Abstract
BACKGROUND
The association between cardiovascular health and salt intake remains controversial. The objective of our study was to assess the association between arterial stiffness and urinary sodium, both cross-sectionally and prospectively.
METHODS
In 630 participants (mean age 40.6 years; 51% women), randomly recruited from a Flemish population, we measured sodium and creatinine in 24-hour urine samples at baseline and follow-up (median, 9.7 years) and the carotid and aortic augmentation indexes (AIs) standardized to heart rate at follow-up only.
RESULTS
From baseline to follow-up, the urinary sodium concentration decreased (117.1 vs. 105.2 mmol/L; P < 0.0001), whereas 24-hour urinary sodium did not change significantly (166.5 vs. 171.5 mmol/L; P = 0.12). In multivariable-adjusted longitudinal analyses, a 40 mmol/L (~1 SD) increase in the urinary sodium concentration was independently and inversely associated with the carotid AI (effect size, −1.38 ± 0.66%; P = 0.04) and aortic AI (−1.54 ± 0.72%; P = 0.02). In cross-sectional analyses of follow-up data, these estimates were −1.26 ± 0.70% (P = 0.07) and −1.52 ± 0.76% (P = 0.04), respectively. In the longitudinal and cross-sectional analyses, the carotid and aortic AIs were unrelated to the 24-hour urinary excretion of sodium.
CONCLUSIONS
Our study showed an inverse association between the central arterial AIs and the urinary sodium concentration. Further research is required to consolidate our findings, to unravel the underlying mechanism, and to establish the role of renal vasodilatation in the maintenance of sodium balance.
Keywords: artery stiffness, blood pressure, cross-sectional, hypertension, prospective, urinary sodium, white population
Pulse wave velocity is a directed measure of arterial stiffness,1 while the augmentation index (AI) is additionally influenced by cardiac output and wave reflections.2 Both aortic pulse wave velocity and systolic AIs predict cardiovascular outcome1,3,4 The evidence relating cardiovascular health to salt intake in the general population remains a matter of debate.5,6 The review by Alderman and Cohen summarized recent publications documenting a J-shape relation between cardiovascular disease and salt intake with higher cardiovascular disease risk observed to be associated with both the higher and lower ends of sodium intake.7 To the best of our knowledge, only 2 previous studies with cross-sectional design assessed the relation between the aforementioned indexes of arterial stiffness and estimated8 or measured9 24-hour urinary sodium excretion. These 2 studies, involving Korean hypertensive patients8 or South African participants of black descent,9 produced contradictory results for the AI8,9 or reported nonsignificant findings for pulse wave velocity.9 In view of scarce evidence currently available, we analyzed the FLEMish Study on Environment, Genes and Health Outcomes to assess the association between indexes of arterial stiffness and urinary sodium, both cross-sectionally and prospectively.
Aortic AI can be measured from the aortic pressure wave derived from the radial artery using a generalized transfer function.10 When a radial-to-aortic transform is applied to a typical radial waveform, there is marked variation in the aortic AI.11 Owing to the proximity of the carotid artery to the aorta, the carotid waveform can be use to estimate aortic AI without the use of a transfer function.12 In the current study, we measured both the carotid and derived aortic AIs.
METHODS
Study population
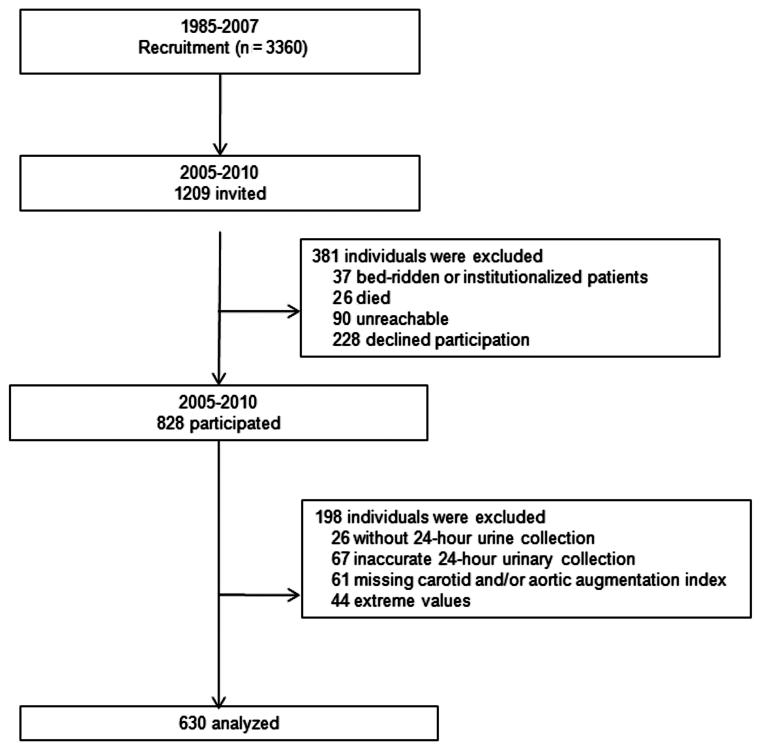
Previous publications describe the recruitment of the FLEMish Study on Environment, Genes and Health Outcomes participants in detail.13 From August 1985 to November 1990, a random sample of the households living in a geographically defined area of Northern Belgium was recruited with the goal to enroll an equal number of participants in each of 6 subgroups by sex and age (20–39, 40–59, and ≥60) (Figure 1). All household members with a minimum age of 20 years were invited to take part, if the quota of their sex–age group had not yet been fulfilled.13 From June 1996 to January 2007, recruitment of families continued, using the former participants (1985–1990) as index persons and including subjects younger than 20 years (Figure 1). At enrollment, the participation rate was 65.0%. The Ethics Committee of the University of Leuven approved the study. All participants or their parents gave informed written consent.
Figure 1.
Flowchart of participants.
From 2005 to 2010, after excluding 37 patients who were bed-ridden or institutionalized, 26 who had died, and 90 who were unreachable, we reinvited 1,056 former participants for arterial phenotyping, of whom 828 renewed their consent (participation rate, 78.4%) (Figure 1). We excluded 131 participants from the present analysis, because a 24-hour urine sample was unavailable at baseline and/or follow-up (n = 26), the carotid and/or aortic AIs had not been measured (n = 61), or the AIs or 24-hour urinary sodium were more than 3 SDs away from the mean (n = 44). In addition, we excluded 67 participants, because their baseline and/or follow-up 24-hour urine sample had a volume of less than 300 mL, or because their 24-hour urine creatinine excretion was less than 4 mmol or more than 25 mmol in women, or less than 6 mmol or more than 30 mmol in men. Thus, the number of participants statistically analyzed totaled 630 (Figure 1).
Arterial phenotyping
For at least 3 hours before the examination, the participants were asked to refrain from heavy exercise, smoking, and intake of alcohol and caffeine-containing beverages. After they had rested in the supine position for at least 15 minutes, 1 trained nurse recorded, during an 8-second period, the radial and carotid arterial waveforms at the right side by applanation tonometry. We used a high-fidelity SPC-301 micromanometer (Millar Instruments, Houston, TX) interfaced with a laptop computer running the SphygmoCor software, version 8.2 (AtCor Medical Pty., West Ryde, New South Wales, Australia). We discarded recordings when the systolic or diastolic variability of consecutive waveforms exceeded 5% or when the amplitude of the pulse wave signal was less than 80 mV. We calibrated the pulse wave by the blood pressure measured at the right arm immediately before the SphygmoCor recordings.
From the radial signal, the SphygmoCor software calculates the aortic AI by means of a validated generalized transfer function.10 The carotid AI was measured directly at the carotid artery. The AI was the ratio of the second to the first peak of the pressure wave expressed as a percentage (Figure 2). Carotid–femoral pulse wave velocity was measured by sequential electrocardiographically-gated recordings of the arterial pressure waveform at the carotid and femoral arteries. We measured the distance from the suprasternal notch to the carotid sampling site (distance A) and from the suprasternal notch to the femoral sampling site (distance B).14 Pulse wave travel distance was calculated as distance B minus distance A. Pulse transit time was the average of 10 consecutive beats. Carotid–femoral pulse wave velocity was calculated as the ratio of the travel distance in meters to the transit time in seconds. The intraobserver intrasession reproducibility of carotid and aortic AIs, and carotid–femoral pulse wave velocity was 5.87%, 2.20%, and 2.61%, respectively.
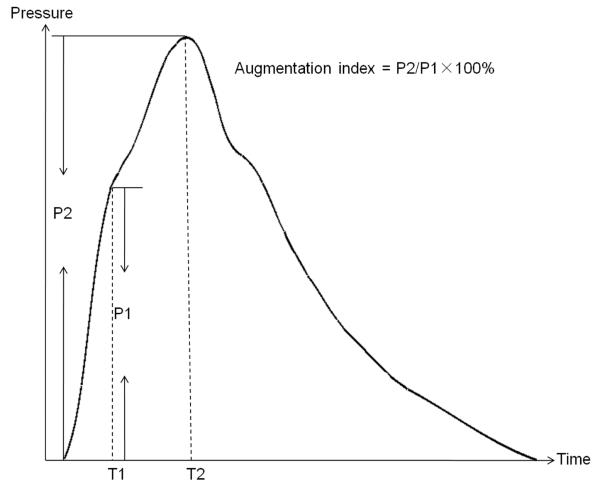
Figure 2.
Derivation of the AI. P1 is the pressure wave rising to an initial peak at T1, where it forms a shoulder. T2 is the second shoulder at T2, which is the peak of the superimposed reflected pulse wave. Abbreviation: AI, augmentation index.
Clinical and biochemical measurements
Trained nurses measured the subjects’ anthropometric characteristics and blood pressure. They administered a questionnaire to collect information about each participant’s recent medical history, smoking and drinking habits, and intake of medications. Body mass index was body weight in kilograms divided by body height in meters squared. Blood pressure was the average of 5 consecutive readings measured after the participants had rested in the sitting position for at least 5 minutes. Hypertension was a blood pressure of at least 140 mm Hg systolic or 90 mm Hg diastolic, and/or use of antihypertensive drugs. Mean arterial pressure was diastolic blood pressure plus one-third of the difference between systolic and diastolic blood pressures.
With the subjects fasting for at least 6 hours, venous blood samples were drawn. Using standardized automated methods, we measured total cholesterol on serum. Diabetes mellitus was a self-reported diagnosis, use of antidiabetic medications, a blood glucose concentration of at least 7.0 mmol/L (126 mg/dL) fasting or 11.1 mmol/L (200 mg/dL) random. Participants collected a 24-hour urine sample in a 2500-mL wide-neck plastic container (Sarstedt article number 77.756, Nümbrecht, Germany). Sodium in urine was determined by flame photometry and creatinine by an automated enzymatic method.
Data analysis
For database management and statistical analysis, we used SAS software (SAS Institute, Cary, NC), version 9.2. We compared means and proportions, using a large-sample z test, and χ2 statistic, respectively, and correlated proportions by McNemar’s test. All arterial measurements were standardized to a heart rate of 75 beats per minute. We tested for collinearity, using the variance inflation factor, and we tested interactions of urinary sodium with anthropometric characteristics, including sex, age, body height and weight, and body mass index. In all analyses, the variance inflation factor associated with explanatory variables was less than 2, and the interactions of urinary sodium with the anthropometric characteristics did not reach significance (P ≥ 0.42). Our statistical methods also included multiple linear regressions. We searched for possible covariables of the arterial traits, using a stepwise regression procedure with the P values for independent variables to enter and to stay in the model set at 0.15. As covariables, we considered sex, age, body height, mean arterial pressure, total cholesterol, urinary sodium concentration and excretion, and design variables (0, 1), coding for current smoking, alcohol intake, and use of antihypertensive drugs.
RESULTS
Characteristics of the participants
Table 1 and 2 list the characteristics of the participants at baseline and follow-up by sex. At baseline and follow-up, women had lower height, weight and body mass index, lower blood pressure values, lower urinary sodium concentration and excretion, and a lower prevalence of hypertension and less frequently reported alcohol consumption compared with men. On the other hand, women had higher heart rate at baseline and follow-up, and a higher serum total cholesterol at follow-up than men did. As shown in Table 2, the carotid and aortic AIs were higher (P < 0.001) in women than in men, whereas the opposite was true for carotid–femoral pulse wave velocity. Standardization to a heart rate of 75 beats per minute did not alter these findings.
Table 1.
Characteristics of Female and Male Participants at Baseline and Follow-up
| Women (N = 321) |
Men (N = 309) |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Baseline | Follow-up | Change | Baseline | Follow-up | Change |
| Anthropometrics measurements | ||||||
| Age (y) | 40.6 ± 13.5 | 52.1 ± 14.4 | 11.5 (11.0–12.0)*** | 40.7 ± 14.0 | 51.9 ± 15.0 | 11.2 (10.7–11.8)*** |
| Height (cm) | 162.2 ± 6.5 | 162.1 ± 6.7 | −0.103 (–0.374 to 0.167) | 175.2 ± 7.21 | 175.2 ± 7.21 | 0.054 (−0.386 to 0.476) |
| Weight (kg) | 64.8 ± 12.2 | 68.4 ± 12.7 | 3.57 (2.72–4.42)*** | 78.6 ± 11.91 | 81.7 ± 11.21 | 3.19 (2.34–4.04)*** |
| Body mass index (kg/m2) |
24.6 ± 4.3 | 26.0 ± 4.4 | 1.39 (1.09–1.69)*** | 25.6 ± 3.61 | 26.6 ± 3.51 | 1.06 (0.81–1.30)*** |
| Urinary measurements | ||||||
| Volume (L/d) | 1.63 ± 0.67 | 1.80 ± 0.64 | 0.162 (0.083–0.241)*** | 1.50 ± 0.571 | 1.76 ± 0.70 | 0.259 (0.17–0.343)*** |
| Sodium concentration (mmol/L) |
101.3 ± 47.4 | 88.4 ± 38.1 | −12.9 (−18.2 to –7.57)*** | 133.6 ± 49.71 | 122.7 ± 47.11 | −10.9 (−17.1 to −4.64)** |
| Sodium excretion (mmol/d) |
147.2 ± 56.3 | 146.7 ± 59.2 | −0.467 (−8.226 to 7.291) | 186.8 ± 71.71 | 197.3 ± 72.71 | 10.6 (0.7–20.5)* |
| Creatinine concentration (mmol/L) |
6.76 ± 2.85 | 6.04 ± 3.09 | −0.716 (−1.067 to −0.365)*** | 10.8 ± 4.21 | 9.71 ± 4.381 | −1.06 (−1.63 to −0.49)** |
| Creatinine excretion (mmol/d) |
9.61 ± 2.28 | 9.52 ± 3.00 | −0.089 (−0.433 to 0.254) | 14.4 ± 3.51 | 14.8 ± 4.21 | 0.453 (−0.060 to 0.966) |
| Risk factors | ||||||
| Total cholesterol (mmol/L) |
5.42 ± 1.16 | 5.41 ± 1.01 | −0.010 (−0.132 to −0.112) | 5.33 ± 1.10 | 5.16 ± 0.921 | −0.165 (−0.286 to −0.044)** |
| Smoking [n (%)] | 88 (27.4) | 80 (24.9) | −2.49 (−4.92 to −0.06)* | 81 (26.2) | 77 (24.9) | −1.29 (−3.48 to 0.90) |
| Drinking alcohol [n (%)] |
50 (15.6) | 101 (31.5) | 15.9 (11.4–20.4)*** | 125 (40.4)1 | 173 (56.0)1 | 15.5 (9.5–21.5)*** |
| Hypertension [n (%)] |
38 (11.8) | 68 (21.2) | 9.35 (5.43–13.3)*** | 75 (24.3)1 | 106 (34.3)1 | 10.0 (5.5–14.5)*** |
| Treatment for hypertension [n (%)] |
22 (6.8) | 38 (11.8) | 4.98 (2.04–7.92)*** | 19 (6.1) | 40 (12.9) | 6.80 (4.00–9.60)*** |
Values are mean (±SD), number of subjects (%), or mean changes from baseline to follow-up (95% confidence interval). Hypertension was a blood pressure (mean of 5 consecutive readings) of at least 140 mm Hg systolic or 90 mm Hg diastolic, or use of antihypertensive drugs.
Indicates a significant difference (P < 0.05) with women.
Significance of the change from baseline to follow-up: P < 0.05;
P < 0.01;
P < 0.001.
Table 2.
Hemodynamic Measurements at Baseline and Follow-up by Sex
| Women (N = 321) |
Men (N = 309) |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Baseline | Follow-up | Change | Baseline | Follow-up | Change |
| Peripheral measurements | ||||||
| Systolic blood pressure (mm Hg) |
119.2 ± 14.2 | 127.8 ± 19.5 | 8.60 (6.74–10.40)* | 126.8 ± 14.31 | 131.6 ± 15.61 | 4.81 (3.09–6.53)* |
| Diastolic blood pressure (mm Hg) |
73.6 ± 9.3 | 77.9 ± 8.9 | 4.21 (3.15–5.27)* | 78.0 ± 11.01 | 82.3 ± 9.31 | 4.31 (3.09–5.53)* |
| Mean arterial pressure (mm Hg) |
88.8 ± 10.1 | 94.5 ± 10.9 | 5.67 (4.51–6.83)* | 94.3 ± 11.01 | 98.7 ± 9.81 | 4.48 (4.51–6.83)* |
| Heart rate (beats/ min) |
70.4 ± 9.4 | 64.6 ± 9.5 | −5.80 (−7.12 to −4.48)* | 67.5 ± 10.41 | 61.9 ± 9.91 | −5.56 (−6.86 to −4.26)* |
| Central measurements | ||||||
| Unstandardized for heart rate | ||||||
| Carotid AI (%) | … | 135.9 ± 26.4 | … | … | 121.0 ± 24.31 | … |
| Aortic AI (%) | … | 150.9 ± 28.6 | … | … | 133.9 ± 25.81 | … |
| Carotid–femoral pulse wave velocity (m/s) |
7.20 ± 1.45 | … | … | 7.60 ± 1.581 | ||
| Standardized to 75 (beats/min) | ||||||
| Carotid AI (%) | … | 130.2 ± 25.4 | … | … | 113.7 ± 23.51 | … |
| Aortic AI (%) | … | 145.7 ± 27.7 | … | … | 127.4 ± 25.31 | … |
| Carotid-femoral pulse wave velocity (m/s) |
7.30 ± 1.44 | … | … | 7.72 ± 1.581 | ||
Values are mean (±SD) or mean changes from baseline to follow-up (95% confidence interval).
Indicates a significant difference (P < 0.05) with women.
Significance of the change from baseline to follow-up: P < 0.001. Hemodynamic measurements were only obtained at follow-up. Carotid–femoral pulse wave velocity was measured in 285 women and 275 men.
Median follow-up was 9.7 years (5.6–20.7 years). Of 41 participants on antihypertensive treatments at baseline, 11 (26.8%) took diuretics, 32 (78.0%) β-blockers, 5 (12.2%) calcium channel blockers, and 3 (7.3%) angiotensin II converting enzyme inhibitors; 10 patients (24.4%) were on multiple drugs. Of 76 subjects on antihypertensive treatments at follow-up, 25 (32.9%) used diuretics, 56 (73.7%) β-blockers, 13 (17.1%) calcium channel blockers, and 10 (13.1%) angiotensin II converting enzyme inhibitors; 32 patients (42.1%) were taking more than 1 drug class.
Correlates of the hemodynamic measurements
Carotid and Aortic AIs
In cross-sectional and longitudinal analyses (Table 3), the carotid and aortic AIs increased with female sex, age, mean arterial blood pressure, and current smoking, but decreased with body height. At follow-up, the aortic AI also increased with total serum cholesterol. The models for the carotid and aortic AIs explained approximately 49.5% and 43.5% of the variance in the cross-sectional and longitudinal analysis, respectively. There was a positive correlation between the carotid and aortic AIs (r = 0.727; P < 0.0001).
Table 3.
Correlates of the Carotid and Aortic AIs
| Carotid AI |
Aortic AI |
|||||
|---|---|---|---|---|---|---|
| Correlates | β ± SE | P | r2 | β ± SE | P | r2 |
| Longitudinal analysis | ||||||
| Being female | 11.3 ± 2.2 | <0.0001 | 10.3 | 14.9 ± 2.4 | <0.0001 | 10.8 |
| Age (+15 years) | 12.4 ± 0.96 | <0.0001 | 28.9 | 13.8 ± 1.14 | <0.0001 | 29.6 |
| Body height (+7 cm) | −3.22 ± 0.84 | <0.0001 | 1.49 | −1.75 ± 0.91 | 0.03 | 0.41 |
| Mean arterial pressure (+10 mm Hg) | 3.41 ± 0.79 | 0.0001 | 1.42 | 2.28 ± 0.87 | 0.01 | 0.66 |
| Smoking at baseline | 6.80 ± 1.76 | 0.0001 | 1.39 | 8.85 ± 1.91 | <0.0001 | 2.14 |
| Cross-sectional analysis | ||||||
| Being female | 10.2 ± 2.2 | <0.0001 | 2.23 | 13.0 ± 2.4 | <0.0001 | 10.4 |
| Age (+15 years) | 10.9 ± 0.90 | <0.0001 | 33.2 | 13.7 ± 0.97 | <0.0001 | 34.8 |
| Body height (+7 cm) | −3.85 ± 0.84 | <0.0001 | 10.3 | −2.45 ± 0.91 | 0.0053 | 0.64 |
| Mean arterial pressure (+10 mm Hg) | 5.59 ± 0.76 | <0.0001 | 3.56 | 3.23 ± 0.84 | <0.0001 | 1.46 |
| Total cholesterol (+1 mmol/L) | … | … | … | 2.54 ± 0.86 | 0.002 | 0.77 |
| Smoking at follow-up | 5.22 ± 1.69 | 0.002 | 0.79 | 8.23 ± 1.85 | <0.0001 | 1.47 |
The AIs were standardized to a heart rate of 75 beats/min. The covariables considered for entry into the multiple regression model were sex, age, body height, mean arterial pressure, total cholesterol, current smoking and drinking, and antihypertensive drug treatment. β ± SE, P, and r2 indicate the effect size ±SE, statistical significance, and the percentage variance of the dependent variable explained by a single covariable, respectively. Effect sizes are expressed for a ~1 SD higher value of the explanatory variables. The percentage of variance explained by the whole model was 50.1% and 49.5% for the carotid and aortic AIs in the cross-sectional analysis, respectively, and 43.5% and 43.6% in the longitudinal analysis, respectively. Abbreviation: AI, augmentation index.
Carotid-Femoral Pulse Wave Velocity
Carotid-femoral pulse wave velocity increased with age, body height and mean arterial pressure (see Supplementary Table S1 online). These covariables explained 35.9% and 40.6% of pulse wave velocity in the longitudinal and cross-sectional analysis, respectively. The carotid–femoral pulse wave velocity was positively correlated (P < 0.0001) with both the carotid (r = 0.311) and aortic AIs (r = 0.340).
Association of hemodynamic measurements with urinary sodium
Figure 3 shows the unadjusted sex-specific associations of the carotid and aortic AIs across quintiles of the urinary sodium concentration at baseline and follow-up. The carotid and aortic AIs decreased with higher urinary sodium concentration at baseline (P ≤ 0.0005) with a similar trend at follow-up (0.046 ≤ P ≤ 0.21). In continuous analyses, with adjustment for the covariables listed in Table 3, the carotid and aortic AIs decreased with higher urinary sodium concentration. For a 40-mmol/L (~1 SD) increase in urinary sodium at baseline (Table 4), the carotid AI decreased by 1.38% (95% confidence interval [CI], 0.09–2.67%; P = 0.04) and the aortic AI by 1.54% (CI, 0.13–2.95%; P = 0.02), respectively. In the cross-sectional analyses, these estimates were 1.26% (CI, −0.11% to 2.63%, P = 0.07) and 1.52% (CI, 0.030–3.01%; P = 0.04), respectively. Urinary sodium concentration explained less than 0.5% of the variance in the AIs that was not yet explained by the other covariables. In sensitivity analyses, from which we excluded patients on antihypertensive drug treatment at baseline or follow-up, the multivariable-adjusted longitudinal and cross-sectional associations between the carotid and aortic AIs and urinary sodium concentration were all significant (0.01 < P < 0.05;Table 5).
Figure 3.
The carotid and aortic AIs by quintiles of the distributions of the urinary sodium concentration at baseline and follow-up. Circles and squares indicate women and men, respectively. Open and closed symbols indicate baseline and follow-up measurements, respectively. P values are for linear trend across quintiles. Abbreviation: AI, augmentation index.
Table 4.
Multivariable-adjusted Correlations of the Carotid and Aortic AIs with Urinary Measurements
| Carotid AI |
Aortic AI |
|||||
|---|---|---|---|---|---|---|
| Correlates | β ± SE | P | r2 | β ± SE | P | r2 |
| Longitudinal analysis | ||||||
| Sodium concentration (+40 mmol/L) | −1.38 ± 0.66 | 0.04 | 0.39 | −1.54 ± 0.72 | 0.02 | 0.50 |
| Sodium excretion (+60 mmol) | 0.10 ± 0.75 | 0.90 | 0.01 | 0.03 ± 0.81 | 0.96 | 0.03 |
| Creatinine concentration (+4 mmol/L) | −0.30 ± 0.90 | 0.74 | 0.02 | −1.22 ± 0.96 | 0.21 | 0.47 |
| Creatinine excretion (+4 mmol) | 1.55 ± 1.14 | 0.17 | 0.28 | 0.92 ± 1.23 | 0.46 | 0.37 |
| Cross-sectional analysis | ||||||
| Sodium concentration (+40 mmol/L) | −1.26 ± 0.70 | 0.07 | 0.26 | −1.52 ± 0.76 | 0.04 | 0.32 |
| Sodium excretion (+60 mmol) | 0.33 ± 0.67 | 0.62 | 0.25 | 0.57 ± 0.73 | 0.43 | 0.04 |
| Creatinine concentration (+4 mmol/L) | −1.79 ± 0.82 | 0.02 | 0.41 | −2.78 ± 0.89 | 0.001 | 0.96 |
| Creatinine excretion (+4 mmol) | −0.77 ± 0.89 | 0.38 | 0.30 | −0.95 ± 0.97 | 0.33 | 0.06 |
The AIs were standardized to a heart rate of 75 beats/min. All estimates were adjusted to the covariables are listed in Table 3. β ± SE, P, and r2 indicate the effect size ±SE, statistical significance, and the percentage variance of the dependent variable explained by a single covariable, respectively. Effect sizes are expressed for a ~1 SD higher value of the explanatory variables. Abbreviation: AI, augmentation index.
Table 5.
Sensitivity Analyses of the Correlations of the Carotid and Aortic AIs with Urinary Sodium Concentration
| Carotid AI |
Aortic AI |
|||||
|---|---|---|---|---|---|---|
| Correlates | β ± SE | P | r2 | β ± SE | P | r2 |
| Longitudinal analysis (n = 589) | −1.66 ± 0.68 | 0.01 | 0.57 | −1.80 ± 0.74 | 0.01 | 0.65 |
| Cross-sectional analysis (n = 552) | −1.64 ± 0.72 | 0.02 | 0.46 | −1.52 ± 0.76 | 0.048 | 0.33 |
The AIs were standardized to a heart rate of 75 beats/min. The covariables considered for entry into the multiple regression model are listed in Table 3. β ± SE, P, and r2 indicate the effect size ±SE, statistical significance, and the percentage variance of the dependent variable explained by a single covariable, respectively. Effect sizes are expressed for ~1 SD higher value of the explanatory variables. The analysis included 589 and 552 subjects at baseline and follow-up, respectively, with patients on antihypertensive drug treatment excluded. Abbreviation: AI, augmentation index.
In the cross-sectional analyses at follow-up, there was an inverse multivariable-adjusted correlation between the carotid and aortic AIs and the urinary creatinine concentration (Table 4). In longitudinal analyses, urinary creatinine concentration did not correlate with the AIs. None of the correlations of the AIs with the 24-hour excretion of sodium or creatinine was significant (Table 4). None of the associations between carotid–femoral pulse wave velocity and any urinary measurement reached significance (P ≥ 0.15; see Supplementary Table S2 online).
DISCUSSION
The key finding of our study was that the carotid and aortic AIs decreased with higher urinary sodium concentration. These results were consistent in the longitudinal and cross-sectional analysis. To our knowledge, only 2 previous reports8,9 addressed the association between the aortic AI and urinary sodium. Park and coworkers8 observed a decrease in pulse pressure amplification and an increase in central pulse pressure, augmented pressure, and the central AI with higher 24-hour urinary sodium and sodium-to-potassium ratio.8 Park’s study has several limitations. He obtained spot urine samples in 515 selected hypertensive patients (63.1% women; mean age 48.5 years) and extrapolated 24-hour urinary sodium and potassium excretion from the concentration in spot urines. The formula applied by Park8 allows estimating 24-hour urinary measurements at the population level, but not in individual subjects.15 In contrast to most literature data and our current observations, in Park’s study,8 women did not have lower estimated 24-hour urinary sodium than men. This discrepancy casts doubt on Park’s results.
Redelinghuys and colleagues9 assessed the independent associations between indexes of arterial stiffness and 24-hour urinary sodium and potassium in 635 randomly recruited black South Africans. In multivariable-adjusted analyses, the central pulse pressure estimated by applanation tonometry and the 24-hour ambulatory pulse pressure, but not carotid–femoral pulse wave velocity, increased with higher urinary sodium-to-potassium ratio. However, these indexes of arterial stiffness were unrelated to the 24-hour urinary sodium or potassium excretion, or to urinary sodium and potassium standardized to creatinine. Redelinghuys and coworkers did neither report the participation rate in their study,9 nor in previous manuscripts to which they refer.16,17 This makes it difficult to assess whether their results are truly representative for a black population.
Several mechanisms might explain the inverse association between systolic AIs and urinary sodium concentration. First, numerous cross-sectional and longitudinal studies demonstrated that arterial stiffness and the systolic AIs increase with advancing age.18,19 The concentrating capacity of the kidney declines with age.20–22 Rowe measured the maximal urine concentrating capability in healthy individuals in 3 age groups: 20–39, 40–59, and 60–79 years. Individuals aged 60–79 years had approximately a 20% reduction in maximum urinary osmolality when compared to the 2 younger age groups.20 In line with Rowe’s observations, we found that over 10 years of follow-up, the urinary concentration of sodium and creatinine decreased, whereas the 24-hour urinary excretion of these solutes remained unchanged. Aging per se might offer a simple explanation for the inverse association between arterial stiffness as exemplified by the systolic AIs and the urinary sodium concentration. However, we do not believe that this hypothesis holds true, because the associations of the systolic AIs with the urinary creatinine concentration were not consistently significant in the longitudinal and cross-sectional analyses and because there was no association between carotid–femoral pulse wave velocity and the urinary sodium concentration.
More plausible mechanisms, possibly explaining the inverse association between the systolic AIs and urinary sodium concentration, might originate from the concept that renal arteries are important reflection sites of the forward arterial pulse wave.23,24 Latham and colleagues investigated the human aorta and its terminal branches in normal subjects during cardiac catheterization.23 They evaluated regional wave travel and arterial wave reflections, using a specially designed catheter with 6 micromanometers equally spaced at 10-cm intervals. They positioned the tip sensor in the distal external iliac artery and the proximal sensor in the aortic arch. Inspection of thoracic aortic pressures revealed a reflected wave originating from the region of the aorta at the level of the renal arterial branches, while abdominal aortic pressures exhibited reflection from a site peripheral to the terminal aortic bifurcation.23 Segers and Verdonck24 confirmed these findings by measuring pressure and flow simultaneously at 6 locations along the aorta of an anatomically correct 1:1 scale hydraulic elastic tube model of the arterial tree of an adult man. Several in vitro experiments25,26 and an in vivo micropuncture studies27 demonstrate that high sodium concentration in the connecting tubules leads to vasodilatation of the afferent arterioles, which account for most of the renal vascular resistance.25 This might attenuate systolic augmentation in the central arteries based on the concept that renal arteries are important reflection sites of the forward arterial wave.
Nowadays, low dietary sodium intake is broadly carried out at a population level to prevent cardiovascular morbidity and mortality.28,29 However, the protective effect of sodium restriction on public health encounters suspicion and challenge. Alderman and Cohen examined the totality of published data linking sodium intake to health outcomes, which included 23 observational studies and 4 randomized trials. They found a “J-shaped” relation of sodium to cardiovascular outcomes.7 People at the low end of intake, less than 2.5 g/day, and those at the high end of intake, more than 6.0 g/day, have increased cardiovascular risk.7 In our study, the 24-hour urinary sodium excretion averaged proximately 4 g/day. In line with Alderman and Cohen’s review, there was an inverse association between central systolic augmentation, an independent cardiovascular factor, and urinary sodium.
The present study must be interpreted within the context of its limitations and strengths. First, to what extent the urinary sodium reflects the sodium concentration in the connecting tubules is not clearly established. However, approximately 87% of the filtered sodium is reabsorbed in the renal tubule proximal of the connecting tubule. Second, as in other studies,8,9 we estimated the aortic AI from the radial pulse wave using a generalized transfer function, which might introduce some degree of inaccuracy.30 However, we computed the aortic AI as the ratio of the second to the first peak of the arterial pressure wave. This removes any inaccuracy that might result from estimating central pressure from a peripheral arterial wave. Moreover, our findings on the directly measured carotid AI in relation to the urinary sodium concentration were confirmatory. Third, we have no certain explanation why the associations between the systolic AIs and the urinary sodium concentration tended to be tighter in the longitudinal than in the cross-sectional analyses. However, the age-related decline in the concentration capability of the kidney might underlie this observation. Our results were largely consistent, irrespective of whether we analyzed our data longitudinally or cross-sectionally, and whether or not we excluded patients on antihypertensive treatment. Finally, we excluded participants with extreme urinary and arterial measurements, and those who died, or were hospitalized or institutionalized. Compared with participants included in the current study, excluded subjects were older, had higher body mass index, systolic blood pressure, total cholesterol, and lower urinary creatinine, and more frequently reported hypertension and antihypertensive drug treatment (see Supplementary Table S3 online). Although excluded and analyzed participants did not differ in urinary sodium measurements, excluding participants with extreme values along with attrition of the cohort might have introduced bias, which, however, is unavoidable in longitudinal population studies.
The classical paradigm is that when the central arteries stiffen with aging, the pulsatile energy is transmitted to the microcirculation of organs with high resting flow, such as the kidneys. Our current study, for the first time, inverses this paradigm by showing that the age-related decline in renal function as reflected by the urinary sodium concentration might change systolic augmentation in the central arteries, likely by moving reflection sites in the renal circulation. Further research is required to consolidate our findings, to unravel the underlying mechanism, and to establish the role of renal vasodilatation in the maintenance of sodium balance.
ACKNOWLEDGMENTS
The European Union (grants IC15-CT98-0329-EPOGH, LSHM-CT-2006-037093-InGenious HyperCare, HEALTH-2007-2.1.1-2-HyperGenes, HEALTH-2011.2.4.2-2-EU-MASCARA and the European Research Council Advanced Researcher Grant-2011-294713-EPLORE) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Brussels, Belgium (grant G.0734.09) gave support to the Studies Coordinating Centre.
Footnotes
SUPPLEMENTARY MATERIAL Supplementary materials are available at the American Journal of Hypertension online (http://www.oxfordjournals.org/our_journals/ajh/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
DISCLOSURE The authors declared no conflict of interest.
REFERENCES
- 1.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 2.Sakurai M, Yamakado T, Kurachi H, Kato T, Kuroda K, Ishisu R, Okamoto S, Isaka N, Nakano T, Ito M. The relationship between aortic augmentation index and pulse wave velocity: an invasive study. J Hypertens. 2007;25:391–97. doi: 10.1097/HJH.0b013e3280115b7c. [DOI] [PubMed] [Google Scholar]
- 3.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflection, and the risk of coronary artery disease. Circulation. 2004;109:184–89. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Nakayama Y, Tsumura K, Yoshimaru K, Ueda H. Reflection in the arterial system and the risk of coronary heart disease. Am J Hypertens. 2002;15:405–9. doi: 10.1016/s0895-7061(02)02260-4. [DOI] [PubMed] [Google Scholar]
- 5.Alderman MH. Reducing dietary sodium: the case for caution. JAMA. 2010;303:448–9. doi: 10.1001/jama.2010.69. [DOI] [PubMed] [Google Scholar]
- 6.Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials (Cochrane review) Am J Hypertens. 2011;24:843–53. doi: 10.1038/ajh.2011.115. [DOI] [PubMed] [Google Scholar]
- 7.Alderman MH, Cohen HW. Dietary sodium intake and cardiovascular mortality: controversy resolved? Am J Hypertens. 2012;25:727–34. doi: 10.1038/ajh.2012.52. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Park JB, Lakatta EG. Association of central hemodynamics with estimated 24-h urinary sodium in patients with hypertension. J Hypertens. 2011;29:1502–9. doi: 10.1097/HJH.0b013e3283486311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redelinghuys M, Norton GR, Scott L, Maseko MJ, Brooksband R, Majane OHI, Sareli P, Woodiwiss AJ. Relationship between urinary salt excretion and pulse pressure and central aortic hemodynamics independent of steady state pressure in the general population. Hypertension. 2010;56:584–90. doi: 10.1161/HYPERTENSIONAHA.110.156323. [DOI] [PubMed] [Google Scholar]
- 10.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–36. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 11.Millasseau SC, Patel SJ, Redwood SR, Ritter JM, Chowienczyk PJ. Pressure wave reflection assessed from the peripheral pulse: is a transfer function necessary? Hypertension. 2003;41:1016–20. doi: 10.1161/01.HYP.0000057574.64076.A5. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Ting CT, Nussbacher A, Nevo E, Kass DA, Pak P, Wang SP, Chang MS, Yin FC. Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension. 1996;27:168–75. doi: 10.1161/01.hyp.27.2.168. [DOI] [PubMed] [Google Scholar]
- 13.Staessen JA, Wang JG, Brand E, Barlassina C, Birkenhager WH, Hermann SM, Fagard R, Tizzoni L, Bianchi G. Effects of three candidate genes on prevalence and incidence of hypertension in a Caucasian population. J Hypertens. 2001;19:1349–58. doi: 10.1097/00004872-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T, Artery Society, European Society of Hypertension Working Group on Vascular Structure and Functioin. European Network for Noninvasive Investigation of Large Arteries Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, Hashimoto T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. doi: 10.1038/sj.jhh.1001307. [DOI] [PubMed] [Google Scholar]
- 16.Norton GR, Maseko M, Libhaber E, Libhaber CD, Majane OHI, Dessein P, Woodiwiss AJ. Is prehypertension an independent predictor of target organ changes in young-to-middle-aged persons of African descent? J Hypertens. 2008;26:2279–87. doi: 10.1097/HJH.0b013e328311f296. [DOI] [PubMed] [Google Scholar]
- 17.Woodiwiss AJ, Molebatsi N, Maseko MJ, Libhaber E, Libhaber CD, Majane OHI, Paicker J, Dessein P, Brooksbank R, Sareli P, Norton GR. Nurse-recorded auscultatory blood pressure at a single visit predicts target organ changes as well as ambulatory blood pressure. J Hypertens. 2009;27:287–97. doi: 10.1097/HJH.0b013e328317a78f. [DOI] [PubMed] [Google Scholar]
- 18.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–7. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 19.Kohara K, Tabara Y, Oshiumi A, Miyawaki Y, Kobayashi T, Miki T. Radial augmentation index: a useful and easily obtainable parameter for vascular aging. Am J Hypertens. 2005;18:115–45. doi: 10.1016/j.amjhyper.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Rowe JW, Shock NW, DeFronzo RA. The influence of age on the renal response to water deprivation in man. Nephron. 1976;17:270–8. doi: 10.1159/000180731. [DOI] [PubMed] [Google Scholar]
- 21.Sporn IN, Lancestremere RG, Papper S. Differential diagnosis of oliguria in aged patients. N Engl J Med. 1962;267:130–2. doi: 10.1056/NEJM196207192670304. [DOI] [PubMed] [Google Scholar]
- 22.O’Neill PA, McLean KA. Water homeostasis and ageing. Med Lab Sci. 1992;49:291–8. [PubMed] [Google Scholar]
- 23.Latham RD, Westerhof N, Sipkema P, Rubal BJ, Reuderink P, Murgo JP. Regional wave travel and reflections along the human aorta: a study with six simultaneous micromanometric pressures. Circulation. 1985;6:1257–69. doi: 10.1161/01.cir.72.6.1257. [DOI] [PubMed] [Google Scholar]
- 24.Segers P, Verdonck P. Role of tapering in aortic wave reflection: hydraulic and mathematical model study. J Biomech. 2000;33:299–303. doi: 10.1016/s0021-9290(99)00180-3. [DOI] [PubMed] [Google Scholar]
- 25.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int. 2007;71:1116–21. doi: 10.1038/sj.ki.5002190. [DOI] [PubMed] [Google Scholar]
- 26.Ren Y, D’Ambrosio MA, Garvin JL, Wang H, Carretero OA. Possible mediators of connecting tubule glomerular feedback. Hypertension. 2009;53:319–23. doi: 10.1161/HYPERTENSIONAHA.108.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Garvin JL, D’Ambrosio MA, Ren Y, Carretero OA. Connecting tubule glomerular feedback antagonizes tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol. 2010;299:F1374–8. doi: 10.1152/ajprenal.00403.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He FJ, MacGregor GA. Salt intake, plasma sodium, and worldwide salt reduction. Ann Med. 2012;44(Suppl 1):127–37. doi: 10.3109/07853890.2012.660495. [DOI] [PubMed] [Google Scholar]
- 29.Panel on Dietary Reference Intakes for Electrolytes and Water, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes . Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. The National Academies Press; DC: 2005. [Google Scholar]
- 30.Segers P, Rietzschel E, Heireman S, De Buyzere M, Gillebert T, Verdonck P, Van Bortel L. Carotid tonometry versus synthesized aorta pressure waves for the estimation of central systolic blood pressure and augmentation index. Am J Hypertens. 2005;18:1168–73. doi: 10.1016/j.amjhyper.2005.04.005. [DOI] [PubMed] [Google Scholar]