Abstract
Purpose
Endoscopic submucosal dissection has recently been practiced on a differentiated type of early gastric cancer. However, there is no clear evidence for endoscopic treatments of signet ring cell carcinoma. The aim of this study is to identify the predictive clinicopathological factors for lymph node metastasis in signet ring cell carcinoma for assisting endoscopic submucosal dissection trials.
Materials and Methods
A total of 186 patients with early signet ring cell carcinoma who underwent radical curative gastrectomy between January 2001 and September 2009 were enrolled in this study. Retrospective reviews of their medical records are being conducted. Several clinicopathologic factors were being investigated in order to identify predictive factors for lymph nodes metastasis: age, gender, tumor size, type of operation, tumor location, gross type, ulceration, Lauren's classification, depth of invasion, and lymphatic invasion.
Results
The lymph node metastasis rate for signet ring cell carcinoma was 4.3% (n=8). Of the 186 lesions with early signet ring cell carcinoma, 91 (48.9%) tumors were larger than 15 mm in size and 40 (21.5%) showed submucosal invasions in the resection specimens. In multivariate analysis, only the lymphatic invasion (P<0.0001) showed an association with lymph node metastasis. To evaluate cutoff values for tumor sizes in the presence of lymph node metastasis, early signet ring cell carcinomas with lymphatic invasions were excluded. In the absence of lymphatic invasion, mucosal cancer with tumor sizes <15 mm had no lymph node metastasis.
Conclusions
Endoscopic submucosal dissection can be performed on patients with early signet ring cell carcinoma limited to the mucosa and less than 15 mm.
Keywords: Stomach neoplasms; Carcinoma, signet ring cell; Endoscopic submucosal dissection; Lymph node metastasis; Predictive factor
Introduction
Endoscopic submucosal dissection (ESD) has been widely accepted as an alternative treatment for early gastric cancer (EGC).1 Due to the higher risk of lymph node metastasis (LNM) in undifferentiated EGC, application of ESD has been limited to EGC of well or moderately differentiated histology smaller than 30 mm in diameter and confined to the mucosa without ulceration.2,3 According to the Japanese Gastric Cancer Association, undifferentiated gastric carcinoma includes poorly differentiated adenocarcinoma and signet ring cell carcinoma (SRC).4
However, a recent study reported that undifferentiated EGC smaller than 15 mm and confined to mucosa or with minimal submucosal infiltration (≤500 µm) had no LNM,5 suggesting that patients with undifferentiated EGC under specific conditions could be considered as candidates for ESD. In addition, EGC with the histological features of SRC is a distinct biologic manifestation of gastric cancer.6 The lower rate of LNM and favorable prognosis of early SRC could be considered for curative ESD.7 However, there is little evidence for effectiveness of this procedure in clinical practice.
Thus, we conducted this study in order to determine the factors related to LNM in early SRC, and to guide the individual application of ESD in a suitable subgroup of patients with early SRC.
Materials and Methods
1. Patients
A total of 186 patients with early SRC, who had undergone radical gastrectomy with D2 lymph node dissection at Kyungpook National University Hospital, Daegu, Korea from January 2001 to September 2009, were enrolled in our study. The patients' medical records were reviewed retrospectively. Mixed type of SRC was excluded. This study was approved by our institutional review board (KNUMC_12-1050).
2. Clinicopathologic factors
Analysis of several clinicopathologic factors including age (<45 years, ≥45 years), sex (male or female), tumor size (maximum diameter <10 mm, or ≥10 mm), type of operation, tumor location (upper, middle, or lower third of the stomach), gross type (elevated, flat, depressed, or mixed), ulceration, Lauren's classification (intestinal, diffuse, mixed), depth of invasion (mucosa or submucosa) and lymphatic invasion was performed for identification of predictive factors for LNM.
3. Pathologic variables
SRC was defined pathologically as an adenocarcinoma in which the predominant component (more than 50 percent of the tumor) is made up of isolated or small groups of malignant cells containing intracytoplasmic mucin. The submucosal layer was graded into three layers (submucosal invasion depth [SM] 1: upper third, SM 2: middle third, SM 3: lower third). All histologic slides were re-examined in a blind manner by one pathologist (J.Y.P).
4. Analysis of endoscopic submucosal dissection on signet ring cell carcinoma
A total of 773 patients with EGCs underwent treatment by ESD in our center between January 2005 and December 2011, five partients (0.64%) had SRC. The mean follow-up duration was 22 months (range: 9~42). We reviewed these cases in order to assess the clinical efficacy of ESD according to our results.
5. Statistical analysis
The statistical software Statistical Package for Social Science (SPSS) version 18.0 for Windows (IBM Co., Armonk, NY, USA) was used in performance of statistical analysis. To identify risk factors predictive of LNM, the data were analyzed using the chi-squared test. Multivariate logistic regression analysis was performed for evaluation of independent risk factors affecting LNM. A P-value of less than 0.05 was considered statistically significant.
Results
1. Clinical features of patients
Of the 186 patients with early SRC who had undergone radical gastrectomy, 91 were male and 95 were female. Their mean age was 51.6 years (range: 26~76). The mean tumor size was approximately 20 mm. The mean number of removed lymph nodes was 31 (range: 6~103). Forty patients (21.5%) had submucosal invasion. According to submucosal layer category, there were 14 (7.5%), 13 (7.0%), and 13 (7.0%) SM 1, SM 2, and SM 3 lesions, respectively. And seven patients (3.8%) had lymphatic involvement. Among them, four patients had submucosal invasion. A summary of the baseline characteristics of enrolled patients is shown in Table 1.
Table 1.
Baseline characteristics of enrolled patients

Values are presented as mean±standard deviation or number (%). M = male; F = female; SM = submucosal invasion depth.
2. Risk Factors for lymph node metastasis by univariate and multivariate analysis
Individual risk factors for LNM were investigated in early SRC. The rate of LNM was 4.3% (n=8). In univariate anlaysis, LNM showed a strongly association with lymphatic involvement (P<0.0001). Tumor size, ulceration and submucosal invasion did not show a significant association with LNM (Table 2).
Table 2.
Relationship between the clinicopathological factors and lymph node metastasis: univariated analysis results
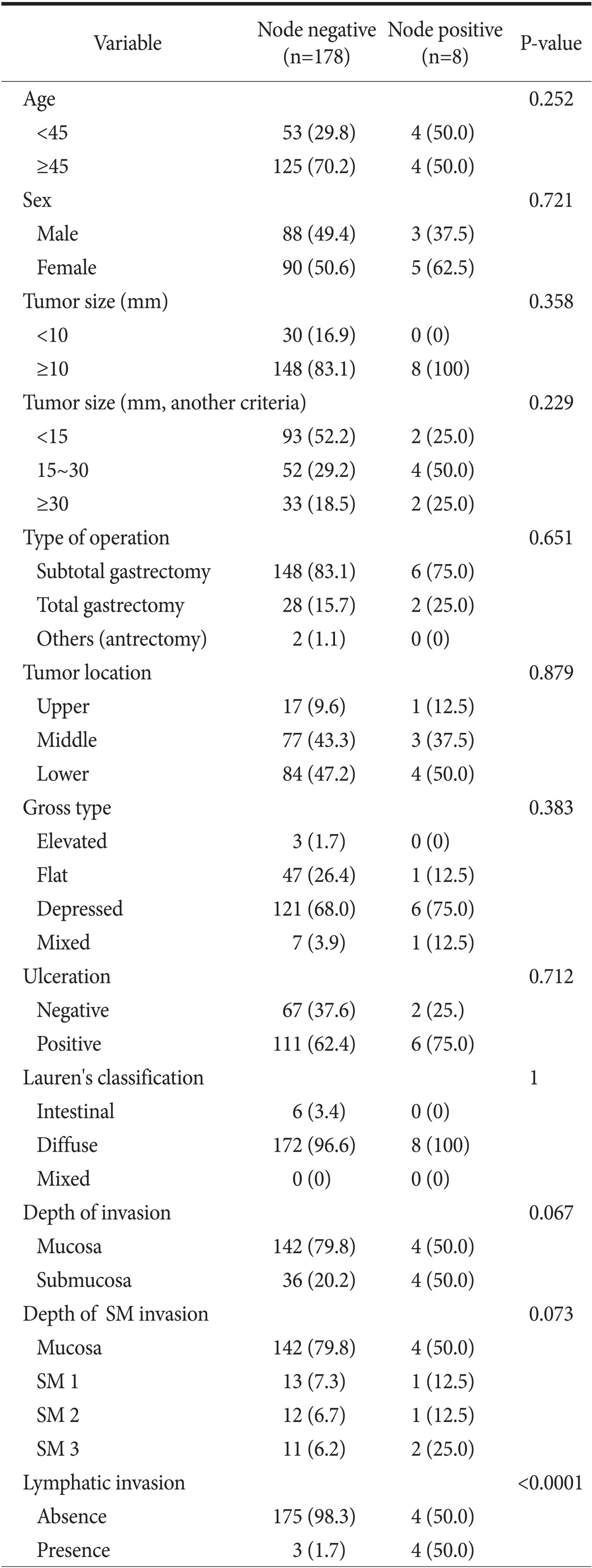
Values are presented as number (%). Chai-squre test was used. SM = submucosal invasion depth.
Multivariate analyses were performed in order to investigate the relationship with depth of invasion, lymphatic invasion, and ulceration. In multivariate analysis, the independent risk factors for LNM was lymphatic involvement (odds ratio: 58.333, 95% confidence interval: 9.6~351.2) (Table 3).
Table 3.
Relationship between the clinicopathological factors and lymph node metastasis: multivariated analysis results
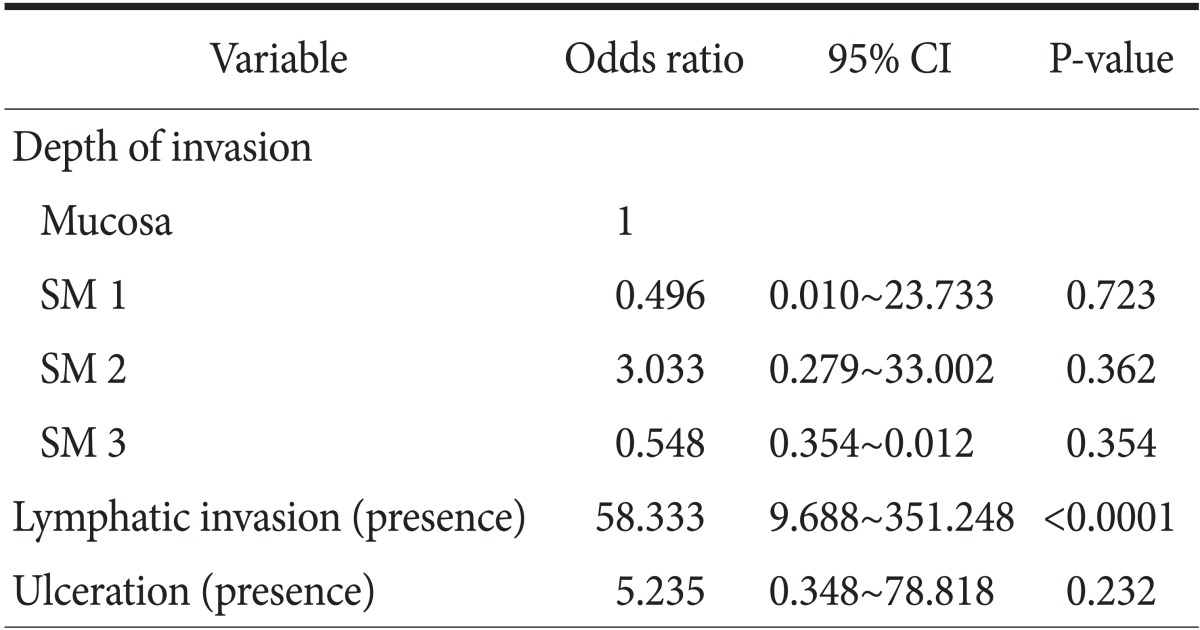
The multivariate logistic regression test was used. CI = confidence interval; SM = submucosal invasion depth.
3. Cutoff tumor size for endoscopic treatment
To evaluate cutoff values for tumor size in the presence of LNM, tumor size and submucosal invasion were stratified. After exclusion of early SRC with lymphatic invasion, a total of four patients were analyzed. In the absence of lymphatic invasion, mucosal cancer with tumor size <15 mm had no LNM.
4. Results of endoscopic submucosal dissection in signet ring cell carcinoma
Five patients with early SRC underwent treatment by ESD in our center from January 2005 to December 2011. We have treated early intramucosal SRCs without ulcer findings <20 mm in size with ESD as the expanded indication. All of the lesions were smaller than 15 mm and intramucosal carcinoma without ulceration and lymphatic invasion. Endoscopic follow-up examinations were performed routinely at 3, 6, 12, and 18 months, and then annually to assess for the completeness of resection and for detection of metachronous lesions. Abdominal computed tomography was performed at 6 and 12 months, and then annually to assess distant metastasis. Overall follow-up period was 22 months, and there was no local or distant recurrence (Table 4).
Table 4.
Results of ESD of early SRC in our center
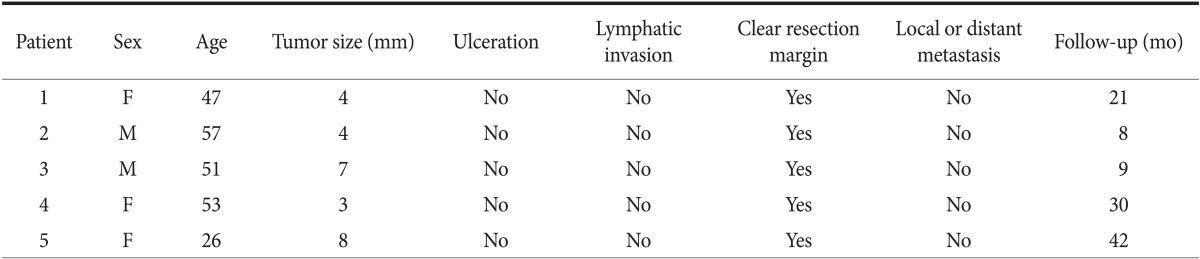
ESD = Endoscopic submucosal dissection; SRC = signet ring cell carcinoma; F = female; M = male.
Discussion
As a result of advances in diagnostic technology, including both the radiologic and endoscopic modalities, the incidence of EGC has increased. Due to the higher rate of LNM in the undifferentiated histologic type, the accepted indications for ESD are limited to differentiated EGC. Recently, according to Japanese gastric cancer treatment guidelines 2010 (ver. 3),8 ESD for undifferentiated type tumors clinically diagnosed as T1a (mucosa confined) and less than 2 cm in diameter without ulceration is regarded as an investigational treatment (expanded indication). However, evidence regarding the curability of ESD under the expanded criteria remains insufficient,9 and should be prospectively evaluated in appropriate clinical research settings. Therefore, in this study, we attempted to establish a criterion for expansion of the possibility of using ESD for treatment.
Early SRC differs significantly from non-SRC, in terms of the depth of invasion, and LNM.10 The prognosis of early SRC is more favorable than that of non-SRC.7,10,11 One study reported a strong association of poorly differentiated EGC with the presence of ulcer, submucosal invasion, and lymphovascular invasion, compared with early SRC. This means that poorly differentiated EGC is more complicated than early SRC when considering endoscopic treatment.12 Thus, we sought to expand the use of ESD to early SRC by retrospective examination to determine predictive factors of LNM.
Previous studies have reported that tumor size, lymphatic invasion, and depth of invasion were significant factors for prediction of LNM.13,14 A recent study12 reported that early SRC with mucosal invasion, size <15 mm, and no lymphatic invasion had no LNM. Another study15,16 reported that early SRC with mucosal invasion, size ≤2 cm, and no lymphatic invasion had no LNM. In our study, in univariate and multivariate analysis, LNM of early SRC showed a strong association with lymphatic invasion. We also analyzed LNM in terms of tumor size and depth of invasion. After exclusion of cases of early SRC with lymphatic involvement, four patients were analyzed. LNM was found only in cases in which the tumor size was greater than 15 mm. In our study, there was no significant finding between depth of invasion and LNM. However, there was a positive tendency for LNM in patients with submucosal invasion (P=0.067). This may be due to the small number of cases.
Based on the findings of our study, we supposed that ESD could be possible in early SRC confined to mucosa, less than 15 mm in size. Actually, in our center, five cases of early SRCs treated by ESD were technically feasible during the follow-up period. Similarly, one study17 reported that complete endoscopic resection was possible in the majority of tumors ≤20 mm in size, as well as in intramucosal carcinomas, and no recurrence and no metastasis occurred after ESD during a median follow-up period of 13 months.
There are several limitations in the design of this study. One potential limitation of our study is that it was conducted as a single center retrospective study with a small number of cases. However, all patients who underwent radical gastrectomy were reviewed and histologic slides were re-examined by one pathologist during the study period in order to obtain a higher accuracy. Another potential limitation was the small number of cases of ESD in early SRC in our clinical setting. A recent study reported that ESD for undifferentiated EGC fulfilling the expanded criteria was achieved in 99.9% (102/103) of en bloc and curative resection and yielded good long-term outcomes.18 Use of ESD for undifferentiated EGC has only begun recently, therefore, the precise usefulness of ESD for early SRC remains to be evaluated prospectively in a large scale trial.
Our results were similar to those of previous studies and outcomes of ESD were favorable in actual clinical settings. Therefore, although there is little information on long-term follow-up, ESD of early SRC may be reasonable under specific tumor conditions. Conduct of further studies is needed in order to validate expanded indications for ESD in patients with early SRCs.
References
- 1.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 2.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe N, Watanabe T, Suzuki K, Machida H, Toda H, Nakaya Y, et al. Risk factors predictive of lymph node metastasis in depressed early gastric cancer. Am J Surg. 2002;183:168–172. doi: 10.1016/s0002-9610(01)00860-1. [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 5.Park YD, Chung YJ, Chung HY, Yu W, Bae HI, Jeon SW, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7–10. doi: 10.1055/s-2007-966750. [DOI] [PubMed] [Google Scholar]
- 6.Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, et al. Signet ring cell carcinoma of the stomach. Cancer. 1992;69:1645–1650. doi: 10.1002/1097-0142(19920401)69:7<1645::aid-cncr2820690702>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Akiyama H. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319–1324. doi: 10.1002/bjs.4637. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Jung HY. Expansion of indication for endoscopic SD in early gastric cancer. J Gastric Cancer. 2010;10:49–54. [Google Scholar]
- 10.Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, et al. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78–83. doi: 10.1002/cncr.10120. [DOI] [PubMed] [Google Scholar]
- 11.Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, et al. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg. 2004;74:1060–1064. doi: 10.1111/j.1445-1433.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim HM, Pak KH, Chung MJ, Cho JH, Hyung WJ, Noh SH, et al. Early gastric cancer of signet ring cell carcinoma is more amenable to endoscopic treatment than is early gastric cancer of poorly differentiated tubular adenocarcinoma in select tumor conditions. Surg Endosc. 2011;25:3087–3093. doi: 10.1007/s00464-011-1674-5. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa S, Togashi A, Inoue M, Honda S, Nozawa F, Toyama E, et al. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer. 2007;10:35–38. doi: 10.1007/s10120-006-0407-2. [DOI] [PubMed] [Google Scholar]
- 14.Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, et al. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009;41:498–503. doi: 10.1055/s-0029-1214758. [DOI] [PubMed] [Google Scholar]
- 15.Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508–513. doi: 10.1245/s10434-007-9660-9. [DOI] [PubMed] [Google Scholar]
- 16.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 17.Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc. 2010;24:509–516. doi: 10.1007/s00464-009-0614-0. [DOI] [PubMed] [Google Scholar]
- 18.Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122–127. doi: 10.1055/s-0031-1291486. [DOI] [PubMed] [Google Scholar]


