Abstract
Chronic kidney disease (CKD) is well known as a strong risk factor for both of end-stage renal disease and cardiovascular disease. To clarify the association of polymorphisms in the genes encoding antioxidant enzymes (SOD2, CAT, GPx, TXNRD, SEPP1, SEP15 and SELS) with the risk of CKD in Japanese, we examined this association using the cross-sectional data of Japan Multi-Institutional Collaborative Cohort (J-MICC) Study. The subjects were 3,285 men and women, aged 35–69 years, selected from J-MICC Study participants for whom genotyping were conducted by multiplex polymerase chain reaction-based Invader assay. The prevalence of CKD was determined for CKD stages 3–5 (eGFR <60 ml/min/1.73 m2). When those with CAT C-262T C/C were defined as reference, those with CAT C-262T C/T demonstrated the OR for CKD of 0.67 (95% CI 0.43–1.06) with the marginally significant trend for decreased odds ratio with increasing numbers of T allele (p = 0.070). There were no significant associations between the other polymorphisms with CKD risk. The present study found a marginally significant trend of the decreased risk of CKD with increasing numbers of T allele of CAT, which may suggest the possibility of personalized risk estimation of this life-limiting disease in the near future.
Keywords: antioxidant enzymes, genetic predisposition to disease, single nucleotide polymorphisms, chronic kidney disease
Introduction
Chronic kidney disease (CKD) is recently gathering attention as a potential risk factor for both of end-stage renal disease (ESRD) and cardiovascular disease (CVD). The prevalence of CKD is increasing in Japan, affecting about 19.1 million adult patients with CKD of stage ≥3.(1) The community-based study in Hisayama town showed that the age-adjusted prevalence of stage 3 and 4 CKD increased from 4.1% in 1974 through 4.8% in 1988 to 8.7% in 2002 in men, from 7.3% in 1974 through 11.2% in 1988, to 10.7% in 2002 in women.(2,3) Although the prevalence of CKD is known to be remarkably higher in our country of Japan compared to those in other countries,(4) it is also reported that about 8 million adults are affected by this disease in the U.S.,(1,5) suggesting that CKD is a major universal health problem, making its prevention an important issue worldwide.
Meanwhile, inflammation is shown to play an important role in the genesis of CKD mainly through the promotion of atherosclerosis; according to recent reports, inflammation is recognized in up to 50% of CKD predialysis and dialysis patients.(6) Increased oxidative stress has been hypothesized to be an important link between inflammation and cellular damage, which may result in the development of cardiovascular disease as well as CKD.(7) Oxidative stress results from increased concentrations of reactive oxygen species and/or a reduction in antioxidants.(8) The antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) constitute the primary defense system against reactive oxygen species (ROS) and oxidative stress. In light of these biological backgrounds, relationships between genetic variations of the genes encoding antioxidant enzymes and various diseases associated with oxidative stress are attracting attention because of the hope that they might be of benefit in screening and possible individualized prevention of these diseases.(8,9) Among them, SOD2 T201C (Val16Pro), CAT C-262T, GPx1 Pro198Leu, GPx4 Ex7 + 77 C>T (Leu220Leu), GPx4 273 bp 3' of STP (stop codon) [C>T], thioredoxin reductase 1 (TXNRD1) IVS1-181 [C>G], selenoprotein P (SEPP1) 1345 G>A (3'UTR), SEPP1 31,174 bp 3' of STP, SEPP1 43,881 bp 3' of STP, SEPP1 44,321 bp of 3' of STP [C>T], selenoprotein S (SELS) G-105A and 15kDa selenoprotein (SEP15) 3'UTR are the representative polymorphisms that are shown to modulate antioxidant enzyme capacities and studied well in association with the risks of various chronic diseases.(8,9)
We conducted the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study, a large genome cohort study to confirm and detect gene-environment interactions in lifestyle-related diseases, mainly cancer, launched in 2005 supported by a research grant for Scientific Research on Special Priority Areas of Cancer from the Japanese Ministry of Education, Culture, Sports, Science and Technology.(10,11)
Considering the crucial role of the antioxidant enzymes in the atherosclerogenesis and the involvement of atherosclerosis in the etiology of CKD, it would be plausible to hypothesize that genetic polymorphisms modulating the defense system against oxidative stress will also affect CKD risk in humans. Accordingly, to clarify the association of polymorphisms in genes encoding antioxidant enzymes (SOD2, CAT, GPx, TXNRD, SEPP1, SEP15 and SELS) with the risk of CKD in Japanese, we examined this association among the Japanese subjects using the cross-sectional data of this J-MICC study.
Materials and Methods
Study subjects
Subjects were the participants of the J-MICC Study, initially conducted in 10 areas of Japan, in which about 75,000 voluntarily enrolled participants aged 35–69 years provided their blood, health checkup data, and their lifestyle data based on the questionnaire after informed consent.(10)
In this analysis, 4,519 randomly selected J-MICC Study participants (about 500 subjects from each of the 10 areas) were analyzed, for whom 108 selected polymorphisms were genotyped.(11) Firstly, six subjects were excluded due to withdrawal from the study. Serum creatinine (SCr) was measured in 3,327 respondents from 8 areas out of 10. Forty-two subjects were excluded because of genotyping error, and the remaining 3,285 subjects were included in the analyses.
Evaluation of lifestyle exposure
Lifestyle exposures were evaluated with a self-administered questionnaire checked by trained staffs. The questionnaire included items on smoking status, alcohol consumption and food consumption. Smoking status was classified as current, former or never, and level of exposure was evaluated in pack-years. Former smokers were defined as people who had quitted smoking for at least 1 year. Alcohol consumption of each type of beverage was determined by average number of drinks per day, and then converted into the Japanese sake unit; ’gou’ (180 ml), which is equivalent to 23 g of ethanol.
Estimated glomerular filtration rate (eGFR) and definitions of CKD
SCr was measured in all participants using an enzymatic method. The eGFR of each participant was calculated based on SCr, age, and sex using the following Japanese eGFR equation proposed by the Japanese Society of Nephrology: eGFR (ml/min/1.73 m2) = 194 × SCr (mg/dl)−1.094 × age−0.287 (×0.739 if female).(12) The prevalence of CKD was determined for CKD stages 3–5 (defined as eGFR <60 ml/min/1.73 m2).
Genotyping of polymorphisms
DNA was extracted from buffy coat with a BioRobot M48 Workstation (QIAGEN Group, Tokyo, Japan). The genotyping of SOD2 T201C (Val16Pro), CAT C-262T, GPx1 Pro198Leu, GPx4 Ex7 + 77 C>T (Leu220Leu), GPx4 273 bp 3' of STP [C>T], TXNRD1 IVS1-181 [C>G], SEPP1 1345 G>A (3'UTR), SEPP1 31,174 bp 3' of STP, SEPP1 43,881 bp 3' of STP, SEPP1 44,321 bp of 3' of STP [C>T], SELS G-105A and SEP15 3'UTR polymorphisms was conducted by the RIKEN institute using multiplex polymerase chain reaction-based Invader assay (Third Wave Technologies, Madison, WI) as described previously.(13) The genotype distributions of all the 108 polymorphisms examined in this cross-sectional study are shown in the recently published data.(11)
Statistical analysis
Logistic regression analysis was performed for estimating age- and sex-adjusted odds ratios (aORs) and 95% CI for CKD by genotype. All the other potential confounders of body mass index (BMI), systolic blood pressure, diastolic blood pressure, use of anti-hypertensive drugs, fasting plasma glucose, hemoglobin A1c (HbA1c), use of glucose-lowering drugs, total cholesterol, high density lipoprotein (HDL) cholesterol, triglyceride, use of lipid-lowering drugs, uric acid, past history of cardiovascular diseases, past history of cerebrovascular diseases, smoking status and drinking status were tested for change in estimate (CIE) to see if any of these covariates produces significant change in estimates.(14) We decided not to include any of these variables because none of them fulfilled the criteria of CIE ≥0.1. Gene-environment interactions were assessed by the logistic model, which included a multiplicative interaction term as well as variables for each genotype, age, sex, and smoking and drinking habits. Age adjustments in the analyses were done with ages regarded as continuous variables. Trend analyses by genotypes were done with genotypes for each polymorphism coded as ordinal-categorical variables. Differences in the distribution of the values of each characteristic variable between two groups (i.e., CKD (+) and CKD (−)) were examined by Student’s t test or by the χ2 test. Accordance with the Hardy-Weinberg’s equilibrium, which indicates an absence of discrepancy between genotype and allele frequencies, was checked using the χ2 test. Haplotype analysis using genotypes in two loci was calculated by the ’haplologit’ command of STATA adjusted for age and sex based on the EM algorhythm.(15) The linkage disequilibrium (LD) between the polymorphisms in two loci (D' and r2) was estimated by the ’pwld’ command of STATA. All the calculations were done using the STATA version 10 (Stata Corp, College Station, TX).
Results
Characteristics of the subjects and allele frequency of the SOD2, CAT, GPx, TXNRD, SEPP, SEP and SELS polymorphisms
The characteristics of the subjects are summarized in Table 1. The mean age ± SD was 56.7 ± 8.6 years, and the males were 48.7% in the whole subjects. Subjects with CKD accounted for 17.3% (568/3,285) of the entire study population. Age, systolic blood pressure, total cholesterol and uric acid were significantly higher in subjects with CKD, and use of anti-hypertensive/lipid-lowering drugs, past history of cardiovascular/cerebrovascular diseases, and current smokers were more frequently observed in subjects with CKD, relative to those without CKD.
Table 1.
Comparison of characteristics between subjects with and without CKD (n = 3,285)
| CKD (+) (n = 568) | CKD (–) (n = 2,717) | p value | |
|---|---|---|---|
| eGFR (ml/min/1.73 m2) | 53.6 ± 6.0 | 78.3 ± 12.5 | <0.001 |
| Age (y) | 60.4 ± 7.2 | 55.9 ± 8.7 | <0.001 |
| Male | 263 (46.3%) | 1,337 (49.2%) | 0.208 |
| Body mass index | 23.5 ± 3.0 | 23.4 ± 3.3 | 0.464 |
| Systolic blood pressure (mmHg) | 130.3 ± 19.8 | 128.2 ± 19.3 | 0.017 |
| Diastolic blood pressure (mmHg) | 79.0 ± 12.4 | 78.7 ± 11.9 | 0.608 |
| Use of anti-hypertensive drugs | 154 (27.1%) | 490 (18.0%) | <0.001 |
| Fasting plasma glucose (mg/dl) | 99.0 ± 22.1 | 100.0 ± 20.9 | 0.351 |
| HbA1c (%) | 5.22 ± 0.69 | 5.22 ± 0.66 | 0.971 |
| Use of glucose-lowering drugs | 32 (5.6%) | 112 (4.1%) | 0.110 |
| Total cholesterol (mg/dl) | 218.5 ± 33.9 | 211.0 ± 33.9 | <0.001 |
| HDL cholesterol (mg/dl) | 61.9 ± 16.0 | 63.3 ± 16.4 | 0.056 |
| Triglyceride (mg/dl) | 107 (77–151) | 104.5 (74–154) | 0.951 |
| Use of lipid-lowering drugs | 73 (12.9%) | 228 (8.4%) | 0.001 |
| Uric acid (mg/dl) | 5.55 ± 1.48 | 5.11 ± 1.33 | <0.001 |
| Cardiovascular diseases | 34 (6.0%) | 80 (2.9%) | 0.001 |
| Cerebrovascular diseases | 31 (5.5%) | 53 (2.0%) | <0.001 |
| Current smokers | 72 (12.7%) | 489 (18.0%) | 0.002 |
| Current drinkers | 298 (52.5%) | 1,522 (56.0%) | 0.126 |
Results are expressed as means ± SD, n (%), or median (interquartile range). CKD = chronic kidney disease. CKD was defined as estimated glomerular filtration rate <60 ml/min/1.73 m2. eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high density lipoprotein.
The genotype frequencies among the genotyped subjects were in accordance with Hardy-Weinberg’s equilibrium for all of the SOD2 T201C (Val16Pro) (C allele = 0.133 [minor allele frequency], χ2 = 1.036, p = 0.3089), CAT C-262T (T allele = 0.030, χ2 = 0.001, p = 0.9777), GPx1 Pro198Leu (T allele = 0.075, χ2 = 4.767, p = 0.0290), GPx4 Ex7 + 77 C>T (Leu220Leu) (T allele = 0.354, χ2 = 1.539, p = 0.2148), GPx4 273 bp 3' of STP [C>T] (T allele = 0.185, χ2 = 0.009, p = 0.9226), TXNRD1 IVS1-181 [C>G] (G allele = 0.025, χ2 = 0.382, p = 0.5365), SEPP1 1345 G>A (3'UTR) (A allele = 0.383, χ2 = 0.033, p = 0.8548), SEPP1 31,174 bp 3' of STP (T allele = 0.352, χ2 = 0.000, p = 0.9878), SEPP1 43,881 bp 3' of STP (A allele = 0.383, χ2 = 0.052, p = 0.8193), SEPP1 44,321 bp of 3' of STP [C>T] (C allele = 0.456, χ2 = 1.432, p = 0.2314), SELS G-105A (A allele = 0.281, χ2 = 0.252, p = 0.6157), and SEP15 3'UTR (T allele = 0.043, χ2 = 0.756, p = 0.3845). Genotype call rate was more than 99.6% for each genotype among those with SCr data (n = 3,327).
SOD2, CAT, GPx, TXNRD, SEPP1, SEP15 and SELS polymorphisms and risk of CKD
When those with CAT C-262T C/C were defined as reference, those with CAT C-262T C/T demonstrated the OR for CKD of 0.67 (95% CI 0.43–1.06) with the marginally significant trend for decreased OR with the increasing number of T allele (p = 0.070). There were no significant associations between the polymorphisms in other polymorphisms in genes encoding antioxidant enzymes (SOD2 T201C (Val16Pro), CAT C-262T, GPx1 Pro198Leu, GPx4 Ex7 + 77 C>T (Leu220Leu), GPx4 273 bp 3' of STP [C>T], TXNRD1 IVS1-181 [C>G], SEPP1 1345 G>A (3'UTR), SEPP1 31,174 bp 3' of STP, SEPP1 43,881 bp 3' of STP, SEPP1 44,321 bp of 3' of STP [C>T], SELS G-105A and SEP15 with the risk of CKD (Table 2).
Table 2.
SOD2, CAT, GPx, TXN, SEPP1, SEP15 and SELS polymorphisms and risk of CKD
| Polymorphism | Genotype | CKD (+) (n = 568) | CKD (–) (n = 2,717) | aOR (95% CI) | Trend p |
|---|---|---|---|---|---|
| SOD2 Val16Pro (T201C) (rs4880) | T/T | 442 (77.8%) | 2,033 (74.8%) | Reference | |
| T/C | 117 (20.6%) | 628 (23.1%) | 0.88 (0.70–1.10) | 0.198 | |
| C/C | 9 (1.6%) | 56 (2.1%) | 0.77 (0.37–1.59) | ||
| CAT C–262T (rs1001179) | C/C | 545 (96.0%) | 2,546 (93.7%) | Reference | |
| C/T | 23 (4.0%) | 168 (6.2%) | 0.67 (0.43–1.06) | 0.070 | |
| T/T | 0 (0.0%) | 3 (0.1%) | 0 (–) | ||
| GPx1 Pro198Leu (rs1050450) | C/C | 489 (86.1%) | 2,332 (85.8%) | Reference | |
| C/T | 73 (12.9%) | 364 (13.4%) | 0.94 (0.72–1.24) | 0.951 | |
| T/T | 6 (1.1%) | 21 (0.8%) | 1.37 (0.54–3.49) | ||
| GPx4 Ex7 + 77C>T (Leu220Leu) (rs713041) | C/C | 247 (43.5%) | 1,140 (42.0%) | Reference | |
| C/T | 253 (44.5%) | 1,217 (44.8%) | 0.97 (0.80–1.19) | 0.340 | |
| T/T | 68 (12.0%) | 360 (13.2%) | 0.85 (0.63–1.14) | ||
| GPx4 273 bp 3' of STP [C>T] (rs2075710) | C/C | 382 (67.3%) | 1,802 (66.3%) | Reference | |
| C/T | 165 (29.0%) | 823 (30.3%) | 0.96 (0.78–1.18) | 0.954 | |
| T/T | 21 (3.7%) | 92 (3.4%) | 1.12 (0.68–1.84) | ||
| TXNRD1 IVS1–181 [C>G] (rs35009941) | C/C | 539 (94.9%) | 2,582 (95.0%) | Reference | |
| C/G | 29 (5.1%) | 132 (4.9%) | 1.17 (0.97–1.41) | 0.879 | |
| G/G | 0 (0.0%) | 3 (0.1%) | 1.02 (0.67–1.56) | ||
| SEPP1 1345G>A (3' UTR) (rs7579) | G/G | 224 (39.4%) | 1,028 (37.8%) | Reference | |
| A/G | 256 (45.1%) | 1,292 (47.6%) | 0.90 (0.73–1.10) | 0.720 | |
| A/A | 88 (15.5%) | 397 (14.6%) | 1.00 (0.76–1.32) | ||
| SEPP1 31,174 bp 3' of STP (rs12055266) | G/G | 242 (42.6%) | 1,138 (41.9%) | Reference | |
| G/A | 245 (43.1%) | 1,253 (46.1%) | 0.90 (0.73–1.10) | 0.745 | |
| A/A | 81 (14.3%) | 326 (12.0%) | 1.00 (0.76–1.32) | ||
| SEPP1 43,881 bp 3' of STP (rs3797310) | G/G | 224 (39.4%) | 1,028 (37.8%) | Reference | |
| A/G | 256 (45.1%) | 1,291 (47.5%) | 0.90 (0.73–1.10) | 0.716 | |
| A/A | 88 (15.5%) | 398 (14.6%) | 1.00 (0.76–1.32) | ||
| SEPP1 44,321 bp of 3' of STP [C>T] (rs2972994) | T/T | 174 (30.6%) | 780 (28.7%) | Reference | |
| C/T | 273 (48.1%) | 1,391 (51.2%) | 0.87 (0.70–1.07) | 0.609 | |
| C/C | 121 (21.3%) | 546 (20.1%) | 0.95 (0.73–1.24) | ||
| SELS G-105A (rs34713741) | G/G | 284 (50.0%) | 1,418 (52.2%) | Reference | |
| A/G | 237 (41.7%) | 1,080 (39.7%) | 1.10 (0.90–1.33) | 0.339 | |
| A/A | 47 (8.3%) | 219 (8.1%) | 1.11 (0.78–1.57) | ||
| SEP15 3'-UTR (rs5859) | G/G | 520 (91.5%) | 2,493 (91.8%) | Reference | |
| G/A | 47 (8.3%) | 217 (8.0%) | 0.99 (0.70–1.38) | 0.900 | |
| A/A | 1 (0.2%) | 7 (0.3%) | 0.85 (0.10–7.07) |
aOR: adjusted odds ratio (adjusted for age and sex); CKD: chronic kidney disease; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; TXNRD, thioredoxin reductase; SEPP, selenoprotein P; SELS, selenoprotein S; SEP15, 15kDa selenoprotein.
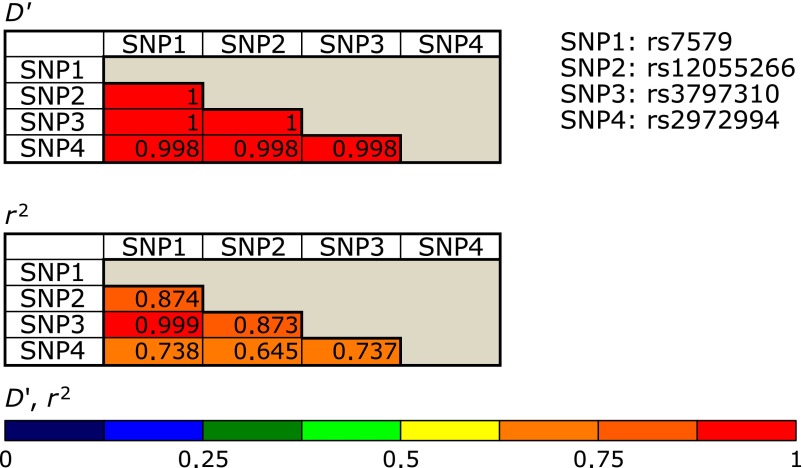
We also estimated the LD within GPx4 or SEPP1 polymorphisms, which revealed that GPx4 Ex7 + 77 C>T (Leu220Leu) and GPx4 273 bp 3' of STP [C>T] were in complete linkage (D' = 1.00 and r2 = 0.12), while almost all of the 4 loci of SEPP1 were also tightly linked to each other (Fig. 1). Haplotype analysis of the GPx4 or SEPP1 polymorphisms did not reveal any significant association of GPx4 or SEPP1 haplotypes with the risk of CKD.
Fig. 1.
Linkage disequilibrium between the 4 SEPP1 polymorphisms.
We tested possible interaction between antioxidant enzyme genotypes and lifestyle/etiologic factors including smoking, alcohol drinking, hypertension, dysglycemia, dyslipidemia and high uric acid, none of which resulted in statistical significance (data not shown). As this study was held in 10 institutions, of which 8 had data for eGFR, we also conducted the analyses adjusted for institutions, the results of which were not substantially different from the unadjusted results. In addition, we conducted our analyses having subjects with medication (use of anti-hypertensive/glucose-lowering/lipid-lowering drugs) excluded, which did not substantially alter the results, either.
Discussion
Oxidative stress along with inflammation is shown to promote kidney and vascular injury, and several factors are demonstrated to induce ROS in renal cells: e.g., inflammatory cytokines, Toll-like receptors, Angiotensin II, bradykinin, arachidonic acid, thrombin, growth factors and mechanical pressure, in which NADPH oxidases, i.e., Nox enzymes are supposed to play key roles as the mediator of the ROS genesis.(16) Especially in ESRD patients, it is well-established that there is a high prevalence of acute-phase inflammation and oxidative stress, which are shown to lead to the high rate of cardiovascular morbidity and mortality,(17) suggesting the importance of oxidative stress in the CKD etiology. The present study suggested that CAT polymorphism, a key polymorphism in genes encoding antioxidant enzymes, is marginally significantly associated with the risk of CKD in Japanese. As far as we know, there is one report from Australia that investigated the association of genetic variations in antioxidant enzymes with CKD risk,(18) but this is the first one that investigated the roles of antioxidant enzyme polymorphisms in the risk of CKD in East Asian population. It is already reported that polymorphisms in both pro- and anti-inflammatory cytokines play important roles in the risk of CKD by way of atherosclerogenesis through the modulation of the cytokine balance.(19) Our study results suggested the trend that the subjects with the T allele of CAT C-262Tpolymorphism was at a decreased risk of CKD, which was in line with our hypothesis and demonstrated the novel evidence that augmented antioxidant function due to this polymorphism may reduce the risk of CKD as well as other vascular diseases.
Superoxide anion, i.e., ROS, is dismutated by superoxide dismutases (SODs) to hydrogen peroxide that is catalyzed to water and oxygen by catalase or glutathione perioxidases (GPx).(20) There are three distinct isoforms of SOD identified and characterized in mammals: copper-zinc superoxide dismutase (Cu/Zn SOD) which is encoded by the SOD1 gene, manganese superoxide dismutase (MnSOD) encoded by the SOD2 gene, and extracellular superoxide dismutase (ECSOD) encoded by the SOD3 gene. These forms of SOD elicit similar functions, although the characteristics of their structure, chromosome position, metal cofactor requirements, gene distribution, and cellular localization are distinct from one another.(21) Namely, SOD1 is present in red blood cells, SOD2 is primarily mitochondrial and SOD3 is extracellular.(22) Regarding GPx, 5 selenium-containing GPx’s have been identified so far in humans,(23) that is, GPx 1-4 and GPx6. All of them can react with H2O2 and soluble fatty acid hydroperoxides. GPx4 is the only GPx that can react with complex lipid hydroperoxides.(24) Of all the GPx family members, accumulated evidence suggests the particular importance of GPx1 and GPx4 in human chronic diseases.(25) GPx4 is a monomer that may facilitate its reactivity with lipids, and is considered to have most important functions of all the GPx family members based on the fact that its knockout mouse is lethal.(26,27) Our study results revealed no significant association of CKD risk with the polymorphisms in these genes encoding antioxidant enzymes, SOD2 T201C (Val16Pro), GPx1 Pro198Leu, GPx4 Ex7 + 77 C>T (Leu220Leu) and GPx4 273 bp 3' of STP [C>T], suggesting that the roles of genetic variation of these antioxidants are limited in Japanese.
Meanwhile, CAT is an important endogenous antioxidant enzyme that detoxifies hydrogen peroxide to oxygen and water, which then helps prevent the formation of carbon dioxide bubbles in the blood, and thus limits the deleterious effects of ROS.(28) CAT also utilizes hydrogen peroxide to breakdown toxins such as alcohol, phenol, formaldehyde, etc. In addition, CAT works in coordination with SOD to further prevent free-radical damage to human body.(28) Based on all of these facts, CAT has been considered to be an important regulator of oxidative stress, which is involved in the genesis of various human chronic diseases. Of the several polymorphisms in CAT gene reported, one common polymorphism in the promoter region, CAT C-262T, is considered to be functional and relatively studied well in association with human diseases.(29) Individuals with homozygous and heterozygous mutant (= T allele) of CAT C-262T polymorphism are demonstrated to have increased levels of CAT mRNA and protein in erythrocytes in some studies,(30–32) whereas one recent report from Egypt showed lower blood CAT activity in those with at least one T allele of CAT C-262T polymorphism compared with those with the CAT C-262T C/C genotype.(28) Catalase is a common enzyme which catalyzes the decomposition of hydrogen peroxide to water and oxygen.(33) Our study results revealed the marginally significant trend of the decreased risk of CKD with the increasing number of T allele of CAT C-262T polymorphism, suggesting that higher CAT activity due to this polymorphism lead to the less renal injury and CKD risk reduction in those with at least one T allele, if the reported association of the T allele of CAT C-262T polymorphism and higher CAT activity is true.
Of the remaining genes encoding antioxidant enzymes, TXNRD, SEPP1, SEP15 and SELS, mainly consists of selenoprotein genes through which selenium (Se) exerts its biological effects; Se is a micronutrient that is essential for human health, and there is an evidence that low Se status is associated with increased risk of colorectal cancer (CRC), whereas the higher Se intake will lower CRC morality, especially when Se status is low prior to supplementation.(34) Among the selenoproteins presented, SEPP1 is a protein that transports Se to tissues, TXNRD1 is the one that functions in redox control, and SEP15 and SELS are the ones that are involved in inflammation.(35) To the best of our knowledge, the present study is the first one that investigated the association of genetic variations in selenoproteins with the risk of human CKD, but it did not reach any statistical significance, suggesting that the roles of polymorphisms of these selenoproteins are limited in the etiology of CKD in Japanese.
There are some potential limitations in this study. At first, all of the CKD cases are diagnosed based on the SCr data, which might potentially be different from the actual GFR based on the renal measurement. Adjustments for multiple comparisons may be another issue; we decided not to adopt these adjustments in this study, because the present study was conducted under the exploratory context. In this study, the genotype frequencies of GPx1 Pro198Leu polymorphism among the entire subjects significantly deviated from the Hardy-Weinberg’s equilibrium, which may be explained by the type-I error randomly caused by the considerably small frequency of the minor allele (T allele = 0.075). Further investigations with better study designs in these aspects will also be required.
In summary, the present study found a marginally significant trend of the reduced risk of CKD with the increasing number of T allele of CAT C-262T polymorphism, which may suggest the future possibility of personalized risk estimation of this life-limiting disease in the near future.
Acknowledgments
The authors wish to thank Mr. Kyota Ashikawa and Ms. Tomomi Aoi at the Laboratory of Genotyping Development, Center for Genomic Medicine, RIKEN for genotyping. The authors also thank Ms. Yoko Mitsuda, and Ms. Keiko Shibata at Nagoya University for their technical assistance. This study was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas of Cancer (No. 17015018) and Scientific Support Programs for Cancer Research, Grant-in-Aid for Scientific Research on Innovative Areas (No. 221S0001) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Abbreviations
- aOR
adjusted odds ratio
- CAT
catalase
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- GPx
glutathione peroxidase
- J-MICC
Japan Multi-Institutional Collaborative Cohort
- LD
linkage disequilibrium
- ROS
reactive oxygen species
- SCr
serum creatinine
- SELS
selenoprotein S
- SEP15
15kDa selenoprotein
- SEPP1
selenoprotein P, plasma, 1
- SOD
superoxide dismutase
- TXNRD1
thioredoxin reductase 1
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Yamamoto R, Kanazawa A, Shimizu T, et al. Association between atherosclerosis and newly classified chronic kidney disease stage for Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2009;84:39–45. doi: 10.1016/j.diabres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Iseki K. Chronic kidney disease in Japan from early predictions to current facts. Nephron Clin Pract. 2008;110:c268–c272. doi: 10.1159/000170094. [DOI] [PubMed] [Google Scholar]
- 3.Ninomiya T, Kiyohara Y. Chronic kidney diseases and other diseases: 1. Cardiovascular diseases [in Japanese] Nihon Naika Gakkai Zasshi. 2007;96:887–893. doi: 10.2169/naika.96.887. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo S, Yasuda Y, Imai E, Horio M. Current status of estimated glomerular filtration rate (eGFR) equations for Asians and an approach to create a common eGFR equation. Nephrology (Carlton) 2010;15 (Suppl 2):45–48. doi: 10.1111/j.1440-1797.2010.01313.x. [DOI] [PubMed] [Google Scholar]
- 5.Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18:629–636. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 6.Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension. 2005;45:1042–1049. doi: 10.1161/01.HYP.0000167121.14254.a0. [DOI] [PubMed] [Google Scholar]
- 7.Del Vecchio L, Locatelli F, Carini M. What we know about oxidative stress in patients with chronic kidney disease on dialysis--clinical effects, potential treatment, and prevention. Semin Dial. 2011;24:56–64. doi: 10.1111/j.1525-139X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- 8.Crawford A, Fassett RG, Geraghty DP, et al. Relationships between single nucleotide polymorphisms of antioxidant enzymes and disease. Gene. 2012;501:89–103. doi: 10.1016/j.gene.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Yuzhalin AE, Kutikhin AG. Inherited variations in the SOD and GPX gene families and cancer risk. Free Radic Res. 2012;46:581–599. doi: 10.3109/10715762.2012.658515. [DOI] [PubMed] [Google Scholar]
- 10.Hamajima N, J-MICC Study Group The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac J Cancer Prev. 2007;8:317–323. [PubMed] [Google Scholar]
- 11.Wakai K, Hamajima N, Okada R, et al. J-MICC Study Group Profile of participants and genotype distributions of 108 polymorphisms in a cross-sectional study to elucidate associations between genotypes and lifestyle and clinical factors: a project in the Japan multi-institutional Collaborative Cohort (J-MICC) Study. J Epidemiol. 2011;21:223–235. doi: 10.2188/jea.JE20100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Ohnishi Y, Tanaka T, Ozaki K, Yamada R, Suzuki H, Nakamura Y. A high-throughput SNP typing system for genome-wide association studies. J Hum Genet. 2001;46:471–477. doi: 10.1007/s100380170047. [DOI] [PubMed] [Google Scholar]
- 14.Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P. Confounder selection in environmental epidemiology: assessment of health effects of prenatal mercury exposure. Ann Epidemiol. 2007;17:27–35. doi: 10.1016/j.annepidem.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Inoue E, Kamatani N. Association test algorithm between a qualitative phenotype and a haplotype or haplotype set using simultaneous estimation of haplotype frequencies, diplotype configurations and diplotype-based penetrances. Genetics. 2004;168:2339–2348. doi: 10.1534/genetics.103.024653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rojas-Rivera J, Ortiz A, Egido J. Antioxidants in kidney diseases: the impact of bardoxolone methyl. Int J Nephrol. 2012:321714. doi: 10.1155/2012/321714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 18.Crawford A, Fassett RG, Coombes JS, et al. Relationship between antioxidant enzyme genotype and activity and kidney function: a case-control study. Clin Nephrol. 2012;78:135–144. doi: 10.5414/cn107421. [DOI] [PubMed] [Google Scholar]
- 19.Okada R, Wakai K, Naito M, et al. Pro-/anti-inflammatory cytokine gene polymorphisms and chronic kidney disease: a cross-sectional study. BMC Nephrol. 2012;13:2. doi: 10.1186/1471-2369-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao L, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47:344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford A, Fassett RG, Coombes JS, et al. Glutathione peroxidase, superoxide dismutase and catalase genotypes and activities and the progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26:2806–2813. doi: 10.1093/ndt/gfq828. [DOI] [PubMed] [Google Scholar]
- 23.Brigelius-Flohé R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790:1555–1568. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 25.Rayman MP. Selenoproteins and human health: insights from epidemiological data. Biochim Biophys Acta. 2009;1790:1533–1540. doi: 10.1016/j.bbagen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Imai H, Hirao F, Sakamoto T, et al. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem Biophys Res Commun. 2003;305:278–286. doi: 10.1016/s0006-291x(03)00734-4. [DOI] [PubMed] [Google Scholar]
- 27.Yant LJ, Ran Q, Rao L, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 28.Ghaly MS, Ghattas MH, Labib SM. Association of catalase gene polymorphisms with catalase activity and susceptibility to systemic lupus erythematosus in the Suez Canal area, Egypt. Lupus. 2012;21:1244–1249. doi: 10.1177/0961203312451505. [DOI] [PubMed] [Google Scholar]
- 29.Hebert-Schuster M, Fabre EE, Nivet-Antoine V. Catalase polymorphisms and metabolic diseases. Curr Opin Clin Nutr Metab Care. 2012;15:397–402. doi: 10.1097/MCO.0b013e328354a326. [DOI] [PubMed] [Google Scholar]
- 30.Forsberg L, Lyrenäs L, de Faire U, Morgenstern R. A common functional C-T substitution polymorphism in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase levels. Free Radic Biol Med. 2001;30:500–505. doi: 10.1016/s0891-5849(00)00487-1. [DOI] [PubMed] [Google Scholar]
- 31.Bastaki M, Huen K, Manzanillo P, et al. Genotype-activity relationship for Mn-superoxide dismutase, glutathione peroxidase 1 and catalase in humans. Pharmacogenet Genomics. 2006;16:279–286. doi: 10.1097/01.fpc.0000199498.08725.9c. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Yu M, Li M, et al. Polymorphic variations in manganese superoxide dismutase (MnSOD), glutathione peroxidase-1 (GPX1), and catalase (CAT) contribute to elevated plasma triglyceride levels in Chinese patients with type 2 diabetes or diabetic cardiovascular disease. Mol Cell Biochem. 2012;363:85–91. doi: 10.1007/s11010-011-1160-3. [DOI] [PubMed] [Google Scholar]
- 33.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson P, Roe DJ, Fales L, et al. Design and baseline characteristics of participants in a phase III randomized trial of celecoxib and selenium for colorectal adenoma prevention. Cancer Prev Res (Phila) 2012;5:1381–1393. doi: 10.1158/1940-6207.CAPR-12-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Méplan C, Hughes DJ, Pardini B, et al. Genetic variants in selenoprotein genes increase risk of colorectal cancer. Carcinogenesis. 2010;31:1074–1079. doi: 10.1093/carcin/bgq076. [DOI] [PubMed] [Google Scholar]