Abstract
This study assessed the endocrine pancreatic responses to liraglutide (0.9 mg once a day) during normal living conditions in Japanese patients with type 2 diabetes. The study included 14 hospitalized patients with type 2 diabetes. Meal tests were performed after improvement of glycemic control achieved by two weeks of multiple insulin injection therapy and after approximately two weeks of liraglutide treatment. Continuous glucose monitoring was performed to compare daily variation in glycemic control between multiple insulin injection therapy and liraglutide treatment. Liraglutide reduced plasma glucose levels after the test meals (60–180 min; p<0.05), as a result of significant increases in insulin secretion (0–180 min; p<0.05) and decreases in the incremental ratio of plasma glucagon (15–60 min; p<0.05). Continuous glucose monitoring showed that liraglutide treatment was also associated with a decrease in glucose variability. We also demonstrated that optimal glycemic control seen as a reduction in 24-h mean glucose levels and variability was obtained only with liraglutide monotherapy. In conclusion, liraglutide treatment increases insulin secretion and suppresses glucagon secretion in Japanese patients with type 2 diabetes under normal living conditions. The main therapeutic advantages of liraglutide are its use as monotherapy and its ability to decrease glucose variability.
Keywords: liraglutide, insulin, glucagon, test meal, continuous glucose monitoring
Introduction
In healthy people, elevated glucose levels have counter effects on α- and β-cells by stimulating insulin secretion and inhibiting glucagon secretion. This results in maintenance of glucose homeostasis after oral ingestion. The normal interaction between α- and β-cells is disrupted in patients with type 2 diabetes and is characterized by loss of the first-phase insulin response to glucose(1) and perturbed inhibition of glucagon release.(2,3) Glucagon-like peptide-1 (GLP-1) is an incretin hormone produced in the gastrointestinal tract and released in response to meals. GLP-1 stimulates insulin secretion and inhibits glucagon secretion in a glucose-dependent manner,(4,5) and effectively inhibits prandial hyperglycemia.(6) Liraglutide is a GLP-1 analogue with 97% structural homology to human GLP-1, and is used in the treatment of type 2 diabetes to regulate glucose levels by stimulating glucose-dependent insulin secretion, suppressing glucagon secretion, delaying gastric emptying, and promoting satiety.
The pathophysiology of type 2 diabetes differs between ethnic groups, resulting in appreciable diversity in clinical presentation. It is therefore important to verify the effect of liraglutide in the Japanese (East Asian) population. Postprandial insulin and glucagon secretion during normal living are important factors which account for amelioration of glycemic control in a clinical setting. However, there have been only a few studies that have measured blood insulin and glucagon concentrations after a practical meal in Japanese patients. The majority of studies have been performed using quite different nutritional or caloric conditions than that used in daily life such as liquid(7) or low-calorie meals,(8) or different doses or administration route of liraglutide(9–11) that are used clinically in Japan. This study therefore assessed the endocrine pancreatic responses to liraglutide during normal living conditions in Japanese patients with type 2 diabetes, by performing a meal test with a practical caloric level (460 kcal) following a subcutaneous injection of 0.9 mg liraglutide, the maximum dose permitted in Japan.
Materials and Methods
Subjects
The studies were carried out in 14 patients with type 2 diabetes who were admitted to the Hospital of Kyoto Prefectural University of Medicine for glycemic control between 1 January 2011 and 30 September 2011. Before admission, all the patients were treated with oral anti-diabetic drugs (OADs) and/or insulin (OADs 3/Insulin 4/OADs + Insulin 7 patients). The decision to treat with liraglutide (Novo Nordisk, Bagsvaerd, Denmark) was made by the physician after consultation with the patients and consideration of the frequency of hypoglycemia, body weight gain, and quality of life. Patients with insulin-dependent diabetes, renal insufficiency (serum creatinine level>1.5 mg/dl), or levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) more than twofold the upper limit of normal were excluded from the study.
Study Protocol
After admission, all patients were treated with multiple insulin injection therapy for 2 weeks (MIIT: prandial rapid-acting insulin immediately before meals and basal long-acting insulin in the morning) to improve blood glucose levels (Fig. 1). The starting dose of insulin was up-titrated to achieve target blood glucose levels of fasting blood glucose (FBG) <8.3 mmol/l and postprandial 2-h blood glucose (2h-PBG) level<11.1 mmol/l. Glycemic variations were measured by continuous glucose monitoring (CGM) in patients who consented to this procedure. The day after CGM, the patients were given a standard test meal (JANEF E460F18®, Q.P. Tokyo, Japan) at 9:00 a.m. without injection of prandial or basal insulin. The test meal consisted of a cracker, pudding and chicken cream stew (460 kcal, 51.4% carbohydrates, 33.3% fat and 17.2% protein). Blood samples for measurement of glucose, C-peptide, insulin, and glucagon were drawn at 0, 15, 30, 45, 60, 90, 120, 150, and 180 min after the patients had started eating.
Fig. 1.
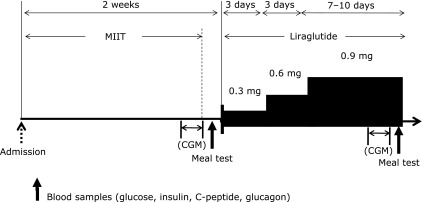
Study protocol. After improvement in glycemic control by multiple insulin injection therapy (MIIT), treatment was switched to liraglutide. Blood samples were collected during meal tests before and after the change in therapy. In addition, patients who gave consent had their 24-h glucose levels measured by continuous glucose monitoring (CGM).
MIIT was then changed to treatment using a single morning injection of liraglutide, with the dose increased from 0.3 mg to 0.9 mg/day by 0.3 mg every 3 days. In Japan, the permitted maximum dose of liraglutide is 0.9 mg/day. Patients not meeting the glycemic goals with liraglutide monotherapy were given rescue therapy of sulfonylurea agents (SU). The criteria for rescue therapy were FPG≥8.3 mmol/l and 2h-PBG≥11.1 mmol/l.
After achieving steady glycemic control with liraglutide monotherapy or combination therapy, variations in glycemic control were monitored by CGM and a repeat standard meal tests was performed as described above.
Analytical methods
Plasma glucose was determined by a standard laboratory assay. Plasma insulin and C-peptide were analyzed by CLEIA, and plasma glucagon measured using RIA kits (Linco Research, St. Charles, MO). CGM was performed using a CGMS® System GoldTM (Medtronic MiniMed, Northridge, CA). The recorded data were analyzed with CGMS Solutions software. Hemoglobin A1c (HbA1c) was assayed using high-performance liquid chromatography and was expressed as a National Glycohemoglobin Standardization Program Unit.(12)
Statistical Analysis
Data in the table are expressed as mean ± SD and data in the figures as mean ± SEM. The effects of liraglutide during the standard meal test and CGM were analyzed using a paired t test. CGM was also examined using a repeated measurement analysis of variance (RM-ANOVA). The Wilcoxon test was used to compare the clinical characteristics of patients on liraglutide monotherapy and combination therapy. All statistical analyses were performed using JMP software (JMP; SAS Institute Inc., Cary, NC).
Results
Patient baseline characteristics
The characteristics of the 14 subjects enrolled in this study are shown in Table 1. The mean (± SD) age was 63.5 ± 15.1 years and mean duration of diabetes was 15.5 ± 8 years. Most patients were slightly obese. The mean level of HbA1c was 8.7 ± 1.7%. Six patients (42.9%) took SU and 11 patients (78.6%) used insulin.
Table 1.
Characteristics of the study subjects with type 2 diabetes
| Number (Male) | 14 (5) |
| Age (years) | 63.5 ± 15.1 |
| Duration (years) | 15.5 ± 8.0 |
| Body weight (kg) | 75.8 ± 17.2 |
| Body mass index (kg/m2) | 29.2 ± 4.22 |
| HbA1c (%)* | 8.7 ± 1.7 |
| Fasting Plasma glucose (mmol/L) | 9.2 ± 2.7 |
| Fasting C-peptide (nmol/L)* | 0.6 ± 0.4 |
| C-peptide index* | 12.2 ± 8.04 |
| Insulin dose(unit)§ | 36.1 ± 8.3 |
| Before treatment | |
| OADs (%) | 21.4 |
| (SU/DPP4-I/BG/TZD/αGI) | (3/2/2/1/1) |
| insulin (%) | 28.6 |
| OADs + insulin (%) | 50.0 |
| (SU/DPP4-I/BG/TZD/αGI) | (3/0/4/3/2) |
Data are expressed as mean ± SD. *On admission; §After glycemic control. C-peptide index = C-peptide(nmol/l)/Glucose(mmol/l) × 100. SU, sulfonylurea; DPP4-I, dipeptidyl peptidase-4 inhibitor; BG, biguanide; TZD, thiazolidinedione; α-GI, α-glucosidase inhibitor.
Meal test
All subjects tolerated the studies well, with no hypoglycemic episodes or major side effects being observed. The mean titrated doses for prandial and basal insulin were 21.2 ± 5.2 and 14.9 ± 7.7 units, respectively. Even at a dose of 0.9 mg/day, liraglutide treatment failed to lower FPG or PPG to the target levels in five patients, so combination therapy with SU was applied.
There was a significant reduction in postprandial plasma glucose levels with liraglutide treatment between 60 and 180 min after ingestion of the meal (60–180 min; p<0.05) (Fig. 2a). This reduction was accompanied by a significant increase in insulin secretion throughout this period. Insulin secretion was increased significantly even in the fasting state (insulin and C-peptide 0–180 min; p<0.05) (Fig. 2 b and c). On the other hand, plasma glucagon concentrations showed no significant differences before and after replacement to liraglutide therapy at all time points (Fig. 2d). However, there was a significant reduction in the incremental ratio of plasma glucagon with liraglutide between 15 and 45 min after ingestion of the meal (15–60 min; p<0.05) (Fig. 2e). The combined effects of the increase in insulin secretion and reduction in early phase of postprandial plasma glucagon excursion may contribute to the amelioration in blood glucose control.
Fig. 2.
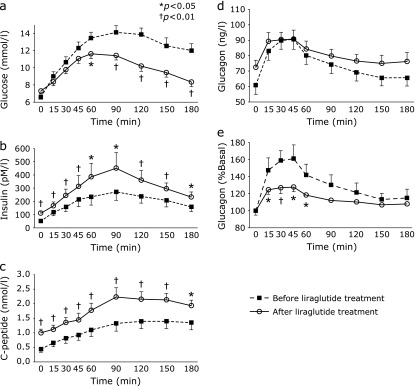
Effect of subcutaneous liraglutide on plasma concentrations of (a) glucose, (b) insulin, (c) C-peptide, (d) glucagon, and (e) incremental ratio of plasma glucagon (%Basal) after the test meals (n = 14). %Basal = 100 × postprandial plasma glucagon (15–180 min)/baseline plasma glucagon (0 min).
CGM
Fig. 3 shows the 48-h blood glucose profiles measured using the CGM system. The mid-night blood glucose levels were significantly lower with MIIT than with liraglutide treatment, although 24-h mean glucose levels were similar between the two treatments (liraglutide, 8.6 ± 1.8 mg/dl; MIIT, 8.1 ± 1.1 mg/dl; p = 0.2). On the other hand, the standard deviation of blood glucose levels (SDs) decreased significantly with liraglutide (liraglutide, 1.9 ± 1.2; MIIT, 2.7 ± 1.4; p<0.05). These data indicated that liraglutide treatment was associated with a decrease in glucose variability.
Fig. 3.
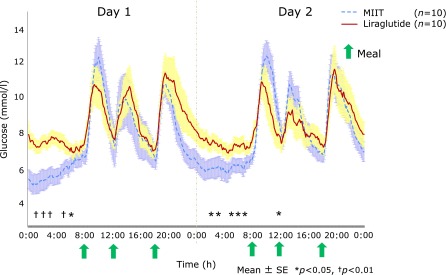
Blood glucose profiles measured by continuous glucose monitoring during multiple insulin injection therapy (MIIT) and liraglutide treatment.
We analyzed the differences in blood glucose profiles measured by CGM between liraglutide monotherapy and combination therapy (Fig. 4). This demonstrated clearly that optimal glycemic control and a reduction in 24-h mean glucose levels and SDs were obtained only in patients receiving liraglutide monotherapy (p<0.0001, by RM-ANOVA). We then analyzed the differences in insulin and glucagon secretion during the meal test between liraglutide monotherapy and combination therapy. No significant differences were detected between the two treatments. There were also no significant differences in the clinical characteristics of the patients including age, duration of diabetes, BMI, HbA1c and fasting C-peptide levels, and insulin dose immediately before liraglutide treatment.
Fig. 4.
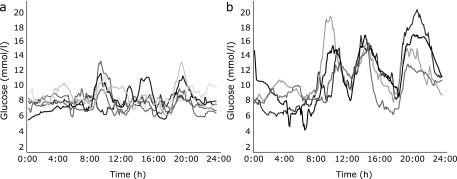
Blood glucose profiles measured by continuous glucose monitoring during (a) liraglutide monotherapy and (b) combination therapy (+SU).
Discussion
This study was conducted under the conditions of practical medical care used in Japan. Our data show that liraglutide treatment at the standard therapeutic dose in Japan of 0.9 mg once a day evaluated under normal living conditions caused a significant increase in insulin secretion and inhibited glucagon secretion. This improvement in islet β- and α-cell function contributed to an improvement in glycemic control. These clinical data are similar to those reported in other ethnic groups.(9,13,14) Liraglutide treatment also decreased glucose variability measured by CGM, with this effect being more marked in patients on liraglutide monotherapy.
Unexpectedly, liraglutide treatment did not cause a significant suppression of fasting or postprandial plasma glucagon levels. This absence of an effect may be related to the functional characteristics of islet α-cells which are affected by factors such as the heterogeneous pathophysiology of diabetes and modes of pretreatment therapy.(3,15,16) The relatively low dose of liraglutide (0.9 mg once a day) may also have been another factor that contributed to the lack of effect we observed. Despite no changes in plasma glucagon levels, the liraglutide treatment used in the present study resulted in a better glycemic profile. In the fasting state, the significant increase in fasting plasma insulin levels was associated with compensatory regulation of hepatic glucose production that resulted in similar fasting blood glucose levels before and after liraglutide treatment. In the postprandial state, liraglutide treatment caused a significant reduction in the incremental ratio of plasma glucagon, but not the crude plasma level. This result suggests that the early phase of glucagon suppression has an important role in regulation of postprandial blood glucose levels.
In agreement with previous studies,(17,18) we observed that liraglutide treatment caused a decrease in glucose variability compared with MIIT. Once-daily administration and a lower incidence of nocturnal hypoglycemia may be therapeutic advantages for liraglutide over MIIT. It was notable that patients receiving liraglutide monotherapy had significantly less glycemic excursions and lower 24-h mean glucose levels than those on MIIT. These findings indicate the best therapeutic advantage occurs with liraglutide monotherapy. It is of clinical interest to predict successful switching from MIIT to liraglutide monotherapy. Previous studies on Japanese patients with type 2 diabetes have shown that fasting blood C-peptide, C-peptide index, insulinogenic index, urine C-peptide, and duration of diabetes may be potentially useful predictors.(19,20) However, in this study, we could not detect any significant predictive factors including parameters related to glucagon secretion. It is possible the small number of the subjects in this study may also have contributed to these different findings. On the other hand, it may be impossible to predict the efficacy of liraglutide treatment using only parameters of insulin secretory capacity, considering that the effect on the central nervous system and gastric motility could significantly affect peripheral blood glucose levels, resulting in regulation of energy balance.(21) Further studies with sufficient statistical power are therefore necessary to determine the clinical parameters that predict the efficacy of liraglutide.
In conclusion, this study in Japanese patients with type 2 diabetes carried out under normal living conditions demonstrated that a dose of 0.9 mg liraglutide increases insulin secretion and suppresses glucagon secretion assessed by the plasma glucagon incremental ratio. These beneficial effects may contribute to an improvement in glycemic control in practical clinical settings. Our data indicate that the best therapeutic advantage can be achieved with liraglutide monotherapy as it decreases glucose variability.
Conflict of Interest
Shinobu Matsumoto, Masahiro Yamazaki, Michiaki Fukui, Goji Hasegawa and Naoto Nakamura have received lecture fees from Novo Nordisk. Another authors have nothing to disclose.
References
- 1.Pfeifer MA, Halter JB, Prote D., Jr Insulin secretion in diabetes mellitus. Am J Med. 1981;70:579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- 2.Müller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal α-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970;283:109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- 3.Dunning BE, Gerich JE. The role of α-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev. 2007;28:253–283. doi: 10.1210/er.2006-0026. [DOI] [PubMed] [Google Scholar]
- 4.Holst JJ, Orskov AC, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987;211:169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- 5.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 7.Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: Effects of exogenous glucogon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 8.Nauck MA, Wollschläger D, Werner J, et al. Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7-36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–1553. doi: 10.1007/s001250050613. [DOI] [PubMed] [Google Scholar]
- 9.Flint A, Kapitza C, Hindsberger C, Zdravkovic M. The once-daily human glucagon-like peptide-1 (GLP-1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients. Adv Ther. 2011;28:213–226. doi: 10.1007/s12325-010-0110-x. [DOI] [PubMed] [Google Scholar]
- 10.Todd JF, Edwards CM, Ghatei MA, Mather HM, Bloom SR. Subcutaneous glucagon-like peptide-1 improves postprandial glycaemic control over a 3-week period in patients with early type 2 diabetes. Clin Sci. 1998;95:325–329. [PubMed] [Google Scholar]
- 11.Nicolaus M, Brödl J, Linke R, Woerle HJ, Göke B, Schirra J. Endogenous GLP-1 regulates postprandial glycemia in humans: relative contributions of insulin, glucagon, and gastric emptying. J Clin Endocrinol Metab. 2011;96:229–236. doi: 10.1210/jc.2010-0841. [DOI] [PubMed] [Google Scholar]
- 12.Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juhl CB, Hollingdal M, Sturis J, et al. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes. 2002;51:424–429. doi: 10.2337/diabetes.51.2.424. [DOI] [PubMed] [Google Scholar]
- 14.Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on β-cell function in normal living conditions. Diabetes Care. 2007;30:2032–2033. doi: 10.2337/dc07-0310. [DOI] [PubMed] [Google Scholar]
- 15.de Heer J, Holst JJ. Sulfonylurea compounds uncouple the glucose dependence of the insulinotropic effect of glucagon-like peptide 1. Diabetes. 2007;56:438–443. doi: 10.2337/db06-0738. [DOI] [PubMed] [Google Scholar]
- 16.D’Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab. 2011;13:126–132. doi: 10.1111/j.1463-1326.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- 17.Flint A, Kapitza C, Hindsberger C, Zdravkovic M. The once-daily human glucagon-like peptide-1 (GLP-1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients. Adv Ther. 2011;28:213–226. doi: 10.1007/s12325-010-0110-x. [DOI] [PubMed] [Google Scholar]
- 18.Mori Y, Taniguchi Y, Sezaki K, Yokoyama J, Utsunomiya K. Liraglutide narrows the range of circadian glycemic variations in Japanese type 2 diabetes patients and nearly flattens these variations in drug-naive type 2 diabetes patients: a continuous glucose monitoring-based study. Diab Technol Ther. 2011;13:1139–1144. doi: 10.1089/dia.2011.0137. [DOI] [PubMed] [Google Scholar]
- 19.Kozawa J, Inoue K, Iwamoto R, et al. Liraglutide is effective in type 2 diabetic patients with sustained endogenous insulin-secreting capacity. J Diab Invest. 2011;3:294–297. doi: 10.1111/j.2040-1124.2011.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwao T, Sakai K, Sata M. Postprandial serum C-peptide is a useful parameter in the prediction of successful switching to liraglutide monotherapy from complex insulin therapy in Japanese patients with type 2 diabetes. J Diabetes Complications. 2013;27:87–91. doi: 10.1016/j.jdiacomp.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7:507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]


