Abstract
Gamma irradiation is a proven sterilization method, but is not widely used on allografts for anterior cruciate ligament (ACL) reconstruction (e.g., patella tendon) due to radiation-induced decreases in mechanical strength. Addressing this drawback would improve the safety and supply of allografts to meet current and future demand. It was hypothesized that genipin-induced collagen cross-linking would increase the tensile modulus of patella tendon tissue such that 5 MRad gamma irradiation would not reduce the tissue mechanical strength below the original untreated values. Optimized genipin treatment increased the tensile modulus of bovine tendons by ~2.4-fold. After irradiation, genipin treated tissue did not significantly differ from native tissue, proving the hypothesis. Optimized genipin treatment of human tendons increased the tensile modulus by ~1.3-fold. After irradiation, both control and genipin-treated tissues possessed ~50–60% of their native tendon modulus, disproving the hypothesis. These results highlight possible age- and species-dependent effects of genipin cross-linking on tendon tissue. Cross-linking of human allografts may be beneficial only in younger donor tissues. Future research is warranted to better understand the mechanisms and applications of collagen cross-linking for clinical use.
Introduction
The anterior cruciate ligament (ACL) is an important stabilizer of the knee, minimizing excessive anterior translation and rotation during physical activity (Beasley et al. 2005). Though the tissue has high mechanical strength (Noyes et al. 1984), ACL tears can occur through traumatic impact to the knee or excessive rotational forces during sudden motion of knee (e.g., playing soccer) (Brown and Trojian 2004; Yu and Garrett 2007). There are approximately 100,000–200,000 ACL ruptures per year in the USA that require surgical intervention. In the general populace, the incidence of this injury is approximately 0.03% per year (Miyasaka et al. 1991) and increases to 1.5% in an athletic population (Souryal and Freeman 1993; LaPrade and Burnett 1994). As the younger sub-population of the USA becomes more active (National Sporting Goods Association 2010; CDC 2010), the total number of ACL injuries is predicted to increase dramatically over the next 10 years, motivating the need for research into improved surgical techniques and biomedical modalities for ACL reconstruction.
An ACL rupture often accompanies additional soft tissue damage and these combined injuries amplify knee instability that can lead to early onset osteoarthritis (Hirshman et al. 1990; Lohmander et al. 2007). Standard surgical treatment to reconstruct a ruptured ACL involves a two-stage approach: the first surgery is used to harvest a tendinous autograft (autologous graft), typically from the patella tendon or hamstring, and a second surgery is used to reconstruct the ruptured ACL with the harvested graft (Jones 1963; Hamner et al. 1999). When implanted into the injured site, cells infiltrate the graft and remodel it over a period of 1–2 years (Falconiero et al. 1998). Though repair with an autograft restores patient mobility, it can lead to donor site morbidity and persistent pain/weakness in the graft-harvested location (Sachs et al. 1989; Nakamura et al. 2002). Due to this drawback, surgeons are increasingly utilizing allografts for ACL reconstruction, which avoids donor site morbidity, has less invasive surgery, and can lead to faster recovery times (Olson et al. 1992; Cole et al. 2005). These benefits in-turn lead to reduced hospitalization time and costs for patients and healthcare providers. Clinical studies have shown comparable long-term clinical outcomes for patients receiving allograft and autograft repairs (Ahn et al. 2008; Krych et al. 2008). The major disadvantage of allografts, however, is the liability of disease transmission that can have clinical ramifications as well as incite fear in patients (Barbour and King 2003). To address this, graft donors are screened prior to allograft harvest and then tissues are exposed to gamma radiation sterilization at a dose up to 2.5 Mrad. While this radiation dose has bactericidal effects, complete virucidal sterilization requires a radiation dosage of 3 – 5 Mrad (Pruss et al. 2002). Such high radiation doses result in tendon matrix damage and decreased mechanical properties (Gibbons et al. 1991; Fideler et al. 1995; Salehpour et al. 1995) that may lead to early mechanical failure.
In light of this limitation in allograft sterilization, improving the mechanical strength of allograft tendon constructs prior to gamma irradiation may facilitate the use of high radiation dosages that can completely eradicate disease carriers. A promising method to enhance the mechanical strength of soft tissues is collagen cross-linking. Cross-linking can be achieved through a range of modalities. Natural collagen cross-linking through sugar (advanced glycation end products) is one possibility, however it leads to increased tissue brittleness (Verzijl et al. 2002). Glutaraldehyde is another cross-linking agent commonly used for prostheses and tissues, but results in increased in vivo cytotoxicity (Huang-Lee et al. 1990). For the research presented in this publication, genipin was chosen as the experimental cross-linking agent. Genipin is a naturally-derived cross-linking agent that possesses low cytotoxicity (Sung et al. 1999; Chen et al. 2005; Ferretti et al. 2006) and is currently used in food and pharmaceutical applications (Paik et al. 2001; Yamazaki et al. 2001; Koo et al. 2004). For treating tissues, genipin has been shown to increase tissue mechanical properties in pericardium, cornea, intervertebral discs, and cartilage in a time- and concentration-dependent fashion (Avila and Navia; Sung et al. 2000; Lima et al. 2007; Yerramalli et al. 2007). The mechanism of genipin is not defined, but is thought to be via introducing intermicrofibrillar crosslinks between adjacent collagen microfibrils (Sung et al. 2000). This cross-linking confers a higher resistance to collagenase breakdown than other cross-linking methods (e.g., 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide [EDC]) (Sung et al. 2003), which may also lead to better resistance to inflammation-induced collagen breakdown post-operatively. Taking all of this into consideration, it was hypothesized that collagen cross-linking of patella tendon tissue [the “gold standard” graft, ~1.5X stronger than native ACL(Noyes et al. 1984)] with genipin would increase the tendon’s tensile modulus to allow the tissue to withstand high dose gamma radiation without loss of functional mechanical strength. The criteria for affirming the hypothesis was whether or not the post-irradiation, genipin-treated tendons possessed a statistically similar modulus to their values pre-treatment and pre-radiation (the state in which the tissue would have been used clinically).
As the time and concentration effects of genipin on tendon tissue are not known, a pilot study using mature bovine patella tendons was first performed with 2 genipin concentrations (0.250% and 0.625%) over 12 hours of incubation time. After these effects were measured and compared with trends established in literature, human patella tendons were harvested and treated with the same concentrations and incubation times (up to 12 hours) to test the study hypothesis. In addition, the cytotoxicity of genipin-treated human tendon was evaluated. Ultimately, by addressing the mechanical weakening of graft after high dose gamma irradiation, this research may have a significant impact on improving allograft supply and safety to meet future demands.
Methods
Patella tendon sample preparation
Patella tendon preparation and mechanical testing are schematically represented in Figure 1. To mimic a surgical graft (Jones 1963), the central 1/3 of patella tendons were isolated from either mature bovine stifle (knee) joints (n = 10, male, age 30–42 months; Max Insel Cohen, Livingston, NJ) or fresh frozen human cadaver knees (n = 10 patella tendons from 5 donors; mixed male/female, age 47 – 56 years old; Table 1; International Institute for the Advancement of Medicine, Jessup, PA). Left and right knee tendons were randomly assigned for experimental groups. The outer 2/3’s of the tendon tissue were frozen and saved for cytotoxicity testing (human only) and biochemical analysis.
Figure 1.
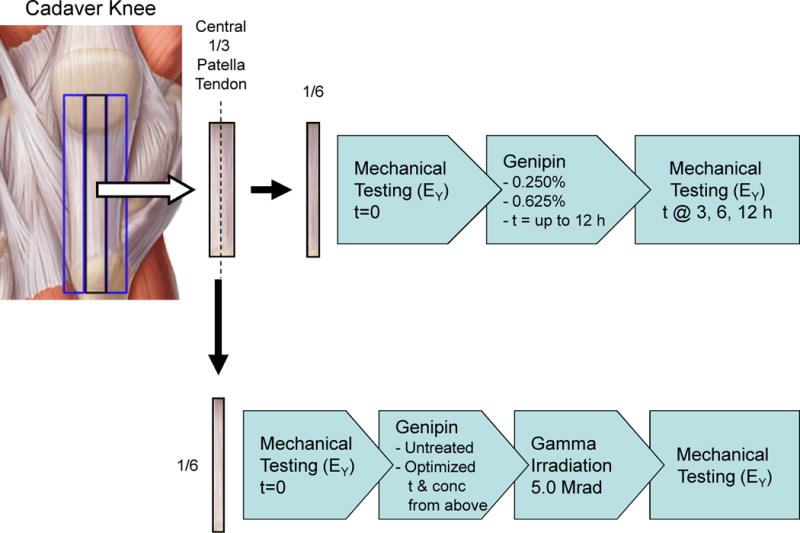
The central 1/3 of the patella tendon was harvested from bovine or human knees and then halved into two central 1/6 pieces. Top: One half was mechanically tested without treatment (t = 0), serving as control tendon values, and then incubated for up to 12 hours in either 0.250% or 0.625% genipin, with testing repeated after t = 3, 6, and 12 hours of incubation. Bottom: The other half was tested for control values, treated with optimized genipin protocol (determined above), gamma irradiated to a dose of 5.0 Mrad, and then retested again.
Table 1.
Human patella tendon specimens were obtained both knees of 5 donors aged 47–56 years old. Left and right patella tendons were randomized into experimental groups (0.250% vs. 0.625% genipin). The absolute values of the tensile modulus data are shown from mechanical testing after 3, 6, and 12 hours of incubation with genipin. Given the variability in tendon properties between donors, all data was normalized to t = 0 values for statistical analysis.
| Tensile Modulus Genipin incubation time (t = 0–12 hours) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Specimen | Age | Sex | Limb | Group | t = 0 (untreated) |
t = 3 | t = 6 | t = 12 |
| 1 | 55 | m | Left | 0.250% | 57.47 | 63.35 | 66.37 | 56.17 |
| 4 | 47 | f | Right | 0.250% | 45.86 | 66.37 | 63.87 | 43.98 |
| 5 | 50 | m | Left | 0.250% | 35.39 | 43.78 | 49.54 | 38.54 |
| 8 | 56 | f | Right | 0.250% | 68.69 | 78.48 | 93.38 | 61.37 |
| 9 | 55 | f | Left | 0.250% | 59.57 | 66.78 | 78.50 | 54.70 |
| 2 | 55 | m | Right | 0.625% | 44.01 | 33.93 | 43.79 | 45.80 |
| 3 | 47 | f | Left | 0.625% | 44.24 | 37.97 | 42.47 | 41.25 |
| 6 | 50 | m | Right | 0.625% | 60.93 | 43.51 | 62.71 | 35.86 |
| 7 | 56 | f | Left | 0.625% | 47.70 | 24.5 | 39.73 | 28.91 |
| 10 | 55 | f | Right | 0.625% | 45.72 | 41.63 | 52.43 | 40.78 |
As the central 1/3 of the patella tendon has been shown to be structurally homogeneous (Yin et al. 1997; Basso et al. 2001), this tissue was halved length-wise, creating specimens that represent the central 1/6 of the original patella tendon. Mature bovine, central 1/6 patella tendon specimens were tested first in a pilot study to confirm the time and concentration behavior of genipin as reported in the literature (Avila and Navia; Sung et al. 2000; Lima et al. 2007; Yerramalli et al. 2007). Then, human specimens were tested using the same protocol.
Genipin cross-linking and mechanical testing
As the human specimens were limited, a repeated measures design was adopted where each specimen would be tested at t = 0 (without any treatment) and retested after genipin treatment at t = 3, 6, and 12 hours. Therefore each specimen would serve as its own control increasing the statistical power of the study design. Data was normalized to the t = 0 values to account for variability in the elastic modulus of the tissue specimens given the variable age and sex of the human donors (Table 1).
A mechanical testing protocol was adopted to keep the tendon in the elastic strain range to allow for repeated testing of the tendon specimen during genipin treatment. Tendon specimens were first prepared for mechanical testing by suturing waterproof sandpaper (grit 40) to the ends of each specimen. The sandpaper ends were gripped using custom, self-tightening, serrated clamps and the tissue was non-destructively tested using a MTS hydraulic load frame (MTS, Eden Prairie, MN) controlled by an Instron 8500 controller (Instron, Norwood, MA) with a 25 lb. load cell (Interface Inc., Scottsdale, AZ). Specimens were first tensioned with a 5 N load for 30s to remove tissue slack and then the load was re-zeroed. Tendons were then preconditioned with 15 cycles of an oscillatory half-sine waveform from 0.1 – 5 N at 0.1 Hz. Immediately after preconditioning, specimens were then ramp loaded from 0.1N to 10 N at a rate of 0.1 N per second (0.1 Hz). Each testing procedure took approximately 5 minutes to complete and samples remained hydrated during testing with a saline drip.
The elastic tensile modulus was determined from the linear part of the ramp-loading curve and the specimen grip-to-grip length and cross-sectional area as determined by repeated caliper measurements. After determining the untreated, baseline moduli (t = 0), one of each central 1/6 bovine or human tendon was frozen for gamma radiation and the other was used as follows to determine the genipin time/concentration effects. Tendon samples were randomly assigned to either Genipin 0.250% or Genipin 0.625% group. Samples were then incubated in 0.250 or 0.625% wt/vol genipin (Waco Chemicals, Richmond, VA) in phosphate-buffered saline (PBS) with antibiotics/mycotics and 1% NaN3 for up to 12 hours at 25°C. At t = 3, 6, 12 hours, samples were removed from the genipin bath, washed 3x in PBS, then retested as above. Then after testing they were restored to their respective genipin bath and incubated at 25°C until the next testing time point.
Gamma irradiation
The frozen central 1/6 of the bovine or human tendon specimens were thawed with specimens randomly assigned for optimized genipin treatment (incubation time and concentration that elicited the highest tensile modulus as determined previously) or remaining untreated (PBS without genipin but with antibiotics/antimycotics and 1% NaN3 for same incubation duration as above) (n = 5 per group). Samples were then refrozen and gamma irradiated to a dose of 5 Mrad. Samples were kept frozen during gamma irradiation using a custom cooler with dry-ice and polymer thermal gel. After irradiation, tensile modulus of the specimens was determined as described above and compared to the t = 0 mechanical properties previously measured. This protocol is schematically represented in the bottom branch of the flowchart in Figure 1.
Cytotoxicity
Three, rectangular biopsies (~5 × 5 mm2) were dissected from each of the remaining outer two thirds of the human patella tendon tissue after thawing (Figure 2). These tissues remained as control (untreated, no genipin) or treated with genipin (concentration and time that resulted in the highest mechanical strength, irradiated to 5 Mrad) or cyanoacrylate glue (positive control to elicit cell death). A no-tissue control was also used to account for any effects of the untreated tendon tissue itself. A cell suspension of 5 × 105 human ACL fibroblasts from 3 separate donors in 5 mL of DMEM with 10% fetal bovine serum and antibiotics was added to the wells of 6 well plates and allowed to attach and spread to confluence after ~48 h in 37°C and 5% CO2. Then, the culture media was changed and one of the tissue biopsies was added to each well and cultured for an additional 48 hours. Following this incubation period, cytotoxicity was assessed by morphological examination as well as trypsinizing and counting the remaining fibroblasts attached to the plate. One well for each fibroblast donor and experimental group was fixed with 10% neutral buffered formalin for representative histological figures.
Figure 2.
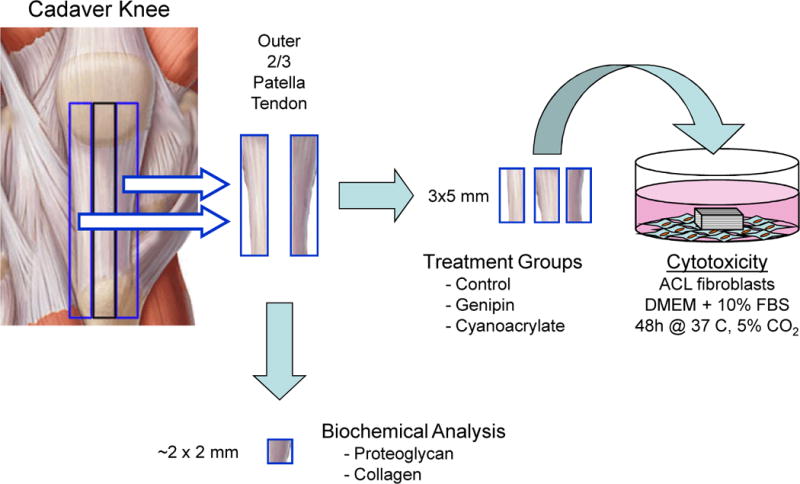
For human specimens, three (3) 3 × 5mm specimens were cut from the outer 2/3’s of the patella tendon and were either left untreated (control) or treated with genipin or cyanoacrylate glue. These specimens were then cultured with confluent ACL fibroblasts for 48 hours to observe any cytotoxic effects of genipin treatment. For both human and bovine specimens, a 2 × 2 mm biopsy (~100 mg) was cut from this tissue for determination of proteoglycan and collagen content.
Biochemical analysis
After isolating specimens for cytotoxicity from the thawed outer 2/3’s of the mature bovine and human patella tendons, a small ~100 mg specimen was dissected through the tendon cross-section, weighed, and digested in a 1 mL volume of of 1 mg/mL protease K (Roche) in 30 mM EDTA overnight at 55°C. Proteoglycan content was measured via the dimethylmethylene blue assay modified for micro-titer plates (Farndale et al. 1982).
For collagen assay determination, orthohydroxyproline (OHP) was measured using a base-hydrolysis assay (Reddy and Enwemeka 1996) that was modified for micro-titer plates. The protocol is as follows:
The following reagents were first created (all from Sigma Aldrich, St. Louis, MO):
Acetate-citrate stock buffer: 50 g of citric acid monohydrate, 12 mL glacial acetic acid, 120 g sodium acetate trihydrate, 34 g sodium hydroxide. Make up to 1 liter with deionized water. pH 6.
Assay buffer: 1:10 dilution of stock buffer above in deioninzed water.
Chloramine T reagent: 0.3525g chloramine T dissolved in 5.2 mL deionized water followed by 6.5 mL 1-proponol (n-proponol) and 13.3 mL of stock buffer. Make fresh before each assay.
p-Dimethylaminobenzaldehyde (Ehrlich’s) reagent: 3.75 g of p-dimethylaminobenzaldehyde in 15 mL 1-proponol followed by 6.5 mL perchloric acid (70% solution) added slowing with gentle mixing. Make fresh before each assay.
First, standards were created in duplicate (0 – 40 μg/mL OHP) with assay buffer. Then a 200 μL aliquot of each standard and tissue digest was mixed with 200 μL of 4N NaOH solution for base hydrolysis and then autoclaved at 110°C for 2 hours. After cooling to room temperature, the NaOH was neutralized by adding 400 μL of 2N HCl. Then, 100 μL of the undiluted tissue hydrolysates were pipetted into a 96-well microtiter plate along with 1:11, 1:121, and 1:1331 serial dilutions (made in the microtiter wells, 100 μL assay buffer + 10 μL previous dilution). Each standard and sample well was then mixed with 75 μL Chloramine T reagent and allowed to stand at RT for 20 minutes. Then 75 μL of Ehrlich’s reagent was added and the plate was covered and incubated at 60°C for 15 minutes followed by cooling for 5 minutes in a tap water bath. Plates were then read at 540nm absorbance using a Tecan SpectraFluor Plus plate-reader (Tecan Group, Durham, NC).
Statistics
Human patella tendon sample size was determined from a power analysis based on the mature bovine pilot data that suggested that 4 samples per group in a repeated measures design would have a power of 0.8 to detect a conservative effect size of 0.4 (obtained with 0.250% genipin at t = 6 hours). Elastic modulus data was analyzed using repeated measures ANOVA comparing the effects of genipin concentration and incubation time, with significance determined via Tukey HSD post-hoc test. For the effects of gamma radiation, elastic modulus data was analyzed using repeated measures ANOVA to compare the effects of pre- and post-gamma radiation and genipin treatment, with significance determined via Tukey HSD post-hoc test. Statistical differences between human and bovine tendon biochemical composition (proteoglycan and collagen) were tested using a two-tailed t-test. Differences in cytotoxicity were determined with ANOVA comparing tendon treatment (control, CA, genipin) groups followed by Tukey HSD post-hoc tests. Significance for all tests was set at α = 0.05.
RESULTS
Effects of genipin cross-linking on bovine patella tendons
Increasing time and concentration of genipin was found to have the greatest effect on the elastic modulus (EY) of mature bovine patella tendon samples (Figure 3, left). Mature bovine patella tendons were initially found to have a tensile modulus of 255.1 ± 44.2 MPa. Treatment with 0.250% genipin resulted in a significant ~1.7 fold increase in the elastic modulus compared to t = 0 values after 12 hours incubation (p < 0.05). Increasing the genipin concentration to 0.625% resulted in a ~2.3-fold higher elastic modulus after 12 hours of incubation than their original t = 0 (untreated) values (p < 0.05). It was also noted that with genipin treatment, the tendon tissue became blue in color as well as more rigid and less flexible during handling.
Figure 3.
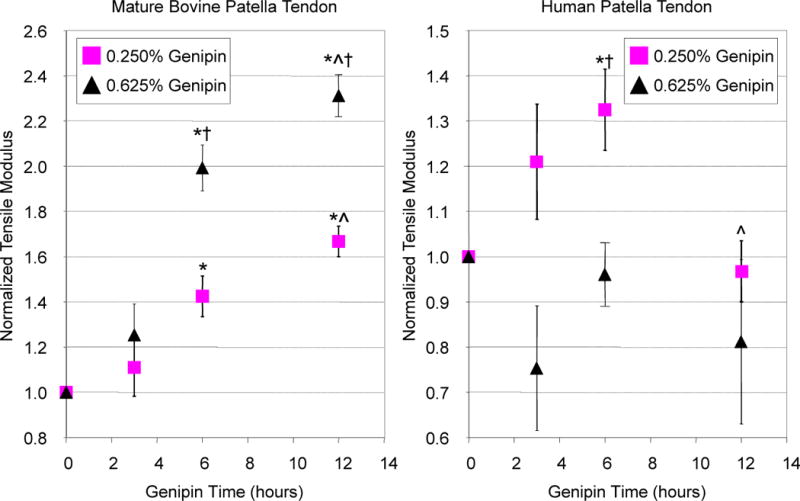
Genipin was found to have a time and concentration effect on increasing the tensile modulus (normalized to t = 0 values) of mature bovine patella tendons (left), with a concentration of 0.625% for 12 hours yielding the highest modulus value. In contrast, no clear trend was observed for genipin cross-linking of human patella tendons (right), with the highest modulus achieved with 0.250% genipin for 6 hours incubation. *p < 0.05 from t = 0 values, ^ p < 0.05 from previous time point, †p < 0.05 from respective 0.250% genipin group.
The optimized combination of concentration and time (0.625%, t = 12 h) prevented the tendon modulus from falling below the values of untreated tendons (normalized EY = 1) after 5.0 Mrad gamma radiation (Figure 4, left). In both untreated and optimized genipin-treated tissue, gamma radiation resulted in a significant ~50% loss of mechanical properties consistent with literature (p < 0.05) (Gibbons et al. 1991; Fideler et al. 1995; Salehpour et al. 1995).
Figure 4.
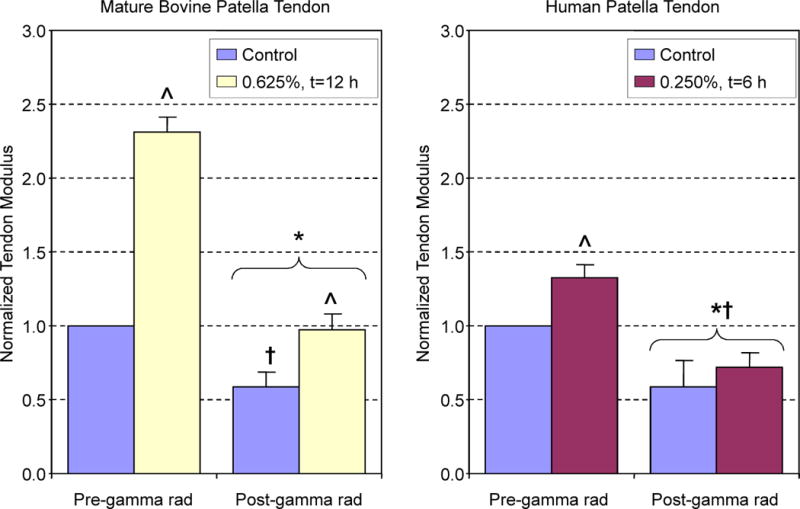
Mature bovine patella tendons (left) that were treated with optimized genipin protocol had ~2.4 fold higher modulus values than initial t = 0 measurements. High dose gamma radiation significantly reduced control and genipin tendon modulus. However, only genipin-treated tissues were not significantly different from their t = 0 modulus values. For human patella tendons (right), optimized genipin treatment increased tensile modulus ~1.4 fold. Gamma radiation significantly reduced the tensile modulus of specimens below their pre-radiation and t = 0 values. *p < 0.05 from pre-radiation values, ^ p < 0.05 from respective control, †p < 0.05 from t = 0 values
Effects of genipin cross-linking on human patella tendons
The relationship between time and concentration on genipin-induced changes in elastic modulus was not as clear for human patella tendon specimens as for bovine tissues (Figure 3, right). Genipin at a concentration of 0.625% was found to have no positive effect on tensile modulus for any incubation time. For a concentration of 0.250%, a statistically significant ~1.3-fold increase in elastic modulus was observed after 6 hours of incubation. Similar to bovine tendons, human specimens became blue in color and more rigid and less pliable compared to untreated tissues. Gamma radiation significantly reduced tendon modulus (p < 0.05) with no significant difference in tensile modulus between untreated control tendons and tendons treated with the optimized genipin conditions (0.250%, t = 6h), with both tendons possessing moduli below t = 0 values (Figure 4, right). Control human tissue (112.7 ± 7.7% cellularity normalized to no-tissue controls) and genipin-treated tissues were found to elicit no cytotoxicity (101.1 ± 8.2% cellularity). In contrast, marked cytotoxicity was noted in the positive-control cyanoacrylate treated tissues with only 58.2 ± 12.1% viable cells compared to no-tissue controls (p < 0.05 vs. control and genipin treated tissues; Figure 5).
Figure 5.
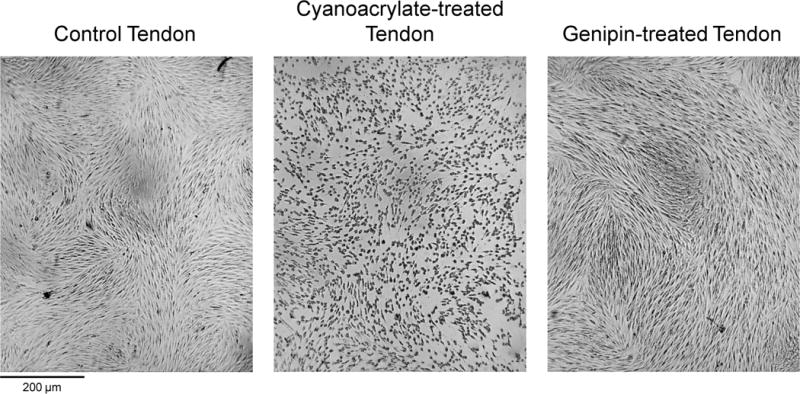
Representative images from cytotoxicity assay. Untreated control (left) and genipin treated (right) tendon biopsies did not have any cytotoxic effect on the plated ACL fibroblasts. Cyanoacrylate-treated tendon tissue (middle) as a positive control lead to ~50% cell death after 48 hours of culture.
Biochemical differences between bovine and human patella tendons
Biochemical analysis revealed significant differences in the collagen content between the mature bovine and human patella tendon samples (p < 0.05). Normalized to wet weight, untreated mature bovine patella tendons possessed a proteoglycan content of 0.37 ± 0.08% ww and collagen content of 35.75 ± 2.73% ww. Human patella tendons possessed a proteoglycan content of 0.56 ± 0.21% ww and collagen content of 27.03 ± 1.30% ww.
DISCUSSION
Gamma irradiation is a penetrating sterilization method with proven bactericidal and virucidal effects (Pruss et al. 2002). High dose gamma irradiation for the sterilization of tendon allografts for orthopedic applications, however, has not been adopted by tissue banks due to the radiation-induced graft weakening (Gibbons et al. 1991; Fideler et al. 1995; Salehpour et al. 1995). Addressing this drawback would improve the safety and supply of allografts to address the current and future surgical demand. The goal of this study was to test the hypothesis that collagen cross-linking of patella tendon tissue with genipin would increase the tendon’s tensile modulus to allow the tissue to withstand high dose gamma radiation without loss of functional mechanical strength below the tissue’s original (untreated) values. The hypothesis was proven in mature bovine patella tendons, where the optimal parameters were determined to be 12 hour incubation with a 0.625% genipin solution. The hypothesis, however, was not proven in human patella tendons, where the optimal parameters of 6 hours with 0.250% genipin did not prevent radiation-induced weakening of the tissue to below the original tensile modulus values (a normalized value of 1). Genipin-treated tendons were found to have no significant cytotoxic effects, consistent with literature (Sung et al. 1999; Chen et al. 2005; Ferretti et al. 2006).
In the literature, genipin was shown to increase the mechanical properties of porcine pericardium, porcine cornea, bovine intervertebral discs, and bovine cartilage in a time- and concentration-dependent fashion (Avila and Navia ; Sung et al. 2000; Lima et al. 2007; Yerramalli et al. 2007). A recent study by Seto, et al. demonstrated that cross-linking with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) combined with free-radical scavengers (mannitol, ascorbate, or riboflavin) protected Achilles tendon grafts (from New Zealand white rabbits) from losses in mechanical strength from 5.0 Mrad gamma radiation (Seto et al. 2009). The experimental results from mature bovine tendons presented above found similar genipin time/concentration effects consistent with the literature and a radio-protective effect that proved the study hypothesis. However, the presented research in this publication is the first (as known by the authors) to study the effects of collagen cross-linking on the mechanical properties of human tissues. The vastly different effect of genipin on human patella tendon exposes the potential danger of extrapolating results in animal tissue models into more clinically relevant human tissue models.
Significant differences in the collagen content between the human and bovine patella tendons allude to a species- or age-dependent mechanism behind the difference in the efficacy of genipin cross-linking. Interspecies differences in collagen properties have been noted in the literature, with Angele, et al. finding differences in structure, collagenase susceptibility, and tensile strength between matrices made from purified equine and bovine collagen (Angele et al. 2004). Age-matching human and animal tissue is difficult given the differences in development/maturation between animals and humans (Moriguchi and Fujimoto 1978; Vogel 1991). The human tendons tested in this study were from individuals aged 47 – 56 years old. The mature bovine tendons were obtained from skeletally mature steers 30–42 months of age. Seto, et al. utilized 5–8 month old New Zealand white rabbits for EDC cross-linking tendon experiments (Seto et al. 2009). However, cattle can have an average lifespan of ~276 months (Crews 2003) and New Zealand rabbits can have a lifespan of ~80 months (Weisbroth 1974; Gibb 1993). Therefore, all of the tendons for the animal experiments come from relatively young adults and are not in the same relative age range of the ~50 year old human specimens tested in this study and used as clinical graft tissue. The human patella tendon specimens themselves possess varying absolute tensile modulus values, likely due to differences in age and exercise level during donor lifetime (Reeves et al. 2003).
Understanding the difference in the relative ages of the human and bovine patella tendons helps to elucidate the disparity in the action of genipin on the tissue. Compared to the young adult bovine tendons, the late-middle aged human tendons had lower collagen content (~27 vs. ~35% ww) and likely greater age-related cross-links (known as advanced glycation endproducts, AGE). Based on the difference in the collagen content, genipin could potentially form more cross-links in the bovine tendon than the human tendon, explaining the greater increase in tendon modulus observed in the results.
Regarding cross-linking of collagen, it is known that lysyl oxidase-based cross-links that form with tissue maturation reinforce the structure of the tissue and improve its strength without losses in elasticity or toughness (Tuite et al. 1997). AGE crosslinks, however, form from the accumulation of sugars in collagenous tissues and also stiffen the tissue, but eventually lead to increased brittleness and reduction of tissue elasticity and mechanical strength (Tuite et al. 1997). Though the exact cross-linking mechanism of genipin is not known, the loss of flexibility and increased rigidity observed during handling from genipin cross-linking imply that the formed cross-links may be related to AGE cross-links than those formed through lysyl oxidase. If this is true, then the additional cross-linking of the human tissue with genipin on top of the AGE cross-links that already existed in the tissue may have exceeded the “ideal” threshold and therefore yielded no positive benefit in increasing tissue strength. In fact, the increasing rigidity and brittleness of heavily cross-linked fibers may lead to irreversible deformation or failure on a microscopic level upon mechanical loading. The occurrence and accumulation of such micro-damage may explain why human tendon that was treated with 0.250% for 6 hours increased in mechanical strength but weakened after 12 hours of treatment. No gross plastic deformation or failure was observed likely due to the inhomogeneity of genipin cross-linking (due to genipin acting on the outer surfaces first and then diffusing inwards over time). These hypothesized mechanisms would have to be confirmed in follow-up studies looking at the lysyl oxidase and AGE cross-linking in the original tissue and measuring the quantity and type of the cross-links formed by genipin.
Though insightful, the presented study is not without limitations. Due to the difficulty in obtaining sufficient human patella tendons for the study, a repeated measures design was adopted for mechanical testing. This allowed each sample to serve as its own control, increasing statistical power, but also required low strain, non-destructive mechanical testing. Our human patella tendon modulus values are consistent with the results of previously published, low load/strain testing (Chandrashekar et al. 2008), though it is known that the patella tendon exhibits different tensile response at low and high strains and strain rates (Staubli et al. 1999; Clemmer et al. 2010). In addition, creep testing and testing to failure would yield more information about the structural and material properties of the genipin-treated tendons (e.g., elongation, toughness, ultimate failure stress/strain), but these were not feasible due to limitations in specimen number. For strain measurements, grip-to-grip strain was used to calculate the tensile modulus. This is known to underestimate the strains that occur in the tendon midsubstance during mechanical loading (Haraldsson et al. 2005). More accurate strain measurements can be made employing an optical marker system (Derwin et al. 1994) for future studies. Tensile modulus was the only property tested, but as the ACL is not only loaded in tension but also has torsional loading, future tests should also examine bending or shear modes. These testing modalities would also highlight any changes in tissue brittleness due to genipin cross-linking.
In conclusion, it was found that the efficacy of genipin cross-linking on increasing tendon mechanical strength appears to have age or species dependence. Though the study hypothesis was proven only for animal tissue and not human tissue, there may still be a subset of human allografts, such from younger donors, where cross-linking can succeed. The results in this publication motivate further research into the basic science and optimization of collagen cross-linking for clinical use. Ultimately, increased efforts into improving safety and supply of allograft tissues will help reduce medical costs while providing improved quality of care for those in need.
Acknowledgments
This research was supported by the NIH via a post-doctoral training award (TL1RR024998 - KW Ng) and facilities grants for the Hospital for Special Surgery (Core Center AR046121, Research Facilities Improvement Program C06-RR12538–01). Special thanks to Dr. Jo Hannafin (Hospital for Special Surgery, New York, NY), Prof. Clark T. Hung (Columbia University, New York, NY), and Prof. Steven B. Nicoll (City College of New York, New York, NY) for their scientific and clinical advice in data analysis and study design.
References
- Ahn JH, Lee YS, Ha HC. Comparison of Revision Surgery With Primary Anterior Cruciate Ligament Reconstruction and Outcome of Revision Surgery Between Different Graft Materials. Am J Sports Med. 2008 doi: 10.1177/0363546508317124. [DOI] [PubMed] [Google Scholar]
- Angele P, Abke J, Kujat R, Faltermeier H, Schumann D, Nerlich M, Kinner B, Englert C, Ruszczak Z, Mehrl R, Mueller R. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials. 2004;25:2831–2841. doi: 10.1016/j.biomaterials.2003.09.066. [DOI] [PubMed] [Google Scholar]
- Avila MY, Navia JL. Effect of genipin collagen crosslinking on porcine corneas. J Cataract Refract Surg. 36:659–664. doi: 10.1016/j.jcrs.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Barbour SA, King W. The safe and effective use of allograft tissue--an update. Am J Sports Med. 2003;31:791–797. doi: 10.1177/03635465030310052801. [DOI] [PubMed] [Google Scholar]
- Basso O, Johnson DP, Amis AA. The anatomy of the patellar tendon. Knee Surg Sports Traumatol Arthrosc. 2001;9:2–5. doi: 10.1007/s001670000133. [DOI] [PubMed] [Google Scholar]
- Beasley LS, Weiland DE, Vidal AF, Chhabra A, Herzka AS, Feng MT, West RV. Anterior cruciate ligament reconstruction: A literature review of the anatomy, biomechanics, surgical considerations, and clinical outcomes. Oper Tech Orthop. 2005;15:5–19. [Google Scholar]
- Brown JR, Trojian TH. Anterior and posterior cruciate ligament injuries. Prim Care. 2004;31:925–956. doi: 10.1016/j.pop.2004.07.004. [DOI] [PubMed] [Google Scholar]
- CDC . U.S. Physical Activity Statistics. Centers for Disease Control and Prevention; US: 2010. 1988–2008 No Leisure-Time Physical Activity Trend Chart. [Google Scholar]
- Chandrashekar N, Hashemi J, Slauterbeck J, Beynnon BD. Low-load behaviour of the patellar tendon graft and its relevance to the biomechanics of the reconstructed knee. Clin Biomech (Bristol, Avon) 2008;23:918–925. doi: 10.1016/j.clinbiomech.2008.03.070. [DOI] [PubMed] [Google Scholar]
- Chen YS, Chang JY, Cheng CY, Tsai FJ, Yao CH, Liu BS. An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material. Biomaterials. 2005;26:3911–3918. doi: 10.1016/j.biomaterials.2004.09.060. [DOI] [PubMed] [Google Scholar]
- Clemmer J, Liao J, Davis D, Horstemeyer MF, Williams LN. A mechanistic study for strain rate sensitivity of rabbit patellar tendon. J Biomech. 2010;43:2785–2791. doi: 10.1016/j.jbiomech.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DW, Ginn TA, Chen GJ, Smith BP, Curl WW, Martin DF, Poehling GG. Cost comparison of anterior cruciate ligament reconstruction: autograft versus allograft. Arthroscopy. 2005;21:786–790. doi: 10.1016/j.arthro.2005.04.102. [DOI] [PubMed] [Google Scholar]
- Crews DE. Human senescence: Evolutionary and biocultural perspectives. Cambridge, UK: Cambridge University Press; 2003. p. 8. [Google Scholar]
- Derwin KA, Soslowsky LJ, Green WD, Elder SH. A new optical system for the determination of deformations and strains: calibration characteristics and experimental results. J Biomech. 1994;27:1277–1285. doi: 10.1016/0021-9290(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Falconiero RP, DiStefano VJ, Cook TM. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14:197–205. doi: 10.1016/s0749-8063(98)70041-6. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Ferretti M, Marra KG, Kobayashi K, Defail AJ, Chu CR. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng. 2006;12:2657–2663. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- Fideler BM, Vangsness CT, Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23:643–646. doi: 10.1177/036354659502300521. [DOI] [PubMed] [Google Scholar]
- Gibb JA. Sociality, time and space in a sparse population of rabbits (oryctolagus cuniculus) J Zoology. 1993;229:581–607. [Google Scholar]
- Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone-patellar tendon-bone allografts. J Orthop Res. 1991;9:209–218. doi: 10.1002/jor.1100090209. [DOI] [PubMed] [Google Scholar]
- Hamner DL, Brown CH, Jr, Steiner ME, Hecker AT, Hayes WC. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am. 1999;81:549–557. doi: 10.2106/00004623-199904000-00013. [DOI] [PubMed] [Google Scholar]
- Haraldsson BT, Aagaard P, Krogsgaard M, Alkjaer T, Kjaer M, Magnusson SP. Region-specific mechanical properties of the human patella tendon. J Appl Physiol. 2005;98:1006–1012. doi: 10.1152/japplphysiol.00482.2004. [DOI] [PubMed] [Google Scholar]
- Hirshman HP, Daniel DM, Miyasaka KC. The fate of unoperated knee ligament injuries. In: Daniel WA D, O’Connor J, editors. Knee Ligaments: Structure, Function, Injury and Repair. New York: Raven; 1990. pp. 481–503. [Google Scholar]
- Huang-Lee LL, Cheung DT, Nimni ME. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J Biomed Mater Res. 1990;24:1185–1201. doi: 10.1002/jbm.820240905. [DOI] [PubMed] [Google Scholar]
- Jones KG. Reconstruction of the Anterior Cruciate Ligament. a Technique Using the Central One-Third of the Patellar Ligament. J Bone Joint Surg Am. 1963;45:925–932. [PubMed] [Google Scholar]
- Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM, Kim SJ, Kim BC, Jin C, Lim CJ, Park EH. Antiinflammatory effects of genipin, an active principle of gardenia. Eur J Pharmacol. 2004;495:201–208. doi: 10.1016/j.ejphar.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Krych AJ, Jackson JD, Hoskin TL, Dahm DL. A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:292–298. doi: 10.1016/j.arthro.2007.08.029. [DOI] [PubMed] [Google Scholar]
- LaPrade RF, Burnett QM., 2nd Femoral intercondylar notch stenosis and correlation to anterior cruciate ligament injuries. A prospective study. Am J Sports Med. 1994;22:198–202. doi: 10.1177/036354659402200208. discussion 203. [DOI] [PubMed] [Google Scholar]
- Lima EG, Tai T, DeFail AJ, Marra KG, Ateshian GA, Hung CT. The use of genipin as a cross-linker to enhance the mechanical properties of tissue-engineered cartilage constructs. Trans Orthop Res. 2007;32:351. [Google Scholar]
- Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- Miyasaka KC, Daniel DM, Stone ML, Hirshman P. The incidence of knee ligament injuries in the general population. Am J Knee Surg. 1991;4:3–8. [Google Scholar]
- Moriguchi T, Fujimoto D. Age-related changes in the content of the collagen crosslink, pyridinoline. J Biochem. 1978;84:933–935. doi: 10.1093/oxfordjournals.jbchem.a132206. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Horibe S, Sasaki S, Kitaguchi T, Tagami M, Mitsuoka T, Toritsuka Y, Hamada M, Shino K. Evaluation of active knee flexion and hamstring strength after anterior cruciate ligament reconstruction using hamstring tendons. Arthroscopy. 2002;18:598–602. doi: 10.1053/jars.2002.32868. [DOI] [PubMed] [Google Scholar]
- National Sporting Goods Association. Ten-Year History of Sports Participation. 2010. [Google Scholar]
- Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66:344–352. [PubMed] [Google Scholar]
- Olson EJ, Harner CD, Fu FH, Silbey MB. Clinical use of fresh, frozen soft tissue allografts. Orthopedics. 1992;15:1225–1232. doi: 10.3928/0147-7447-19921001-13. [DOI] [PubMed] [Google Scholar]
- Paik Y, Lee C, Cho M, Hahn T. Physical stability of the blue pigments formed from geniposide of gardenia fruits: effects of pH, temperature, and light. J Agric Food Chem. 2001;49:430–432. doi: 10.1021/jf000978f. [DOI] [PubMed] [Google Scholar]
- Pruss A, Kao M, Gohs U, Koscielny J, von Versen R, Pauli G. Effect of gamma irradiation on human cortical bone transplants contaminated with enveloped and non-enveloped viruses. Biologicals. 2002;30:125–133. doi: 10.1006/biol.2002.0326. [DOI] [PubMed] [Google Scholar]
- Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003;548:971–981. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17:760–765. doi: 10.1177/036354658901700606. [DOI] [PubMed] [Google Scholar]
- Salehpour A, Butler DL, Proch FS, Schwartz HE, Feder SM, Doxey CM, Ratcliffe A. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone-patellar tendon-bone allografts. J Orthop Res. 1995;13:898–906. doi: 10.1002/jor.1100130614. [DOI] [PubMed] [Google Scholar]
- Seto A, Gatt CJ, Jr, Dunn MG. Improved tendon radioprotection by combined cross-linking and free radical scavenging. Clin Orthop Relat Res. 2009;467:2994–3001. doi: 10.1007/s11999-009-0934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souryal TO, Freeman TR. Intercondylar notch size and anterior cruciate ligament injuries in athletes. A prospective study. Am J Sports Med. 1993;21:535–539. doi: 10.1177/036354659302100410. [DOI] [PubMed] [Google Scholar]
- Staubli HU, Schatzmann L, Brunner P, Rincon L, Nolte LP. Mechanical tensile properties of the quadriceps tendon and patellar ligament in young adults. Am J Sports Med. 1999;27:27–34. doi: 10.1177/03635465990270011301. [DOI] [PubMed] [Google Scholar]
- Sung HW, Chang WH, Ma CY, Lee MH. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res A. 2003;64:427–438. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- Sung HW, Chang Y, Liang IL, Chang WH, Chen YC. Fixation of biological tissues with a naturally occurring crosslinking agent: fixation rate and effects of pH, temperature, and initial fixative concentration. J Biomed Mater Res. 2000;52:77–87. doi: 10.1002/1097-4636(200010)52:1<77::aid-jbm10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sung HW, Huang RN, Huang LL, Tsai CC. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J Biomater Sci Polym Ed. 1999;10:63–78. doi: 10.1163/156856299x00289. [DOI] [PubMed] [Google Scholar]
- Tuite DJ, Renstrom PA, O’Brien M. The aging tendon. Scand J Med Sci Sports. 1997;7:72–77. doi: 10.1111/j.1600-0838.1997.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Ben ZC, Brau-Benjamin O, Maroudas A, Bank RA, Mizrahi J, Schalkwijk CG, Thorpe SR, Baynes JW, Bijlsma JW, Lafeber FP, TeKoppele JM. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Vogel HG. Species differences of elastic and collagenous tissue--influence of maturation and age. Mech Ageing Dev. 1991;57:15–24. doi: 10.1016/0047-6374(91)90021-q. [DOI] [PubMed] [Google Scholar]
- Weisbroth SH. Neoplastic diseases. In: Weisbroth SH, Flatt RE, Kraus AL, editors. The biology of the laboratory rabbit. New York, USA: Academic Press; 1974. p. 333. [Google Scholar]
- Yamazaki M, Sakura N, Chiba K, Mohri T. Prevention of the neurotoxicity of the amyloid beta protein by genipin. Biol Pharm Bull. 2001;24:1454–1455. doi: 10.1248/bpb.24.1454. [DOI] [PubMed] [Google Scholar]
- Yerramalli CS, Chou AI, Miller GJ, Nicoll SB, Chin KR, Elliott DM. The effect of nucleus pulposus crosslinking and glycosaminoglycan degradation on disc mechanical function. Biomech Model Mechanobiol. 2007;6:13–20. doi: 10.1007/s10237-006-0043-0. [DOI] [PubMed] [Google Scholar]
- Yin C, Wayne JS, Jiranek WA, Zuelzer WA. Biochemical and molecular homogeneity in the patellar tendon of the immature pig. J Orthop Res. 1997;15:712–718. doi: 10.1002/jor.1100150513. [DOI] [PubMed] [Google Scholar]
- Yu B, Garrett WE. Mechanisms of non-contact ACL injuries. Br J Sports Med. 2007;41(Suppl 1):i47–51. doi: 10.1136/bjsm.2007.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]


