Abstract
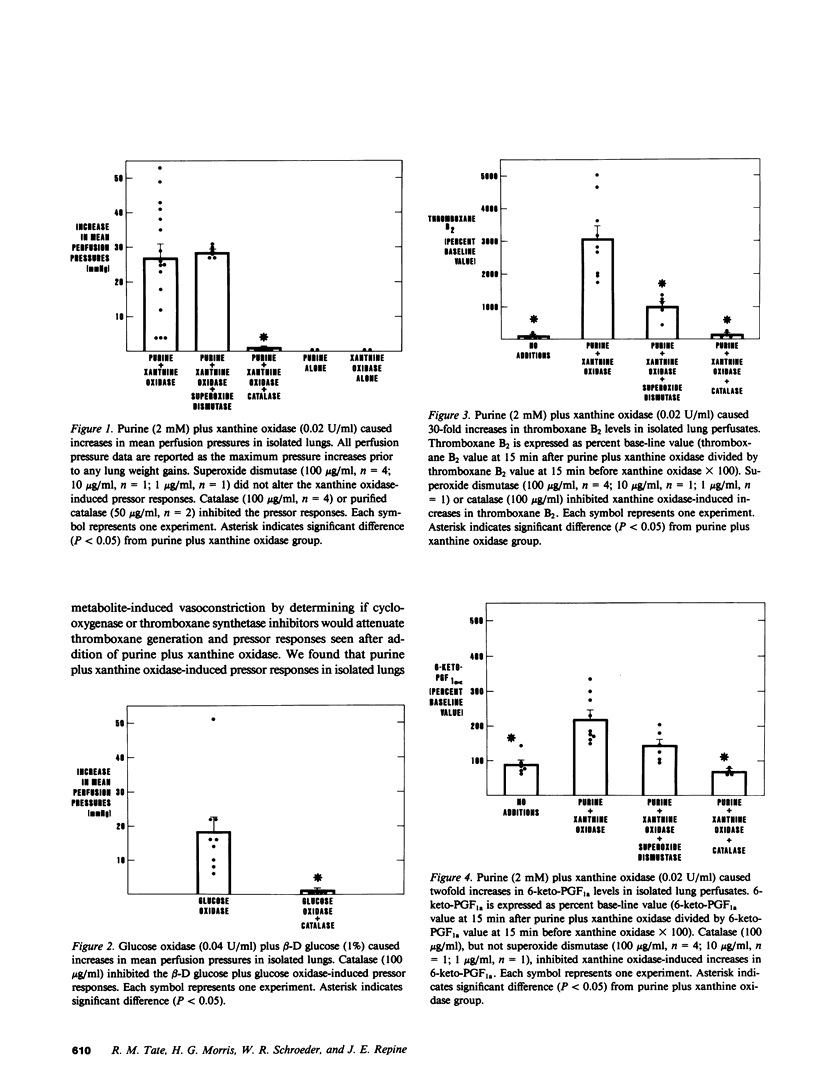
Generation of reactive oxygen metabolites, thromboxane increases, and vasoconstriction have been implicated in the pathogenesis of acute edematous lung injury, such as that seen in patients with the Adult Respiratory Distress Syndrome (ARDS), but their interactions are unknown. We hypothesized that reactive O2 products would stimulate arachidonic acid metabolism in lungs and that vasoactive products of arachidonate, such as the potent vasoconstrictor thromboxane A2, might then mediate O2-metabolite-induced pulmonary vasoconstriction. We found that O2 metabolites generated by injection of purine plus xanthine oxidase caused increases in mean pulmonary artery perfusion pressures (27 +/- 4 mmHg) in isolated perfused lungs. In addition, purine plus xanthine oxidase also caused 30-fold increases in perfusate levels of thromboxane B2 (the stable metabolite of thromboxane A2) compared with only twofold increases in 6-keto-PGF1a (the stable metabolite of prostacyclin). Moreover, prior addition of catalase inhibited both vasoconstriction and the thromboxane B2 production seen in isolated lungs following injection of purine plus xanthine oxidase. Similarly, pretreatment with cyclooxygenase inhibitors, either aspirin or indomethacin, also completely blocked thromboxane generation and markedly attenuated pressor responses usually seen after purine plus xanthine oxidase (increase in mean pulmonary artery perfusion pressures, 4.4 +/- 1.5 mmHg). Furthermore, imidazole, a thromboxane synthetase inhibitor, also decreased O2-metabolite-induced thromboxane generation and vasoconstriction. These results suggested that thromboxane generation might participate in O2-metabolite-induced vasoconstriction. However, since a significant correlation between thromboxane levels and the degree of vasoconstriction could not be demonstrated, and since addition of superoxide dismutase reduced thromboxane generation but did not affect the intensity of vasoconstriction, it is possible that thromboxane is not the only vasoactive mediator in this model. We conclude that exposing lungs to O2 metabolites results in thromboxane generation and that thromboxane is a major mediator of oxidant-induced vasoconstriction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhle Y. S. Biosynthesis of prostaglandins and thromboxanes in lung. Bull Eur Physiopathol Respir. 1981;17(4):491–508. [PubMed] [Google Scholar]
- Brigham K. L. Mechanisms of lung injury. Clin Chest Med. 1982 Jan;3(1):9–24. [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Demling R. H. Role of prostaglandins in acute pulmonary microvascular injury. Ann N Y Acad Sci. 1982;384:517–534. doi: 10.1111/j.1749-6632.1982.tb21397.x. [DOI] [PubMed] [Google Scholar]
- Fridovich S. E., Porter N. A. Oxidation of arachidonic acid in micelles by superoxide and hydrogen peroxide. J Biol Chem. 1981 Jan 10;256(1):260–265. [PubMed] [Google Scholar]
- Frölich J. C., Ogletree M., Peskar B. A., Brigham K. L. Pulmonary hypertension correlated to pulmonary thromboxane synthesis. Adv Prostaglandin Thromboxane Res. 1980;7:745–750. [PubMed] [Google Scholar]
- Hales C. A., Sonne L., Peterson M., Kong D., Miller M., Watkins W. D. Role of thromboxane and prostacyclin in pulmonary vasomotor changes after endotoxin in dogs. J Clin Invest. 1981 Aug;68(2):497–505. doi: 10.1172/JCI110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. R., Bohr D. F. Hypertension, transmural pressure, and vascular smooth muscle response in rats. Circ Res. 1975 May;36(5):590–598. doi: 10.1161/01.res.36.5.590. [DOI] [PubMed] [Google Scholar]
- Heffner J. E., Shoemaker S. A., Canham E. M., Patel M., McMurtry I. F., Morris H. G., Repine J. E. Acetyl glyceryl ether phosphorylcholine-stimulated human platelets cause pulmonary hypertension and edema in isolated rabbit lungs. Role of thromboxane A2. J Clin Invest. 1983 Feb;71(2):351–357. doi: 10.1172/JCI110776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Cook H. W., Lands W. E. Prostaglandin biosynthesis can be triggered by lipid peroxides. Arch Biochem Biophys. 1979 Apr 1;193(2):340–345. doi: 10.1016/0003-9861(79)90038-9. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Graff G., Lands W. E. Accelerative autoactivation of prostaglandin biosynthesis by PGG2. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1325–1331. doi: 10.1016/0006-291x(78)91148-8. [DOI] [PubMed] [Google Scholar]
- Hüttemeier P. C., Watkins W. D., Peterson M. B., Zapol W. M. Acute pulmonary hypertension and lung thromboxane release after endotoxin infusion in normal and leukopenic sheep. Circ Res. 1982 May;50(5):688–694. doi: 10.1161/01.res.50.5.688. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- Lands W. E., Sauter J., Stone G. W. Oxygen requirement for prostaglandin biosynthesis. Prostaglandins Med. 1978 Aug;1(2):117–120. doi: 10.1016/0161-4630(78)90037-x. [DOI] [PubMed] [Google Scholar]
- Lands W. E. The biosynthesis and metabolism of prostaglandins. Annu Rev Physiol. 1979;41:633–652. doi: 10.1146/annurev.ph.41.030179.003221. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd, Gadek J. E., Hunninghake G. W., Crystal R. G. Oxidant injury of lung parenchymal cells. J Clin Invest. 1981 Nov;68(5):1277–1288. doi: 10.1172/JCI110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Bunting S., Mullane K., Thorogood P., Vane J. R., Raz A., Needleman P. Imidazole: a selective inhibitor of thromboxane synthetase. Prostaglandins. 1977 Apr;13(4):611–618. doi: 10.1016/0090-6980(77)90232-5. [DOI] [PubMed] [Google Scholar]
- Morris H. G., Sherman N. A., Shepperdson F. T. Variables associated with radioimmunoassay of prostaglandins in plasma. Prostaglandins. 1981 May;21(5):771–788. doi: 10.1016/0090-6980(81)90234-3. [DOI] [PubMed] [Google Scholar]
- Ogletree M. L., Brigham K. L. Effects of cyclooxygenase inhibitors on pulmonary vascular responses to endotoxin in unanesthetized sheep. Prostaglandins Leukot Med. 1982 May;8(5):489–502. [PubMed] [Google Scholar]
- Panganamala R. V., Brownlee N. R., Sprecher H., Cornwell D. G. Evaluation of superoxide anion and singlet oxygen in the biosynthesis of prostaglandins from eicosa-8,11,14-trienoic acid. Prostaglandins. 1974 Jul 10;7(1):21–28. doi: 10.1016/s0090-6980(74)80074-2. [DOI] [PubMed] [Google Scholar]
- Panganamala R. V., Sharma H. M., Sprecher H., Geer J. C., Cornwell D. G. A suggested role for hydrogen peroxide in the biosynthesis of prostaglandins. Prostaglandins. 1974 Oct 10;8(1):3–11. doi: 10.1016/0090-6980(74)90031-8. [DOI] [PubMed] [Google Scholar]
- Piper P., Vane J. The release of prostaglandins from lung and other tissues. Ann N Y Acad Sci. 1971 Apr 30;180:363–385. doi: 10.1111/j.1749-6632.1971.tb53205.x. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Vanbenthuysen K. M., Tate R. M., Shasby S. S., McMurtry I., Repine J. E. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis. 1982 Apr;125(4):443–447. doi: 10.1164/arrd.1982.125.4.443. [DOI] [PubMed] [Google Scholar]
- Smedegård G., Hedqvist P., Dahlén S. E., Revenäs B., Hammarström S., Samuelsson B. Leukotriene C4 affects pulmonary and cardiovascular dynamics in monkey. Nature. 1982 Jan 28;295(5847):327–329. doi: 10.1038/295327a0. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Gunther R., Gee M., Flynn J., Demling R. H. Leukocytes, platelets, and thromboxane A2 in endotoxin-induced lung injury. Surgery. 1981 Jul;90(1):102–107. [PubMed] [Google Scholar]
- Tappel A. L. Lipid peroxidation damage to cell components. Fed Proc. 1973 Aug;32(8):1870–1874. [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Vanbenthuysen K. M., Shasby D. M., McMurtry I. F., Repine J. E. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982 Nov;126(5):802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- Watkins W. D., Hüttemeier P. C., Kong D., Peterson M. B. Thromboxane and pulmonary hypertension following E. coli endotoxin infusion in sheep: effect of an imidazole derivative. Prostaglandins. 1982 Mar;23(3):273–285. doi: 10.1016/0090-6980(82)90073-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., Renzetti A. D., Hill H. R. Functional and metabolic activity of granulocytes from patients with adult respiratory distress syndrome. Evidence for activated neutrophils in the pulmonary circulation. Am Rev Respir Dis. 1983 Mar;127(3):290–300. doi: 10.1164/arrd.1983.127.3.290. [DOI] [PubMed] [Google Scholar]



