Abstract
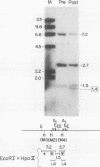
Hydroxyurea, a widely used cytotoxic/cytostatic agent that does not influence methylation of DNA bases, increases fetal hemoglobin production in anemic monkeys. To determine its effect in sickle cell anemia, we treated two patients with a total of four, 5-d courses (50 mg/kg per d, divided into three oral doses). With each course, fetal reticulocytes increased within 48-72 h, peaked in 7-11 d, and fell by 18-21 d. In patient I, fetal reticulocytes increased from 16.0 +/- 2.0% to peaks of 37.7 +/- 1.2, 40.0 +/- 2.0, and 32.0 +/- 1.4% in three successive courses. In patient II the increase was from 8.7 +/- 1.2 to 50.0 +/- 2.0%. Fetal hemoglobin increased from 7.9 to 12.3% in patient I and from 5.3 to 7.4% in patient II. Hemoglobin of patient I increased from 9.0 to 10.5 g/dl and in patient II from 6.7 to 9.9 g/dl. Additional single-day courses of hydroxyurea every 7-20 d maintained the fetal hemoglobin of patient I t 10.8-14.4%, and the total hemoglobin at 8.7-10.8 g/dl for an additional 60 d. The lowest absolute granulocyte count was 1,600/mm3; the lowest platelet count was 390,000/mm3. The amount of fetal hemoglobin per erythroid burst colony-forming unit (BFU-E)-derived colony cell was unchanged, but the number of cells per BFU-E-derived colony increased. Although examination of DNA synthesis in erythroid marrow cells in vitro revealed no decreased methylcytidine incorporation, Eco RI + Hpa II digestion of DNA revealed that hypomethylation of gamma-genes had taken place in vivo after treatment. This observation suggests that hydroxyurea is a potentially useful agent for the treatment of sickle cell anemia and that demethylation of the gamma-globin genes accompanies increased gamma-globin gene activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation--how important in gene control? Nature. 1984 Feb 9;307(5951):503–504. doi: 10.1038/307503a0. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- CHERNOFF A. I., HORTON L. L. Determinations of fetal hemoglobin in whole blood, using a modified alkali denaturation test. Am J Clin Pathol. 1958 Sep;30(3):204–208. doi: 10.1093/ajcp/30.3.204. [DOI] [PubMed] [Google Scholar]
- Charache S., Dover G., Smith K., Talbot C. C., Jr, Moyer M., Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone J., Heller P., Hall L., Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H., Bell W. R. Microscopic method for assaying F cell production: illustrative changes during infancy and in aplastic anemia. Blood. 1978 Oct;52(4):664–672. [PubMed] [Google Scholar]
- Ebert P. S., Wars I., Buell D. N. Erythroid differentiation in cultured Friend leukemia cells treated with metabolic inhibitors. Cancer Res. 1976 May;36(5):1809–1813. [PubMed] [Google Scholar]
- Felsenfeld G., McGhee J. Methylation and gene control. Nature. 1982 Apr 15;296(5858):602–603. doi: 10.1038/296602a0. [DOI] [PubMed] [Google Scholar]
- HERMAN E. C., Jr, CONLEY C. L. Hereditary persistence of fetal hemoglobin. A family study. Am J Med. 1960 Jul;29:9–17. doi: 10.1016/0002-9343(60)90003-6. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Javid J., Pettis P. K., Miller J. E. Radio-ligand immunoassay for human hemoglobin variants. J Immunol Methods. 1981;41(2):247–255. doi: 10.1016/0022-1759(81)90247-7. [DOI] [PubMed] [Google Scholar]
- Kufe D. W., Major P. P., Egan E. M., Beardsley G. P. Correlation of cytotoxicity with incorporation of ara-C into DNA. J Biol Chem. 1980 Oct 10;255(19):8997–8900. [PubMed] [Google Scholar]
- Letvin N. L., Linch D. C., Beardsley G. P., McIntyre K. W., Nathan D. G. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984 Apr 5;310(14):869–873. doi: 10.1056/NEJM198404053101401. [DOI] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Anagnou N. P., Keller G. H., Humphries R. K., Turner P. H., Young N. S., Keller P., Nienhuis A. W. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982 Dec 9;307(24):1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Schimke R. T. Gene amplification in a single cell cycle in Chinese hamster ovary cells. J Biol Chem. 1984 Feb 10;259(3):1901–1910. [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Torrealba de Ron A., Veith R., Knitter G., Stamatoyannopoulos G. Arabinosylcytosine induces fetal hemoglobin in baboons by perturbing erythroid cell differentiation kinetics. Science. 1984 May 11;224(4649):617–619. doi: 10.1126/science.6200940. [DOI] [PubMed] [Google Scholar]
- Pembrey M. E., Wood W. G., Weatherall D. J., Perrine R. P. Fetal haemoglobin production and the sickle gene in the oases of Eastern Saudi Arabia. Br J Haematol. 1978 Nov;40(3):415–429. doi: 10.1111/j.1365-2141.1978.tb05813.x. [DOI] [PubMed] [Google Scholar]
- Powars D. R., Weiss J. N., Chan L. S., Schroeder W. A. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood. 1984 Apr;63(4):921–926. [PubMed] [Google Scholar]
- Rencricca N. J., Morse B. S., Monette F. C., Howard D., Stohlman F., Jr Hydroxyurea-induced erythroid differentiation. Proc Soc Exp Biol Med. 1975 Sep;149(4):1052–1054. doi: 10.3181/00379727-149-38956. [DOI] [PubMed] [Google Scholar]
- Scott W. J., Ritter E. J., Wilson J. G. DNA synthesis inhibition and cell death associated with hydroxyurea teratogenesis in rat embryos. Dev Biol. 1971 Oct;26(2):306–315. doi: 10.1016/0012-1606(71)90129-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Timson J. Hydroxyurea. Mutat Res. 1975;32(2):115–132. doi: 10.1016/0165-1110(75)90002-0. [DOI] [PubMed] [Google Scholar]
- Torrealba-de Ron A. T., Papayannopoulou T., Knapp M. S., Fu M. F., Knitter G., Stamatoyannopoulos G. Perturbations in the erythroid marrow progenitor cell pools may play a role in the augmentation of HbF by 5-azacytidine. Blood. 1984 Jan;63(1):201–210. [PubMed] [Google Scholar]
- Wilson J. T., Wilson L. B., deRiel J. K., Villa-komaroff L., Efstratiadis A., Forget B. G., Weissman S. M. Insertion of synthetic copies of human globin genes into bacterial plasmids. Nucleic Acids Res. 1978 Feb;5(2):563–581. doi: 10.1093/nar/5.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]