Abstract
In order to identify new markers of vascular cell senescence with potential in vivo implications, primary cultured endothelial cells, including human umbilical vein endothelial cells (HUVECs), human aortic endothelial cells (HAECs), human coronary artery endothelial cells (HCAECs) and ex vivo circulating angiogenic cells (CACs), were analysed for microRNA (miR) expression. Among the 367 profiled miRs in HUVECs, miR-146a, miR-9, miR-204 and miR-367 showed the highest up-regulation in senescent cells. Their predicted target genes belong to nine common pathways, including Toll-like receptor signalling (TLR) that plays a pivotal role in inflammatory response, a key feature of senescence (inflammaging). MiR-146a was the most up-regulated miR in the validation analysis (>10-fold). Mimic and antagomir transfection confirmed TLR’s IL-1 receptor-associated kinase (IRAK1) protein modulation in both young and senescent cells. Significant correlations were observed among miR-146a expression and β-galactosidase expression, telomere length and telomerase activity. MiR-146a hyper-expression was also validated in senescent HAECs (>4-fold) and HCAECs (>30-fold). We recently showed that CACs from patients with chronic heart failure (CHF) presented a distinguishing feature of senescence. Therefore, we also included miR-146a expression determination in CACs from 37 CHF patients and 35 healthy control subjects (CTR) for this study. Interestingly, a 1,000-fold increased expression of miR-146a was observed in CACs of CHF patients compared to CTR, along with decreased expression of IRAK1 protein. Moreover, significant correlations among miR-146a expression, telomere length and telomerase activity were observed. Overall, our findings indicate that miR-146a is a marker of a senescence-associated pro-inflammatory status in vascular remodelling cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-012-9440-8) contains supplementary material, which is available to authorized users.
Keywords: Vascular senescence, MiR-146a, Circulating angiogenic cells, Congestive heart failure, Toll-like receptor pathway
Introduction
Multiple factors, including vascular cell senescence, may contribute to impaired endothelial functions in elderly people (Kovacic et al. 2011). The endothelial senescence status induced by ageing appears to be accelerated in the presence of pro-inflammatory age-related pathological conditions, including coronary artery disease and chronic heart failure (CHF) (Minamino et al. 2002; Minamino and Komuro 2007). Although specific pathways of vascular cell senescence have been identified, a complete understanding of this intricate process is still limited (Passos et al. 2009). Senescent endothelial cells are characterised by telomere shortening which occurs as a consequence of cellular replication and can be accelerated by harmful environmental factors, such as oxidative stress (Voghel et al. 2007). When telomeres reach a critical threshold, endothelial cells become dysfunctional with an increased pro-inflammatory factors expression, resulting in a profile defined as ‘senescence-associated secretory phenotype’ (SASP) (Donato et al. 2008; Sikora et al. 2011; Saliques et al. 2010). Secreted pro-inflammatory proteins acting in both autocrine and paracrine ways contribute to the senescent phenotype (Passos et al. 2009). The acquisition of SASP reduces endothelial regenerative properties which in turn contribute to the development of pro-inflammatory pathological conditions (Freund et al. 2010; Testa et al. 2011; Olivieri et al. 2009, 2012). Although the evidence of endothelial cell senescence in the vasculature is gaining increasing recognition, the clinical relevance of this process is currently hampered by the unavailability of non-invasive techniques and biomarkers assessing endothelial cell senescence in vivo. Thus, the identification of new cellular and/or circulating markers of vascular senescence may hold important clinical relevance.
MicroRNAs (miRs) are regulatory elements influencing gene expression throughout the lifetime (Liang et al. 2009; Martinez et al. 2011). Several independent lines of evidence have indicated a critical role for miRs, as biomarkers of cellular senescence, in both replicative and stress-induced modulation of in vitro cellular senescence (Lafferty-Whyte et al. 2009; Poliseno et al. 2008; Wagner et al. 2008; Li et al. 2009, 2010; Olivieri et al. 2009). Human umbilical vein endothelial cells (HUVECs) have been extensively used as an in vitro endothelial model representing common functional and morphological features of the in vivo endothelial cells (Unterluggauer et al. 2007). This model was recently used to identify miRs and their relative target proteins associated with replicative and/or stress-induced senescence (Ito et al. 2010; Menghini et al. 2009; Hackl et al. 2010; Vasa-Nicotera et al. 2011; Magenta et al. 2011). However, these recent reports have not been conclusive on identifying specific miRs as markers of vascular ageing-associated dysfunction in vivo.
The present research focused on the identification of miRs associated with senescence status in vascular remodelling cells. For this purpose, we identified specific miRs strongly associated with the senescent phenotype in different in vitro cultured endothelial cells, such as HUVECs, human aortic endothelial cells (HAECs) and human coronary artery endothelial cells (HCAECs).
Furthermore, since in vivo data on the role of senescence-associated miRs in vascular remodelling are still scarce, a vascular progenitor cell subpopulation of circulating angiogenic cells (CACs) was analysed. Even if CACs derive from the monocyte–macrophage lineage and are characterised by a reduced capacity to form blood vessels in vivo, they contribute to vascular homeostasis by secreting angiogenic growth factors in vascular injury sites (Rehman et al. 2003). Thus, CACs are ‘cells involved in vascular remodelling’ easily obtainable from peripheral blood. Since we previously demonstrated that CACs from CHF patients showed the distinguishing feature of senescence, such as telomere attrition, reduced telomerase activity and increased pro-inflammatory status, compared to CACs from healthy control subjects (CTR), we used the same study population to validate the expression levels of miRs associated with senescent phenotype of cultured endothelial cells (Olivieri et al. 2012).
Experimental procedures
Cell culture
HUVECs, HAECs and HCAECs were purchased from Clonetics Corporation (Lonza, Basel, Switzerland) and cultured in endothelial growth medium EGM-2 (Lonza) (see Supplementary material and method). Endothelial cell replicative senescence was studied by subjecting endothelial cells to subsequent passages until XIII, as previously described (Haendeler et al. 2004). Cumulative population doubling (CPD) was calculated as the sum of all the changes in PD (Supplementary material and method). SA-β-gal activity was assessed as reported in Supplementary material and method.
Cytokines production
The cell supernatants were collected at the end of each passage before tripsinisation, centrifuged and stored at −20°C until the assays. Interleukin (IL)-1β, IL-1α, IL-2, IL-6, IL-8, IL-10, IL-12, tumour necrosis factor (TNF)-a, interferon (INF)-γ and myeloperoxidase (MPO) concentration were measured by means of commercially available high-sensitivity enzyme-linked immunosorbent assay kits (Search Light, Thermo Scientific, Rockford, IL, USA).
Telomere length determination
Telomere length analysis was performed by using both the traditional absolute quantification method (TRF) and relative telomere length analysis (assessed as T/S ratio, telomeric template (T) vs. a single gene copy (S)) based on quantitative polymerase chain reaction (PCR) Cawthon’s method (Cawthon 2002). Detailed methods were reported in Supplementary material and method.
Telomerase (TERT) activity assay
Quantitative determination of telomerase activity was performed using a validated quantitative telomerase detection kit (Allied Biotech, Inc., Ijamsville, MD, USA) (high-sensitive real-time quantitative Telomeric Repeat Amplification Protocol) according to the manufacturer’s protocol (Wege et al. 2003). Detailed methods are reported in Supplementary material and method.
H2O2 exposure
HUVEC cells were seeded at 80 % density and cultured for 12 h, then H2O2 200 or 400 μM was added for 1 h and cells cultured for further 16 h. The optimum concentration of H2O2 was selected according to the results of preliminary experiments performed to establish the cytotoxicity and survival effects of a broad range of concentrations of the reagent. H2O2 was diluted in fresh EGM-2 medium just prior to each experiment. Three independent experiments were performed.
RNA isolation
Total RNA from cells (HUVECs, HCAECs, HAECs and CACs) was isolated using RNA purification kit (Norgen Biotek Corporation, Thorold, ON, Canada) which allows isolation of both enriched miRs and larger RNA species. The RNA was stored at −80°C until use.
Microarray analysis of mature microRNAs
About 150–200 ng of total RNA was converted to cDNA by priming with a mixture of looped primers using the manufacturer’s instructions (MegaPlex kit, Applied Biosystems, Foster City, CA, USA). Pre-amplification was performed using 3 μl of input RNA with PreAmp kit (Applied Biosystems). Nine microlitres of pre-amplified cDNA was used for mature miRs profiling by real-time PCR instrument equipped with a 348-well reaction plate (7900 HT, Applied Biosystems) and human miR Array pool A (Applied Biosystems) containing 367 different human miR assays in addition to selected small nucleolar RNAs.
Quantitative RT-PCR of mature microRNAs
MiRs were quantified using TaqMan MicroRNA Assay (Applied Biosystems) with some modifications. Briefly, total RNA was reverse-transcribed with the TaqMan MicroRNA reverse transcription kit. Five microlitres of RT reactions contained 1 μl of each miR-specific stem-loop primers, 1.67 μl of input RNA, 0.4 μl of 10 mM dNTPs, 0.3 μl of reverse transcriptase, 0.5 μl of 10× buffer, 0.6 μl of RNAse inhibitor diluted 1:10 and 0.5 μl of H2O2. The mixture was incubated at 16°C for 30 min, 42°C for 30 min and 85°C for 5 min. Subsequently, quantitative real-time PCR was performed in 20 μl of PCR reaction containing 1 μl of 20× Taqman miR Assay in which PCR primers and probes (5′-FAM) were contained, 10 μl of 2× TaqMan Universal Master mix no UNG (Applied Biosystems) and 5 μl of RT product. The reaction was first incubated at 95°C for 2 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Data were analysed with real-time PCR OpticonMonitor version 2 (MJ Research, Bio-Rad, Hercules, CA, USA), with the automatic Ct setting for adapting baseline. Detection thresholds were set to 35 Ct. The relative amount of each miRNA was calculated using the comparative threshold (Ct) method with ΔCt = Ct(miRNA) − Ct(RNU44). We used different normalisation methods for arrays and real-time quantitative PCR (RT-qPCR). Relative quantification of miRNA expression was calculated with the 2−ΔΔCt method. In this way, all the reported miR expression values obtained with RT-qPCR are normalised using either RNU44 and RNU48 expression.
Moreover, miR-199b-5p, a stable miR expressed in young and senescent HUVECs, was also used. Independent samples t test was used to determine statistical significance between samples. P values less than 0.05 were considered significant.
Computational prediction of microRNA target genes
In order to increase the efficiency of identifying common mRNA targets to more than one miR and identify new miRs in the senescence pathway, a previously developed computer programme, named SID1.0 (simple String IDentifier), was used (Albertini et al. 2011). This programme is based on the strategy of exhaustive search and specifically designed to screen shared data (target genes, miRs and pathways) available from PicTar and DIANA-MicroT 3.0 databases. For a defined miR name, target genes can be automatically retrieved from the DIANA-MicroT 3.0. The list IDs are indexed using SID1.0 that looks for KEGGs pathway database IDs shared by the predicted miRs of the different datasets. In this way, we were able to obtain the common pathways of specific miRs.
As described by Papadopoulos et al. (2009), the input of DIANA-mirPath is a list of miRs target genes defined in a user-friendly web interface by simply selecting the miR name and, in our case, the target prediction software TargetScan (Lewis et al. 2005). In the DIANA-mirPath output page, all pathways are sorted according to a descending enrichment statistical score (−lnP) along with the number and names of each miR’s target genes involved in each KEGG pathway. The input dataset enrichment in each KEGG pathway is represented by the negative natural logarithm of the P value (−lnP).
Protein extraction and immunoblotting
Cells were washed twice in cold PBS. Total protein was extracted using RIPA buffer (150 mM NaCl, 10 mM Tris, pH 7.2, 0.1 % SDS, 1.0 % Triton X-100, 5 mM EDTA, pH 8.0) containing protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Protein concentration was determined using Bradford reagent (Sigma-Aldrich, Milan, Italy). Total protein extracts (40 μg) were separated by 10 % SDS-PAGE and transferred to PVDF membrane (Bio-Rad). Membranes were incubated overnight with primary antibodies anti-IRAK1 (MBL, International Corporation Inc. Woburn, MA, USA) or anti-TRAF6 (Santa Cruz Biotechnology, Sta. Cruz, CA, USA). Then, they were incubated with a secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. Immunoreactive proteins were visualised using chemiluminescence substrate (ECL Plus, GE Healthcare, Pittsburgh, PA, USA). As endogenous control, the membrane was incubated with anti-β-actin (Santa Cruz Biotechnology).
Cell transfection
Transient transfection of miRs was performed with FuGENE transfection reagent (Roche Applied Science), according to the manufacturer’s instructions. In brief, 8 × 104 cells were plated in six-well plates and kept overnight for attachment. The next day, cells were transfected with miR-146a mimic (146aM), inhibitor (anti-miR-146a) or negative miR control mimic (ConM) (Dharmacon, Inc. Chicago, IL, USA). The FuGENE transfection method was optimised testing different quantities of reagent and miR. In particular, FuGENE (microlitre)/miR (microgram) ratios of 3:1 were found as optimal. It is important to underline that the transfection FuGENE–miR complex was prepared in serum-free medium. Analyses were performed 72 h after transfection.
MiR-146a expression in CACs and plasma obtained from CHF and CTR
CAC isolation and characterisation have been recently reported in Olivieri et al. (2012). Briefly, CACs were isolated from approximately 14 ml of heparinised peripheral blood after density-gradient centrifugation. PBMCs (5 × 106) were plated on 24-well fibronectin-coated plate and maintained in endothelial basal medium supplemented with EGM SingleQuots and 20 % FCS for 4 days. After 4 days in culture, non-adherent cells were removed by PBS wash, while adherent cells were lysed directly in the culture wells. To confirm the CAC phenotype, each sample was tested for the ability to incorporate 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labelled acetylated LDL (DiLDL) and bind endothelial-specific lectins, such as Ulex europaeus agglutinin-1. More than 95 % of adherent cells were bound to UEA-1 and endocytosed DiLDL and consequently regarded as CACs.
Total RNA was extracted from CACs and 100 μl of plasma. MiRs were quantified by RT-qPCR using TaqMan miRNA assays (Applied Biosystems), according to the manufacturer’s protocol. All RT-qPCR data were analysed as unadjusted Ct values and standardised to miR-17, a miR which was previously validated (D’Alessandra et al. 2010) and fulfilled the following criteria: detectable in all samples, low dispersion of expression levels and null association with CHF. The Ct values from RT-qPCR assays greater than 35 were treated as not expressed. MiR relative expression distribution values were calculated as 2−ΔCt, (ΔCt = Ct miR-X − Ct miR-17). MiR relative fold changes were calculated using the 2−ΔΔCt method setting 1 as an arbitrary value for the control group.
Statistical analysis
Statistical analysis of microarray data: miRs expressed at detectable level in more than 80 % of samples were compared based on their relative expression to the overall miR expression on each array, using median normalisation analysis. The ΔΔCT for each miR was defined as the difference of expression between senescent and young cells. It was calculated with the following equation: [(CT senescent miR − median Ct values obtained in the profiling of senescent cells) − (CT young miR − median Ct values obtained in the profiling of young cells)]. A 2-fold or greater difference was considered a significant finding. The mean values were compared either by two-tailed t test or F test, as appropriate.
Results
Characterisation of replicative senescence and cytokine release in HUVEC cells
HUVECs were maintained in culture until growth arrest (XIII passage). Population doubling, senescence-associated β-galactosidase (SA-β-gal) staining, telomere length and telomerase activity indicated a progressive acquisition of senescence status (Supplementary Fig. 1a–d). To verify if HUVECs acquired a SASP during replicative senescence, the release of IL-1β, IL-1α, IL-2, IL-6, IL-8, IL-10, IL-12, TNF-α, INF-γ and MPO were assessed at different passages. Cytokine release was significantly increased in senescent vs. younger HUVECs (F test, P < 0.05 for all comparison) (Supplementary Table 1). All analysed cytokines increased by approximately 25- to 350-fold in senescent compared to young HUVECs (Supplementary Fig. 2 and Supplementary Table 1). Key pro-inflammatory mediators, IL-6 and IL-8, were mostly secreted by both young (II passage) and senescent (XIII passage) HUVEC cells (IL-6 = 3.2 ± 0.13 vs. 80.12 ± 10.5 pg/ml and IL-8 = 3.1 ± 0.18 vs. 100.12 ± 15.68 pg/ml; t test, P < 0.05). Furthermore, MPO release, a marker of oxidative stress, increased in senescent compared to younger HUVECs (0.027 ± 0.003 vs. 0.445 ± 0.014 pg/ml; t test, P < 0.05) (Supplementary Fig. 2 and Table 1).
MicroRNA profile in replicative senescence HUVEC cells
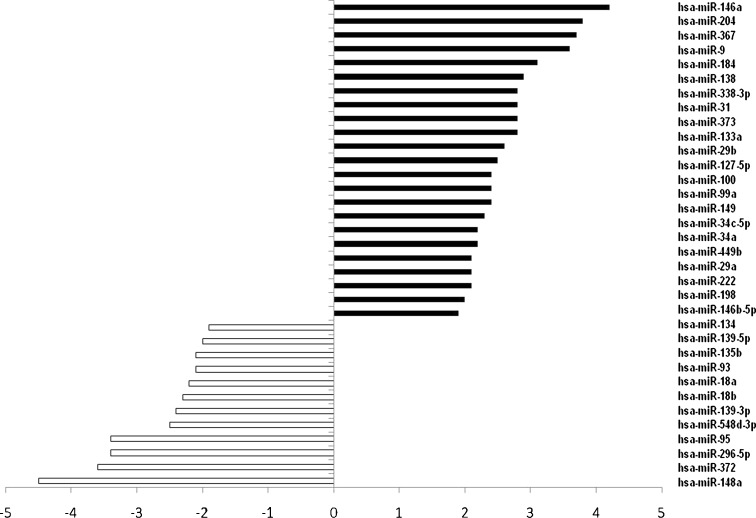
To identify different miR expressions in young and replicative senescent HUVEC cells, 367 human miRs were profiled in cells at the II (young) and XIII (senescent) passages. Up to 265 miRs were expressed at a detectable level in almost all of the samples tested (more than 80 %) and were included in the final analysis. Thirty-four miRs were differentially expressed in senescent compared to young cells. Among them, 23 miRs were up-regulated, while 11 miRs were down-regulated (Fig. 1). Complete profiling results are reported in Supplementary Table 2. Moreover, profiling data have been deposited in NCBI’s Gene Expression Omnibus (Edgar et al. 2002) and are accessible through GEO Series accession number GSE37972 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE37972).
Fig. 1.
MiRs expression profiling in senescent (XIII passage) vs. young HUVECs (II passage). Results were reported as fold change in specific miR expression. Each bar corresponds to the expression fold difference, calculated as ∆∆Ct, of miR listed in the figure. Results were analysed using the ΔΔCt method with normalisation against the median value of each sample. The ∆∆CT value was calculated with the following equation: [(CT senescent miR − median Ct values obtained in the profiling of senescent cells) − (CT young microRNA − median Ct values obtained in the profiling of young cells)]. Data are reported as the mean value (three young vs. three senescent samples) of three independent experiments. A 2-fold or greater difference was considered significant, and only miRs with a ∆∆CT higher than 2 or lower than −2 were reported in the figure
Identification of the common pathways and target genes of up-regulated microRNAs in senescent HUVEC cells
The number of up-regulated miRs in senescent HUVECs was greater than the number of down-regulated ones (Fig. 1). We focused on the former, in order to identify protein targets involved in down-regulated common pathways during the senescence process.
MiR-146a, miR-204, miR-367 and miR-9 were the highest up-regulated miRs in senescent compared to young HUVECs, with an increase of more than 3.5-fold. SID1.0 analysis identified nine pathways common to miR-146a, miR-204, miR-367 and miR-9 (Table 1) with a −ln(P value) higher than 1.5, including Toll-like receptor (TLR) signalling pathway. In Fig. 2, miR-146a, miR-204, miR-367 and miR-9 predicted target genes belonging to the TLR pathway are shown.
Table 1.
Common pathways shared by miR-146a, miR-204, miR-367 and miR-9, the most hyper-expressed miRs in senescent HUVEC cells
| KEGG pathway name | KEGG pathway ID |
|---|---|
| MAPK signalling | hsa04010 |
| Small cell lung cancer | hsa05222 |
| Glycan structures—biosynthesis 1 | hsa01030 |
| Axon guidance | hsa04360 |
| Neuroactive ligand–receptor interaction | hsa04080 |
| Cell adhesion molecules (CAMs) | hsa04514 |
| Erb signalling | hsa04012 |
| Ubiquitin-mediated proteolysis | hsa04120 |
| Toll-like receptor signalling | hsa04620 |
Pathway name and the identification ID used by KEGG database were reported. The database used for this analysis was DIANA-MicroT 3.0
Fig. 2.
Putative mRNA targets of miR-146a (TRAF6; IRAK1; CD80), miR-204 (MAPK1), miR-367 (MP2K4; TRAF3; PIK3R3) and miR-9 (CXL11; MAP2K7; PIK3R3) belong to the Toll-like receptor pathway. Putative mRNAs are marked with red circle. Figure modified from the DIANA-MicroT 3.0 databases (Papadopoulos et al. 2009)
Among deregulated miRs in senescent HUVECs, miRNA-146a was the highest up-regulated miR, showing a −ln(P value) of 4.9 for its predicted target genes: IL-1 receptor-associated kinase (IRAK1), TNF receptor-associated factor 6 (TRAF6) and CD80, all involved in TLR signalling (Table 2). Interestingly, miR-146a is a validated modulator of IRAK1 and TRAF6 (Nahid et al. 2011), which are key proteins involved in cytokine production (Table 2). Thus, we validated IRAK1 and TRAF6 as protein targets of miR-146a in endothelial cells.
Table 2.
List of pathways that includes miR-146a predicted target genes with a −ln(P value) higher than 1.5
| KEGG pathway name | Predicted genes | −ln(P value) |
|---|---|---|
| Toll-like receptor signalling | TRAF6, CD80, IRAK1 | 4.9 |
| SNARE interactions in vesicular transport | GOSR1, STX3 | 4.5 |
| Notch signalling pathway | NUMB, LFNG | 4.3 |
| Colorectal cancer | APPL1, SMAD4 | 1.8 |
| Erb signalling | ABL2, ERBB4 | 1.6 |
Names and −ln(P value) of miR-146a predicted target genes found in each pathway were reported
Validation of microRNA-146a up-regulation in replicative senescent endothelial cells
The hyper-expressed miR-146a, miR-204, miR-367, miR-9 and the hypo-expressed miR-148 were validated with RT-qPCR in senescent cells (data not shown). Moreover, miR-146a expression was also validated by RT-qPCR in HAECs and HCAECs, confirming the up-regulation of this miR in senescent cells compared to the younger ones (Fig. 3). MiR-146a was about 10-fold, 30-fold and 4-fold increased in senescent HUVECs, HCAECs and HAECs, respectively (Fig. 3).
Fig. 3.
Average fold change expression difference of miR-146a in HUVECs, HCAECs and HAECs during replicative senescence. Real-time quantitative PCR (RT-qPCR) expression was performed with RNA extracted from cells at cultured passages reported in the figure. Results were normalised against RNU44. MiR-146a expression at first passage was arbitrarily set to 1. Data are reported as means + SD obtained from three independent experiments in duplicates
All reported miR expression values obtained with RT-qPCR were normalised using the RNU44 expression. Moreover, to reduce a possible bias of the normalisers implied, RNU48 transcript was also used as an alternative normaliser, confirming results obtained with RNU44 (data not shown). In addition, miR-199-5p, one of the most stably expressed miR in young and senescent HUVECs (as shown by profiling data; Supplementary Table 2) was also used for normalisation, confirming an increased miR-146a expression in senescent HUVECs (data not shown). Moreover, significant correlations were observed between miR-146a expression levels and CPD (Pearson correlation, 0.86, P < 0.01), SA-β-gal activity (Pearson correlation, 0.85, P < 0.01), TRF (Pearson correlation, −0.78, P < 0.01) and TERT activity (Pearson correlation, −0.54, P < 0.05) during HUVECs in vitro senescence.
MicroRNA-146a expression in H2O2-stressed HUVEC cells
To address the involvement of miR-146a either on senescence phenotype or oxidative stress status, we investigated miR-146a expression after H2O2 treatment of young and senescent HUVEC cells. The treatment induced an expression peak for miR-200c and co-transcribed miR-141 (Fig. 4), but no significant change in miR-146a expression was found (Fig. 4). These results confirmed those recently reported by Magenta et al. (2011).
Fig. 4.
MiR-146a, miR-200c and miR-141 fold change expression in H2O2-treated HUVEC cells. Data reported are means + SD obtained from three independent experiments in duplicates. MiR expression in untreated cells was arbitrarily set to 1. Young and senescent HUVECs were treated with H2O2 (200 and 400 μM) for 1 h and then cultured for further 16 h. t test, *P < 0.05
MicroRNA-146a protein targets expression in HUVECs
IRAK1 expression was highly reduced in senescent (XIII passage) compared with young (II passage) HUVECs, while TRAF6 was not significantly modulated (Fig. 5a). Similarly, the over-expression of miR-146a in HUVEC senescent cells correlated with the down-regulation of its protein target IRAK1 but not with TRAF6. In order to directly correlate miR-146a expression with IRAK1 and TRAF6 protein expression levels, SA-β-gal activity and IL-6 release, young and senescent HUVEC endothelial cells were transfected with miR-146a mimic and antagomir. Young and senescent transfected cells showed miR-146a increased expression of approximately 27- and 25-fold vs. non-transfected cells, respectively. Transfection-induced over-expression of mature miR-146a significantly inhibited IRAK1 protein. As expected, knockdown of endogenous miR-146a with an oligonucleotide antimir (anti-miR-146a) significantly increased IRAK1 protein expression both in young and senescent HUVEC cells, while any effect on TRAF6 protein expression was observed (Fig. 5b). IL-6 release and SA-β-gal activity were not different in young and senescent HUVECs transfected with miR-146a mimic (146aM) and antagomir (anti-miR-146a) (data not shown). Together, these data establish that the transient modulation of miR-146a has an independent effect on IRAK1 regulation without affecting TRAF6 expression, IL-6 release and SA-β-gal activity in HUVEC cells.
Fig. 5.
IRAK1 and TRAF6 protein expression in HUVECs: a IRAK1 and TRAF6 protein levels in young (II passage) and senescent (XIII passage) HUVECs. b IRAK1 and TRAF6 protein expressions in young (left panel) and senescent (right panel) HUVECs transfected with miR-146a (146aM), miR-146a inhibitor (anti-miR-146a) and negative miR control mimic (ConM). The experiment was performed three times. Densitometric analysis was performed with Quantity-One software (Bio-Rad). Protein expression values were reported as percentage vs. actin. t test, *P < 0.05
MicroRNA-146a expression in CAC and plasma of CHF patients and healthy CTR
We recently reported that senescence-associated biomarkers, such as telomere attrition and telomerase-reduced activity, were increased in CACs from CHF patients compared to healthy CTR (Olivieri et al. 2012). Therefore, we checked whether miR-146a could be also modulated in cells and plasma from CHF patients vs. CTR. In particular, miR-146a expression was measured in CACs, leukocytes and plasma obtained from a subset of 35 CHF patients and 37 healthy CTR from a previous study (Olivieri et al. 2012) and reported in Table 3. MiR-146a expression increased approximately 1,000-fold in CACs (Fig. 6a) and 2-fold in plasma of CHF patients compared to CTR (Fig. 6b), but did not reach statistical significance in CHF compared to CTR leukocytes (data not shown).
Table 3.
Biochemical and clinical parameters of CTR subjects (n = 35) and CHF patients (n = 37)
| Variables | CTR | CHF | P |
|---|---|---|---|
| Median age (IQR), years | 80 (76–86) | 81 (76–89) | 0.13 |
| Males, N (%) | 15 (43) | 16 (43) | 0.98 |
| Median troponin T (IQR), ng/ml | 0.01 (0.01–0.02) | 0.06 (0.02–0.14) | 0.04 |
| Mean (SD) LVEF, % | 57.76 (13.89) | 38.56 (13.99) | 0.03 |
| Mean (SD) NT proBNP, pg/ml | 812.7 (333.67) | 10,063.1 (11,745.76) | <0.01 |
| Median hs-CRP (IQR), mg/l | 0.57 (0.18–1.50) | 1.88 (0.88–2.79) | 0.03 |
| Mean (SD) CAC T/S | 0.61 (0.39) | 0.34 (0.15) | <0.01 |
| Mean (SD) CAC TERT activity | 0.014 (0.007) | 0.0007 (0.0006) | <0.01 |
| T2DM, N (%) | 5 (14) | 12 (32) | <0.01 |
| Arterial hypertension, N (%) | 5 (14) | 13 (35) | <0.01 |
| Use of statins, N (%) | 5 (14) | 19 (51) | <0.01 |
CAC TERT activity was reported as amol/microgram proteins, amol = attomole: 10–18 mol. P from the general linear model, adjusted for age and sex (for continuous variables) and from χ2 test (for dichotomous variables)
CTR healthy control subjects, CHF congestive heart failure, CACs circulating angiogenic cells, hs-CRP high sensitivity C-reactive protein, LVEF left ventricular ejection fraction, NT proBNP N-terminal prohormone brain natriuretic peptide, T2DM type 2 diabetes mellitus, IQR interquartile range, SD standard deviation
Fig. 6.
MiR-146a expression in CACs and plasma from CHF patients (n = 37) and CTR subjects (n = 35). CACs circulating angiogenic cells, CTR healthy control subjects, CHF congestive heart failure. *P from general linear model, age and sex adjusted, P < 0.05. Box: interquartile range; horizontal line in the box: median value; whiskers: minimum and maximum values
According to senescent HUVEC cells, IRAK1 expression was highly reduced in CACs of CHF patients compared to those of healthy subjects (Fig. 7). Furthermore, significant correlations were observed between miR-146a expression levels and telomere length, as T/S (Pearson correlation, −0.19, P < 0.05) and TERT activity (Pearson correlation, −0.16, P < 0.05) in CACs from CHF patients and CTR subjects.
Fig. 7.
IRAK1 and TRAF6 protein expressions in CACs from CHF and CTR subjects. Reported data are means + SD obtained from four CHF patients vs. four CTR subjects. Densitometric analysis was performed with Quantity-One software (Bio-Rad). Protein expression values were reported as percentage vs. actin. t test, *P < 0.05
Discussion
Human endothelial cells undergo senescence both in vivo and in vitro, providing a useful model for the identification of new specific and sensitive markers of vascular cell senescence. Using a microarray approach, we found that the expression of several miRs is modulated in HUVEC senescent cells. Interestingly, there was an almost 2-fold increase in up-regulated miRs compared to down-regulated ones in senescent cells. This finding is in accordance with data reported regarding in vitro cultured cells and tissues of aged animals and humans, suggesting that an over-expression of miRs might counteract the age-related increase in the expression of protein-encoding genes (Maes et al. 2009; Bates et al. 2010; Li et al. 2009). Among the 367 profiled miRs, miR-146a, miR-9, miR-204 and miR-367 were significantly up-regulated in senescent vs. young cells with miR-146a showing the highest expression. Our data correlated with those of previous reports on trabecular meshwork cells and human foreskin fibroblasts (BJ) (Li et al. 2010; Bonifacio and Jarstfer 2010; Christoffersen et al. 2010; Bhaumik et al. 2009). However, previous data on miR-146a expression in HUVECs during replicative senescence showed conflicting results (Hackl et al. 2010; Vasa-Nicotera et al. 2011). In particular, Hackl et al. (2010) reported no significant change of miR-146a expression in senescent HUVECs, whereas Vasa-Nicotera et al. (2011) observed a significant decrease of miR-146a expression in senescent cells. Interestingly, we found an up-regulation of miR-146a expression not only in HUVECs, but also in HAEC and in HCAEC senescent cells, confirming that its up-regulation is associated with senescent phenotype in different vascular cell types. We cannot exclude that contrasting results on miR-146a expression in senescent HUVEC cells could depend on the different strains used in previous investigations. In fact, a HUVEC strain-specific expression of inflammatory cytokines during in vitro senescence was previously reported, suggesting that young HUVECs of different strains could be characterised by different pro-inflammatory status, blunting the acquisition of a pro-inflammatory phenotype during replicative senescence (Garfinkel et al. 1994). Another possibility for discrepancies could be due to the choice of different normalisers. At present, there is no consensus on normalisation for either hybridisation microarray or RT-qPCR and the choice of a normalisation method could be a bias (Meyer et al. 2010). For these reasons, we used different normalisation methods for arrays and RT-qPCR. In particular, RNU44 and RNU48 were used for their abundance and low variability across different normal tissues and cell lines (Wong et al. 2007). Moreover, miR-199b-5p, which is stably expressed in young and senescent HUVECs (as confirmed by our profiling results), was also used. In all cases, the up-regulation of miR-146a in senescent cells was confirmed.
In order to render a fast and efficient approach for the identification of common pathways of the most up-regulated miRs, such as miR-146a, miR-204, miR-367 and miR-9, in senescent cells, we used the SID1.0 computer programme, which has been recently developed by our group (Albertini et al. 2011). SID1.0 analysis identified nine common pathways, including the pro-inflammatory TLR signalling pathway. As inflammation is a key feature of ageing, we focused on the TLR pathway including miR-146a target genes with an high prediction score (−ln(P value) higher than 1.5). In particular, we investigated two proteins involved in this pathway: TRAF6 and IRAK1. MiR-146a was previously reported to regulate an inflammatory response by repressing TRAF6 and IRAK1 expression in fibroblast and meshwork cells (Freund et al. 2010; Li et al. 2010). Both proteins are involved in the transcription of pro-inflammatory molecules, including IL-6, acting in an autocrine feedback loop to reinforce the senescence growth arrest and the acquisition of SASP (Freund et al. 2010; Ma et al. 2011).
Our results show that miR-146a hyper-expression mediates IRAK1 repression in human senescent endothelial cells (HUVECs): miR-146a transfection in HUVECs induced a down-regulation of IRAK1 protein, whereas knockdown of endogenous miR-146a increased its level, extending previous findings on fibroblasts (Taganov et al. 2006; Campisi and d’Adda di Fagagna 2007; Li et al. 2010) even if miR-146a modulation of TRAF6 was not confirmed in our endothelial cell model.
Furthermore, we observed an increased cytokine release in senescent compared to younger HUVECs, suggesting that IRAK1 down-regulation is not sufficient to counteract the acquisition of SASP in endothelial cells. Indeed, when senescent HUVECs were transiently transfected with miR-146a mimic, IL-6 production was not consistently modified. These data suggest that the acquisition of SASP during replicative senescence is not fully controlled by miR-146a. Further studies are in progress to clarify the role of the other three up-regulated miRs in senescent cells, involved both in the TLR pathway and inflammatory response (Bazzoni et al. 2009; Li et al. 2011).
We also observed an increased release of MPO in HUVEC senescent cells, indicative of an increased oxidative stress. However, our findings clearly show that miR-146a is not involved in oxidative stress response in neither younger nor senescent HUVECs. These results are in accordance with previous data showing that robust expression of miR-146a is not associated with growth arrest or stress-induced stasis (Bhaumik et al. 2009; Magenta et al. 2011; Li et al. 2009). We also confirmed that miR-200c and miR-141 were up-regulated in younger and senescent HUVECs in response to oxidative stress (Magenta et al. 2011; Li et al. 2009).
Interestingly, miR-146a expression was validated in CACs obtained from CHF patients and CTR subjects. This ex vivo cellular model was chosen because CACs are involved in vascular renewal and can be easily purified from peripheral blood, and CACs obtained from CHF patients have a distinguishing feature of senescence (Olivieri et al. 2012; Fadini et al. 2008; Rehman et al. 2003). A 1,000-fold increase of miR-146a expression along with a strong reduction of IRAK1 expression was observed in CACs of CHF patients compared to CTR. Interestingly, significant anti-correlations between miR-146a expression and telomere length and telomerase activity were observed. These data reinforce the hypothesis that cellular senescence associated with a pro-inflammatory status could play a role in vascular dysfunction involved in age-related diseases, such as CHF. Interestingly, the increased expression of miR-146a did not reach significant levels in leukocytes obtained from the same setting of patients, which may be explained by the heterogeneity of leukocyte subpopulations (Lin et al. 2010). We also observed a 2-fold over-expression of plasma levels of miR-146a in CHF patients.
It was hypothesised that an up-regulation of miR-146a in senescent cells may serve to prevent an excessive production of inflammatory mediators, thus limiting some of the potentially deleterious effects of the SASP (Li et al. 2010). Interestingly, it was recently reported that the inflammatory response of the innate immune system, especially through macrophages, promotes arterial remodelling (Nazari-Jahantigh et al. 2012).
Obviously, we cannot exclude that the difference of miR-146a levels between CHF patients and healthy individuals may be attributed to factors far beyond vascular senescence. Moreover, the present work involved a limited number of patients and only large-scale multicentre studies will determine the potential use of circulating miRs (plasma and subpopulation of circulating cells) as senescence-associated biomarkers. Overall, our investigation suggests that miR-146a is a marker of a senescence-associated pro-inflammatory status in vascular remodelling cells.
Electronic supplementary material
(DOC 46 kb)
IL-1 β, 1-α, -2, -6, -8, -10, -12, TNF-a, INF-γ and mieloperoxidase (MPO) concentration were measured at II, III, V, IX, XI, XII and XIII passages. Values were reported as pg/ml (DOC 41 kb)
MiRNAs profiling results in senescent (XIII) vs. young (II) HUVECs. Each value corresponds to the fold difference expression of single microRNA, calculated as ∆∆Ct. ∆∆CT for each miR was defined as expression changes of senescent vs. young HUVEC, calculated with the following equation: [(CT senescent microRNA- median Ct values obtained in the profiling of senescent cells)-(CT young microRNA- median Ct values obtained in the profiling of young cells)] (DOC 264 kb)
Markers of cellular senescence in HUVEC cells until replicative proliferation arrest: a growth curve; cell population doubling (CPD) from I to XIII passages. b SΑ−β-gal staining; percentage of positive SA-β-gal cells. c Telomere length; telomere restriction fragment (TRF) length. d Telomerase activity (TERT) (JPEG 193 kb)
IL-1 β, 1-α, -2, -6, -8, -10, -12, TNF-α, INF-γ and mieloperoxidase (MPO) release in young (II) and senescent (XIII) HUVECs (values were reported as pg/ml). T-test * p < 0.05 for all comparisons (JPEG 227 kb)
Contributor Information
Fabiola Olivieri, Phone: +39-71-2206242, FAX: +39-71-2206240, Email: f.olivieri@yhaoo.it.
Raffaella Lazzarini, Email: raffaellalazzarini@hotmail.com.
Rina Recchioni, Email: r.recchioni@inrca.it.
Fiorella Marcheselli, Email: f.marcheselli@inrca.it.
Maria Rita Rippo, Email: m.r.rippo@univpm.it.
Silvia Di Nuzzo, Email: silviadinuzzo@libero.it.
Maria Cristina Albertini, Email: maria.albertini@uniurb.it.
Laura Graciotti, Email: l.graciotti@univpm.it.
Lucia Babini, Email: l.babini@univpm.it.
Serena Mariotti, Email: s.mariotti@inrca.it.
Angela Marie Abbatecola, Email: angela_abbatecola@libero.it.
Roberto Antonicelli, Email: r.antonicelli@inrca.it.
Claudio Franceschi, Email: claudio.franceschi@unibo.it.
Antonio Domenico Procopio, Email: a.d.procopio@univpm.it.
References
- Albertini MC, Olivieri F, Lazzarini R, Pilolli F, Galli F, Spada G, Accorsi A, Rippo MR, Procopio AD. Predicting microRNA modulation in human prostate cancer using a simple String IDentifier (SID1.0) J Biomed Inform. 2011;44:615–620. doi: 10.1016/j.jbi.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Bates DJ, Li N, Liang R, Sarojini H, An J, Masternak MM, Bartke A, Wang E. MicroRNA regulation in Ames dwarf mouse liver may contribute to delayed aging. Aging Cell. 2010;9:1–18. doi: 10.1111/j.1474-9726.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci U S A. 2009;106:5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, Lithgow GJ, Campisi J. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging. 2009;1:402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacio LN, Jarstfer MB. MiRNA profile associated with replicative senescence, extended cell culture, and ectopic telomerase expression in human foreskin fibroblasts. PLoS One. 2010;5(9):e12519. doi: 10.1371/journal.pone.0012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 15: 30(10):e47 [DOI] [PMC free article] [PubMed]
- Christoffersen NR, Shalgi R, Frankel LB, Leucci E, Lees M, Klausen M, Pilpel Y, Nielsen FC, Oren M, Lund AH. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ. 2010;17:236–245. doi: 10.1038/cdd.2009.109. [DOI] [PubMed] [Google Scholar]
- D'Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M, Micheli B, Biglioli P, Achilli F, Martelli F, Maggiolini S, Marenzi G, Pompilio G, Capogrossi MC. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel S, Brown S, Wessendorf JH, Maciag T. Post-transcriptional regulation of interleukin 1 alpha in various strains of young and senescent human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 1994;91:1559–1563. doi: 10.1073/pnas.91.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl M, Brunner S, Fortschegger K, Schreiner C, Micutkova L, Mück C, Laschober GT, Lepperdinger G, Sampson N, Berger P, Herndler-Brandstetter D, Wieser M, Kühnel H, Strasser A, Rinnerthaler M, Breitenbach M, Mildner M, Eckhart L, Tschachler E, Trost A, Bauer JW, Papak C, Trajanoski Z, Scheideler M, Grillari-Voglauer R, Grubeck-Loebenstein B, Jansen-Dürr P, Grillari J. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 2010;9:291–296. doi: 10.1111/j.1474-9726.2010.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM, Dimmeler S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular senescence, vascular disease, and aging: part 1 of a 2-part review. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- Lafferty-Whyte K, Cairney CJ, Jamieson NB, Oien KA, Keith WN. Pathway analysis of senescence-associated miRNA targets reveals common processes to different senescence induction mechanisms. Biochim Biophys Acta. 2009;1792:341–352. doi: 10.1016/j.bbadis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress-induced cellular senescence. Mech Ageing Dev. 2009;130:731–741. doi: 10.1016/j.mad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Modulation of inflammatory markers by miR-146a during replicative senescence in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2010;6:2976–2985. doi: 10.1167/iovs.09-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:2999–3007. doi: 10.1167/iovs.10-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Bates DJ, Wang E. Epigenetic control of microRNA expression and aging. Curr Genomics. 2009;10:184–193. doi: 10.2174/138920209788185225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-{kappa}B signaling. J Mol Cell Biol. 2011;3:159–166. doi: 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes OC, Sarojini H, Wang E. Stepwise up-regulation of microRNA expression levels from replicating to reversible and irreversible growth arrest states in WI-38 human fibroblasts. J Cell Physiol. 2009;221:109–119. doi: 10.1002/jcp.21834. [DOI] [PubMed] [Google Scholar]
- Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011;18(10):1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I, Almstead LL, Di Maio D. MicroRNAs and senescence. Aging. 2011;3:77–78. doi: 10.18632/aging.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- Meyer SU, Pfaffl MW, Ulbrich SE. Normalization strategies for microRNA profiling experiments: a 'normal' way to a hidden layer of complexity? Biotechnol Lett. 2010;32:1777–1788. doi: 10.1007/s10529-010-0380-z. [DOI] [PubMed] [Google Scholar]
- Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100(1):15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105(13):1541–1544. doi: 10.1161/01.CIR.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011;8:388–403. doi: 10.1038/cmi.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari-Jahantigh M, Wei Y, Schober A. The role of microRNAs in arterial remodelling. Thromb Haemost. 2012;107(4):611–618. doi: 10.1160/TH11-12-0826. [DOI] [PubMed] [Google Scholar]
- Olivieri F, Lorenzi M, Antonicelli R, Testa R, Sirolla C, Cardelli M, Mariotti S, Marchegiani F, Marra M, Spazzafumo L, Bonfigli AR, Procopio A. Leukocyte telomere shortening in elderly Type2DM patients with previous myocardial infarction. Atherosclerosis. 2009;206:588–593. doi: 10.1016/j.atherosclerosis.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Olivieri F, Antonicelli R, Recchioni R, Mariotti S, Marcheselli F, Lisa R, Spazzafumo L, Galeazzi R, Caraceni D, Testa R, Latini R, Procopio AD (2012) Telomere/telomerase system impairment in circulating angiogenic cells of geriatric patients with heart failure. Int J Cardiol. doi:10.1016/j.ijcard.2011.06.091 [DOI] [PubMed]
- Papadopoulos GL, Alexiou P, Maragkakis M, Reczko M, Hatzigeorgiou AG. DIANA-mirPath: Integrating human and mouse microRNAs in pathways. Bioinformatics. 2009;25:1991–1993. doi: 10.1093/bioinformatics/btp299. [DOI] [PubMed] [Google Scholar]
- Passos JF, Simillion C, Hallinan J, Wipat A, von Zglinicki T. Cellular senescence: unravelling complexity. Age. 2009;31:353–363. doi: 10.1007/s11357-009-9108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliseno L, Pitto L, Simili M, Mariani L, Riccardi L, Ciucci A, Rizzo M, Evangelista M, Mercatanti A, Pandolfi PP, Rainaldi G. The proto-oncogene LRF is under post-transcriptional control of MiR-20a: implications for senescence. PLoS One. 2008;2:2542. doi: 10.1371/journal.pone.0002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;4:1164–1169. doi: 10.1161/01.CIR.0000058702.69484.A0. [DOI] [PubMed] [Google Scholar]
- Saliques S, Zeller M, Lorin J, Lorgis L, Teyssier JR, Cottin Y, Rochette L, Vergely C. Telomere length and cardiovascular disease. Arch Cardiovasc Dis. 2010;103:454–459. doi: 10.1016/j.acvd.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Sikora E, Arendt T, Bennett M, Narita M. Impact of cellular senescence signature on ageing research. Ageing Res Rev. 2011;10:146–152. doi: 10.1016/j.arr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2481–2486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa R, Olivieri F, Sirolla C, Spazzafumo L, Rippo MR, Marra M, Bonfigli AR, Ceriello A, Antonicelli R, Franceschi C, Castellucci C, Testa I, Procopio AD. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet Med. 2011;28(11):1388–1394. doi: 10.1111/j.1464-5491.2011.03370.x. [DOI] [PubMed] [Google Scholar]
- Unterluggauer H, Hütter E, Voglauer R, Grillari J, Vöth M, Bereiter-Hahn J, Jansen-Dürr P, Jendrach M. Identification of cultivation-independent markers of human endothelial cell senescence in vitro. Biogerontology. 2007;8:383–397. doi: 10.1007/s10522-007-9082-x. [DOI] [PubMed] [Google Scholar]
- Vasa-Nicotera M, Chen H, Tucci P, Yang AL, Saintigny G, Menghini R, Mahè C, Agostini M, Knight RA, Melino G, Federici M. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis. 2011;217:326–330. doi: 10.1016/j.atherosclerosis.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Voghel G, Thorin-Trescases N, Farhat N, Nguyen A, Villeneuve L, Mamarbachi AM, Fortier A, Perrault LP, Carrier M, Thorin E. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech Ageing Dev. 2007;128(11–12):662–671. doi: 10.1016/j.mad.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;21:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H, Chui MS, Le HT, Tran JM, Zern MA. SYBR Green real-time telomeric repeat amplification protocol for the rapid quantification of telomerase activity. Nucleic Acids Res. 2003;31:E3–3. doi: 10.1093/nar/gng003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L, Lee K, Russell I, Chen C (2007) Endogenous controls for real-time quantitation of miRNA using TaqMan® microRNA assays. Applied Biosystems Application Note, Publication 127AP11-01, available at: www.appliedbiosystems.com
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 46 kb)
IL-1 β, 1-α, -2, -6, -8, -10, -12, TNF-a, INF-γ and mieloperoxidase (MPO) concentration were measured at II, III, V, IX, XI, XII and XIII passages. Values were reported as pg/ml (DOC 41 kb)
MiRNAs profiling results in senescent (XIII) vs. young (II) HUVECs. Each value corresponds to the fold difference expression of single microRNA, calculated as ∆∆Ct. ∆∆CT for each miR was defined as expression changes of senescent vs. young HUVEC, calculated with the following equation: [(CT senescent microRNA- median Ct values obtained in the profiling of senescent cells)-(CT young microRNA- median Ct values obtained in the profiling of young cells)] (DOC 264 kb)
Markers of cellular senescence in HUVEC cells until replicative proliferation arrest: a growth curve; cell population doubling (CPD) from I to XIII passages. b SΑ−β-gal staining; percentage of positive SA-β-gal cells. c Telomere length; telomere restriction fragment (TRF) length. d Telomerase activity (TERT) (JPEG 193 kb)
IL-1 β, 1-α, -2, -6, -8, -10, -12, TNF-α, INF-γ and mieloperoxidase (MPO) release in young (II) and senescent (XIII) HUVECs (values were reported as pg/ml). T-test * p < 0.05 for all comparisons (JPEG 227 kb)