrective sub-movements are presumably related to an inaccuracy of the initial motor command in older adults which is also supported by the decreased end-point performance in primary sub-movements (Ketcham et al. 2002; Pratt et al. 1994). As the motor control is based on information arising from internal models, the observed increased need for corrective sub-movements supported an age-related alteration of these models. However, other studies evidenced an absence of age-related differences in the number of sub-movements per movement (Seidler-Dobrin and Stelmach 1998), the proportion of movements containing a secondary sub-movement (Pratt et al. 1994) and the spatial location of the end of the primary sub-movement (Seidler-Dobrin and Stelmach 1998; Welsh et al. 2007). Nevertheless, due to the knowledge of the result inherent in aiming movements to visual targets, a learning effect that could enable older adults to compensate for a possible alteration of the internal models cannot be ruled out from the discussion of these latter studies. Removing visual information on target location upon movement initiation (Elliott et al. 1991; Seidler-Dobrin and Stelmach 1998) would solve this problem but produce other limitations associated with the quick degradation of visual information related to the movement environment after visual occlusion (Elliott 1988; Elliott and Madalena 1987). This removal also implies sensory reweighting processes which have shown to be altered in adult ageing (Eikema et al. 2012; Speers et al. 2002).
Adaptation of a method classically used to test the proprioceptive perception could remove the aforementioned limits in internal models assessment: the contralateral concurrent joint position matching task (Adamo et al. 2007; Goble and Brown 2007). In this task, a subject’s limb is displaced to a reference position. The task consists in reproducing this reference position with the contralateral limb, the control of this active movement being based on proprioceptive information. The reference position can be seen as a proprioceptive target which allows the absence of visual feedback and sensory reweighting.
The present study proposed to assess the effects of physiological adult ageing on the proprioceptive control of movement and the related internal models. To this aim, one group of young adults and one group of older adults performed an ankle contralateral concurrent matching task in two speed conditions (self-selected and fast). These conditions intended to assess age-related differences in the absence and presence of temporal constraints, respectively. It was hypothesised that (1) the ability to accurately match the proprioceptive target in the self-selected speed condition was not different between young and older adults. Indeed, although it was against an increased cognitive cost and additional neural resources, previous studies showed that older adults were able to reach levels of proprioceptive control of movement comparable to those of young adults (Batavia et al. 1999; Boisgontier et al. 2012; Deshpande et al. 2003; Goble et al. 2012a, b; Heuninckx et al. 2008; Marks 1996; Pickard et al. 2003). However, on the basis of the differences observed in motor imagery (Personnier et al. 2010, 2008; Skoura et al. 2005) and kinematic studies (Elliott and Hansen 2010; Goggin and Meeuwsen 1992; Rey-Robert et al. 2012; Seidler-Dobrin and Stelmach 1998), we hypothesised that (2) older adults were not able to reach the same level of end-point performance as the young adults in the fast speed condition due to the temporal constraints, making it impossible to use additional sub-movements to compensate for the presumably altered internal models. We also hypothesised that (3) older adults required a greater number of corrective sub-movements reflecting an intermittent control movement used to compensate for a presumed alteration of the internal models.
Methods
Participants
Twelve older adults (age 76 ± 8 years; weight 57 ± 11 kg; height 163 ± 12 cm; mean ± SD) and 12 young adults (age 28 ± 3 years; weight 65 ± 8 kg; height 173 ± 8 cm) without history of neurological disease, diabetes or lower limb injuries participated in the study. To ensure that this study assessed physiological adult ageing, participants had to validate different criteria to be involved in the experiment. They all had a normal pre-testing examination to exclude peripheral vascular disease and peripheral neuropathy. Participants lived independently in their own accommodation and reported no history of falls in the past year. A fall was defined as an event resulting in a person inadvertently coming to rest on the ground or another lower level. To assess the cognitive function, all participants took a Mini Mental State Examination (Folstein et al. 1975) and the minimum score for inclusion was set at 27 out of 30. To identify leg dominance which was an inclusion criterion, participants were asked their preference for kicking a ball toward a target (Peters 1988). All participants indicated their right leg as their dominant leg. They all gave written informed consent and their rights were protected as required by the Helsinki Declaration (1964) and the local Ethics Committee.
Setup and procedure
Participants were comfortably seated barefoot with their feet secured with Velcro straps onto two rotating lightweight paddles (Fig. 1). A panel was placed above their legs to ensure the absence of ankle visual feedback. Position of the lower extremities was standardised and maintained for all conditions with the thighs horizontal and the knees bent at approximately 70°. Ankle movements were restricted to the ankle in the sagittal plane. Precision linear potentiometers attached on both paddles provided an analogue voltage signal which was converted into angular displacements proportional to the ankle joint angle. At the beginning of each experiment, potentiometer outputs for both paddles were checked and, when calibrated, gave an angular resolution of 0.01°. Participants held a switch in the dominant hand to record the trial. Signals from the potentiometers and the switch were sampled at 100 Hz.
Fig. 1.

Apparatus and setup for the contralateral concurrent matching task
The reference foot and the matching foot were the non-dominant and dominant feet, respectively. To perform the contralateral concurrent ankle position matching task, one experimenter passively positioned the participant’s reference foot on a fixed support at 10 ± 0.1° above horizontal, corresponding to a 10° plantarflexion position. Participants were instructed to maintain the muscles of their leg relaxed throughout the duration of ten trials. Immediately following the positioning of the reference limb, a verbal ‘ready’ command alerted participants of the start of the trial. Following a 2-s delay and the verbal command ‘go’, participants’ task was to actively reproduce the reference position with the matching foot. Participants were informed that this was not a reaction time task. Therefore, they could start moving whenever they liked after the ‘go’ signal. Participants were instructed to indicate that they had achieved a subjective satisfactory matching by pressing the switch that registered the trial. Participants did not receive any feedback about their matching performance neither during nor after the matching performance. Once the matching had been validated, the participant repositioned the matching foot in the initial position (5 ± 0.1° under horizontal). This matching task was performed in two experimental conditions which were counterbalanced across participants. In the self-selected speed condition, participants were instructed to produce an accurate matching at a self-selected speed. This was explained to them as being the speed at which they felt most comfortable: the speed they would choose by preference. This condition aimed at assessing participants’ ability to accurately match a proprioceptive position and to evidence possible age-related differences in the number of sub-movements required to reach the targeted position. In the fast speed condition, participants were instructed to perform the matching as quickly and as accurately as possible in one single movement. This condition aimed at imposing a minimum number of sub-movements. Before each condition, participants received specific instructions on how to perform the experimental task and then performed three trials of familiarisation. For each condition, ten blocked trials were recorded for a total of 20 trials per participant.
Dependent variables
Three error, three temporal and two kinematic dependent variables were used to assess the matching performance. The error-dependent variables were the total error, the variable error and the constant error (Schmidt and Lee 2005). The total error (TE) was used to measure the end-point overall performance. Combining accuracy and variability (namely, TE² = variable error² + constant error²), its formula is 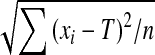 , where xi is the score on trial i, T is the target (10 ± 0.1° above horizontal) and n is the number of trials the participant performed (n = 10). The variable error (VE) was a measure of the spread about participant’s own average. Its formula is
, where xi is the score on trial i, T is the target (10 ± 0.1° above horizontal) and n is the number of trials the participant performed (n = 10). The variable error (VE) was a measure of the spread about participant’s own average. Its formula is 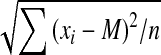 , where M is the participant’s average score. The constant error (CE) was a measure of response bias. Its formula is
, where M is the participant’s average score. The constant error (CE) was a measure of response bias. Its formula is 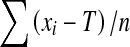 . This variable represents the amount and direction of deviation relative to the target. Negative CE indicated that the matching foot undershot the reference position whereas positive CE indicated that the matching foot overshot the reference position. For the temporal dependent variables, the total matching time was defined as the mean time elapsed between movement onset and the moment the participant pressed the switch to record the trial. A movement was determined to have started when the ankle position exceeded the initial position by more than 0.1°. In an attempt to discriminate (1) the time required to reach a stabilised position and (2) the time required to validate this position, the total matching movement was divided into two phases. The algorithm which was used to determine the end of the positioning phase and the onset of the validation phase worked backward from the final matching position and stopped at the first sample strictly superior or inferior to the final matching position plus or minus 1.5° (i.e. 10 % of the distance between the initial position of the matching foot and the reference position), respectively. As the foot position was stable during the validation phase, the kinematic analysis only assessed the positioning phase by measuring the peak velocity and computing the number of times the mean acceleration profile crossed the zero line. This latter variable was used to examine corrective sub-movements which are designed to reduce the errors made in the previous sub-movements (Elliott et al. 1991; Keele 1968; van Donkelaar and Franks 1991; Yan et al. 2000).
. This variable represents the amount and direction of deviation relative to the target. Negative CE indicated that the matching foot undershot the reference position whereas positive CE indicated that the matching foot overshot the reference position. For the temporal dependent variables, the total matching time was defined as the mean time elapsed between movement onset and the moment the participant pressed the switch to record the trial. A movement was determined to have started when the ankle position exceeded the initial position by more than 0.1°. In an attempt to discriminate (1) the time required to reach a stabilised position and (2) the time required to validate this position, the total matching movement was divided into two phases. The algorithm which was used to determine the end of the positioning phase and the onset of the validation phase worked backward from the final matching position and stopped at the first sample strictly superior or inferior to the final matching position plus or minus 1.5° (i.e. 10 % of the distance between the initial position of the matching foot and the reference position), respectively. As the foot position was stable during the validation phase, the kinematic analysis only assessed the positioning phase by measuring the peak velocity and computing the number of times the mean acceleration profile crossed the zero line. This latter variable was used to examine corrective sub-movements which are designed to reduce the errors made in the previous sub-movements (Elliott et al. 1991; Keele 1968; van Donkelaar and Franks 1991; Yan et al. 2000).
Results
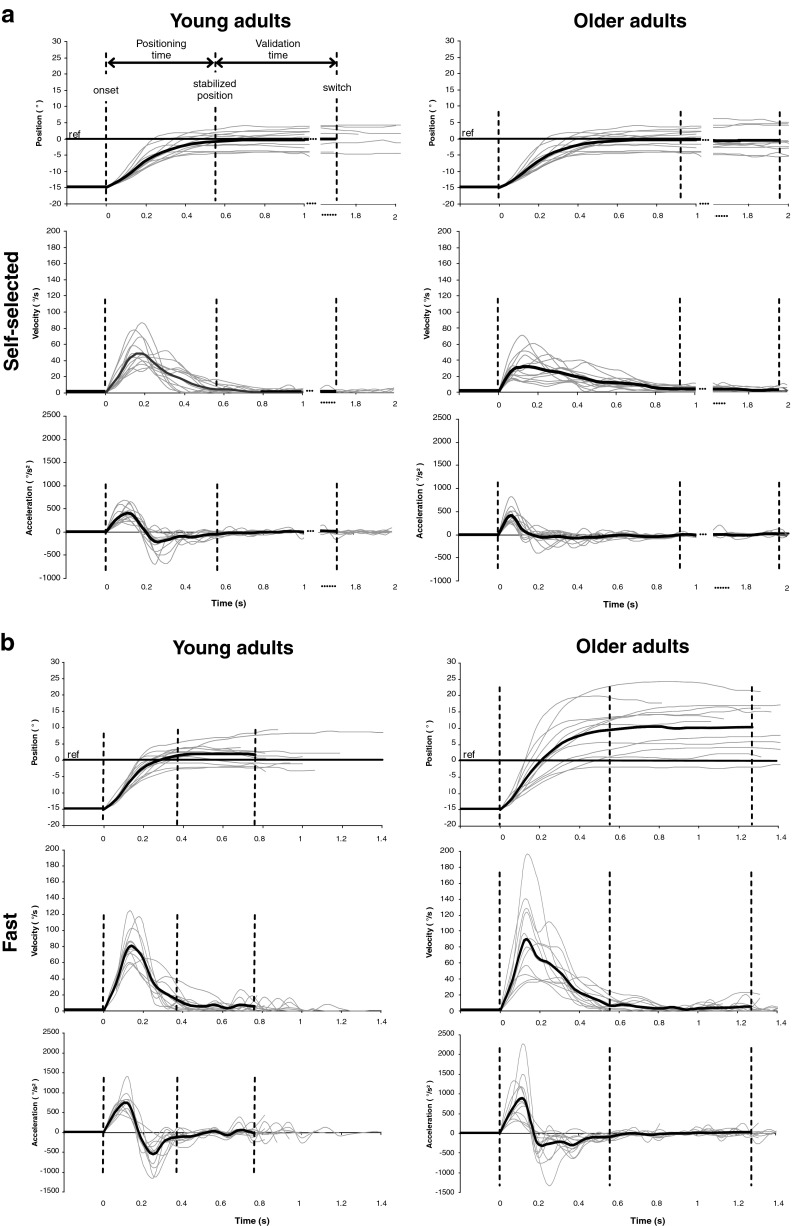
To describe the behaviours in the matching task, different age groups and conditions, the position, velocity and acceleration profiles have been illustrated in Fig. 2 for each participant and the mean of all participants. For the statistical analysis of the ankle matching performance, 2 age groups (young adults vs. older adults) × 2 speeds (self-selected vs. fast) analyses of variance (ANOVAs) with repeated measures on the last factor were applied to the different dependent variables. The level of significance was set at P < 0.05. The purpose of these ANOVAs was to assess the effects of physiological adult ageing on internal models in proprioceptive control. A correlation analysis was also performed between peak velocity and constant error to test whether the response bias was only explained by the initially planned trajectory (a strong correlation would validate this hypothesis) or also by compensatory adjustments to this trajectory (no correlation would validate this hypothesis) (Messier and Kalaska 1999). Indeed, if the trajectory was predetermined before movement initiation, the initial kinematics of the matching illustrated by peak velocity should predict the end-point distribution illustrated by the constant error. Conversely, if patterns of movement amplitude and peak velocity are different, this would suggest that processes occurring during movement execution influenced the distribution of end-point positions.
Fig. 2.
a, b Mean of the ten trials for each participant (grey lines) and all participants (black bold line) for the position, velocity and acceleration profiles as a function of age group (young adults; older adults) and speed condition (self-selected; fast). The horizontal solid line stands for the reference position, i.e. the absence of matching error. The vertical left, central and right hatched lines respectively stand for the mean movement onset, the moment the mean matching position was stabilised and the switch which validated the mean matching position
As illustrated in Fig. 3a, analysis of TE showed significant main effects of age group (F1, 11 = 25.14, P < 0.001) and speed condition (F1, 11 = 14.78, P = 0.003) with greater TE for the older adults and the fast speed condition. The interaction of group × speed was also significant (F1, 11 = 9.51, P = 0.010). The decomposition of the interaction into its simple main effects showed that the older adults exhibited a greater TE than the young adults in the fast speed condition (3.8 ± 0.6 vs. 11.7 ± 2.0°, P = 0.003) but not in the self-selected speed condition (3.0 ± 0.3 vs. 3.8 ± 0.5°, P = 0.957).
Fig. 3.
Mean total errors (a), variable errors (b) and constant errors (c) of each participant in degrees as a function of age group (young adults in white; older adults in black) and speed condition (self-selected; fast). The hatched line, the bottom and the top of the box, the lower and upper whiskers and the circles respectively depict the median, the 25th and 75th percentile, the smallest and the largest mean and the outliers
As illustrated in Fig. 3b, analysis of VE showed a significant main effect of age group (F1, 11 = 8.48, P = 0.014) with greater VE for the older adults. No significant main effect of speed (F1, 11 = 2.38, P = 0.151) nor significant interaction of group × speed (F1, 11 = 2.15, P = 0.171) were evidenced.
As illustrated in Fig. 3c, analysis of CE showed significant main effects of age group (F1, 11 = 17.49, P = 0.002) and speed (F1, 11 = 11.68, P = 0.006) with greater CE for the older adults and the fast speed condition. The interaction of group × speed was also significant (F1, 11 = 6.64, P = 0.026). The decomposition of the interaction into its simple main effects showed that the older adults exhibited a greater CE than the young adults in the fast speed condition (2.2 ± 1.0 vs. 10.6 ± 2.1°, P = 0.004) but not in the self-selected speed condition (0.4 ± 0.8 vs. 1.5 ± 1.0°, P = 0.756). Analyses were also performed for the error values at the end of the positioning time and showed similar results due to the backward method used to determine the positioning time.
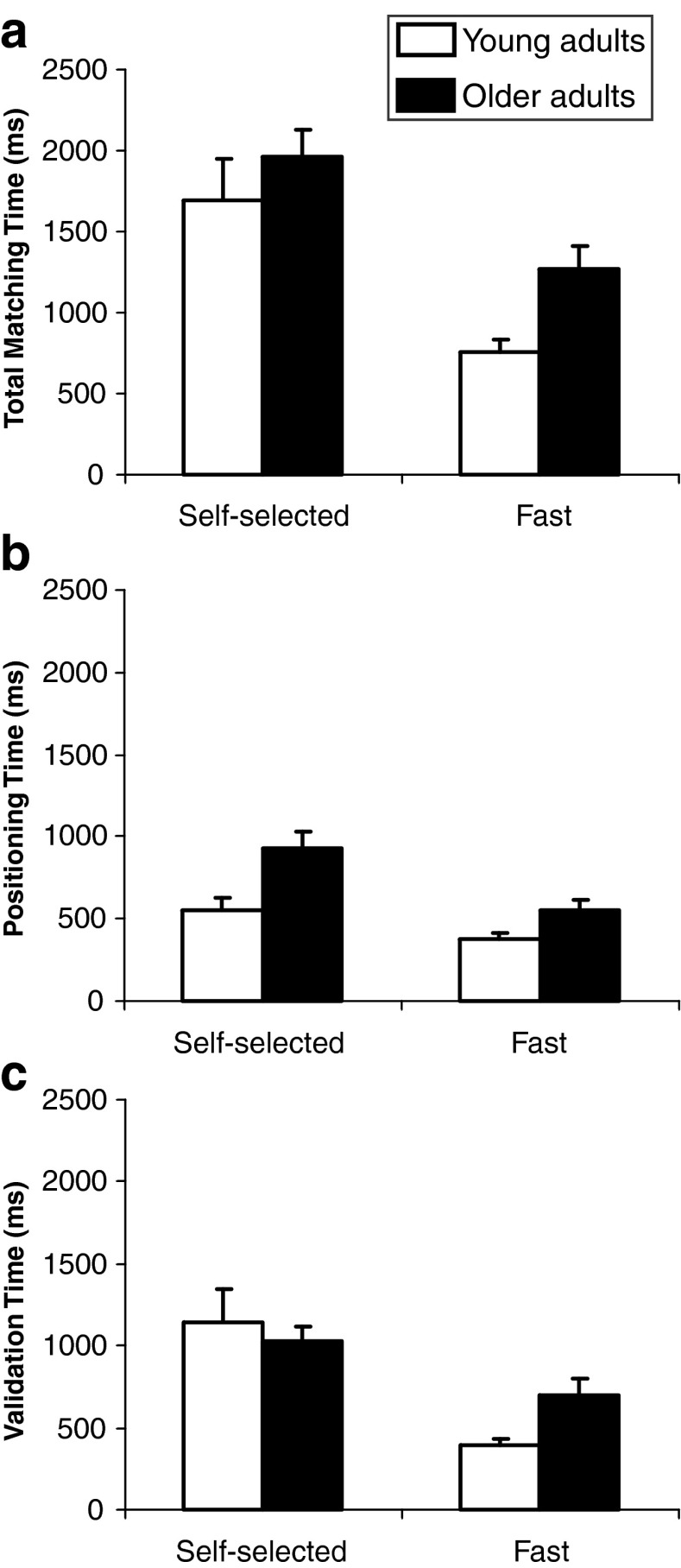
As illustrated in Fig. 4a, analysis of the total matching time showed significant main effects of age group (F1, 11 = 5.93, P = 0.033) and speed (F1, 11 = 21.33, P < 0.001) with longer matching times for the older adults and the self-selected speed condition. The interaction of group × speed was not significant (F1, 11 = 0.47, P = 0.506).
Fig. 4.
Mean total matching time (a), positioning time (b) and validation time (c) in millisecond as a function of age group (young adults; older adults) and speed condition (self-selected; fast)
As illustrated in Fig. 4b, analysis of the positioning time showed significant main effects of age group (F1, 11 = 10.79, P = 0.007) and speed (F1, 11 = 13.35, P = 0.004) with a longer positioning time for the older adults and the self-selected speed condition. The interaction of group × speed was not significant (F1, 11 = 1.20, P = 0.296).
As illustrated in Fig. 4c, analysis of the validation time showed no significant main effect of age group (F1, 11 = 0.74, P = 0.406) but a significant main effect of speed (F1, 11 = 21.46, P < 0.001) with a longer validation time in the self-selected speed condition. The interaction of group × speed was not significant (F1, 11 = 3.21, P = 0.101).
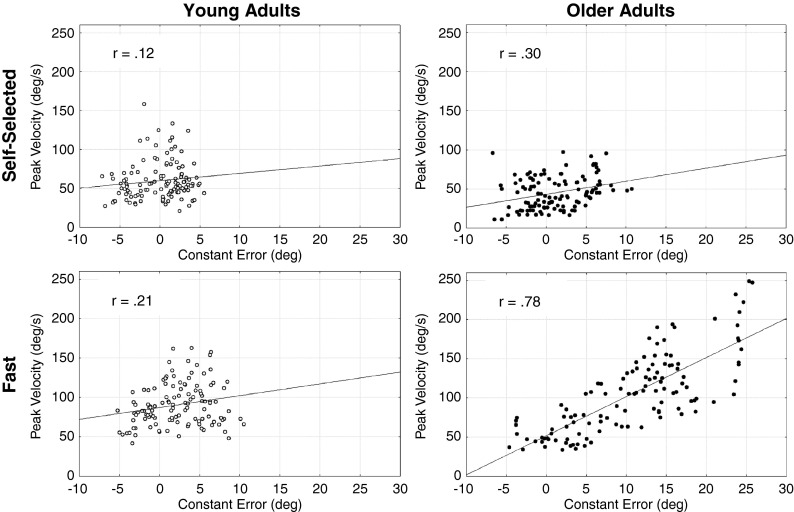
Analysis of peak velocity showed no significant main effects of age group (F1, 11 = 0.12, P = 0.734) but a significant main effect of speed (F1, 11 = 25.08, P < 0.001) with a greater peak velocity in the fast speed condition (52.6 ± 6.3 vs. 100.7 ± 11.4°/s). The interaction of group × speed was not significant (F1, 11 = 3.97, P = 0.07). As illustrated in Fig. 5, analysis of the correlation between the peak velocity and the constant error showed weak correlation coefficients for the young adults in both self-selected (r = 0.12, P < 0.05) and fast speed (r = 0.21, P < 0.05) conditions as well as for the older adults in the self-selected speed condition (r = 0.30, P < 0.05). The present results suggested that in these conditions, the end-point position was related to adjustments subsequent to the initial motor command. For the older adults in the fast speed condition, the correlation coefficient was strong (r = 0.78, P < 0.05) and suggested that the end-point position was mainly related to the initial motor command.
Fig. 5.
Relation between peak velocity and constant error as a function of age group (white circles for young adults; black circles for older adults) and speed condition (self-selected; fast). The solid lines represent the regression lines for data point related to each individual trial. The r-value is the correlation coefficient at P < 0.05
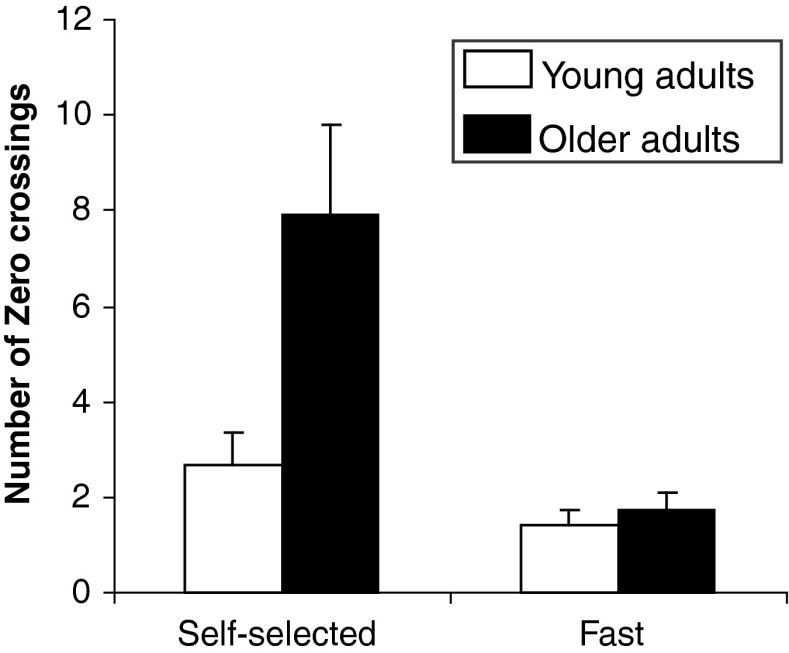
As illustrated in Fig. 6, analysis of the number of zero-crossings in the acceleration profiles during the positioning time showed significant main effects of age group (F1, 11 = 5.42, P = 0.040) and speed (F1, 11 = 16.77, P = 0.002) with a greater number of zero-crossings for the older adults and the self-selected speed condition. The interaction group × speed was also significant (F1, 11 = 5.16, P = 0.044). The decomposition of the interaction into its simple main effects showed that the older adults exhibited a greater number of zero-crossings than the young adults in the self-selected speed condition (2.7 ± 0.7 vs. 7.9 ± 1.9, P = 0.002) but not in the fast speed condition (1.4 ± 0.3 vs. 1.8 ± 0.4, P = 0.996). These results suggested that older adults used adjustments subsequent to the initial motor command in the self-selected speed condition but not in the fast speed one.
Fig. 6.
Mean of zero-crossings in the acceleration profile during the positioning time as a function of age group (young adults; older adults) and speed condition (self-selected; fast)
Discussion
In this study, one group of young adults and one group of older adults performed a contralateral concurrent ankle matching task in self-selected and fast speed conditions to assess age-related alterations in the proprioceptive control of movement and the related internal models. For the sake of clarity, the following discussion has been divided in seven sub-sections.
Working memory
As the target angle was the same for all trials, the present results could have reflected an age-related difference in a working memory mechanism that builds a representation of the target position over trials. Indeed, working memory has shown to be impaired in normal ageing (e.g. Gazzaley et al. 2005). However, Goble et al. (2010b) outlined that a longer time of reference limb presentation affords participants an increased opportunity to develop neural representations of the reference limb position to be matched. Furthermore, the literature suggests that presenting the reference limb for an extended period of time to older adults enabled them to reach a performance of proprioceptive control similar to the young adults' one. Indeed, three of the studies which assessed the proprioceptive control of movement of young and older adults in a contralateral concurrent matching task showed that older adults exhibited greater absolute errors than young ones (Adamo et al. 2007, 2009; Meeuwsen et al. 1993), whereas the other study did not evidence significant errors between young and older adults (Boisgontier et al. 2012). The only difference between the first three studies and the last one was the time spent in the reference position. In the first three studies, the reference foot was maintained for one trial only whereas in the last one it was maintained for ten successive trials. In other words, when performing a contralateral concurrent matching task, older adults took more advantage from the working memory mechanism than young adults. It could be assumed that young adults did not need additional time of presentation of the reference limb position to reach the best performance of proprioceptive control. As a consequence, young adults would not benefit from a longer time of presentation of the reference limb with a ceiling effect. Therefore, as the presumed working memory mechanisms implicated in a contralateral concurrent matching task reduced the effects of ageing on the proprioceptive control performance, the differences observed in the present study were likely not reflecting an age-related difference in working memory.
Origin of the sub-movements
Despite the fact that previous studies assumed that the number of zero-line crossings in the acceleration profile could be used to infer the number of corrective sub-movements (Elliott et al. 1991; Keele 1968; van Donkelaar and Franks 1991; Yan et al. 2000), we explored the other potential origins of sub-movements to test whether they could be ruled out from the discussion. First, physiological tremor could also produce sub-movements. However, its frequency declines after the age of 40 years from 10 to 6 Hz in older adults (Marshall 1961). As the mean positioning time in the self-selected speed condition was near 0.5 s for the young adults and near 1 s for the older adults, the number of sub-movements which could be related to the physiological tremor would have been approximately the same for the young and older adults (approximately five vs. six sub-movements, respectively). Therefore, the increased number of sub-movements observed in older adults was likely not related to physiological tremor. Second, as pointed out by Loram et al. (2011), ‘responses may be constructed and executed in a serial fashion in which case sensory information may be assimilated continuously but only responded to at particular times when actions are executed’. However, given the short duration and the simplicity of the matching task performed in the present study, such a construction and its related sub-movements was unlikely. Finally, sub-movements could originate from a strategy aiming at propelling the limb near to the target in an initial sub-movement and consequently performing a secondary sub-movement to reach the target. In this latter case, even if they were a consequence of an initial strategy, sub-movements were still related to a correction, or at least to an adjustment of the matching movement. The zero-line crossings in the acceleration profile observed in the present study were thus assumed to mainly reflect the number of corrective sub-movements necessary to adjust the matching performance.
End-point performances
In the self-selected speed condition, no difference in the overall end-point performance of proprioceptive control (total error) was observed between the two age groups (hypothesis 1). This absence of significant age-related difference supported previous studies showing that older adults were able to reach levels of proprioceptive control performance comparable to those observed in young adults (Batavia et al. 1999; Boisgontier et al. 2012; Deshpande et al. 2003; Goble et al. 2012a, b; Marks 1996; Pickard et al. 2003). The splitting of the total error into variable and constant errors confirmed this similar pattern of end-point results between age groups.
In the fast speed condition, older adults degraded their overall end-point performance (total error) as compared to young ones. This result showed that older adults were not able to reach levels of end-point performance comparable to the young adults when they were submitted to a temporal constraint (hypothesis 2). The splitting of the total error into variable and constant errors showed that this decrease in the overall end-point performance was mainly related to the constant error, evidencing an overshooting behaviour in the older adults as compared to the young ones. This result supported the study of Boisgontier et al. (2012) which showed that older adults overshot the proprioceptive target when the cognitive load increased. Together, these results suggested that when they have to deal with a stressor (temporal or cognitive) in a proprioceptive task, older adults overshoot rather than undershoot the target. In the present study, the overshoot was unlikely a deliberate strategy because participants were instructed to match the position in one single movement. It could rather reflect an age-related alteration of the proprioceptive control of movement. This overshoot also supported previous postural results of older adults in quiet standing tasks which could be seen as a succession of small reaching movements toward a vestibulo-proprioceptive target, i.e. the body equilibrium. In these studies, older adults exhibited a greater tendency to overshoot the body equilibrium over short-term intervals which are supposed to reflect an open-loop mode of control (Collins et al. 1995). This correspondence between results in ankle proprioceptive control and postural control was consistent with the observed correlation between ankle proprioception and postural balance performance (Goble et al. 2011).
The total matching time and positioning time extensions observed in older adults as compared to young ones supported previous studies of self-selected speed matching tasks (Boisgontier et al. 2012) and fast speed goal-directed aiming movements (Cooke et al. 1989; Ketcham et al. 2002; Seidler-Dobrin and Stelmach 1998; Welford et al. 1969; Welsh et al. 2007) which showed a longer movement time in older than in young adults. These time extensions observed in older adults could be seen as an attempt to increase the presentation time of the reference limb. Indeed, a longer time of reference limb presentation affords individuals an increased opportunity to develop neural representations of the reference position to be matched (Goble et al. 2010b).
Finally, analysis of the validation time showed that older adults needed a greater time to validate the stabilised position in the fast speed condition. Duration of the validation time increased by 300 ms on average. As the predicted difference for simple reaction time measure between the two age groups of this study was approximately 100 ms (Der and Deary 2006), the remaining 200 ms probably reflected additional cognitive processes required to validate the proprioceptive performance of older adults in the fast speed condition.
Continuous vs. intermittent models
Whether a continuous (e.g. Kleinman 1969) or an intermittent (e.g. Gawthrop et al. 2011) model of control is more appropriate to explain the motor behaviour has been widely discussed in the literature. Results of the present study suggested that the control of movement was mainly continuous in young adults and mainly intermittent in older adults.
Intermittency is characterised by sub-movements which are triggered by an error crossing a threshold (Asai et al. 2009; Hanneton et al. 1997; Miall et al. 1993; Wolpert et al. 1992). In both self-selected and fast speed conditions, the weak correlations observed between the peak velocity and the constant error suggested that the young adults used adjustments to reach the end-point position (Messier and Kalaska 1999). However the number of zero-crossings in the acceleration profile of the self-selected and fast speed conditions (2.7 ± 0.7 and 1.4 ± 0.3, respectively) showed that young adults did not use sub-movements. Together, these results suggested that the movement trajectory of young adults was adjusted by a continuous model of control.
The correlation between the peak velocity and the constant error was also weak for the older adults in the self-selected speed condition. This result suggested that the older adults used adjustments to reach the end-point position (Messier and Kalaska 1999). Concurrently to this weak correlation, the older adults showed secondary sub-movements as evidenced by the number of zero-crossings in the acceleration profile (7.9 ± 1.9) (hypothesis 3). The sub-movements observed in the older adults were consistent with previous results in aiming arm movements (Yan et al. 2000). Together, these results suggested that the movement trajectory of older adults was adjusted by an intermittent model of control. Conversely, the strong correlation observed between the peak velocity and the constant error in the fast condition for the older adults suggested that there was no adjustment in this condition (Messier and Kalaska 1999). Results suggested, in the fast condition, that the continuous and intermittent modes of control failed to correct the trajectory errors. If the temporal constraint may have prevented the intermittent control to adjust the trajectory through a sub-movement, there is no explanation for the absence of continuous corrections except the absence of a continuous mode of control. The internal models theory may explain this potential absence of a continuous control in older adults.
Models of control and internal models
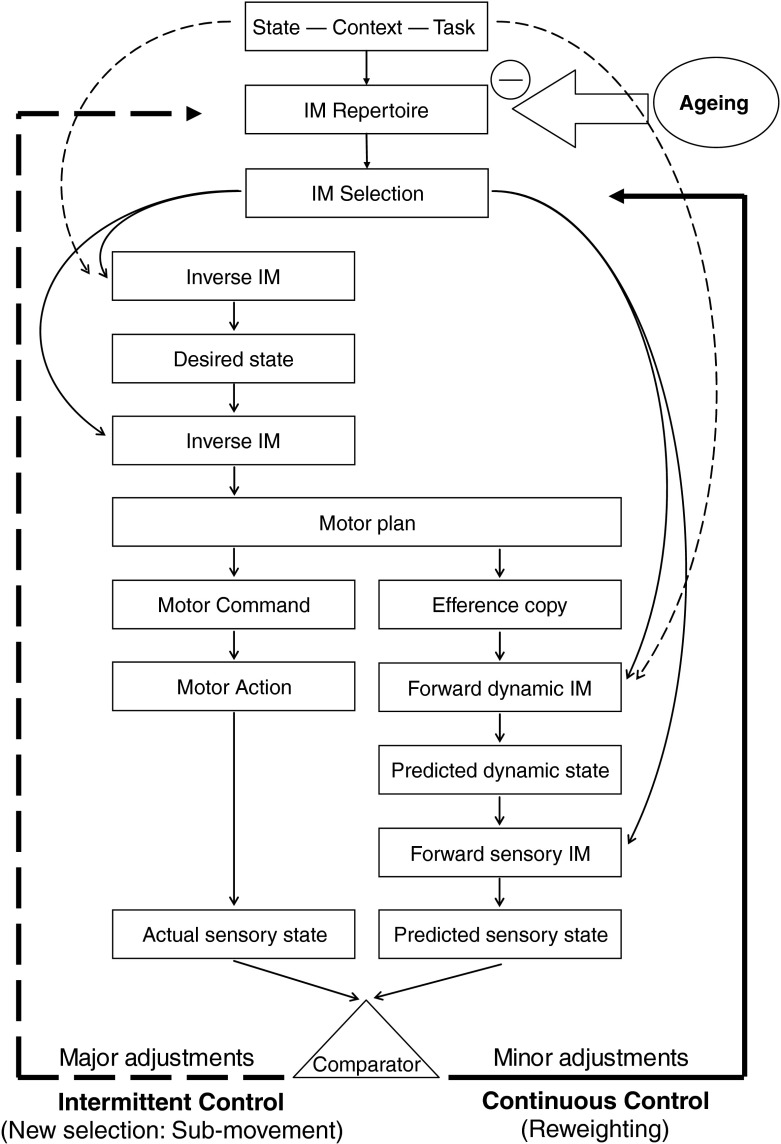
Both continuous and intermittent models acknowledge the necessity of internal models to control human movements. The basic idea of the theory of multiple internal models (Wolpert and Kawato 1998) is that a set of multiple pairs of corresponding forward and inverse models is selected to control a given motor behaviour, a greater responsibility being attributed to pairs exhibiting low prediction errors and therefore best capturing the current dynamics of the system. The pairs of internal models used for a single movement differ from each other on the basis of the specific experience they account for. Therefore, to control the movement of a joint, a single pair of internal models would provide the system with a weaker prediction than the one resulting from the weighting of multiple pairs. The exact number of pairs which are selected to control a movement would depend on the participant’s sensory–motor experience. As suggested by the present results, both types of models of control were consistent with the multiple internal models theory (Fig. 7).
Fig. 7.
Algorithm that describes continuous (bold solid arrow) and intermittent (bold hatched arrow) motor control processes. On the basis of previous experience, the central nervous system selects internal models (IM) that will be responsible for the task to be performed. Depending on the necessity for making minor or major adjustments on the initial trajectory, the central nervous system either reweights the responsibilities between the initially selected models (continuous control) or perform a new selection of models (intermittent control), respectively. Ageing reduces the variety of internal models available in the repertoire
The continuous control observed in young adults could result from a reweighting process within a single selection of pairs of internal models. This reweighting would result in a corrected predictor which prevents the movement trajectory to reach the error threshold that requires a new selection of internal models to be corrected. This new selection of internal models would imply a discontinuity in the control process, i.e. a sub-movement.
The switch from a continuous to an intermittent mode of control observed in the older adults could be explained by an age-related decreased variety of internal models rather than by an age-related degradation of internal models. Indeed, for the older adults in the fast speed condition, degradation of the internal models would have resulted in a degraded control, which was not the case as there was no control at all. Potential reasons for the impoverishment of the older adults' repertoire of internal models are discussed in the following sub-section. In this context of impoverished repertoire, older adults no longer had the specific set of internal models which fit to the desired movement. Therefore, the only way to control the system was to successively use multiple sets of internal models resulting in an increased number of sub-movements. The limit of this intermittent control was evidenced by older adults' performance in the fast speed condition. When there was not enough time to perform a sub-movement, the movement was not adjusted and the end-point position was the result of the initial set of internal models' selection, only.
Origins of age-related alteration of the system of internal models
Taken together, the results of the present and previous studies showed that the older adults' proprioceptive control of movement was different from the young ones and suggested that the system of internal models related to this control was altered in physiological adult ageing. Multiple and non-exclusive hypotheses can explain this system’s alteration. Knowing the body state, for example the position and velocity of a body segment relative to other segments and to the environment, is fundamental for an efficient proprioceptive control. This knowledge is based on two types of information: one issued from sensory signals and the other one from internal models’ prediction when the movement has already begun. With regard to the sensory signals, age-related alterations in muscle, joint and skin receptors are well documented (e.g. Bolton et al. 1966; Salo and Tatton 1993; Swash and Fox 1972). These changes lead to a decreased resolution of the sensory inputs coming from these proprioceptive receptors (Levin and Benton 1973; Miwa et al. 1995). Additionally, a deficient neuromodulation is suspected to cause less distinct internal representations (Li and Sikstrom 2002). Age-related alterations of internal representations of space and body position are presumably a direct consequence of these decreased resolution of sensory inputs and distinctiveness of internal representations (Barbieri et al. 2010; Ghafouri and Lestienne 2000; Lepelley et al. 2010). As a consequence of these decreases, inverse models and paired forward models become impoverished. The increased number of sub-movements observed for older adults in the self-selected speed condition could be a consequence of this decrease variety of paired internal models. Additionally, the age-related reorganisation of motor units (Erim et al. 1999) and increasing muscular noise (Enoka et al. 2003) may alter the ability of the central nervous system to finely mimic the direct relationship between motor commands and their consequences. Finally, as sensory reweighting has shown to be altered with ageing (Eikema et al. 2012; Speers et al. 2002), it could be extrapolated that reweighting of responsibilities between the pairs of internal models would also be altered in adult ageing. Such an alteration could partly explain the observed shift from a continuous mode of control in young adults toward an intermittent one in older adults.
Frailty in the proprioceptive control of movement
At a more general level, the suggested decrease in pairs of internal models in older adults would be consistent with the perspective of Thaler (2002) which proposed that physiological ageing is associated with a decline in the variety of available system states and responses. This decline does not necessarily result in a degraded end-point performance in older adults as compared to young ones. Indeed, recent studies showed that older adults could reach levels of motor performance comparable to those obtained in young adults. However, to reach such levels of performance, older adults had to recruit additional attentional and cortical resources which could be interpreted as a compensating mechanism (Boisgontier et al. 2012; Goble et al. 2010a, b; Heuninckx et al. 2004, 2005, 2008; Hutchinson et al. 2002; Mattay et al. 2002; Naccarato et al. 2006). This additional recruitment reduced the remaining attentional and cortical resources as evidenced by a degraded behavioural performance in older adults when the task complexity increased (Boisgontier et al. 2012; Goble et al. 2012b; Heuninckx et al. 2004; Seidler et al. 2002). The present study is in line with previous ones supporting the idea that motor control is also affected by the frailty syndrome, i.e. a decreased resistance to stressors such as time constraint, which characterises older adults (Fried et al. 2001).
Conclusion
Since comparison between actual and predicted states of the system could not be performed by the central nervous system in the motor imagery studies, the increased time required to perform the movement observed in older adults as compared to young ones supported an age-related alteration of the inverse models (e.g. Personnier et al. 2008; Skoura et al. 2005). However, the absence of end-point differences in goal-directed aiming movements to visual target (e.g. Welsh et al. 2007; Rey-Robert et al. 2012) suggested that the presumed alteration of inverse internal models could be compensated for through online adjustments of the trajectory. However, due to the visual knowledge of the result, these adjustments could have also been the consequence of a learning effect. The similar level of proprioceptive control performance observed in young and older adults in the present and previous studies confirmed that these adjustments were not related to a learning effect (e.g. Boisgontier et al. 2012; Goble et al. 2012a, b; Heuninckx et al. 2008) but to a compensation from the system of control.
In the present study, the contralateral matching task was used as a goal-directed aiming movement task, the reference foot being seen as a proprioceptive target. This paradigm allowed elimination of visual feedback and sensory reweighting which were potential confounders of age-related alterations in the proprioceptive control of movement. For the first time, this study evidenced that proprioceptive-based movements of older adults were controlled differently than young adults' equivalent movements, that is, intermittently vs. continuously, respectively. We proposed to explain these results in light of the multiple internal models theory. Specifically, we proposed that the continuous control of movement was performed through reweighting of responsibilities within a single set of internal models whereas the intermittent control of movement was performed through the selection of new sets of internal models resulting in additional sub-movements.
The necessity for an age-related switch from a continuous to an intermittent mode of control could be explained by an age-related decrease of internal models' variety. Indeed, the results of the present study suggested that ageing does not specifically affect inverse models, forward models or responsibility generators. As the human system ages, some of its sensory receptors become deficient (e.g. Kararizou et al. 2005; Liu et al. 2005) or disappear (e.g. Morisawa 1998). As a result, the ability to perceive different sensations decreases, which also induces a loss of variety in the repertoire of responses. The age-related decrease of the variety of sensations and responses may directly impact the variety of internal models which are based on sensori-motor experience (Wolpert and Kawato 1998). This impoverished repertoire of internal models prevents the system from performing fine adjustments through a continuous mode of control. As a result, in order to still be able to control the movement, the system needs to switch toward an intermittent mode of control.
Obviously, the present study did not totally unmask all the facets of motor control and internal models of ageing. However, the original way the matching task has been used and in which the results have been analysed provided interesting information for better understanding the ageing process of the proprioceptive control of movement and its inseparable internal models.
Acknowledgments
The study was supported by the Cluster Handicap Vieillissement Neurosciences of the Région Rhône-Alpes, France. We thank the anonymous reviewers for their stimulating comments about the present work and Professor Alain Franco for his valuable advice.
References
- Adamo DE, Alexander NB, Brown SH. The influence of age and physical activity on upper limb proprioceptive ability. J Aging Phys Act. 2009;17:272–293. doi: 10.1123/japa.17.3.272. [DOI] [PubMed] [Google Scholar]
- Adamo DE, Martin BJ, Brown SH. Age-related differences in upper limb proprioceptive acuity. Percept Mot Skills. 2007;104:1297–1309. doi: 10.2466/pms.104.4.1297-1309. [DOI] [PubMed] [Google Scholar]
- Asai Y, Tasaka Y, Nomura K, Nomura T, Casadio M, Morasso P. A model of postural control in quiet standing: robust compensation of delay-induced instability using intermittent activation of feedback control. PLoS One. 2009;4:e6169. doi: 10.1371/journal.pone.0006169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkeson CG. Learning arm kinematics and dynamics. Annu Rev Neurosci. 1989;12:157–183. doi: 10.1146/annurev.ne.12.030189.001105. [DOI] [PubMed] [Google Scholar]
- Barbieri G, Gissot AS, Perennou D. Ageing of the postural vertical. Age. 2010;32:51–60. doi: 10.1007/s11357-009-9112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard C, Turrell Y, Fleury M, Teasdale N, Lamarre Y, Martin O. Deafferentation and pointing with visual double-step perturbations. Exp Brain Res. 1999;125:410–416. doi: 10.1007/s002210050697. [DOI] [PubMed] [Google Scholar]
- Batavia M, Gianutsos JG, Ling W, Nelson AJ. The effects of circumferential wrist pressure on reproduction accuracy of wrist placement in healthy young and elderly adults. J Gerontol A Biol Sci Med Sci. 1999;54:M177–183. doi: 10.1093/gerona/54.4.M177. [DOI] [PubMed] [Google Scholar]
- Bock O. Components of sensorimotor adaptation in young and elderly subjects. Exp Brain Res. 2005;160:259–263. doi: 10.1007/s00221-004-2133-5. [DOI] [PubMed] [Google Scholar]
- Boisgontier MP, Olivier I, Chenu O, Nougier V (2012) Presbypropria: the effects of physiological ageing on proprioceptive control. Age. doi:10.1007/s11357-011-9300-y [DOI] [PMC free article] [PubMed]
- Bolton CF, Winkelmann RK, Dyck PJ. A quantitative study of Meissner's corpuscles in man. Neurology. 1966;16:1–9. doi: 10.1212/WNL.16.1.1. [DOI] [PubMed] [Google Scholar]
- Branch Coslett H, Buxbaum LJ, Schwoebel J. Accurate reaching after active but not passive movements of the hand: evidence for forward modeling. Behav Neurol. 2008;19:117–125. doi: 10.1155/2008/972542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learn Mem. 2003;10:55–63. doi: 10.1101/lm.50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, De Luca CJ, Burrows A, Lipsitz LA. Age-related changes in open-loop and closed-loop postural control mechanisms. Exp Brain Res. 1995;104:480–492. doi: 10.1007/BF00231982. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Brown SH, Cunningham DA (1989) Kinematics of arm movements in elderly humans. Neurobiol Aging 10:159–165 [DOI] [PubMed]
- Cooke JD, Diggles VA. Rapid error correction during human arm movements: evidence for central monitoring. J Mot Behav. 1984;16:348–363. doi: 10.1080/00222895.1984.10735326. [DOI] [PubMed] [Google Scholar]
- Darling WG, Cooke JD, Brown SH. Control of simple arm movements in elderly humans. Neurobiol Aging. 1989;10:149–157. doi: 10.1016/0197-4580(89)90024-9. [DOI] [PubMed] [Google Scholar]
- Dean P, Porrill J, Ekerot CF, Jörntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;11:30–43. doi: 10.1038/nrn2756. [DOI] [PubMed] [Google Scholar]
- Der G, Deary IJ. Age and sex differences in reaction time in adulthood: results from the United Kingdom Health and Lifestyle Survey. Psychol Aging. 2006;21:62–73. doi: 10.1037/0882-7974.21.1.62. [DOI] [PubMed] [Google Scholar]
- Deshpande N, Connelly DM, Culham EG, Costigan PA. Reliability and validity of ankle proprioceptive measures. Arch Phys Med Rehabil. 2003;84:883–889. doi: 10.1016/S0003-9993(03)00016-9. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci. 1999;2:563–567. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Eikema DJ, Hatzitaki V, Tzovaras D, Papaxanthis C (2012) Age-dependent modulation of sensory reweighting for controlling posture in a dynamic virtual environment. Age. doi:101007/s11357-011-9310-9 [DOI] [PMC free article] [PubMed]
- Elliott D. The influence of visual target and limb information on manual aiming. Can J Psychol. 1988;42:57–68. doi: 10.1037/h0084172. [DOI] [PubMed] [Google Scholar]
- Elliott D, Carson RG, Goodman D, Chua R. Discrete vs continuous visual control of manual aiming. Hum Mov Sci. 1991;10:393–418. doi: 10.1016/0167-9457(91)90013-N. [DOI] [Google Scholar]
- Elliott D, Hansen S. Visual regulation of manual aiming: a comparison of methods. Behav Res Methods. 2010;42:1087–1095. doi: 10.3758/BRM.42.4.1087. [DOI] [PubMed] [Google Scholar]
- Elliott D, Madalena J. The influence of premovement visual information on manual aiming. Q J Exp Psychol A. 1987;39:541–559. doi: 10.1080/14640748708401802. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13:1–12. doi: 10.1016/S1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- Erim Z, Beg MF, Burke DT, de Luca CJ. Effects of aging on motor-unit control properties. J Neurophysiol. 1999;82:2081–2091. doi: 10.1152/jn.1999.82.5.2081. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Paillard J, Jeannerod M. The role of proprioception in action recognition. Conscious Cogn. 2003;12:609–619. doi: 10.1016/S1053-8100(03)00047-3. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci. 1997;17:1519–1528. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Gawthrop P, Loram I, Lakie M, Gollee H. Intermittent control: a computational theory of human control. Biol Cybern. 2011;104:31–51. doi: 10.1007/s00422-010-0416-4. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Ghafouri M, Lestienne FG. Altered representation of peripersonal space in the elderly human subject: a sensorimotor approach. Neurosci Lett. 2000;289:193–196. doi: 10.1016/S0304-3940(00)01280-5. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Task-dependent asymmetries in the utilization of proprioceptive feedback for goal-directed movement. Exp Brain Res. 2007;180:693–704. doi: 10.1007/s00221-007-0890-7. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, De Vos J, Wenderoth N, Swinnen SP. The neural control of bimanual movements in the elderly: brain regions exhibiting age-related increases in activity, frequency-induced neural modulation, and task-specific compensatory recruitment. Hum Brain Mapp. 2010;31:1281–1295. doi: 10.1002/hbm.20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Doumas M, Wenderoth N, Swinnen SP. Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. J Neurosci. 2011;31:16344–16352. doi: 10.1523/JNEUROSCI.4159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Van Hecke W, Sunaert S, Wenderoth N, Swinnen SP. The neural basis of central proprioceptive processing in older versus younger adults: an important sensory role for right putamen. Hum Brain Mapp. 2012;33:895–908. doi: 10.1002/hbm.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Mousigian MA, Brown SH. Compromised encoding of proprioceptively determined joint angles in older adults: the role of working memory and attentional load. Exp Brain Res. 2012;216:35–40. doi: 10.1007/s00221-011-2904-8. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Noble BC, Brown SH. Where was my arm again? Memory-based matching of proprioceptive targets is enhanced by increased target presentation time. Neurosci Lett. 2010;481:54–58. doi: 10.1016/j.neulet.2010.06.053. [DOI] [PubMed] [Google Scholar]
- Goggin NL, Meeuwsen HJ. Age-related differences in the control of spatial aiming movements. Res Q Exerc Sport. 1992;63:366–372. doi: 10.1080/02701367.1992.10608758. [DOI] [PubMed] [Google Scholar]
- Goggin NL, Stelmach GE. Age-related differences in a kinematic analysis of precued movements. Can J Aging. 1990;9:371–385. doi: 10.1017/S0714980800007480. [DOI] [Google Scholar]
- Hanneton S, Berthoz A, Droulez J, Slotine JJE. Does the brain use sliding variables for the control of movements? Biol Cybern. 1997;77:381–393. doi: 10.1007/s004220050398. [DOI] [PubMed] [Google Scholar]
- Hansen S, Elliott D. Three-dimensional manual responses to unexpected target perturbations during rapid aiming. J Mot Behav. 2009;41:16–29. doi: 10.1080/00222895.2009.10125917. [DOI] [PubMed] [Google Scholar]
- Hegele M, Heuer H. Adaptation to a direction-dependent visuomotor gain in the young and elderly. Psychol Res. 2010;74:21–34. doi: 10.1007/s00426-008-0221-z. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Debaere F, Wenderoth N, Verschueren S, Swinnen SP. Ipsilateral coordination deficits and central processing requirements associated with coordination as a function of aging. J Gerontol B Psychol Sci Soc Sci. 2004;59:P225–232. doi: 10.1093/geronb/59.5.P225. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005;25:6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson S, Kobayashi M, Horkan CM, Pascual-Leone A, Alexander MP, Schlaug G. Age-related differences in movement representation. Neuroimage. 2002;17:1720–1728. doi: 10.1006/nimg.2002.1309. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14:S103–109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Jordan MI, Rumelhart DE. Forward models: supervised learning with a distal teacher. Cogn Sci. 1992;16:307–354. doi: 10.1207/s15516709cog1603_1. [DOI] [Google Scholar]
- Kararizou E, Manta P, Kalfakis N, Vassilopoulos D. Morphometric study of the human muscle spindle. Anal Quant Cytol Histol. 2005;27:1–4. [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/S0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Kawato M, Furukawa K, Suzuki R. A hierarchical neural-network model for control and learning of voluntary movement. Biol Cybern. 1987;57:169–185. doi: 10.1007/BF00364149. [DOI] [PubMed] [Google Scholar]
- Keele SW. Movement control in skilled motor performance. Psych Bull. 1968;70:387–403. doi: 10.1037/h0026739. [DOI] [Google Scholar]
- Ketcham CJ, Seidler RD, Van Gemmert AW, Stelmach GE. Age-related kinematic differences as influenced by task difficulty, target size, and movement amplitude. J Gerontol B Psychol Sci Soc Sci. 2002;57:P54–P64. doi: 10.1093/geronb/57.1.P54. [DOI] [PubMed] [Google Scholar]
- Kleinman D. Optimal control of linear systems with time-delay and observation noise. IEEE Trans Autom Control. 1969;14:524–527. doi: 10.1109/TAC.1969.1099242. [DOI] [Google Scholar]
- Lepelley MC, Thullier F, Bolmont B, Lestienne FG. Age-related differences in sensorimotor representation of space in drawing by hand. Clin Neurophysiol. 2010;121:1890–1897. doi: 10.1016/j.clinph.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Levin HS, Benton AL. Age effects in proprioceptive feedback performance. Gerontol Clin. 1973;15:161–169. doi: 10.1159/000245447. [DOI] [PubMed] [Google Scholar]
- Li SC, Sikstrom S. Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neurosci Biobehav Rev. 2002;26:795–808. doi: 10.1016/S0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- Liu JX, Eriksson PO, Thornell LE, Pedrosa-Domellöf F. Fiber content and myosin heavy chain composition of muscle spindles in aged human biceps brachii. J Histochem Cytochem. 2005;53:445–454. doi: 10.1369/jhc.4A6257.2005. [DOI] [PubMed] [Google Scholar]
- Loram ID, Gollee H, Lakie M, Gawthrop PJ. Human control of an inverted pendulum: is continuous control necessary? Is intermittent control effective? Is intermittent control physiological? J Physiol. 2011;589:307–324. doi: 10.1113/jphysiol.2010.194712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J, Elliott D, Swanson LR, Chua R. The use of vision and manual aiming by young and older adults. J Aging Phys Act. 1996;4:165–178. [Google Scholar]
- Marks R. Further evidence of impaired position sense in knee osteoarthritis. Physiother Res Int. 1996;1:127–136. doi: 10.1002/pri.6120010208. [DOI] [PubMed] [Google Scholar]
- Marshall J. The effect of ageing upon physiological tremor. J Neurol Neurosurg Psychiatry. 1961;24:14–17. doi: 10.1136/jnnp.24.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/WNL.58.4.630. [DOI] [PubMed] [Google Scholar]
- Meeuwsen HJ, Sawicki TM, Stelmach GE. Improved foot position sense as a result of repetitions in older adults. J Gerontol. 1993;48:P137–P141. doi: 10.1093/geronj/48.3.P137. [DOI] [PubMed] [Google Scholar]
- Messier J, Kalaska JF. Comparison of variability of initial kinematics and endpoints of reaching movements. Exp Brain Res. 1999;125:139–152. doi: 10.1007/s002210050669. [DOI] [PubMed] [Google Scholar]
- Miall RC, King D. State estimation in the cerebellum. Cerebellum. 2008;7:572–576. doi: 10.1007/s12311-008-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Intermittency in human manual tracking tasks. J Mot Behav. 1993;25:53–63. doi: 10.1080/00222895.1993.9941639. [DOI] [PubMed] [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/S0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Miwa T, Miwa Y, Kanda K. Dynamic and static sensitivities of muscle spindle primary endings in aged rats to ramp stretch. Neurosci Lett. 1995;201:179–182. doi: 10.1016/0304-3940(95)12165-X. [DOI] [PubMed] [Google Scholar]
- Morisawa Y. Morphological study of mechanoreceptors on the coracoacromial ligament. J Orthop Sci. 1998;3:102–110. doi: 10.1007/s007760050029. [DOI] [PubMed] [Google Scholar]
- Murrell KFH, Entwisle DG. Age differences in movement pattern. Nature. 1960;185:948–949. doi: 10.1038/185948a0. [DOI] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage. 2006;32:1250–1256. doi: 10.1016/j.neuroimage.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Personnier P, Ballay Y, Papaxanthis C. Mentally represented motor actions in normal aging: III. Electromyographic features of imagined arm movements. Behav Brain Res. 2010;206:184–191. doi: 10.1016/j.bbr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Personnier P, Kubicki A, Laroche D, Papaxanthis C. Temporal features of imagined locomotion in normal aging. Neurosci Lett. 2008;476:146–149. doi: 10.1016/j.neulet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Peters M. Footedness: asymmetries in foot preference and skill and neuropsychological assessment of foot movement. Psychol Bull. 1988;103:179–192. doi: 10.1037/0033-2909.103.2.179. [DOI] [PubMed] [Google Scholar]
- Pickard CM, Sullivan PE, Allison GT, Singer KP. Is there a difference in hip joint position sense between young and older groups? J Gerontol A Biol Sci Med Sci. 2003;58:631–635. doi: 10.1093/gerona/58.7.M631. [DOI] [PubMed] [Google Scholar]
- Pratt J, Chasteen AL, Abrams RA. Rapid aimed limb movements: age differences and practice effects in component submovements. Psychol Aging. 1994;9:325–334. doi: 10.1037/0882-7974.9.2.325. [DOI] [PubMed] [Google Scholar]
- Rey-Robert B, Temprado JJ, Lemaire P, Berton E. Combining movement kinematics, efficiency functions, and Brinley plots to study age-related slowing of sensorimotor processes: insights from Fitts' task. Gerontology. 2012;58:171–180. doi: 10.1159/000329347. [DOI] [PubMed] [Google Scholar]
- Salo PT, Tatton WG. Age-related loss of knee joint afferents in mice. J Neurosci Res. 1993;35:664–677. doi: 10.1002/jnr.490350609. [DOI] [PubMed] [Google Scholar]
- Saltzman E. Levels of sensorimotor representation. J Math Psychol. 1979;20:97–163. doi: 10.1016/0022-2496(79)90020-8. [DOI] [Google Scholar]
- Scarchilli K, Vercher JL. The oculomanual coordination control center takes into account the mechanical properties of the arm. Exp Brain Res. 1999;124:42–52. doi: 10.1007/s002210050598. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. Motor control and learning: a behavioral emphasis. Champaign: Human Kinetics; 2005. [Google Scholar]
- Seidler RD, Alberts JL, Stelmach GE. Changes in multi-joint performance with age. Motor Control. 2002;6:19–31. doi: 10.1123/mcj.6.1.19. [DOI] [PubMed] [Google Scholar]
- Seidler-Dobrin RD, Stelmach GE. Persistence in visual feedback control by the elderly. Exp Brain Res. 1998;119:467–474. doi: 10.1007/s002210050362. [DOI] [PubMed] [Google Scholar]
- Skoura X, Papaxanthis C, Vinter A, Pozzo T. Mentally represented motor actions in normal aging. I. Age effects on the temporal features of overt and covert execution of actions. Behav Brain Res. 2005;165:229–239. doi: 10.1016/j.bbr.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Speers RA, Kuo AD, Horak FB. Contributions of altered sensation and feedback responses to changes in coordination of postural control due to aging. Gait Posture. 2002;16:20–30. doi: 10.1016/S0966-6362(02)00003-6. [DOI] [PubMed] [Google Scholar]
- Swash M, Fox KP. The effect of age on human skeletal muscle. Studies of the morphology and innervation of muscle spindles. J Neurol Sci. 1972;16:417–432. doi: 10.1016/0022-510X(72)90048-2. [DOI] [PubMed] [Google Scholar]
- Thaler DS. Design for an aging brain. Neurobiol Aging. 2002;23:13–15. doi: 10.1016/S0197-4580(01)00262-7. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Franks IM. Preprogramming vs on-line control in simple movement sequences. Acta Psychol. 1991;77:1–19. doi: 10.1016/0001-6918(91)90061-4. [DOI] [PubMed] [Google Scholar]
- van Sonderen JF, Gielen CC, Denier van der Gon JJ. Motor programmes for goal-directed movements are continuously adjusted according to changes in target location. Exp Brain Res. 1989;78:139–146. doi: 10.1007/BF00230693. [DOI] [PubMed] [Google Scholar]
- von Holst E. Relations between the central nervous system and the peripheral organs. Br J Anim Behav. 1954;2:89–94. doi: 10.1016/S0950-5601(54)80044-X. [DOI] [Google Scholar]
- Welford AT, Norris AH, Shock NW. Speed and accuracy of movement and their changes with age. Acta Psychol. 1969;30:3–15. doi: 10.1016/0001-6918(69)90034-1. [DOI] [PubMed] [Google Scholar]
- Welsh TN, Higgins L, Elliott D. Are there age-related differences in learning to optimize speed, accuracy, and energy expenditure? Hum Mov Sci. 2007;26:892–912. doi: 10.1016/j.humov.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Whitney DE. The mathematics of coordinated control of prosthetic arms and manipulators. J Dyn Syst Meas Control. 1972;94:303–309. doi: 10.1115/1.3426611. [DOI] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3(Suppl):1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Kawato M. Multiple paired forward and inverse models for motor control. Neural Netw. 1998;11:1317–1329. doi: 10.1016/S0893-6080(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/S1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Winter JL, Stein JF. Evidence for an error deadzone in compensatory tracking. J Mot Behav. 1992;24:299–308. doi: 10.1080/00222895.1992.9941626. [DOI] [PubMed] [Google Scholar]
- Yan JH, Thomas JR, Stelmach GE, Thomas KT. Developmental features of rapid aiming arm movements across the lifespan. J Mot Behav. 2000;32:121–140. doi: 10.1080/00222890009601365. [DOI] [PubMed] [Google Scholar]