Abstract
Purpose
The effects of hepatic resection on patients with metastatic tumors from gastric adenocarcinomas are unclear. Therefore, we analyzed early clinical outcomes in patients who underwent surgical resection for hepatic metastases from gastric adenocarcinomas.
Materials and Methods
From January 2003 to December 2010, 1,508 patients with primary gastric cancers underwent curative gastric resections at the Korea Cancer Center Hospital. Of these patients, 12 with liver-only metastases underwent curative hepatic resection. Their clinical data were analyzed retrospectively.
Results
The median follow-up period was 12.5 months (range, 1~85 months); no operative mortalities or major complications were observed. Three patients underwent synchronous resections, and 9 underwent metachronous resections. In the latter group, the median interval between gastrectomy and hepatectomy for hepatic metastasis was 10.5 months (range, 5~47 months). The overall 1- and 5-year survival rates of these 12 patients were 65% and 39%, respectively, with a median overall survival of 31.0 months; 2 patients survived for >5 years.
Conclusions
Hepatic resection can be a feasible procedure for treating hepatic metastases from gastric adenocarcinomas. Although this study was small and involved only selected cases, the outcomes of the hepatic resections were comparable and long-term (>5 years) survivors were identified. Surgical resection of the liver can be considered a feasible option in managing hepatic metastases from gastric adenocarcinomas.
Keywords: Hepatectomy, Hepatic metastases, Stomach neoplasms
Introduction
Gastric adenocarcinomas are common malignancies worldwide. Over several decades, the management of gastric adenocarcinoma has advanced, and patient prognosis has improved. The proportion of patients with early stage cancers who are being treated is increasing due to the prevalence of more comprehensive surveillance programs. In addition, the advancement of surgical techniques and the development of chemotherapy involving various molecular targeting agents have led to improved prognoses for these patients. However, several challenging problems remain in the management of gastric cancer.
The liver is the most common site of metastasis in gastrointestinal malignancies and the management of liver metastasis is difficult, and the outcomes are still poor. After curative resection of primary gastric adenocarcinomas, 3.5~14.0% of patients experience intrahepatic recurrence.1-9 Most of these patients have multiple forms of intrahepatic and extrahepatic recurrences, including peritoneal carcinomatosis, lymph node metastasis, and direct tumor invasion of other organs.10,11 To manage hepatic metastases from gastric adenocarcinomas, various treatment modalities have been introduced, such as systemic chemotherapy and local treatments. Although systemic chemotherapy with the new molecular targeting agents is the standard treatment modality, the outcomes remain disappointing and the management of hepatic metastasis remains challenging.5-18
Over the past 3 decades, the management of hepatic metastases associated with colorectal adenocarcinomas has advanced, markedly. Many studies have reported the role of hepatic resection for these metastases, and hepatic resection is now associated with a 5-year survival rate of 30~50%.19,20 Thus, hepatic resection for hepatic metastases from colorectal adenocarcinoma is currently regarded as one of the standard modalities in multi-disciplinary treatments.
Only a few reports of hepatic resection have been reported for hepatic metastases in cases of gastric adenocarcinomas. Some of these have reported long-term survival and outcomes comparable to those associated with colorectal adenocarcinoma, even though they were small case studies.5,6,12-15 However, surgical resection is not commonly performed in patients with hepatic metastases from gastric cancers, because of relatively high morbidity associated with hepatic resection, mostly due to the multiple intrahepatic and extrahepatic lesions. Recent advances in the hepatic resection technique have improved the safety of this type of resection, and the development of imaging techniques such as positron emission tomography (PET) and liver magnetic resonance imaging (MRI) may help to identify the proper indications for hepatic resection, preoperatively. Regardless, the role and proper indications for surgical resection of hepatic metastases from gastric cancers are challenging topics. Thus, to clarify the benefits of surgical treatment and find factors affecting survival, we retrospectively analyzed patients who underwent hepatic resection for hepatic metastases from gastric adenocarcinomas.
Materials and Methods
From January 2003 to December 2010, a total 1,508 patients with primary gastric cancers underwent curative gastric resections at the Korea Cancer Center Hospital. Of these patients, 258 (17.1%) experienced recurrence during the follow-up period, and 67 (4.4%) patients developed metachronous hepatic metastases. Of these 67 patients, 22 patients had liver-only metastases. Nine of these 22 patients underwent hepatic resection, and the remaining 13 patients did not undergo hepatic resection because of resection being contraindicated or refused. Another 3 synchronous hepatic metastases patients were added to the analysis of post-hepatic resection prognosis. The medical records of all 12 patients were retrospectively reviewed.
The indications for hepatic resection as a result of hepatic metastasis from a gastric adenocarcinoma were as follows: a solitary liver lesion, the absence of other distant metastases, favorable liver function, good performance status, and the informed consent of the patient. However, one case did not meet the hepatic resection indication because of a relatively long disease-free interval and had 2 intrahepatic lesions; one was small and suited for radiofrequency ablation. Because this study was documenting early experiences, we included this patient.
Ultrasonography (US), computed tomography (CT), MRI and PET were used to diagnose hepatic metastases. After gastric resection for primary gastric cancer, the patients were routinely examined for recurrence every 6 months for 5 years. The examinations included evaluation of tumor markers, endoscopy, and CT scans. If a suspicious lesion was detected by CT scan, the patient underwent additional imaging studies, including US, MRI, or PET. In some patients, US-guided percutaneous needle aspirations were performed for pathologic confirmation of the hepatic lesions.
SPSS for Windows, Korean version 14.0 (SPSS Inc., Chicago, IL, USA), was used to perform statistical analyses. Survival rates were calculated using the Kaplan-Meier method. Categorical variables were compared using the chi-square test and logistic regression analysis. A P<0.05 was considered statistically significant.
Results
A total of 12 patients (11 men and 1 woman) underwent hepatic resection for hepatic metastases from gastric adenocarcinomas. Their median age, at the time of hepatic resection, was 61 years (range, 51~74 years), and their median post-resection follow-up period was 12.5 months (range, 1~85 months) (Table 1).
Table 1.
Summary of 12 patients underwent curative hepatic resection for hepatic metastases from gastric adenocarcinoma
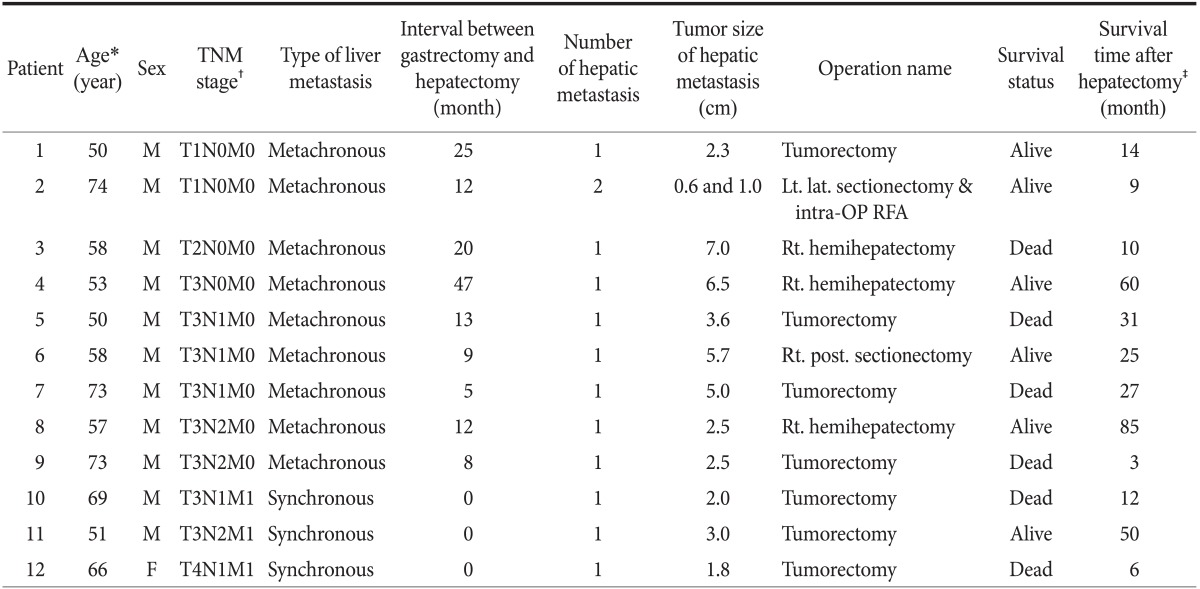
*Age at the time of hepatic resection. †TNM stage: TNM classification of American Joint Committee on Cancer (AJCC) 7th edition at the time of gastric resection. ‡Durations from hepatic resection to death or last follow-up. M = male; F = female; Lt = left; lat = lateral; intra-OP RFA = intraoperative radiofrequency ablation; Rt = right; post. = posterior.
Eleven patients had a single hepatic metastasis and underwent hepatic resection, only; one patient had 2 intrahepatic lesions and underwent both hepatic resection and intraoperative radiofrequency ablation. None of the patients died or experienced major complications because of the surgery. Synchronous resections were performed on 3 patients, metachronous resections were performed 9 patients. Five patients received systemic adjuvant chemotherapy after the resection, and 7 did not (Table 2).
Table 2.
Clinicopathological characteristics of patients with hepatic metastases from gastric adenocarcinoma
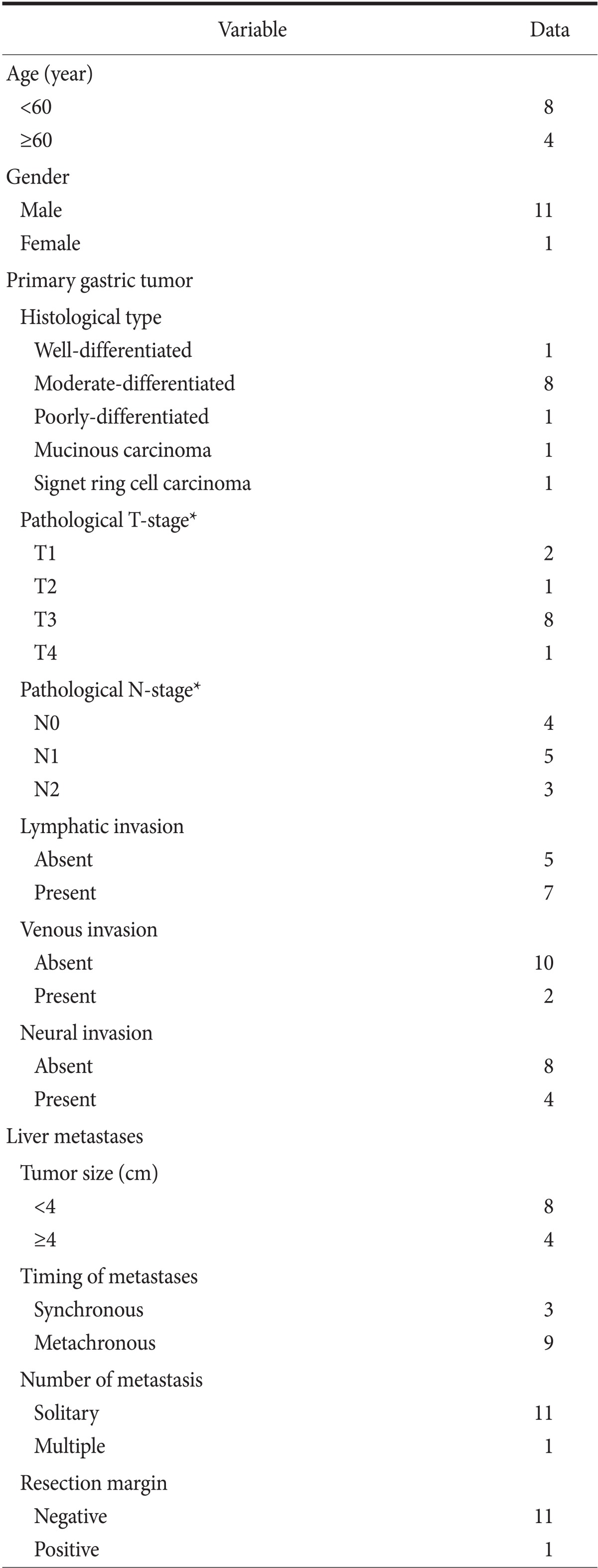
*TNM classification of American Joint Committee on Cancer (AJCC) 7th edition.
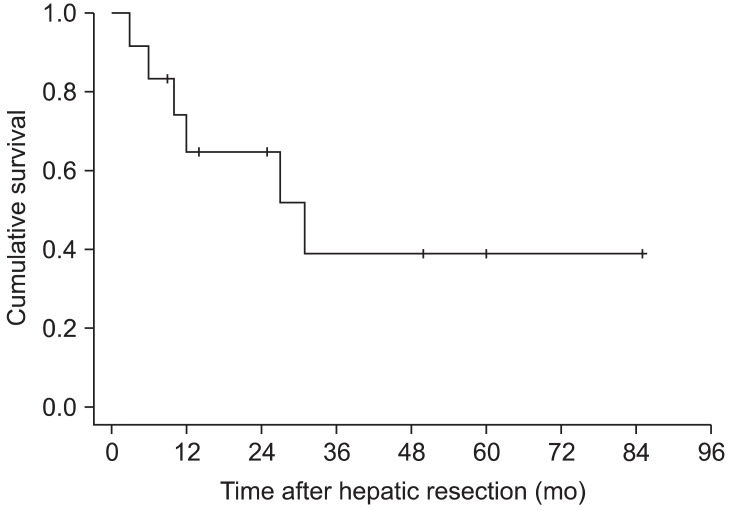
For the 9 patients who underwent metachronous resection, the median interval between gastrectomy and hepatectomy was 10.5 months (range, 5~47 months). The overall 1- and 5-year survival rates of the 12 patients were 65% and 39%, respectively, with a median survival time of 31.0 months (Fig. 1). The overall 3-year survival rates for patients who underwent synchronous and metachronous resections were 33% and 38%, respectively; this difference was not statistically significant (P=0.596, Fig. 2).
Fig. 1.
Overall survival rate for 12 patients who underwent hepatic resection for metastases from gastric adenocarcinoma. The 5-year survival rate was 39% with a median survival time of 31.0 months.
Fig. 2.
Survival rates among patients with synchronous (n=3) and metachronous (n=9) hepatic metastases. The difference was not statistically significant (P=0.596).
To determine the prognostic factors, a univariate analysis was performed. The TNM stage of the primary gastric cancer, the size of metastatic liver tumor, the type of metastasis (synchronous or metachronous), and the use of systemic adjuvant chemotherapy after hepatic resection did not have a significant impact on survival among the 12 patients (Table 3).
Table 3.
Univariate analysis of factors predictive of patient survial
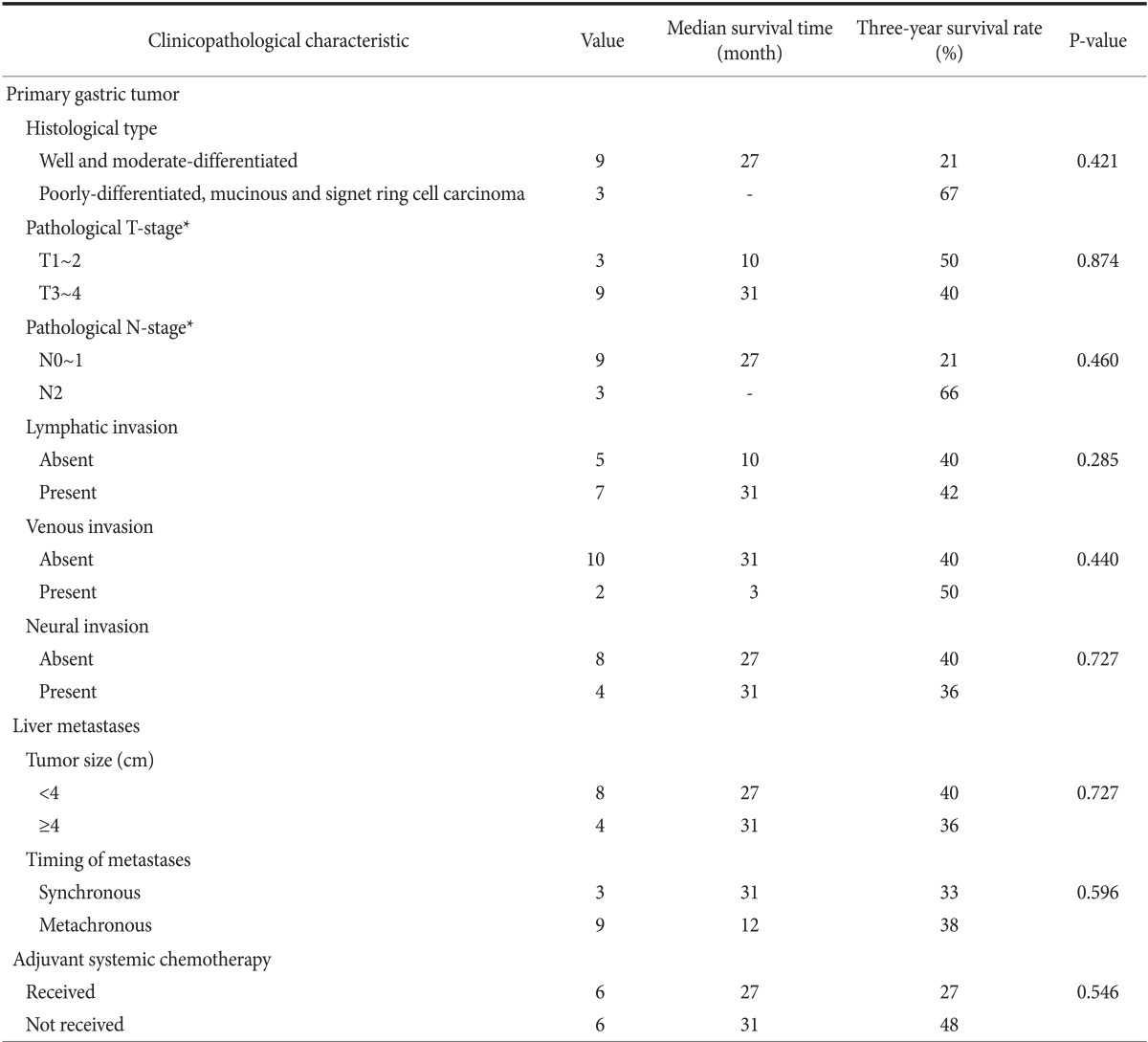
*TNM classification of American Joint Committee on Cancer (AJCC) 7th edition.
Two of the 12 patients survived for more than 5 years after hepatic resection. Both had undergone metachronous hepatic resection, with intervals between gastrectomy and hepatectomy of 12 or 47 months. The pathologic stages of these primary gastric cancers were T3N0M0 and T3N2M0. Both patients had solitary hepatic lesion and both underwent right hemihepatectomy.
One patient who underwent hepatic resection plus intraoperative radiofrequency ablation experienced a new intrahepatic recurrence 8 months later. This patient underwent additional radiofrequency ablation for this lesion and was still living 12 months after the initial hepatic resection.
Discussion
As with other gastrointestinal malignancies, the liver is a common metastatic site for gastric adenocarinomas. After curative resection of a primary gastric adenocarcinoma, 3.5~14.0% of patients experience an intrahepatic recurrence.1-9 Various treatment modalities have been applied to the management of hepatic metastases from gastric adenocarcinomas, but the outcomes have not been satisfactory. Most hepatic metastatic lesions are multiple, bi-lobar lesions that occur in combination with extrahepatic metastases. Therefore, systemic chemotherapy is considered the standard of care, but the median survival time is less than 6 months and long-term survivors are rare.4,6 Other treatment modalities have also been applied, such as radiofrequency ablation, hepatic resection, and radiation, but their reported use has been infrequent, and their outcomes have also been disappointing.5-18
There have been some recent changes in the management of liver metastases associated with gastrointestinal malignancies, especially for those associated with colorectal malignancies. Over several decades, advancements in surgical techniques and postoperative management have improved the safety of liver resections, and newly developed chemotherapeutic molecular targeing agents changed the role of the surgical resection. Liver resection is now one of the multi-disciplinary treatments and play a role in the standard treatment of colorectal liver metastases.19,20
Regardless of the use of liver resection for hepatic metastases associated with colorectal cancers, liver resection is still rarely indicated for patients with hepatic metastases from gastric cancers. In previous reports, only 10~20% of patients with hepatic metastases were found to be suitable for surgical treatment, and the outcomes have been also disappointing (median survival, 5~8 months).2,11,14,16 Recently, a few reports have shown the feasibility of hepatic resections associated with gastric cancer metastases and have noted some long-term survivors.5,6,12,15 Therefore, selected group of patients, with the proper indications could be helpful in establishing the indications for hepatic resection. The experiences obtained from the management of colorectal liver metastases and the development of new imaging methods might help identify the proper indications for hepatic resection. For this reason, we chose initially narrow indications: solitary lesions, relatively long disease-free intervals, and the absence of PET-identified extrahepatic lesion. We allowed some exceptions because this study was designed to provide some initial experience and consisted of a retrospective review of patient medical records. The most significant exception was the one patient who included depite having 2 liver lesions indentified. The exception was permitted because the patient had a relatively long disease-free interval, and one intrahepatic lesion was small and the other lesion was suitable for radiofrequency ablation. We also included 3 patients with synchronous liver metastatic lesions.
Although our study included 12 patients, none of the patients experienced major complicatins or died because of their surgery. The overall 5-year survival rate, after hepatic resection, was 39%, with a median survival of 31 months; 2 patients survived for >5 years. These results suggest that hepatic resection can be a feasible procedure for the managements of hepatic metastases from gastric adenocarcinomas.
The management of hepatic metastases from colorectal cancers and hepatocellular carcinomas has shown that, radiofrequency ablation is a promising method with outcomes similar to those observed following resection of small-sized tumors.21,22 As previously mentioned, we included one patient who did not meet our initial inclusion criteria. This patient had 2 intrahepatic lesions, one in each of segments 8 and 2 of the liver. The lesion in segment 2 was removed by hepatic resection, whereas that in segment 8 was suitable for radiofrequency ablation. After the hepatic resection and intraoperative radiofrequency ablation, this patient experienced another intrahepatic recurrence 8 months later in segment 6. The patient underwent an additional radiofrequency ablation of this lesion and has remained alive without further recurrence. Hepatic resection with intraoperative radiofrequency ablation may be an alternative treatment option for multiple intrahepatic tumors in selected patients.
Post-resection prognoses for hepatic metastases from gastric cancers have not been well evaluated. At the start of this study, we suspected that the presence of a solitary hepatic lesion and a long disease-free interval may be good prognostic factors for hepatic resection of liver metastases from gastric adenocarcinomas, based on the early studies of similar therapy for liver metastases from colorectal adenocarcinomas. In some reports regarding hepatic resection of liver metastases from gastric adenocarcinomas, solitary and metachronous metastases have been found to be significant determinants of favorable prognoses after hepatic resection.6-8 However, we could not find any significant factors that affected survival (Table 3). Our inability to determine any specific factors that influence survival may have been related to our study's limitations, including the small number of patients and the study's retrospective design. Most of patients had solitary and metachronous hepatic metastases with a long disease-free interval. There were two long-term (>5 years) survivors in our study. Although not early stage, they had a long interval between gastrectomy and hepatectomy. This observation may provide a clue for at least one of the indications for resection. Regardless, additional studies are necessary to clarify the role of hepatic resection for metastases from gastric adenocarcinomas.
Hepatic resection can be a feasible procedure for treating hepatic metastases from gastric adenocarcinomas. Although this study was small and involved selected cases, the outcomes of hepatic resections were comparable and long-term (>5 years) survivors were identified. Surgical resection of the liver can be considered a feasible option in managing hepatic metastases from gastric adenocarcinomas.
References
- 1.Marrelli D, Roviello F, De Stefano A, Fotia G, Giliberto C, Garosi L, et al. Risk factors for liver metastases after curative surgical procedures for gastric cancer: a prospective study of 208 patients treated with surgical resection. J Am Coll Surg. 2004;198:51–58. doi: 10.1016/j.jamcollsurg.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Ochiai T, Sasako M, Mizuno S, Kinoshita T, Takayama T, Kosuge T, et al. Hepatic resection for metastatic tumours from gastric cancer: analysis of prognostic factors. Br J Surg. 1994;81:1175–1178. doi: 10.1002/bjs.1800810832. [DOI] [PubMed] [Google Scholar]
- 3.Saiura A, Umekita N, Inoue S, Maeshiro T, Miyamoto S, Matsui Y, et al. Clinicopathological features and outcome of hepatic resection for liver metastasis from gastric cancer. Hepatogastroenterology. 2002;49:1062–1065. [PubMed] [Google Scholar]
- 4.Zacherl J, Zacherl M, Scheuba C, Steininger R, Wenzl E, Mühlbacher F, et al. Analysis of hepatic resection of metastasis originating from gastric adenocarcinoma. J Gastrointest Surg. 2002;6:682–689. doi: 10.1016/s1091-255x(01)00075-0. [DOI] [PubMed] [Google Scholar]
- 5.Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. 2002;235:86–91. doi: 10.1097/00000658-200201000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakamoto Y, Ohyama S, Yamamoto J, Yamada K, Seki M, Ohta K, et al. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery. 2003;133:507–511. doi: 10.1067/msy.2003.147. [DOI] [PubMed] [Google Scholar]
- 7.Koga R, Yamamoto J, Ohyama S, Saiura A, Seki M, Seto Y, et al. Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol. 2007;37:836–842. doi: 10.1093/jjco/hym113. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. 2007;95:534–539. doi: 10.1002/jso.20739. [DOI] [PubMed] [Google Scholar]
- 9.Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19:1146–1153. doi: 10.1093/annonc/mdn026. [DOI] [PubMed] [Google Scholar]
- 10.Koga S, Kawaguchi H, Kishimoto H, Tanaka K, Miyano Y, Kimura O, et al. Therapeutic significance of noncurative gastrectomy for gastric cancer with liver metastasis. Am J Surg. 1980;140:356–359. doi: 10.1016/0002-9610(80)90167-1. [DOI] [PubMed] [Google Scholar]
- 11.Okuyama K, Isono K, Juan IK, Onoda S, Ochiai T, Yamamoto Y, et al. Evaluation of treatment for gastric cancer with liver metastasis. Cancer. 1985;55:2498–2505. doi: 10.1002/1097-0142(19850515)55:10<2498::aid-cncr2820551032>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Ambiru S, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Yoshidome H, et al. Benefits and limits of hepatic resection for gastric metastases. Am J Surg. 2001;181:279–283. doi: 10.1016/s0002-9610(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 13.Shirabe K, Shimada M, Matsumata T, Higashi H, Yakeishi Y, Wakiyama S, et al. Analysis of the prognostic factors for liver metastasis of gastric cancer after hepatic resection: a multi-institutional study of the indications for resection. Hepatogastroenterology. 2003;50:1560–1563. [PubMed] [Google Scholar]
- 14.Bines SD, England G, Deziel DJ, Witt TR, Doolas A, Roseman DL. Synchronous, metachronous, and multiple hepatic resections of liver tumors originating from primary gastric tumors. Surgery. 1993;114:799–805. [PubMed] [Google Scholar]
- 15.Hirai I, Kimura W, Fuse A, Isobe H, Hachiya O, Moriya T, et al. Surgical management for metastatic liver tumors. Hepatogastroenterology. 2006;53:757–763. [PubMed] [Google Scholar]
- 16.Miyazaki M, Itoh H, Nakagawa K, Ambiru S, Shimizu H, Togawa A, et al. Hepatic resection of liver metastases from gastric carcinoma. Am J Gastroenterol. 1997;92:490–493. [PubMed] [Google Scholar]
- 17.Roh HR, Suh KS, Lee HJ, Yang HK, Choe KJ, Lee KU. Outcome of hepatic resection for metastatic gastric cancer. Am Surg. 2005;71:95–99. [PubMed] [Google Scholar]
- 18.Fujii K, Fujioka S, Kato K, Machiki Y, Kutsuna Y, Ishikawa A, et al. Resection of liver metastasis from gastric adenocarcinoma. Hepatogastroenterology. 2001;48:368–371. [PubMed] [Google Scholar]
- 19.Nakamura S, Suzuki S, Baba S. Resection of liver metastases of colorectal carcinoma. World J Surg. 1997;21:741–747. doi: 10.1007/s002689900300. [DOI] [PubMed] [Google Scholar]
- 20.Cady B, Stone MD, McDermott WV, Jr, Jenkins RL, Bothe A, Jr, Lavin PT, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992;127:561–568. doi: 10.1001/archsurg.1992.01420050085011. [DOI] [PubMed] [Google Scholar]
- 21.Kazemier G. Unresectable colorectal cancer liver metastases treated by intraoperative radiofrequency ablation with or without resection (Br J Surg 2012; 99: 558-565) Br J Surg. 2012;99:566. doi: 10.1002/bjs.8724. [DOI] [PubMed] [Google Scholar]
- 22.Cheung TT, Ng KK, Chok KS, Chan SC, Poon RT, Lo CM, et al. Combined resection and radiofrequency ablation for multifocal hepatocellular carcinoma: prognosis and outcomes. World J Gastroenterol. 2010;16:3056–3062. doi: 10.3748/wjg.v16.i24.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]