Abstract
We evaluated the antioxidative effects of astaxanthin through the changes in superoxide scavenging activity, levels of hydrogen peroxide and total hydroperoxides in human aqueous humor. The study subjects were 35 patients who underwent bilateral cataract surgery on one side before and the other side after intake of astaxanthin (6 mg/day for 2 weeks). Their aqueous humor was taken during the surgery and subjected to measurements of the three parameters. After astaxanthin intake, the superoxide scavenging activity was significantly (p<0.05) elevated, while the level of total hydroperoxides was significantly (p<0.05) lowered. There was a significant negative correlation between the superoxide scavenging activity and the level of total hydroperoxides (r = −0.485, p<0.01), but no correlations between the hydrogen peroxide level and the other two parameters. Astaxanthin intake clearly enhanced the superoxide scavenging activity and suppressed the total hydroperoxides production in human aqueous humor, indicating the possibility that astaxanthin has suppressive effects on various oxidative stress-related diseases.
Keywords: astaxanthin, aqueous humor, superoxide, hydrogen peroxide, oxidation
Introduction
As factors that contribute to the development and progression of various pathologies, including changes that accompany infarct diseases of the brain and heart or development of tumors, the involvement of active oxygen and free radicals has been elucidated. At the same time, studies on antioxidants have been actively implemented. The field of ophthalmology is no exception, and many studies have examined the relationships between oxidative stress and various ophthalmic diseases.(1,2,3) In the present study, we focused on an antioxidative substance, astaxanthin (AX), which is a kind of carotenoid.(4,5) Carotenoids are broadly categorized into carotenes and xanthophylls. Among the xanthophylls, lutein, fucoxanthin, AX, tunaxanthin and zeaxanthin are known.(6) AX is a red pigment found in crustaceans, such as prawns and crabs, as well as fish, such as salmon, trout and sea bream, and was first isolated by Kuhn and Soerensen in 1937 (Fig. 1).(4) It is regarded as a strong antioxidant that is about 1000 and 40 times more potent than vitamin E and β-carotene, respectively.(7,8) The existence of AX has been known for a long time, and it is frequently used as a food pigment. Since it is ingested with various foods, its safety has been well established.(9) At present, AX is not considered to be effective against all reactive oxygen species (ROS).(10) The extents to which AX affects ROS generation and the entire reaction-suppressing pathways as the body’s defense system (including peroxidation reactions and antioxidation mechanisms) remain unknown.
Fig. 1.
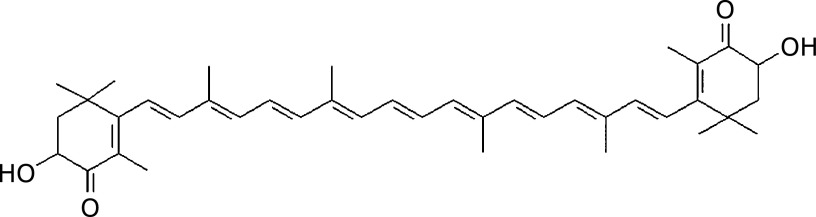
Structural formula of astaxanthin.
Superoxide, a typical kind of free radical, is dismutated by superoxide dismutase (SOD), a scavenging enzyme, into hydrogen peroxide (H2O2), a type of ROS, which is further detoxified by the H2O2 scavenging enzymes such as catalase or glutathione peroxidase into water (H2O). For scavenging of superoxide, not only SOD but also L-ascorbic acid (L-AsA) and reduced glutathione (GSH) are involved. It is known that the superoxide scavenging activities of these substances can work individually, but when they work coordinately, their functions are augmented and show persistent and continuous scavenging effects.
When the reactive oxygen scavenging process of superoxide → H2O2 → H2O is blocked for some reason, superoxide or H2O2 accumulates, and there is a possibility that hydroxyl radicals (HO•) will be generated from H2O2 in the presence of iron or copper ions. Among the ROS, H2O2 is not a free radical and is relatively stable. However, superoxide and hydroxyl radicals are free radicals and are extremely unstable and highly reactive, and it is known that they instantly and nonspecifically induce peroxidation reactions with lipids, proteins, and nucleic acids, thereby generating lipid peroxides, and denatured proteins and nucleic acids by oxidation.
For determination of the point at which AX exerts antioxidative effects in the superoxide → H2O2 → H2O pathway and the magnitude of these effects, we considered that it is necessary to measure the levels of ROS or their scavenging enzymes at each stage. In other words, since free radicals have extremely short half-lives and are unstable, we decided to measure typical parameters in peroxidation reactions that can be steadily measured with small amounts of specimens, namely i) the scavenging activity of superoxide ions generated in the early stage of the reaction, ii) the level of H2O2 as an intermediate metabolite generated as a result of the dismutation reaction from superoxide, and iii) the level of total hydroperoxides including not only lipid peroxides and H2O2 but also peroxides of proteins, amino acids, and nucleic acids at the end stage of the reaction. We measured these three parameters in human aqueous humor and evaluated the changes between before and after intake of AX and their relationships.
Liquid represents about 60% of the adult body, comprising 40% intracellular fluid and 20% extracellular fluid. Extracellular fluid is further categorized into interstitial fluid, plasma and transcellular fluid. It is referred to as the internal environment of the body, because ions and nutrients required for the maintenance of homeostasis in cells and tissues to sustain life are supplied and metabolized through and in the extracellular fluid. The aqueous humor is a kind of transcellular fluid, but has a unique composition and function that are different from those of other extracellular fluids, such as plasma, or interstitial fluids, like cerebrospinal fluid, which is composed of substances that have passed through the blood–brain barrier. It directly exchanges substances with the lens and corneal cells like other extracellular fluids. Although each cell in the human body contains various components, their compositions are similar among the cells throughout the body. Thus, it is considered that the changes in the aqueous humor reflect the changes occurring in the whole body. In the present study, we evaluated the effects of AX intake on the living body by measuring the above-mentioned three peroxidation-related parameters in the aqueous humor. The effects of AX on ophthalmic diseases strongly related to the aqueous humor, such as cataracts, are shown most clearly, but these changes are considered to reflect the effects on the whole body.
The effects of AX on the human body have been actively studied recently, (11) but no reports have described its effects on the lipid peroxidation reactions in the human aqueous humor. This is the first study to examine the effects of AX on the human body through measurements of certain peroxidation-related parameters in human aqueous humor.
Subjects and Methods
Subjects
This study was performed according to the principles of the Declaration of Helsinki. The study protocol was approved by the Ethical Committee of Dokkyo Medical University. Informed consent was obtained from all participants. The study subjects were 35 patients aged 70.9 ± 6.8 years who underwent bilateral cataract surgery at Tsukuba Hashimoto Optical Clinic. Patients with diseases prone to inflammation, such as uveitis, patients with high degrees of refractive error of 8 diopters or above, patients with marked hardening of the nucleus, patients with poor mydriasis, patients in whom difficult surgery was anticipated, and patients who had been taking other supplements were excluded. For intake of AX, the subjects received a full explanation of the purpose of the study and provided consent before the start of the study. Patients who did not want to continue taking AX for any reason during the period in which AX intake was required for the study were allowed to immediately stop the intake and were excluded from the analyses.
In this study, the levels of the parameters were compared between before and after the intake of AX. Since sex and diabetes (DM) are also known as factors that affect peroxidation reactions, we conducted comparative studies by categorizing subjects based on sex and presence of DM. Sixteen patients aged 71.3 ± 6.4 years were in the male group and 19 patients aged 70.6 ± 7.4 years were in the female group, while 16 patients aged 70.3 ± 6.2 years were in the DM group and 19 patients aged 71.5 ± 7.6 years were in the non-DM group.
For AX, we used a commercially available supplement, Astavita (Fuji Chemical Industry Co. Ltd., Toyama, Japan) at a dose of 6 mg/day, which is the recommended dose for the supplement. This recommended dose was set on the basis of a dose-finding study in healthy adults,(12) in which AX at 6 mg/day or above improved the accommodation ability of the eyes and subjective symptoms in asthenopia.
Methods
The patients started to take AX just after the surgery for one eye, and underwent surgery for the other eye after 2 weeks. During each surgery, the primary aqueous humor was sampled and kept at −40°C by immediately filling the test tube with nitrogen gas. The superoxide scavenging activity in the aqueous humor was measured using a nitroblue tetrazolium (NBT) reduction method,(13) and the level of H2O2 was measured by a titanium colorimetric method.(14) As the reagent for the nitroblue tetrazolium reduction method, a SOD Test Wako R (Wako Pure Chem. Ind. Ltd., Osaka, Japan) was used. This method measures the total superoxide scavenging activity including the superoxide scavenging activity for GSH and L-AsA in addition to SOD.
Total hydroperoxides was measured by a microassay using the Free d-ROMs reagent (Diacron Srl, Grosseto, Italy).(15) N,N-diethylparaphenylenediamine, the chromogen pigment in the Free d-ROMs reagent, reacts with H2O2, lipid peroxides, peroxidated nucleic acids and nucleotides, and peroxides of proteins, peptides and amino acids, and thus the level of total hydroperoxides measures the total amount of peroxidated substances (-OOH).(16) Since this method involves a multiple number of objects to be measured, the results are expressed in an arbitrary unit called Carrateli Units (U.CARR). In this study, 20 µl of aqueous humor and 125 µl of buffer were mixed together and left for 5 min at ambient temperature. Next, 2 µl of the above-mentioned chromogen solution was added to the mixture, and the reaction was allowed to proceed at 37°C after 1–2 seconds of agitation. The changes in the OD545 with time were measured. At the same time, standard solutions were measured to create a calibration curve. The level of total hydroperoxides in the aqueous humor was determined based on the calibration curve.
Statistical analysis
Regarding the statistical analyses, Wilcoxon’s signed rank sum test was used for comparisons of the levels between before and after AX intake, the Mann–Whitney U test was used for comparisons between the two groups of sex and DM status, and Pearson’s correlation coefficient was used to examine the relationships among the three parameters of superoxide scavenging activity, H2O2 level and level of total hydroperoxides. For each test, values of p<0.05 were considered significant.
Results
Changes between before and after AX intake in all subjects
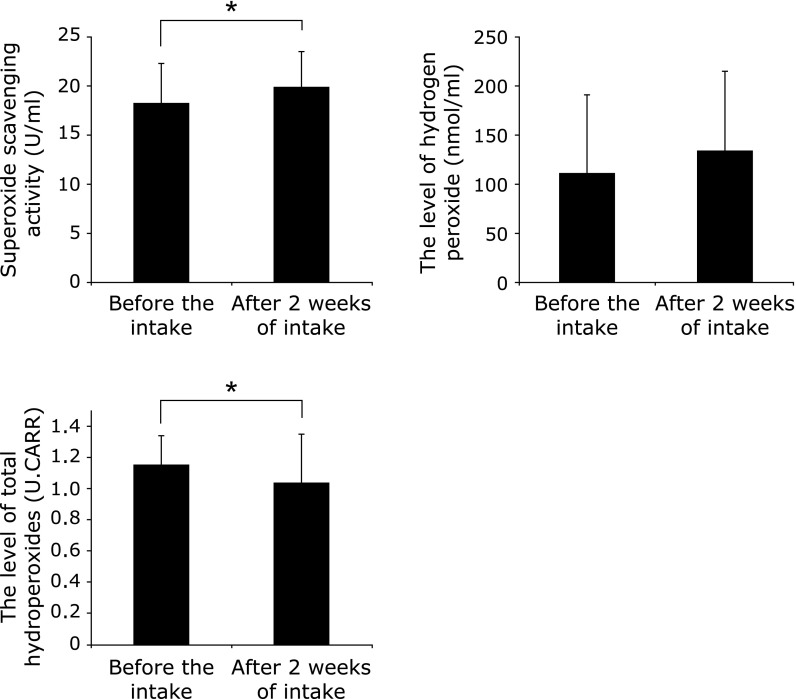
The changes between before and after AX intake for all subjects are shown in Fig. 2. The superoxide scavenging activity was significantly (p<0.05) higher after the intake (18.2 ± 4.1 vs 19.9 ± 3.6 U/ml before and after the intake, respectively). The H2O2 levels were 110 ± 81 and 134 ± 81 nmol/ml before and after the intake, respectively, with no significant difference. The level of total hydroperoxides was significantly (p<0.05) lower after the intake (1.16 ± 0.18 vs 1.04 ± 0.31 U.CARR before and after the intake, respectively).
Fig. 2.
Changes between before and after AX intake in all subjects (n = 35), *p<0.05.
Changes between before and after AX intake by sex
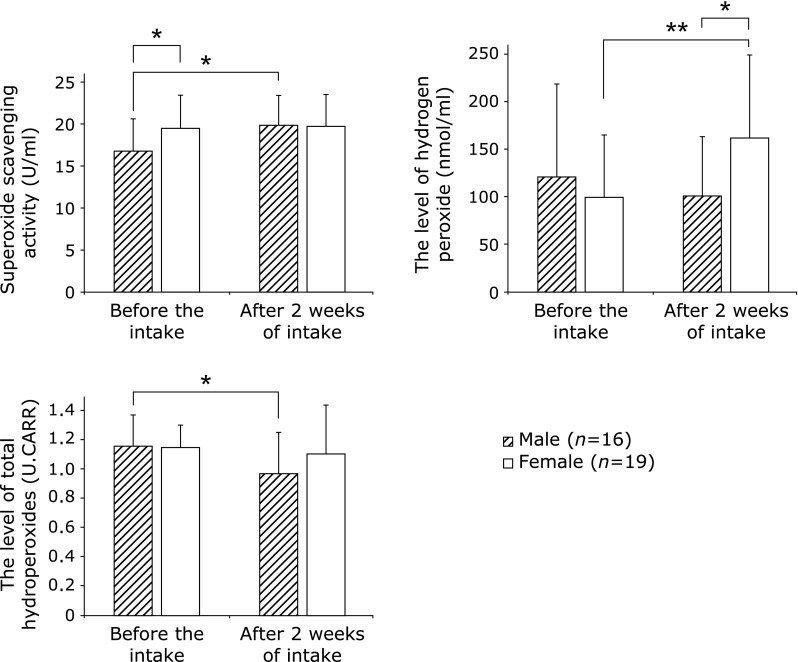
The changes between before and after AX intake according to sex are shown in Fig. 3. The superoxide scavenging activity in the male group was 16.8 ± 3.9 U/ml before and 20.0 ± 3.5 U/ml after the intake, while that in the female group was 19.5 ± 4.0 U/ml before and 19.8 ± 3.8 U/ml after the intake. The activity after the intake was significantly (p<0.05) higher in the male group, but there was no change in the female group. There was already a significant difference in the superoxide scavenging activity before the intake between the two groups, with that in the female group being significantly (p<0.05) higher.
Fig. 3.
Changes between before and after AX intake according to sex. *p<0.05, **p<0.01.
The H2O2 level in the male group was 121 ± 98 nmol/ml before and 101 ± 62 nmol/ml after the intake, and that in the female group was 100 ± 66 nmol/ml before and 162 ± 87 nmol/ml after the intake. The level showed a significant (p<0.05) increase after the intake in the female group. After the intake, there was a significant difference between the two groups, with a significantly (p<0.05) higher value in the female group.
The level of total hydroperoxides in the male group was 1.16 ± 0.21 U.CARR before and 0.97 ± 0.28 U.CARR after the intake, and that in the female group was 1.15 ± 0.15 U.CARR before and 1.10 ± 0.34 U.CARR after the intake. The level was significantly (p<0.05) decreased in the male group.
Changes between before and after AX intake by the DM status
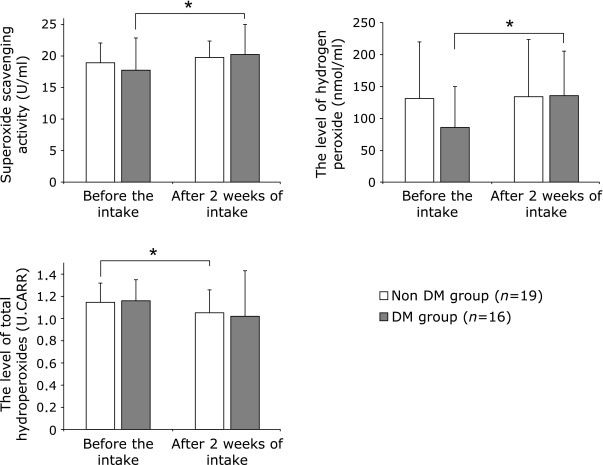
The changes between before and after AX intake according to the DM status are shown in Fig. 4. The superoxide scavenging activity in the non-DM group was 18.8 ± 3.1 U/ml before and 19.7 ± 2.5 U/ml after the intake, and that in the DM group was 17.6 ± 5.1 U/ml before and 20.1 ± 4.7 U/ml after the intake. The activity was significantly (p<0.05) increased after the intake in the DM group.
Fig. 4.
Changes between before and after AX intake according to the DM status *p<0.05.
The H2O2 level in the non-DM group was 130 ± 90 nmol/ml before and 133 ± 91 nmol/ml after the intake, and that in the DM group was 86 ± 64 nmol/ml before and 136 ± 70 nmol/ml after the intake. The level was significantly (p<0.05) increased after the intake in the DM group.
The level of total hydroperoxides in the non-DM group was 1.15 ± 0.17 U.CARR before and 1.05 ± 0.21 U.CARR after the intake, and that in the DM group was 1.16 ± 0.19 U.CARR before and 1.02 ± 0.41 U.CARR after the intake. The level was significantly (p<0.05) decreased in the non-DM group.
Correlations between the three parameters of superoxide scavenging activity, H2O2 level, and level of total hydroperoxides
The correlations between the three parameters are shown in Table 1. Before the intake, there were no significant correlations between the superoxide scavenging activity, H2O2 level, and level of total hydroperoxides. However, after the intake, a significant negative correlation with r = −0.485 (p<0.01) was observed between the superoxide scavenging activity and the level of total hydroperoxides. There were no significant correlations after the intake between the H2O2 level and the superoxide scavenging activity and between the H2O2 level and the level of total hydroperoxides.
Table 1.
Correlations between superoxide scavenging activity, H2O2 level and level of total hydroperoxides in the aqueous humor before and after AX intake
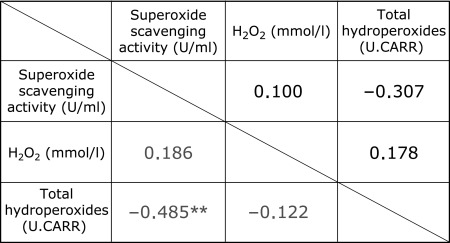
<Pearson’s product-moment correlation coefficient>, **p<0.01. Black; Before AX intake, Gray; After AX intake.
Discussion
AX is lipophilic in its free state. Miki et al.(8,9) reported that it has strong quenching effects against singlet oxygen in non-aqueous lipophilic solutions and that it also possesses strong activity as an inhibitor of lipid peroxidation. In experiments on rats, AX was found to cross the brain–blood barrier and exhibit effects on the brain.(17) It was reported that AX exists as a combined form with water-soluble substances like proteins in aqueous solutions, and that AX in its combined form shows superoxide scavenging effects and is considered to function directly and indirectly for the scavenging of this ROS.(18–20)
The measurements in the present study were performed in the aqueous solution of aqueous humor. Since it has been confirmed that lipoproteins exist in the aqueous humor and lenses, it is considered that AX existed as the combined form and worked directly for the scavenging of superoxide through its antioxidative function. There is also a possibility that the overall superoxide scavenging activity was promoted by a secondary effect through the lowered proportion of GSH, which is required for the scavenging of lipid peroxides by glutathione peroxidase (Gpx) through the quenching of singlet oxygen or the suppression of lipid peroxide generation by AX. Based on these aspects, we consider that the increased superoxide scavenging activity in the aqueous humor observed after AX intake in this study was brought about by both direct and indirect effects of AX.
It is known that H2O2 scavenging mechanisms through catalase or the glutathione cycle exist in the aqueous humor and lenses to detoxify or eliminate H2O2 by changing it into water. However, AX intake did not significantly lower the H2O2 level in any of the groups. Thus, it is considered that AX did not have a direct H2O2 scavenging capacity. Regarding an indirect H2O2 scavenging or generating capacity of AX, it may induce the production of cytochrome P-450, an inducible enzyme (data not shown), in the lenses or peripheral tissues of the aqueous humor including the ciliary body, iris or cornea, and this affect the H2O2 level in the aqueous humor. It has been shown in another study(21) that AX and canthaxanthin, which are 4-oxocartonoids, have strong cytochrome P-450-inducing activities in the liver. Not only the aqueous humor but also the lens and other peripheral tissues of the aqueous humor, including the cornea, have respective H2O2 scavenging mechanisms. However, as an immediate detoxifying function against free radicals, there may be a pathway for excreting H2O2 into the aqueous humor when the H2O2 level exceeds the scavenging capacity in avascular tissues like the lenses or cornea owing to enhanced generation of H2O2 as a result of the augmented superoxide scavenging capacity brought about by AX intake. The increased H2O2 level in the aqueous humor may reflect the transportation process of H2O2 for detoxification in other organs like the liver, or a process for directly excreting H2O2 to the outside the body through urine. For example, it was reported that after intake of coffee polyphenol, which also has antioxidative activity similar to AX, the concentration of H2O2 excreted in the urine as well as the amount of urine itself increased.(22) After intake of AX, the amount of H2O2 excreted into the urine may also increase in a similar manner.
In this study, we chose to use the Free d-ROMs test reagent for the measurement of lipid peroxides. This reagent measures not only lipid peroxides but the total amount of peroxides of proteins, amino acids and nucleic acids. These peroxides are substances generated through peroxidation reactions with extremely unstable radicals, such as superoxide and hydroxyl radicals. They are relatively stable compared with superoxide and hydroxyl radicals, and can thus serve as a good index for the overall effects of AX on the total peroxidation reactions. It has also been reported that AX has an inhibitory activity toward lipid peroxidation.(6) However, in the present study, it was considered that AX intake had inhibitory effects against not only lipid peroxidation but also the generation of peroxides as a whole, since the level of total hydroperoxides in the aqueous humor was significantly decreased in the entire subjects after intake of AX.
In particular, although no correlations were observed between any of the parameters before the intake, a significant negative correlation of r = −0.485 was shown between the superoxide scavenging activity and the level of total hydroperoxides after AX intake. This means that a higher superoxide scavenging activity is associated with a lower level of total hydroperoxides. It is considered that the total hydroperoxides scavenging effect in the aqueous humor after AX intake may be attributable to an increase in the entire antioxidative capacity owing to the increased superoxide scavenging activity in the aqueous humor.
The comparison between the sexes showed great differences for each parameter. Estrogen, one of the female hormones, is known to have a superoxide scavenging capacity or an antioxidative function including the suppression of lipid peroxidation,(23,24) and it is thought that peroxidation reactions tend to progress in postmenopausal women owing to their lowered estrogen level. In this study, the superoxide scavenging activity before AX intake was significantly higher in the female group than in the male group. The female subjects in this study were at ages when their estrogen levels are already lowered (70.6 ± 7.4 years). This finding may reflect a feedback reaction to cope with the higher superoxide generation in females than in males. The H2O2 level after AX intake was significantly higher in the female group. It is considered that there was remaining superoxide that exceeded the elimination capacity before AX intake, and that the augmented superoxide scavenging activity after AX intake dismutated this excess superoxide, resulting in a significantly higher H2O2 level. The level of total hydroperoxides in the female group showed a large variation and no significant decrease was observed. We inferred that this arose because of the individually different aging statuses or because the lowered hormone levels affected the antioxidative capacity, rate of cytochrome P-450 induction or anaerobic status. Other than these, the involvement of smoking status was expected to be a factor that caused sex differences. However, we concluded that the effect of smoking on our data analysis was small because there was only one female patient who habitually smoked among all the study subjects and she smoked less than 20 cigarettes per day.
In the male group, the superoxide scavenging activity was significantly increased, the H2O2 level remained the same, and the level of total hydroperoxides was significantly decreased after AX intake. It appears that the process of total hydroperoxides peroxidation through the increased superoxide scavenging activity in the aqueous humor after AX intake did not accompany the increase in the H2O2 level in the aqueous humor. It was inferred that the H2O2 generated by the elimination of superoxide by SOD and others was metabolized by H2O2 scavenging enzymes in the aqueous humor or immediately eliminated outside of the eyes.
The comparison between the DM statuses showed significant increases in the superoxide scavenging activity and H2O2 level after AX intake in the DM group. It was inferred that this result may reflect the DM patients’ lowered antioxidative activity by enhanced glycation of antioxidative enzymes owing to persistent hyperglycemia.(25) It is thought that in DM patients, the activities of antioxidative enzymes, including SOD, are lowered through glycation.(26) AX intake may have improved the superoxide scavenging activity to the level in the non-DM group and its effect may have been reflected in the increased H2O2 level. This means that when the body has sufficient superoxide scavenging activity, AX intake has little effect, but when the body lacks superoxide scavenging activity, it is expected to function for the mutation of superoxide to H2O2 for the elimination of superoxide. We considered that the H2O2 scavenging activity was also lowered in the DM group, which led to the increased H2O2 level. The level of total hydroperoxides also tended to be affected by the glycation, so the value after AX intake varied widely, although the average value was decreased after AX intake compared with before the intake.
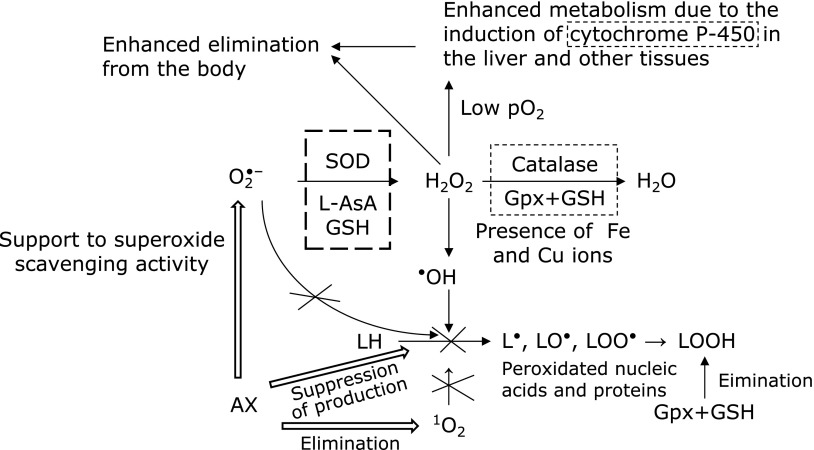
Fig. 5 shows a scheme for the ROS scavenging effects of AX according to our current considerations based on the results of the present study.
Fig. 5.
Changes between before and after AX intake according to the DM status. 1O2; Singlet oxygen, LH; Lipid, O2•−; Superoxide, L•; Lipid radical, H2O2; Hydrogen peroxide, LO•; Alkoxyl radical, •OH; Hydroxyl radical, LOO•; Peroxyl radical, AX; Astaxanthine, LOOH; Lipid peroxide, SOD; Superoxide dismutase, GSH; Reduced glutathione, L-AsA; L-Ascorbic acid, Gpx; Glutathione peroxidase,  ; Eliminates superoxide,
; Eliminates superoxide,  ; Eliminates H2O2,
; Eliminates H2O2,  ; Points where suppression of peroxidation reaction by AX is expected.
; Points where suppression of peroxidation reaction by AX is expected.
The dose of AX at 6 mg/day used in this study was based on the dose that induces improvement of the accommodation ability of the eyes and subjective symptoms in asthenopia in healthy adults. If the dose of AX is changed, it is possible that the efficacy will be improved in the DM group or the female group. To lower ROS in a balanced manner and reduce the damage caused by oxidative stresses, further studies are needed for combinations of AX with other substances with antioxidative capacities that have different mechanisms from AX, since AX is not effective for all ROS, or by employing doses of AX other than 6 mg/day.
AX intake evidently increased the superoxide scavenging activity and lowered the level of total hydroperoxides in the aqueous humor. The possibility was suggested that AX works in a suppressive manner for the development and progression of systemic diseases and conditions that are related to oxidative stresses, like diabetes and aging, or in the ophthalmological field, such as cataract or age-related macular degeneration.
Abbreviations
- AX
astaxanthin
- DM
diabetes mellitus
- Gpx
glutathione peroxidase
- GSH
glutathione
- HO•
hydroxyl radicals
- H2O2
hydrogen peroxide
- L-AsA
L-ascorbic acid
- NBT
nitroblue tetrazolium
- O2•−
superoxide
- OD
optical density
- -OOH
peroxidated substances
- ROMs
reactive oxygen metabolites
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Augusti PR, Quatrin A, Somacal S, et al. Astaxanthin prevents changes in the activities of thioredoxin reductase and paraoxonase in hypercholesterolemic rabbits. J Clin Biochem Nutr. 2012;51:42–49. doi: 10.3164/jcbn.11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohira A, Ueda T, Ohishi K, Hiramitsu T, Akeo K, Obara Y. Oxidative stress in ocular disease. J Nihon Ganka Gakkai Zasshi. 2008;112:22–29. [PubMed] [Google Scholar]
- 3.Moeller SM, Parekh N, Tinker L, et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124:1151–1162. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn R, Soerensen NA. The coloring matters of the lobster (Astacus gammarus L.) Z Angew Chem. 1938;51:465–466. [Google Scholar]
- 5.Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 6.Miki W, Yamaguchi K, Konosu S. Comparison of carotenoids in the ovaries of marine fish and shellfish. Comp Biochem Physiol B. 1982;71:7–11. doi: 10.1016/0305-0491(82)90167-5. [DOI] [PubMed] [Google Scholar]
- 7.Miki W. Biological functions and activities of animal carotenoids. Pure Appl Chem. 1991;63:141–146. [Google Scholar]
- 8.Shimizu N, Goto M, Miki W. Carotenoids as singlet oxygen quenchers marine organisms. Fish Sci. 1996;62:134–137. [Google Scholar]
- 9.Takahashi J, Tsukahara H, Minato S. Toxicological studies of astaxanthin from Haematococcus pluvialis—Ames test, oral single dose and 90-days subchronic toxicity studies in rats—. J Clin Therap Med. 2004;20:867–881. [Google Scholar]
- 10.Miki W. Carotenoids as effective “Antioxidants” in foods. ILSI. 2003;76:27–35. [Google Scholar]
- 11.Katagiri M, Satoh A, Tsuji S, Shirasawa T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: a randomised, double-blind, placebo-controlled study. J Clin Biochem Nutr. 2012;51:102–107. doi: 10.3164/jcbn.D-11-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiratori K, Ohgami K, Nitta T, et al. Effects of astaxanthin on accommodation and asthenopia—efficacy identification study in healthy volunteers—. J Clin Therap Med. 2005;21:637–650. [Google Scholar]
- 13.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 14.Witerbourn CC, Garcia RC, Segal AW. Production of the superoxide adduct of myeloperoxidase (compound III) by stimulated human neutrophils and its reactivity with hydrogen peroxide and chloride. Biochem J. 1985;228:583–592. doi: 10.1042/bj2280583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesarone MR, Belcaro G, Carratelli M, et al. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–130. [PubMed] [Google Scholar]
- 16.Alberti A, Bolognini L, Macciantelli D, Caratelli M. The radical cation of N,N-diethyl-para-phenylendiamine: A possible indicator of oxidative stress in biological samples. Res Chem Intermed. 2000;26:253–267. [Google Scholar]
- 17.Nishigaki I, Dmitrovskii AA, Miki W, Yagi K. Suppressive effect of astaxanthin on lipid peroxidation induced in rats. J Clin Biochem Nutr. 1994;16:161–166. [Google Scholar]
- 18.Zsila F, Fitos I, Bikádi Z, Simonyi M, Jackson HL, Lockwood SF. In vitro plasma protein binding and aqueous aggregation behavior of astaxanthin dilysinate tetrahydrochloride. Bioorg Med Chem Lett. 2004;14:5357–5366. doi: 10.1016/j.bmcl.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Lockwood SF, Penn MS, Hazen SL, Bikádi Z, Zsila F. The effects of oral Cardax (disodium disuccinate astaxanthin) on multiple independent oxidative stress markers in a mouse peritoneal inflammation model: influence on 5-lipoxygenase in vitro and in vivo. Life Sci. 2006;79:162–172. doi: 10.1016/j.lfs.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 20.Sueishi Y, Ishikawa M, Yoshioka D, et al. Oxygen radical absorbance capacity (ORAC) of cyclodextrin-solubilized flavonoids, resveratrol and astaxanthin as measured with the ORAC-EPR method. J Clin Biochem Nutr. 2012;50:127–132. doi: 10.3164/jcbn.11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno M, Darwish WS, Ikenaka Y, Miki W, Ishizuka M. Astaxanthin can alter CYP1A-dependent activities via two different mechanisms: induction of protein expression and inhibition of NADPH P450 reductase dependent electron transfer. Food Chem Toxicol. 2011;49:1285–1291. doi: 10.1016/j.fct.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Long LH, Halliwell B. Coffee drinking increases levels of urinary hydrogen peroxide detected in healthy human volunteers. Free Rad Res. 2000;32:463–467. doi: 10.1080/10715760000300461. [DOI] [PubMed] [Google Scholar]
- 23.Yagi K, Komura S. Inhibitory effect of female hormones on lipid peroxidation. Biochem Int. 1986;13:1051–1055. [PubMed] [Google Scholar]
- 24.Buyon JP, Korchak HM, Rutherford LE, Ganguly M, Weissmann G. Female hormones reduce neutrophil responsiveness in vitro. Arthritis Rheum. 1984;27:623–630. doi: 10.1002/art.1780270604. [DOI] [PubMed] [Google Scholar]
- 25.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 26.Obara Y. The oxidative stress in the cataract formation. Nihon Ganka Gakkai Zasshi. 1995;99:1303–1341. [PubMed] [Google Scholar]