Abstract
S-equol is a natural metabolite of the soy isoflavone, daidzein, produced by intestinal bacteria. S-equol has been shown to have greater estrogenic activity than other soy isoflavones and prevent bone loss in post-menopausal women. Estrogen regulates both bone remodeling and hemopoiesis in the bone marrow, these processes that communicate closely with each other. In this study, we investigated the effect of S-equol on bone mass and gene expression of bone marrow cells in ovariectomized (OVX) mice. Female ddY strain mice, aged 12 weeks, were either sham operated or OVX. The OVX mice were randomly divided into two groups: (1) OVX control and (2) OVX fed a 0.06% (w/w) S-equol supplemented diet. After 2 weeks, the trabecular bone volume of the femoral distal metaphysis was markedly reduced in OVX mice. However, treatment with equol was observed to ameliorate this. Expression of inflammatory-, osteoclastogenesis- and adipogenesis-related genes was increased in OVX mice compared with sham mice, and equol was observed to suppress their expression. The present study demonstrates that equol might ameliorate bone loss caused by estrogen deficiency through regulating hemopoiesis and production of inflammatory cytokines in bone marrow cells.
Keywords: S-equol, isoflavone, bone loss, inflammatory related gene, ovariectomized mice
Introduction
Osteoporosis, a disorder of inadequate skeletal strength predisposing to fracture, is one of the most common human conditions associated with advancing age. A number of factors, including hormones, nutrition, physical activity and genetics, are involved in the pathogenesis of osteoporosis.(1,2) Estrogen deficiency results in marked bone loss due to increased bone resorption by osteoclasts, and estrogen replacement prevents such loss.(3) There is a close relationship between bone remodeling and hemopoiesis in the bone marrow in estrogen deficient states. Masuzawa et al.(4) reported that estrogen deficiency, caused by ovariectomy, stimulates B-lymphopoiesis, resulting in marked accumulation of pre-B cells in murine bone marrow. In contrast, newly formed B cells and pre-B cells are noticeably depressed in pregnant or estrogen-treated mice.(5) Furthermore, various cytokines, such as interleukin (IL)-6, IL-7, tumor necrosis factor (TNF)α and TNFβ are involved in the growth and differentiation of hemopoietic cells, and most of these cytokines are also involved in bone remodeling.(6) Miyaura et al.(7) showed that changes in both B-lymphopoiesis and bone mass in IL-7-treated female mice were similar to those in ovariectomized (OVX) mice. T cells are also key inducers of bone wasting because ovariectomy resulted in expansion of a population of TNF-producing T cells in bone marrow, but failed to induce bone loss in T-cell-deficient nude mice.(8,9) These findings suggest a complex link between hormones and hemopoiesis in bone marrow, is involved in bone loss induced by estrogen deficiency.
Soy isoflavones have structural similarities to 17β-estradiol, exhibiting weakly estrogenic action by binding to estrogen receptors (ERs). These have attracted wide attention because of their potential beneficial effects in preventing menopausal symptoms, osteoporosis, cardiovascular diseases and cancers.(10) Equol, a gut bacterial metabolite of a soy isoflavone, daidzein, binds to ERs and induces transcription more strongly than other soy isoflavonoids.(11) Moreover, equol is a chiral molecule, which exists as enantiomers R-equol and S-equol. In humans, the intestinal bacterial metabolism of daidzein to equol results in S-equol production only.(12) Setchell et al.(13) reported that S-equol, but not R-equol, has a relatively high affinity for ERs. Epidemiologic studies suggest that high equol producers are at lower risk of breast cancer than low equol producers.(14) It is noteworthy that postmenopausal high equol producers had smaller bone loss changes than low producers.(15) In addition, S-equol supplementation prevented a decrease in bone mineral density (BMD) of postmenopausal women without adverse effects.(16) Interestingly, equol inhibits bone loss, apparently without estrogenic activity in the reproductive organs of OVX mice.(17) These results suggest that equol is similar to selective ER modulators with respect to preventing bone loss without estrogenic activity in the reproductive organs.
Although estrogen regulates bone metabolism, at least in part via bone marrow cells, it is unclear how equol affects bone and bone marrow. In this study, we examined the effect of S-equol on bone loss caused by estrogen deficiency, including the mechanism by which equol exerts its effect on bone mass, using analysis of gene expression in bone marrow cells.
Materials and Methods
Animals and chemicals
Female ddY strain mice, aged 12 weeks, were purchased from the Shizuoka Laboratory Animal Center. Mice were housed in individual cages in a temperature- and humidity-controlled room (23 ± 1°C and 60 ± 5% relative humidity) with a 12-h light/dark cycle. Mice were given free access to an AIN-93M diet with corn oil instead of soybean oil for 5 days before performing the operation. Mice were either sham operated (sham) or OVX on the same day. OVX mice were randomly divided into two groups: 1) OVX control (OVX); and 2) OVX fed a 0.06% S-equol supplemented diet (OVX + Equol). We confirmed that the bone loss in OVX animal fed a 0.06% S-equol containing diet was effectively suppressed without causing notable effects in reproductive organs (unpublished data). Thus, in order to examine the mechanism of suppressive effect of S-equol on bone loss caused by estrogen deficiency, we investigated how 0.06% S-equol affects gene expression in bone marrow cell in OVX mice. Table 1 shows the composition of the experimental diets, which were prepared according to the AIN-93M formulation. (18) Corn oil was used to eliminate any possible contamination from isoflavones in soybean oil. S-equol was added to the diet instead of cornstarch. Mice were pair-fed their respective diets for 2 weeks with free access to distilled water during this period. After 2 weeks of treatment, mice were euthanized by exsanguination under anesthesia and weighed. The uterus, thymus and spleen were removed and these tissue wet weights were measured. The right and left tibiae were removed to extract total RNA from the bone marrow cells. For BMD measurement, the right femur was removed and stored in 70% ethanol. All procedures were undertaken in accordance with the National Institute of Health and Nutrition Guidelines for the Care and Use of Laboratory Animals.
Table 1.
Composition of the experimental dieta
| Ingredient | Controlb | Equolc |
|---|---|---|
| g/100 g diet | ||
| Casein | 14 | 14 |
| L-cystine | 0.18 | 0.18 |
| Cornstarch | 46.6 | 46.5 |
| Pregelatinized cornstarch | 15.5 | 15.5 |
| Sucrose | 10 | 10 |
| Corn oil | 4 | 4 |
| Cellulose powder | 5 | 5 |
| AIN-93M mineral mixture | 3.5 | 3.5 |
| AIN-93 vitamin mixture | 1 | 1 |
| Choline bitartrate | 0.25 | 0.25 |
| tert-butylhydroquinone | 0.0008 | 0.0008 |
| S-equol | — | 0.064 |
aPrepared according to the AIN-93M formulation. bControl diet. cEquol containing diet.
Radiographic analysis of the femur
Femurs were excised and BMD was measured by dual-energy X-ray absorptiometry (model DCS-600EX-R, Aloka, Tokyo, Japan). BMD was calculated using the bone mineral content (BMC) of the measured area. The BMC of the mouse femur was closely correlated with its ash weight (r = 0.978). The scanned area of the mouse femur was equally divided into the following three parts: proximal femur, midshaft and distal femur.
Three-dimensional (3D) analysis of trabecular microarchitecture by microcomputed tomography (µCT)
µCT (inspeXio SMX-90CT; Shimadzu, Japan) was used to assess trabecular bone morphology in the distal femur using a 15-µm isotropic voxel size with 90 kV of tube voltage and 110 µA of tube current. 3D CT images were reconstituted and analyzed using TRI/3D-BON analysis software (Ratoc, Tokyo, Japan). Trabecular morphometry was characterized by measuring the bone volume fraction (bone volume per tissue volume; BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular spacing (Tb.Sp).
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from bone marrow of the tibia using Isogen II (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instruction. The complementary DNA (cDNA) was synthesized from 1 µg of total RNA using Prime Script RT Master Mix (Takara, Shiga, Japan). cDNA was quantified by RT-PCR on MiniOpticon Real-Time PCR System (Bio-Rad, CA) using SYBR Premix Ex Taq II (Takara, Shiga, Japan). Cycling conditions were 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. Table 2 shows the primer sequences.
Table 2.
Sequences of primers used for real-time PCR
| Gene | Forward primer (5'to3') | Reverse Primer (5'to3') |
|---|---|---|
| Oct-2 | ATCAAGGCTGAAGACCCCAGTG | TGGAGGAGTTGCTGTATGTCCC |
| TNFSF13B | GGCAGGTACTACGACCATCTC | TGGGCCTTTTCTCACAGAAGT |
| IL-7 | TCCTCCACTGATCCTTGTTC | CTTCAACTTGCGAGCAGCAC |
| IL-7R | GCGGACGATCACTCCTTCTG | AGCCCCACATATTTGAAATTCCA |
| CD40L | TCGGGAGCCTTCGAGTCA | GATCCACTGCTGGGCTTCAG |
| CD28 | CTGGCCCTCATCAGAACAAT | GGCGACTGCTTTACCAAAATC |
| RANKL | TGAAGACACACTACCTGACTCCTG | CCACAATGTGTTGCAGTTCC |
| NFATc1 | GCTTCACCCATTTGCTCCAG | ATGGTGTGGAAATACGGTTGGTC |
| CTSK | CACCCAGTGGGAGCTATGGAA | GCCTCCAGGTTATGGGCAGA |
| PPARγ | TTTTCAAGGGTGCCAGTTTC | AATCCTTGGCCCTCTGAGAT |
| C/EBPα | TTGAAGCACAATCGATCCATCC | GCACACTGCCATTGCACAAG |
| FAS | TTGCTGGCACTACAGAATGC | AACAGCCTCAGAGCGACAAT |
| β-actin | CCACAGCTGAGAGGGAAATC | AAGGAAGGCTGGAAAAGAGC |
TNFSF13B, tumor necrosis factor superfamily, member 13b; IL-7, interleukin-7; IL-7R, interleukin-7 receptor; CD40L, CD40 ligand; RANKL, receptor activator of nuclear factor-κB ligand; NFATc1, nuclear factor of activated T cells c1; CTSK, cathepsin K; PPARγ, peroxidase proliferator–activated receptor-γ; C/EBPα, CCAAT/enhancer-binding protein-α; FAS, fatty acid synthase.
RNA isolation and cDNA microarray analysis
Femora were harvested, immediately frozen in liquid nitrogen and stored at –80°C until required. Frozen bones were homogenized using a Polytron homogenizer (Kinematica, Lucerne, Switzerland). Total RNA was isolated and purified using the RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA target preparation for microarray analysis was performed using the GeneChip® 3’ IVT Express Kit (Affymetrix Japan K.K., Tokyo, Japan) according to the manufacturer’s instructions. Briefly, double-stranded cDNA was synthesized from the total RNA (500 ng) of each mouse with a T7-Oligo (dT) primer. After in vitro transcription to synthesize biotin-labeled aRNA, purification using Magnetic Stand-96 (Life Technologies, Tokyo, Japan) and fragmentation of the labeled aRNA were performed. Fifteen-microgram aliquots of fragmented aRNA were hybridized to an array (Mouse Genome 430 2.0 array, Affymetrix) at 45°C for 16 h. After hybridization, the gene chips were washed and stained using a GeneChip Fluidics Station 450 (Affymetrix) and then scanned (GeneChip Scanner; Affymetrix) with the GeneChip Operation Software ver. 1.4 (Affymetrix). Analysis of the DNA microarray data was performed using Microarray Suite and GeneSpring ver. 11.5 (Agilent Technologies, Santa Clara, CA). The expression level of each gene was expressed as an average of those of 5 mice in each group. The genes up- or down-regulated more than 1.5 fold in the mice femur of OVX group as compared to sham group were analyzed using Ingenuity Pathway Analysis. (Ingenuity® Systems, http://www.ingenuity.com).
Statistics
Data are expressed as means ± SEM. The significance of difference for BMD was determined by single-factor analysis of covariance and post hoc Bonferroni’s multiple-comparison tests (SPSS ver. 15.0; SPSS, Chicago, IL). Body weight was used as a covariate in the analysis of BMD and trabecular morphologic parameters to adjust for possible confounding effects. The remaining data were analyzed using analysis of variance. Differences between treatment groups were assessed by Tukey’s test. Differences were considered significant at p<0.05.
Results
Body and tissue weight
Initial and final body weights did not differ significantly among the three groups (Table 3). Uterine weight was lower in the OVX group than in the sham group (p<0.05), and it was not significantly different between the OVX and OVX + Equol groups. Spleen weight was higher in the OVX group than in the sham group. In contrast, there was no significant difference in spleen weight between the OVX + Equol and sham groups.
Table 3.
Body and organ weight in OVX mice fed with control and equol diets
| Sham | OVX | OVX + Equol | |
|---|---|---|---|
| Uterus (mg) | 120.1 ± 11.6a | 36.6 ± 3.7b | 65.3 ± 10.4b |
| Thymus (mg) | 67.8 ± 6.9 | 59.6 ± 3.2 | 58.7 ± 2.6 |
| Spleen (mg) | 96.8 ± 5.7b | 151.3 ± 20.1a | 116.5 ± 10.8ab |
| First body weight (g) | 28.6 ± 0.5 | 28.3 ± 0.3 | 28.3 ± 0.4 |
| Final body weight (g) | 31.6 ± 0.5 | 33.5 ± 0.6 | 31.7 ± 0.5 |
Values are means ± SEM (n = 9–10); Means with different letters differ significantly; p<0.05.
Effect of equol on bone mass in OVX mice
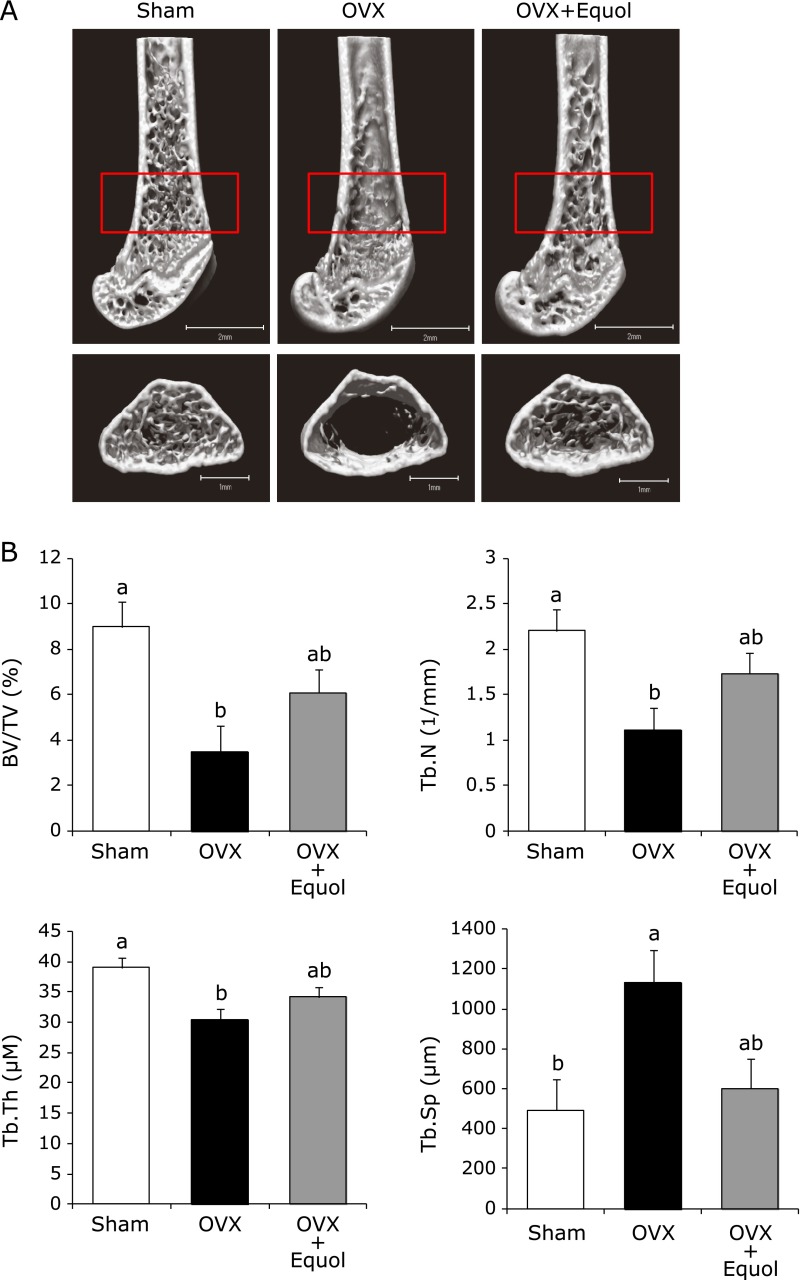
BMD of the distal femur in OVX mice was significantly lower than in the sham mice, whereas treatment with equol for 2 weeks in OVX mice was inclined to inhibit bone loss in the distal femur (Table 4). To confirm the amelioration of cancellous bone mass by equol treatment in OVX mice, bone morphometric analysis was performed using µCT in the trabecular bone of the distal femoral metaphysis. The connecting rods were well maintained in the sham group. In the OVX group, however, many of the connecting rods were missing. Treatment with equol prevented trabecular bone loss in OVX mice, and the 3D trabecular bone architecture was maintained and similar to that of the sham group (Fig. 1A). In the OVX group, BV/TV, Tb.Th and Tb.N were significantly reduced, whereas Tb.Sp was increased compared with the sham group. The increase in Tb.Sp indicates that osteoclastic bone resorption was stimulated, resulting in enhanced inter-trabecular space. Treatment with equol tended to prevent the decrease in BV/TV, Tb.Th and Tb.N, and the increase in Tb.Sp (Fig. 1B). These results indicate that treatment with equol for 2 weeks prevents bone loss caused by estrogen deficiency.
Table 4.
Effect of equol on BMD of femora in OVX mice
| Sham | OVX | OVX + Equol | |
|---|---|---|---|
| BMD of femur (mg/cm2) | |||
| Whole femur | 46.55 ± 0.95 | 43.21 ± 1.02 | 45.72 ± 0.90 |
| Proximal region | 48.57 ± 0.86 | 46.61 ± 0.91 | 49.14 ± 0.80 |
| Middle region | 38.82 ± 0.95 | 37.19 ± 1.01 | 38.15 ± 0.89 |
| Distal region | 52.26 ± 1.24a | 45.84 ± 1.32b | 49.88 ± 1.17ab |
Values are expressed as means ± SEM (n = 9–10). Significant differences in BMD were determined by single-factor analysis of covariance and post hoc Bonferroni’s multiple-comparison tests. Body weight was used as a covariate in the analysis of BMD to adjust for a possible confounding effect. Means with different letters differ significantly; p<0.05.
Fig. 1.
µCT scanning of trabecular bone from sham mice (Sham), OVX mice (OVX) and OVX mice treated for 2 weeks with equol (OVX + Equol). (A) 3D image of the distal femora of representative mice. The lower figure is a trabecular-rich region of the distal femur, surrounded in a red frame on the upper figure. B, 3D microstructural parameters using µCT shown in A. Microstructural parameters of bone structure include bone volume per tissue volume (BV/TV, %), trabecular number (Tb.N, 1/mm), trabecular thickness (Tb.Th, µm) and trabecular separation (Tb.Sp, µm). Values are expressed as means ± SEM (n = 9–10). Means with different letters differ significantly; p<0.05.
Quantitation of mRNA expression in bone marrow cells from the tibia
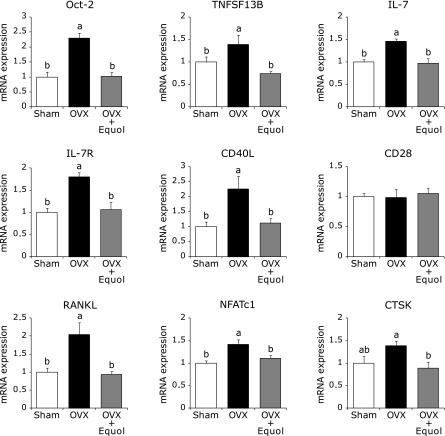
We determined gene expression in bone of sham, OVX or OVX-equol mice using DNA microarrays. Probably because a bone consists of several types’ cells, such as osteocytes, osteoblasts, osteoclasts and bone marrow cells, a one-way ANOVA of the gene expression data did not detect any significant differences in bone gene expression of these three groups. Then we analyzed the group of genes that average expression was more than 1.5-fold different between sham and OVX groups using Ingenuity Pathway Analysis. The result showed that the gene group significantly contained the genes related to inflammatory response. Expression of immune response genes increased in OVX mice, and most of these genes were unaltered in equol-treated OVX mice compared with sham mice (data not shown). In light of the cDNA microarray results, we investigated the effect of equol on mRNA expression of bone marrow cells from the tibia, including B cells, T cells and stromal cells, all of which participate in the immune system. In the OVX group, mRNA expression of Oct-2, expressed predominantly in B cells,(19) was significantly increased compared with the sham group, and treatment with equol suppressed this increase to the same level as the sham group. TNF superfamily member, 13b (TNFSF13B), also known as B cell activation factor (BAFF), is capable of inducing osteoclast formation.(20) Expression of this gene was increased in the OVX group compared with the sham and OVX + Equol groups. mRNA expression of IL-7 was significantly increased in OVX mice compared with sham mice, and treatment with equol reduced expression of this gene to the same level as sham mice. In addition, the increase of IL-7 receptor (IL-7R) expression in OVX mice was suppressed by equol treatment. CD40-ligand (CD40L) is a molecule expressed on activated T cells during antigen presentation by antigen-presenting cells, including B cells.(21) Expression of this gene was induced by OVX, but not by equol treatment in OVX mice. However, expression of CD28, which is constitutively expressed on all T cells,(22) did not differ among the three groups.
Receptor activator of nuclear factor-kappa B ligand (RANKL) stimulates osteoclastogenesis through the nuclear factor of activated T cells cytoplasmic 1 (NFATc1), which is well known as a master transcription factor for osteoclastogenesis.(23) Expression of RANKL and NFATc1 was increased in the OVX group; however, there was no difference in expression of these genes between the OVX + Equol and sham groups. Cathepsin K (CTSK) is a marker of osteoclast differentiation. CTSK expression tended to increased in the OVX group compared with the sham group, and this increase was suppressed by treatment with equol (Fig. 2). These results suggest that increased expression of genes involved in the inflammatory response in OVX mice is suppressed by treatment with equol.
Fig. 2.
Effect of equol on mRNA expression of bone marrow cells collecting from the tibia. Mice were treated with equol for 2 weeks and cells were isolated. Expression of Oct-2, TNFSF13B, IL-7, IL-7R, CD40L, CD28, RANKL, NFATc1 and CTSK were determined by qRT-PCR. The ordinate axis indicates the relative amount of mRNA compared with sham mice. Gene expression levels were normalized with β-actin. Values are expressed as means ± SEM (n = 8). Means with different letters differ significantly; p<0.05.
Expression of adipogenesis-related genes in bone marrow
We investigated the effect of equol on adipogenesis-related gene expression of bone marrow cells in OVX mice. Peroxidase proliferator-activated receptor-γ (PPARγ) and CCAAT /enhancer-binding protein-α (C/EBPα) are known to play a critical role in adipogenic differentiation.(24) As shown in Fig. 3, PPARγ mRNA level in bone marrow cells was higher in the OVX group than in the other groups, and was similar between the OVX + Equol and the sham group. Expression of C/EBPα was lower in the OVX + Equol group compared with the OVX group. Fatty acid synthase (FAS), which is a key enzyme on adipogenesis and is regulated by PPARγ, indicates differentiation and maturation of adipocyte.(25) FAS mRNA expression was higher in the OVX group compared with the sham group, and this increase was suppressed by treatment with equol. These results suggest that equol treatment suppresses expression of adipogenesis-related genes induced by estrogen deficiency in bone marrow cells.
Fig. 3.
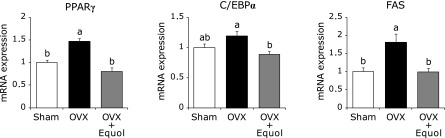
Effect of equol on adipogenesis-related gene expression by tibia bone marrow cells. Mice were treated for 2 weeks with equol, and bone marrow cells were isolated. Expression of PPARγ, C/EBPα or FAS were determined by qRT-PCR. The ordinate axis indicates the relative amount of mRNA compared with sham mice. Gene expression levels were normalized by β-actin. Values are expressed as means ± SEM (n = 8). Means with different letters differ significantly; p<0.05.
Discussion
This study demonstrates that equol treatment for 2 weeks in OVX mice prevents bone loss and changes in bone marrow cell gene expression induced by estrogen deficiency. When we analyzed the effect of equol on gene expression of the whole femur in OVX mice using cDNA micro-array analysis, some inflammatory response genes were up-regulated in OVX mice compared with sham mice, but were unchanged in equol-treated mice (data not shown). The effect of equol on bone marrow cell gene expression analyzed by qRT-PCR reflected the cDNA micro-array results.
Equol is present in humans and animals only as the S-equol which has higher ERs affinity than R-equol.(13) Kimira et al.(26) indicated that the preventive effect of S-equol on bone loss caused by OVX was greater than that of racemic equol. In addition, Yoneda et al.(27) reported that S-equol improved the increased tail-skin temperature in a rat model of hot flushes. S-equol has been shown to be rapidly absorbed and excreted from the body in humans and rats.(28,29) Equol has been reported that almost 50% circulates unbound to serum protein, which is significantly higher than daidzein.(30) In addition, S-equol is a highly stable molecule that undergoes no further metabolism, since the plasma clearance rates of S-equol was slower than the rates for daidzein or genistein.(12,28) These findings suggest that S-equol may exert physiological effects at relatively lower doses than other isoflavones.
In this study, uterine weight of the OVX + Equol group was not significantly higher compared with that of the OVX group. Schwen et al.(29) reported that orally administered S-equol, amount of which was higher than that of dairy intake of S-equol in the present study, did not affect uterine weight and its morphology in normal female rats. In addition, cytotoxicity or genotoxicity of S-equol was not observed in the bone marrow micronucleus test in rats dosed at levels up to the standard limit of 2,000 mg/kg body weight.(31) Although further studies are needed for safety of S-equol, it is seemed that the amount of S-equol used in this study does not show adverse effects in normal animals. The spleen weight, but not the thymus weight, increased in OVX mice and equol treatment was observed to ameliorate this change (Table 1). Ovariectomy is known to increase the weight and immune cell population in spleen and thymus, and to change distribution of the cells in these organs.(32) On the other hand, since estrogen replacement recovers these changes, it is possible that equol also modulates the alteration of B cell maturation and T cell output induced by OVX.
We observed expression of inflammatory-related genes, including Oct-2, TNFSF13B, IL-7 and IL-7R, increased in the OVX group compared with the sham group, and these gene increases were suppressed by treatment with equol. In addition, the increase in osteoclastogenesis-related gene expression in OVX mice, including RANKL, NFATc1 and CTSK, were also inhibited by equol treatment (Fig. 2).
IL-7 regulates T cell and B cell homeostasis via IL-7R signaling.(33) Although IL-7R expression is down-regulated by IL-7 stimulation, regulation of this receptor occurs at the transcriptional level by multiple mechanisms.(34) IL-7 causes bone resorption through the activation of T cells and the T-cell dependent augmentation of osteoclastogenesis.(35) A previous study reported that bone loss induced by IL-7 was related to expansion of the B cell lineage, in particular B220-positive pre-B cells, as well as that caused by ovariectomy.(7) Furthermore, in vivo IL-7 blockade, using neutralizing antibodies, prevented OVX-induced bone destruction.(36) These findings suggest that IL-7 plays a key role in bone resorption stimulated by estrogen deficiency. Estrogen is a potent regulator of B lymphopoiesis at a very early stage.(5) Because B cells at multiple stages of differentiation can express bone-remodeling related cytokines, including RANKL,(37) changes in early B cells with estrogen deficiency may affect osteoclastogenesis and subsequent bone loss. Ishimi et al.(38) reported that genistein completely inhibited the increase in bone loss and B-lymphopoiesis induced by ovariectomy. Since equol and genistein have relatively strong estrogenic effect compared with other isoflavonoids, equol might prevent bone loss caused by estrogen deficiency via regulation of B-lymphopoiesis in bone marrow, similar to genistein.
In this study, although equol treatment suppressed increased mRNA expression of CD40L in the OVX group compared with the sham group, expression of CD28 was not different among the three groups (Fig. 2). CD40L, expressed on activated T cells, binds to CD40 and several integrins, and the CD40L/CD40 system drives crosstalk between T cells and stromal cells. CD40L expressing T cells increase the osteoclastogenic activity of stromal cells by blunting their secretion of osteoprotegerin (OPG) and augmenting their production of RANKL, macrophage colony-stimulating factor, and other proinflammatory factors.(39) On the other hand, CD40 and CD40L knockout mice display diminished BMD and bone mass, enhanced bone resorption and an elevated RANKL/OPG ratio, due to reduced bone marrow OPG production.(40) These finding suggest that CD40L plays a complex role on bone homeostasis via osteoclastgenesis and production of OPG. Tyagi et al.(41,42) reported that ovariectomy leads to the generation of premature senescent CD4+CD28-null T cells, and treatment with daidzein increases mRNA level of CD28 in bone marrow T cells in OVX mice. Because we used whole bone marrow cells, including stromal cells, B cells and other cells from the tibia, the effect of OVX on CD28 mRNA expression might not be confirmed.
We have demonstrated treatment with equol represses increased PPARγ, C/EBPα and FAS mRNA expression in OVX mice, suggesting equol suppresses adipogenesis in bone marrow caused by estrogen deficiency (Fig. 3). Osteoblasts and adipocytes share the same precursor within bone marrow, and therefore adipogenesis increases at the expense of osteoblastogenesis.(43) Heterozygous PPARγ-deficient mice exhibited high bone mass with increased osteoblastogenesis from bone marrow progenitors.(44) In construct, various PPARγ ligands not only induce murine bone marrow stromal cell adipogenesis but also inhibit osteogenesis.(45) In vitro studies using mouse bone marrow cells have found that estrogen reciprocally promotes osteoblastogenesis while inhibiting adipogenesis.(46) In addition, Dang et al.(47) reported that estrogen directly inhibited the differentiation of progenitor cells into adipocyte via down-regulation of PPARγ in vitro. Elbaz et al.(48) reported that ovariectomy induced high levels of both adipogenesis and PPARγ expression in bone marrow, while estrogen replacement inhibited their induction. It has been reported that adipocyte contributes to immune system via secreting adipokines and fatty acids.(49) Thus, the adipogenesis enhanced by OVX in bone marrow might not only suppress osteoblastogenesis, but also might promote bone resorption by induction of inflammatory cytokines which can recruit osteoclast.(50) Equol might prevent bone loss caused by ovariectomy by inhibiting adipogenesis in bone marrow to some degree.
In conclusion, equol ameliorated bone loss and changes in immune system-related genes caused by estrogen deficiency, without exhibiting a substantial effect on the uterus in OVX mice. Equol might affect bone metabolism via hemopoiesis and inflammatory cytokine production in bone marrow. Further studies are necessary to define the mechanism of action of equol in bone and bone marrow in osteoporosis.
Acknowledgments
This study was supported by Grants for project research from the Ministry of Agriculture, Forestry and Fisheries (Development of fundamental technology for analysis and evaluation of functional agricultural products and functional foods). We thank Otsuka Pharmaceutical Co., Ltd for provision of diet containing S-equol of mice.
Abbreviations
- BMC
bone mineral content
- BMD
bone mineral density
- BV
bone volume
- C/EBPα
CCAAT/enhancer-binding protein-α
- CD40L
CD40-ligand
- CTSK
cathepsin K
- ER
estrogen receptor
- FAS
fatty acid synthase
- IL
interleukin
- IL-7R
IL-7 receptor
- NFATc1
nuclear factor of activated T cells cytoplasmic 1
- OPG
osteoprotegerin
- OVX
ovariectomized
- PPARγ
peroxisome proliferator-activated receptor-γ
- RANKL
receptor activator of nuclear factor-kappa B ligand
- Tb.N
trabecular number
- Tb.Sp
trabecular spacing
- Tb.Th
trabecular thickness
- TNF
tumor necrosis factor
- TNFSF13B
TNF superfamily member, 13b
- TV
tissue volume
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Stránský M, Rysavá L. Nutrition as prevention and treatment of osteoporosis. Physiol Res. 2009;58 (Suppl 1):S7–S11. doi: 10.33549/physiolres.931858. [DOI] [PubMed] [Google Scholar]
- 2.Brown LB, Streeten EA, Shapiro JR, et al. Genetic and environmental influences on bone mineral density in pre- and post-menopausal women. Osteoporos Int. 2005;16:1849–1856. doi: 10.1007/s00198-005-1948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christiansen C, Lindsay R. Estrogens, bone loss and preservation. Osteoporos Int. 1990;1:7–13. doi: 10.1007/BF01880410. [DOI] [PubMed] [Google Scholar]
- 4.Masuzawa T, Miyaura C, Onoe Y, et al. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94:1090–1097. doi: 10.1172/JCI117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kincade PW, Medina KL, Payne KJ, et al. Early B-lymphocyte precursors and their regulation by sex steroids. Immunol Rev. 2000;175:128–137. [PubMed] [Google Scholar]
- 6.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 7.Miyaura C, Onoe Y, Inada M, et al. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc Natl Acad Sci U S A. 1997;94:9360–9365. doi: 10.1073/pnas.94.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cenci S, Weitzmann MN, Roggia C, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-α. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roggia C, Gao Y, Cenci S, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J Nutr. 2010;140:1350S–1354S. doi: 10.3945/jn.109.118315. [DOI] [PubMed] [Google Scholar]
- 11.Morito K, Hirose T, Kinjo J, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 12.Ishimi Y. Dietary equol and bone metabolism in postmenopausal Japanese women and osteoporotic mice. J Nutr. 2010;140:1373S–1376S. doi: 10.3945/jn.110.124842. [DOI] [PubMed] [Google Scholar]
- 13.Setchell KD, Clerici C, Lephart ED, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 14.Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3:364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 15.Vatanparast H, Chilibeck PD. Does the effect of soy phytoestrogens on bone in postmenopausal women depend on the equol-producing phenotype? Nutr Rev. 2007;65:294–299. doi: 10.1301/nr.2007.jun.294-299. [DOI] [PubMed] [Google Scholar]
- 16.Tousen Y, Ezaki J, Fujii Y, Ueno T, Nishimuta M, Ishimi Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: a pilot randomized, placebo-controlled trial. Menopause. 2011;18:563–574. doi: 10.1097/gme.0b013e3181f85aa7. [DOI] [PubMed] [Google Scholar]
- 17.Fujioka M, Uehara M, Wu J, et al. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J Nutr. 2004;134:2623–2627. doi: 10.1093/jn/134.10.2623. [DOI] [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 19.Staudt LM, Clerc RG, Singh H, LeBowitz JH, Sharp PA, Baltimore D. Cloning of a lymphoid-specific cDNA encoding a protein binding the regulatory octamer DNA motif. Science. 1988;241:577–580. doi: 10.1126/science.3399892. [DOI] [PubMed] [Google Scholar]
- 20.Hemingway F, Taylor R, Knowles HJ, Athanasou NA. RANKL-independent human osteoclast formation with APRIL, BAFF, NGF, IGF I and IGF II. Bone. 2011;48:938–944. doi: 10.1016/j.bone.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 22.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima T, Takayanagi H. Osteoclasts and the immune system. J Bone Miner Metab. 2009;27:519–529. doi: 10.1007/s00774-009-0089-z. [DOI] [PubMed] [Google Scholar]
- 24.Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122S–3126S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 25.Paulauskis JD, Sul HS. Cloning and expression of mouse fatty acid synthase and other specific mRNAs. Developmental and hormonal regulation in 3T3-L1 cells. J Biol Chem. 1988;263:7049–7054. [PubMed] [Google Scholar]
- 26.Kimira Y, Katsumata S, Suzuki K, et al. Comparative activities of the S-enantiomer and racemic forms of equol on bone fragility in ovariectomized mice. Biosci Biotechnol Biochem. 2012;76:1018–1021. doi: 10.1271/bbb.110973. [DOI] [PubMed] [Google Scholar]
- 27.Yoneda T, Ueno T, Uchiyama S. S-equol and the fermented soy product SE5-OH containing S-equol similarly decrease ovariectomy-induced increase in rat tail skin temperature in an animal model of hot flushes. Menopause. 2011;18:814–820. doi: 10.1097/gme.0b013e318208fb0d. [DOI] [PubMed] [Google Scholar]
- 28.Setchell KD, Zhao X, Jha P, Heubi JE, Brown NM. The pharmacokinetic behavior of the soy isoflavone metabolite S-(−)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am J Clin Nutr. 2009;90:1029–1037. doi: 10.3945/ajcn.2009.27981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwen RJ, Nguyen L, Plomley JB, Jackson RL. Toxicokinetics and lack of uterotropic effect of orally administered S-equol. Food Chem Toxicol. 2012;50:1741–1748. doi: 10.1016/j.fct.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 30.Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. 2010;140:1363S–1368S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwen R, Jackson R, Proudlock R. Genotoxicity assessment of S-equol in bacterial mutation, chromosomal aberration, and rodent bone marrow micronucleus tests. Food Chem Toxicol. 2010;48:3481–3485. doi: 10.1016/j.fct.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 32.Kalu DN, Salerno E, Liu CC, Ferarro F, Arjmandi BN, Salih MA. Ovariectomy-induced bone loss and the hematopoietic system. Bone Miner. 1993;23:145–161. doi: 10.1016/s0169-6009(08)80050-5. [DOI] [PubMed] [Google Scholar]
- 33.Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Semin Immunol. 2006;18:20–30. doi: 10.1016/j.smim.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24:209–217. doi: 10.1016/j.smim.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000;96:1873–1878. [PubMed] [Google Scholar]
- 36.Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110:1643–1650. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horowitz MC, Fretz JA, Lorenzo JA. How B cells influence bone biology in health and disease. Bone. 2010;47:472–479. doi: 10.1016/j.bone.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishimi Y, Miyaura C, Ohmura M, et al. Selective effects of genistein, a soybean isoflavone, on B-lymphopoiesis and bone loss caused by estrogen deficiency. Endocrinology. 1999;140:1893–1900. doi: 10.1210/endo.140.4.6663. [DOI] [PubMed] [Google Scholar]
- 39.Pacifici R. Role of T cells in ovariectomy induced bone loss—revisited. J Bone Miner Res. 2012;27:231–239. doi: 10.1002/jbmr.1500. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Toraldo G, Li A, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyagi AM, Srivastava K, Kureel J, et al. Premature T cell senescence in Ovx mice is inhibited by repletion of estrogen and medicarpin: a possible mechanism for alleviating bone loss. Osteoporos Int. 2012;23:1151–1161. doi: 10.1007/s00198-011-1650-x. [DOI] [PubMed] [Google Scholar]
- 42.Tyagi AM, Srivastava K, Sharan K, Yadav D, Maurya R, Singh D. Daidzein prevents the increase in CD4+CD28null T cells and B lymphopoesis in ovariectomized mice: a key mechanism for anti-osteoclastogenic effect. PLoS One. 2011;6:e21216. doi: 10.1371/journal.pone.0021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duque G, Troen BR. Understanding the mechanisms of senile osteoporosis: new facts for a major geriatric syndrome. J Am Geriatr Soc. 2008;56:935–941. doi: 10.1111/j.1532-5415.2008.01764.x. [DOI] [PubMed] [Google Scholar]
- 44.Akune T, Ohba S, Kamekura S, et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 46.Okazaki R, Inoue D, Shibata M, et al. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology. 2002;143:2349–2356. doi: 10.1210/endo.143.6.8854. [DOI] [PubMed] [Google Scholar]
- 47.Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Löwik CW. Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res. 2002;17:394–405. doi: 10.1359/jbmr.2002.17.3.394. [DOI] [PubMed] [Google Scholar]
- 48.Elbaz A, Rivas D, Duque G. Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology. 2009;10:747–755. doi: 10.1007/s10522-009-9221-7. [DOI] [PubMed] [Google Scholar]
- 49.de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 50.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]