Abstract
Recent studies have suggested that decrease in Helicobacter pylori infection may predispose to allergic diseases. However, there are few reports of the relationships of eosinophilic gastrointestinal disorders (EGIDs), especially eosinophilic gastroenteritis (EGE), with H. pylori infection. We investigated the possible influence of H. pylori infection on EGIDs in Japanese patients. We performed a case-control study to investigate the prevalence of H. pylori infection in patients with EGIDs. Eighteen with eosinophilic esophagitis (EoE) and 22 with EGE were enrolled. For each patient, 3 age- and gender-matched normal controls (n = 120) were randomly selected from a population who received a medical check-up between April 2010 and December 2011 at the Shimane Institute of Health Science. The mean ages of the EoE and EGE patients were 50.9 ± 17 and 49.2 ± 20 years, respectively. Males were more frequently seen in the EoE group, while there was no significant gender difference in regard to EGE. Of the patients with EoE, 22.3% were infected with H. pylori, as compared to 55.5% of their age- and sex-matched normal controls. The odds ratio for EoE patients to have an H. pylori infection was 0.22 (p<0.05). In addition, 22.7% of the patients with EGE and 48.5% of their matched controls were infected with H. pylori, with odds ratio for EGE patients to have an H. pylori infection shown to be 0.31 (p<0.05). In conclusion, the prevalence of H. pylori infection was significantly lower in EGE and EoE patients in Japan as compared to normal control subjects.
Keywords: eosinophilic esophagitis, eosinophilic gastroenteritis, Helicobacter pylori infection, Th2
Introduction
Eosinophilic gastrointestinal disorders (EGIDs) such as eosinophilic esophagitis (EoE) and eosinophilic gastroenteritis (EGE) have recently shown an increasing trend in Japan.(1,2) EGIDs are characterized by chronic mucosal inflammation with dense infiltration of eosinophils in esophageal squamous epithelium and gastrointestinal mucosal epithelium,(3,4) and considered to be caused by local allergic reactions, possibly to foods or airborne antigens.(5,6) The reason for the recent increase in EGID incidence has not been clarified.
Helicobacter pylori (H. pylori) infection is accepted as an important pathogenic factor in chronic gastritis, peptic ulcer diseases, gastric cancers, and mucosa-associated lymphoid tissue (MALT) lymphoma in humans.(7–9) In Japan, the rate of H. pylori infection has steadily decreased, partly due to improved sanitation.(10–13)
EGIDs are frequently accompanied by various types of allergic diseases such as bronchial asthma, and considered to be related to over-activation or dysregulation of Th2-mediated immune reactions. On the other hand, for protection against bacterial infection, Th1-type immune response is believed to be important and activated by a bacterial infection. However, because of recent improvements in environmental cleanness, the chance of bacterial infections has remarkably decreased in Japanese children. In cases of H. pylori infection, Th1-type immune activation occurs, though its prevalence has been rapidly decreasing.(14–18) The reduced chance of bacterial infection may result in failure to activate Th1 immune response, thus causing an imbalance between that and Th2 immune response.(19) A Th2 predominant response is prone to cause various allergic reactions. This concept is termed the hygiene hypothesis.(20) When considering this background, the decreasing rate of H. pylori infection may have some link to the increasing prevalence of EGIDs in Japan. There are few reports on the relationship between EGIDs (especially EGE) and H. pylori infection,(21,22) thus we performed a case-control study to clarify that infection rates in Japanese patients with EGIDs.
Subjects and Methods
Eighteen patients with EoE and 22 with EGE diagnosed based on consensus diagnostic guidelines in Japan were enrolled.(23,24) For each, 3 normal age- and gender-matched control subjects (n = 120) were randomly selected from individuals who received an annual medical check-up between April 2010 and December 2011 at the Shimane Institute of Health Science. Levels of the anti-H. pylori antibody were measured in serum samples obtained from all patients and controls using EIA, according to the supplier’s instructions (E-PLATE ’Eiken’ H. pylori Antibody, Eiken Chemical Co., Ltd., Tokyo, Japan).
This study was approved by the ethical committee of Shimane University Faculty of Medicine and carried out in accordance with the Declaration of Helsinki. Statistical analysis was performed using a χ2 test. Differences at p<0.05 were considered to be statistically significant.
Results
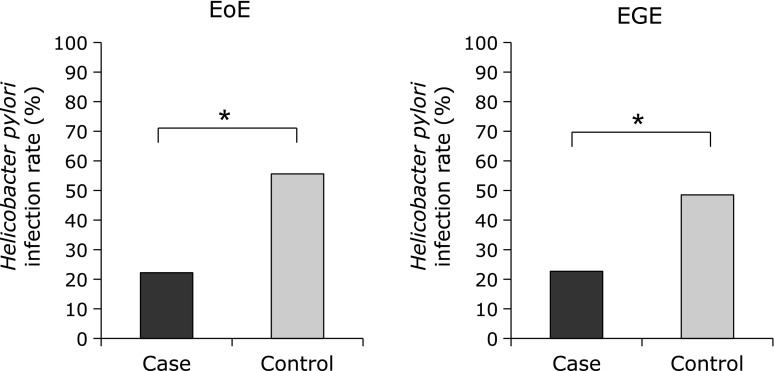
The mean ages of the EoE and EGE patients were 50.9 ± 17 and 49.2 ± 20 years, respectively, similar to previously reported studies in Japan.(2) The percentages of male patients were higher, especially in the EoE group (Table 1). None of the patients enrolled in this study had been treated by administration of systemic glucocorticoids. Among the EoE cases, 22.3% were diagnosed as H. pylori-positive, while 55.5% of the matched controls were positive. Thus, the rate of H. pylori infection was significantly lower in patients with EoE as compared to the normal controls, while the odds ratio (OR) for those patients to be infected was 0.22 (p<0.05). As for EGE cases, 22.7% were diagnosed as H. pylori-positive, while 48.5% of the matched controls were positive, again showing a significantly lower rate of infection in patients with EGE as compared to the normal controls (Fig. 1). The OR for EGE patients to have an H. pylori infection was 0.31 (p<0.05).
Table 1.
Clinical characteristics of enrolled subjects
| EoE | EGE | |||
|---|---|---|---|---|
| Cases | Control | Cases | Control | |
| No. of cases | 18 | 54 | 22 | 66 |
| Male/Female | 11/7 | 33/21 | 12/10 | 36/30 |
| Mean age ± SD (years) | 50.9 ± 17.4 | 50.5 ± 16.5 | 49.2 ± 20.0 | 49.4 ± 18.4 |
Fig. 1.
The H. pylori infection rate was significantly lower in patients with EoE and EGE as compared to their respective age- and gender-matched controls. *Significantly different as compared with control (p<0.05).
Discussion
EoE and EGE are allergic digestive diseases characterized by chronic inflammation along with dense mucosal infiltration of eosinophiles.(3,4) Increasing numbers of patients with EoE have been reported in Western countries,(25–28) while many cases of EGE have been reported in Japan.(1,2) It is considered that Th2-mediated allergic response and Th2-type cytokines, including IL-5, 13, 15, and eotaxines, are involved in development of these diseases.(29–33)
During an immune response, different groups of T helper (Th) cells, termed Th1 and Th2, release different patterns of cytokines. Th1 cells mediate cell-mediated immunity, as well as production of opsonizing and complement-fixing antibodies by B cells, while Th2 cells induce production of high levels of antibodies, including IgE, as well as eosinophilia.
It has been speculated that particular allergic conditions such as bronchial asthma and allergic rhinitis are increasing because of an imbalance between Th1 and Th2 type immune responses due to a modern clean lifestyle,(19) which is termed the hygiene hypothesis.(20) Th2 cytokines suppress Th1 responses, while Th1 cytokines inhibit Th2 responses and vice versa. These systems must remain in balance to maintain a healthy condition. The immune system of neonatal humans is thought to have a Th2 bias that gradually diminishes during the first 2 years of life in non-allergic individuals.(34) For development of a normal well-balanced immune system, Th1 stimulus from the environment is necessary. Without a balanced immune system, allergic diseases are prone to occur because of the continuing Th2 bias. Many bacterial infections including mycobacteria can provide such Th1 stimulus.(35) However, because of improved sanitation and the reduced chance to have a bacterial infection during childhood, individuals have a higher chance to continue to with a skewed Th2 immune response.(36)
H. pylori infection is considered to be an important pathogenic factor in chronic gastritis, peptic ulcer disease, gastric cancer, and MALT lymphoma.(7–9) Due to improved sanitation and the reduced chance of infection during childhood, H. pylori infection is rapidly decreasing in Japan,(10–13) along with peptic ulcer diseases and gastric cancers. However, this decreased rate of H. pylori infection has led to increases in cases of gastric acid secretion and gastroesophageal reflux diseases.(37,38) Moreover, recent studies have suggested that a decrease in H. pylori infections may predispose individuals to various allergic diseases.(39) Th1-mediated response is activated in cases of infection, thus Th2 type immune response is expected to be continuously skewed in infected individuals, who are likely to become prone to Th2 type allergic responses. Common pathological conditions including exaggerated Th2 response to environmental and food allergens are considered to play important roles in the pathogenesis of EGIDs, and Dellon et al. recently reported that H. pylori infection was inversely associated with esophageal eosinophilic infiltration.(21)
In summary, we confirmed the decreased rate of H. pylori infection in Japanese patients with EoE as compared to the control subjects. In addition, our findings are the first to show that the H. pylori infection rate is lower in EGE as well as in EoE patients. Our results are limited by the relatively small sample size, and the fact that other bacterial and viral infections were not investigated. In addition, H. pylori infection was only determined with a serum antibody test. The specific role of the decreased rate of H. pylori infection should be confirmed in a larger scale prospective study.
Acknowledgments
This study was funded in part by a Grant-in-Aid for Science Research from the Ministry of Health, Labour and Welfare of Japan (22141101).
Conflict of interest
No potential conflicts of interest were disclosed.
References
- 1.Fujishiro H, Amano Y, Kushiyama Y, Ishihara S, Kinoshita Y. Eosinophilic esophagitis investigated by upper gastrointestinal endoscopy in Japanese patients. J Gastroenterol (in italic type) 2011;46:1142–1144. doi: 10.1007/s00535-011-0435-5. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita Y, Furuta K, Ishimaura N, et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis J Gastroenterol 2012. DOI: 10.1007/s00535-012-0640-x [DOI] [PubMed] [Google Scholar]
- 3.Straumann A. Idiopathic eosinophilic gastrointestinal diseases in adults. Best Pract Res Clin Gastroenterol. 2008;22:481–496. doi: 10.1016/j.bpg.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Spergel JM, Book WM, Mays E, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52:300–306. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363–368. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 6.Liacouras CA. Eosinophilic esophagitis: treatment in 2005. Curr Opin Gastroenterol. 2006;22:147–152. doi: 10.1097/01.mog.0000203863.40632.ba. [DOI] [PubMed] [Google Scholar]
- 7.Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asaka M, Kato M, Graham DY. Prevention of gastric cancer by Helicobacter pylori eradication. Intern Med. 2010;49:633–636. doi: 10.2169/internalmedicine.49.3470. [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamura A, Adachi K, Takashima T, et al. Prevalence of functional dyspepsia and its relationship with Helicobacter pylori infection in a Japanese population. J Gastroenterol Hepatol. 2001;16:384–388. doi: 10.1046/j.1440-1746.2001.02454.x. [DOI] [PubMed] [Google Scholar]
- 11.Fujisawa T, Kumagai T, Akamatsu T, Kiyosawa K, Matsunaga Y. Changes in seroepidemiological pattern of Helicobacter pylori and hepatitis A virus over the last 20 years in Japan. Am J Gastroenterol. 1999;94:2094–2099. doi: 10.1111/j.1572-0241.1999.01283.x. [DOI] [PubMed] [Google Scholar]
- 12.Tamura T, Morita E, Kondo T, et al. Prevalence of Helicobacter pylori infection measured with urinary antibody in an urban area of Japan, 2008–2010. Nagoya J Med Sci. 2012;74:63–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Takashima T, Iwakiri R, Sakata Y, et al. Endoscopic reflux esophagitis and Helicobacter pylori infection in young healthy Japanese volunteers. Digestion. 2012;86:55–58. doi: 10.1159/000338849. [DOI] [PubMed] [Google Scholar]
- 14.D’Elios MM, Manghetti M, Almerigogna F, et al. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur J Immunol. 1997;27:1751–1755. doi: 10.1002/eji.1830270723. [DOI] [PubMed] [Google Scholar]
- 15.Amedei A, Cappon A, Codolo G, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092–1101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor JM, Ziman ME, Huff JL, Moroski NM, Vajdy M, Solnick JV. Helicobacter pylori lipopolysaccharide promotes a Th1 type immune response in immunized mice. Vaccine. 2006;24:4987–4994. doi: 10.1016/j.vaccine.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 17.D’Elios MM, Andersen LP. Helicobacter pylori inflammation, immunity, and vaccines. Helicobacter. 2007;12 (Suppl 1):15–19. doi: 10.1111/j.1523-5378.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 18.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez FD, Holt PG. Role of microbial burden in aetiology of allergy and asthma. Lancet. 1999;354 (Suppl 2):SII12–SII15. doi: 10.1016/s0140-6736(99)90437-3. [DOI] [PubMed] [Google Scholar]
- 20.Okada H, Kuhn C, Feillet H, Bach JF. The ’hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology. 2011;141:1586–1592. doi: 10.1053/j.gastro.2011.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi Y, Mine T, Yasuzaki H, Kusakabe A, Kawana I, Umemura S. Eosinophilic gastroenteritis cured with Helicobacter pylori eradication. J Clin Gastroenterol. 2008;42:1063–1064. doi: 10.1097/MCG.0b013e31805d7ef1. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita Y, Ishihara S, Amano Y, Fujishiro H. Diagnosis and treatment of eosinophilic esophagitis. Gastroenterological Endoscopy. 2011;53:3–15. [Google Scholar]
- 24.Kinoshita Y, Ishihara S, Amano Y, Shimura S, Tada Y, Maruyama R. Diagnosis and treatment of eosinophilic gastroenteritis. Gastroenterological Endoscopy. 2012;54:1797–1805. [Google Scholar]
- 25.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–1061. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:1305–1313. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316–1321. doi: 10.1053/j.gastro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther. 2010;32:712–719. doi: 10.1111/j.1365-2036.2010.04411.x. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita Y, Furuta K, Ishimura N, Ishihara S. Elevated plasma cytokines in Japanese patients with eosinophilic esophagitis and gastroenteritis. Digestion. 2012;86:238–243. doi: 10.1159/000341421. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Wang M, Mavi P, et al. Interleukin-15 expression is increased in human eosinophilic esophagitis and mediates pathogenesis in mice. Gastroenterology. 2010;139:182–193. doi: 10.1053/j.gastro.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Zuo L, Fulkerson PC, Finkelman FD, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R α2-inhibited pathway. J Immunol. 2010;185:660–669. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchard C, Stucke EM, Burwinkel K, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol. 2010;184:4033–4041. doi: 10.4049/jimmunol.0903069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prescott SL, Macaubas C, Holt BJ, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160:4730–4737. [PubMed] [Google Scholar]
- 35.Barrios C, Brawand P, Berney M, Brandt C, Lambert PH, Siegrist CA. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–1496. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 36.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 37.Haruma K. Influence of Helicobacter pylori on gastro-oesophageal reflux disease in Japan. Aliment Pharmacol Ther. 2004;20:40–44. doi: 10.1111/j.1365-2036.2004.02228.x. [DOI] [PubMed] [Google Scholar]
- 38.Fixa B, Komárková O, Nozicka Z. Changing prevalence of some selected gastrointestinal diseases vis-à-vis H. pylori infection. Hepatogastroenterology. 2011;58:1062–1066. [PubMed] [Google Scholar]
- 39.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]