Abstract
Background
About one half of patients with HF have preserved rather than reduced ejection fraction (HFPEF; HFREF). The differences in risk factors predisposing to the two subtypes of HF are poorly understood. We sought to identify clinical predictors of new-onset HF, and to explore differences in HFPEF versus HFREF.
Methods and Results
We studied new-onset HF cases between 1981 and 2008 in Framingham Heart Study participants, classified into HFPEF and HFREF (EF > 45% vs ≤ 45%). We used Cox multivariable regression to examine predictors of 8-year risk of incident HF, and competing-risks analysis to identify predictors that differed between HFPEF and HFREF. Among 6,340 participants (60 ± 12 years) with 97,808 person-years of follow up, 512 developed incident HF. Of 457 participants with LVEF evaluation at the time of HF diagnosis, 196 (43%) were classified as HFPEF and 261 (56%) as HFREF. Fourteen predictors of overall HF were identified. Older age, diabetes mellitus, and a history of valvular disease predicted both types of HF (p ≤ 0.0025 for all). Higher BMI, smoking, and atrial fibrillation predicted HFPEF only, whereas male sex, higher total cholesterol, higher heart rate, hypertension, cardiovascular disease, left ventricular hypertrophy, and left bundle branch block predicted risk of HFREF.
Conclusions
While multiple risk factors preceded overall HF, distinct clusters of risk factors determine risk for new-onset HFPEF versus HFREF. This knowledge may enable the design of clinical trials of targeted prevention and the introduction of therapeutic strategies for prevention of HF and its two major subtypes.
Keywords: heart failure, epidemiology, risk factors, ejection fraction
Heart failure (HF) is a major and growing public health problem in the United States that accounts for more than 1 million hospital admissions per year1. The identification of at-risk individuals may provide important opportunities to intervene early in the disease process. Of patients presenting with new-onset HF in epidemiological studies, 40–71% have HF with preserved, rather than reduced ejection fraction (HFPEF versus HFREF).2–8 While the clinical course and survival after HF onset have been ascertained for both HFPEF and HFREF,3, 5 clinical characteristics prior to the onset of HF have not been assessed systematically in relation to these established HF categories. Differences in risk factors preceding the onset of HFPEF versus HFREF could provide important insights into distinct pathophysiologic pathways that differ between the two entities. Furthermore, for persons at risk for the development of HFREF, such as those with asymptomatic left ventricular (LV) dysfunction, there is trial evidence that early treatment can improve outcomes.9 In contrast, little is known about treatments that specifically benefit individuals at risk for, or with the diagnosis of HFPEF.10
Few prior studies to our knowledge, have examined risk factors that specifically differ in the prediction of incident HFPEF versus HFREF. Current American College of Cardiology/American Heart Association (ACC/AHA) HF guidelines classify at-risk individuals as stage A HF,11 but this group of individuals remains ill-defined with regard to type of HF. The aim of our study was first, to examine risk factors of incident HF, and second, to contrast clinical risk factors for incident HF with preserved versus reduced ejection fraction in a large community-based cohort with the belief that the identification of distinct risk factor clusters for HFPEF versus HFREF could have important implications for the design of clinical trials and for future therapies to prevent or treat HF.
Methods
Study Sample
The Framingham Heart Study (FHS) original and offspring cohorts have been described previously.12, 13 Men and women in the original cohort underwent periodic cardiovascular examinations approximately every 2 years, whereas those in the offspring cohort were examined approximately every 4 years. At each visit, health history updates, physical examinations, and blood laboratory tests were performed. In total, 6,340 participants (n = 12,631 examinations) who did not have prevalent HF at the time of original cohort examination 16 (1979–1982, n = 2000), examination 20 (1986–1990, n = 1054), or examination 24 (1995–1998, n = 529), or offspring examination 2 (1979–1983, n = 2359), examination 4 (1987–1991, n = 3385), or examination 6 (1995–1998, n = 3304) were included in analyses (for details see Supplemental Table I). From the beginning of each follow-up period, participants were monitored for the first HF hospitalization event occurring up to 8 years later. Written informed consent was obtained from study participants, and the research protocol was approved by the institutional review board of Boston Medical Center.
Clinical Assessment
Potential clinical predictors were assessed at the baseline examination of each 8-year follow-up period. Blood pressure (BP) was the average of 2 seated measurements obtained by a FHS physician, and hypertension was defined as a systolic BP ≥ 140 mmHg, a diastolic BP ≥ 90 mmHg, or current use of antihypertensive medication. Cardiovascular events were adjudicated by a 3-physician panel after review of medical records. History of myocardial infarction (MI) was based on diagnostic ECG, or the combination of cardiac enzymes, and clinical presentation. History of coronary heart disease (CHD, excluding definite MI) was defined as prior clinically unrecognized MI, acute coronary insufficiency (prolonged ischemic symptoms with new ECG abnormalities in the absence of biomarker elevations indicative of infarction), or angina pectoris. Prior cerebrovascular disease was defined as prior stroke or transient ischemic attack. Occurrence of atrial fibrillation was determined after examining all available ECGs from FHS clinic visits and medical records. Valvular heart disease was defined as a systolic murmur ≥ grade 3/6 or any diastolic murmur. Total cholesterol levels were obtained, and diabetes was defined as a fasting glucose ≥ 126 mg/dL, non-fasting blood glucose ≥ 200 mg/dL, or the use of insulin or oral hypoglycemic medications. Participants regularly smoking cigarettes during the year before the baseline examination were considered current smokers. Left ventricular hypertrophy was defined using previously reported ECG criteria.14 Participants with missing covariates were excluded from the study.
Definition of Initial HF Hospitalization
At each examination, interim cardiovascular disease events were identified and medical records obtained. Initial HF hospitalization was confirmed by a panel of 3 physicians after systematic review of outpatient and hospital records using established protocols and FHS criteria (Supplemental Table I).15 In the present study, we included participants with initial HF hospitalization occurring between 1980 and 2008 when an evaluation of left ventricular ejection fraction (LVEF) by echocardiography (85%), radionuclide angiography (10%), or ventriculography (5%) was available near the time of hospitalization.16 Each incident HF event was classified as HFPEF (EF > 45%) or HFREF (EF ≤ 45%) when sufficient data were available.
Statistical Analysis
Baseline clinical characteristics were summarized separately for participants who did and did not develop HF, and were further broken down by participants with HFPEF and HFREF. For HF subtype analyses, participants who developed HF that was unclassified with regard to ejection fraction were included in the group without HF. We examined cumulative incidence of HFPEF and HFREF using a Kaplan-Meier-like method while accounting for competing risks (death, other HF type).17 Analyses were conducted separately in men and women and also in subgroups with/without prior MI. Proportional hazards regression was used to model associations between clinical characteristics and incident HF. Clinical characteristics considered in multivariable models are listed in Table 1. First, age- and sex-adjusted models were fitted for each clinical covariate and incident HF. Next, stepwise multivariable models were constructed with age and sex forced into the model. Hazards ratios for continuous variables are represented as the risk associated with a one-standard deviation change in the predictor. Similar models were fitted for HFREF and HFPEF. We accounted for multiple testing using Bonferroni correction: for each HF subtype, 20 covariates were tested, and retained in the model at a significance level of p ≤ 0.0025 (= 0.05/20). With all covariates that entered into either HFPEF or HFREF in separate models, we used the Lunn-McNeil method, to test whether variables were associated with differential risk for HFPEF versus HFREF.18 In secondary analyses, eGFR was considered in the multivariable model for overall HF. All statistical analyses were conducted with SAS version 9.2 for Windows.
Table 1.
Sample characteristics of participants with and without heart failure
| Characteristic | Men (n = 5,775 examinations) |
Women (n = 6,856 examinations) |
||
|---|---|---|---|---|
|
| ||||
| HF | No HF | HF | No HF | |
| n = 272 | n = 5,503 | n = 240 | n = 6,616 | |
| Demographics | ||||
| Age, years | 72 (10) | 59 (11) | 76 (9) | 61 (12) |
| Clinical | ||||
| Systolic blood pressure, mmHg | 143 (21) | 132 (18) | 148 (23) | 131 (21) |
| Diastolic blood pressure, mmHg | 76 (12) | 80 (10) | 73 (11) | 76 (10) |
| Heart rate, bpm | 68 (13) | 64 (11) | 73 (13) | 67 (11) |
| Body-mass index, kg/m2 | 27 (4) | 28 (4) | 28 (6) | 26 (5) |
| Prior myocardial infarction, % | 23 | 5 | 6 | 1 |
| Prior coronary heart disease, % | 37 | 9 | 21 | 6 |
| Cerebrovascular disease, % | 11 | 3 | 9 | 2 |
| Hypertension, % | 76 | 47 | 83 | 44 |
| Diabetes mellitus, % | 28 | 8 | 25 | 5 |
| Current smoker, % | 16 | 22 | 16 | 22 |
| Valvular disease, % | 9 | 1 | 8 | 1 |
| Taking antihypertensive meds, % | 52 | 26 | 65 | 27 |
| Laboratory | ||||
| Total cholesterol, mg/dl | 203 (41) | 208 (39) | 220 (49) | 218 (41) |
| HDL cholesterol, mg/dl | 40 (12) | 43 (12) | 50 (16) | 56 (16) |
| ECG criteria | ||||
| Atrial fibrillation | 14 | 3 | 8 | 1 |
| Left ventricular hypertrophy | 24 | 9 | 21 | 5 |
| Left bundle branch block | 3 | 1 | 3 | 0 |
| Right bundle branch block | 10 | 3 | 5 | 2 |
Abbreviations: HDL, high-density lipoprotein; HFPEF, heart failure with preserved ejection fraction; HFREF, heart failure with reduced ejection fraction;
Data are presented as mean (SD) unless otherwise indicated.
Results
There were 6,340 unique participants who contributed 12,631 eight-year observation periods in our analysis, with a total follow-up time of 97,808 person-years. The mean age was 60 ± 12 years and 54% of participants were female. A total of 512 participants developed incident HF; 55 (11%) did not undergo evaluation of LVEF – these participants did not differ from participants whose HF was classified by subtype (data not shown). Of 457 participants with HF and known LVEF, 196 (43%) were classified as HFPEF and 261 (56%) as HFREF. The overall incidence of HF was 5 cases per 1,000 person-years (2 per 1,000 HFPEF, and 3 per 1,000 HFREF). The mean follow-up time was 7.7 ± 1.7 years. Baseline clinical characteristics are displayed by incident HF status in Table 1 (sample characteristics by HFPEF and HFREF are displayed in Supplemental Table II).
Risk factors for Incident HF
In multivariable analyses, increasing age, male sex, hypertension, higher body-mass index, increasing heart rate, coronary heart disease, diabetes, smoking, valvular heart disease, lower HDL cholesterol, atrial fibrillation, and the presence of LV hypertrophy or left bundle branch block all were associated with increased risk of incident HF (Table 2). In secondary analyses, eGFR was considered as a predictor of incident HF. Baseline kidney function was available in 8,116 individuals (64% of total sample size), of whom 349 developed subsequent HF. In multivariable analyses, eGFR did not factor into the final model.
Table 2.
Multivariable-adjusted risk factors of incident heart failure
| Overall Heart Failure (512 events/12,631 examinations) |
|||
|---|---|---|---|
| HR | 95% CI | p value | |
| Demographics | |||
| Age, per year | 1.10 | (1.09–1.11) | < 0.001 |
| Female | 0.76 | (0.62–0.93) | 0.01 |
| Clinical | |||
| Hypertension | 1.58 | (1.26–1.98) | < 0.001 |
| Body-mass index, per 4.7 kg/m2 | 1.16 | (1.05–1.27) | 0.002 |
| Heart rate, per 12 bpm | 1.28 | (1.19–1.38) | < 0.001 |
| Prior myocardial infarction | 2.25 | (1.71–2.96) | < 0.001 |
| Prior coronary heart disease | 1.70 | (1.37–2.12) | < 0.001 |
| Diabetes mellitus | 2.50 | (2.03–3.08) | < 0.001 |
| Current smoker | 1.64 | (1.28–2.01) | < 0.001 |
| Valvular disease | 3.17 | (2.31–4.36) | < 0.001 |
| HDL, per 15.6 mg/dl | 0.83 | (0.75–0.93) | 0.001 |
| ECG criteria | |||
| Atrial fibrillation | 1.89 | (1.42–2.51) | < 0.001 |
| Left ventricular hypertrophy | 2.30 | (1.85–2.85) | < 0.001 |
| Left bundle branch block | 2.48 | (1.47–4.19) | < 0.001 |
Hazard ratios are per 1 standard deviation change in continuous variables, and for the presence vs. absence of dichotomous variables. Variables considered but not included in the multivariable model included: systolic and diastolic blood pressure, cerebrovascular disease, total cholesterol, right bundle branch block. All covariates listed in Table 1 were considered in a stepwise selection model with retention of covariates at p < 0.0025.
Risk factors for HFPEF and HFREF
Age- and sex-adjusted clinical predictors of new-onset HFPEF and HFREF are shown in Table 3. With the exception of diastolic BP and cholesterol level, the majority of risk factors including hypertension, previous MI, and diabetes mellitus were significant predictors of both HFPEF and HFREF in age- and sex-adjusted analyses.
Table 3.
Age- and sex-adjusted risk factors of HFPEF and HFREF
| HFPEF (196 events/12,631 examinations) | HFREF (261 events/12,631 examinations) | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Demographics | ||||||
| Age, per year | 1.12 | (1.10–1.14) | < 0.001 | 1.11 | (1.09–1.12) | < 0.001 |
| Female | 0.96 | (0.72–1.29) | 0.79 | 0.35 | (0.28–0.46) | < 0.001 |
| Clinical | ||||||
| Systolic blood pressure | 1.17 | (1.03–1.34) | 0.02 | 1.30 | (1.16–1.46) | < 0.001 |
| Diastolic blood pressure | 0.92 | (0.80–1.05) | 0.22 | 0.97 | (0.86–1.10) | 0.65 |
| Hypertension | 1.87 | (1.33–2.64) | 0.004 | 2.45 | (1.79–3.34) | < 0.001 |
| Heart rate, per 12 bpm | 1.23 | (1.08–1.40) | 0.002 | 1.25 | (1.12–1.39) | < 0.001 |
| Prior myocardial infarction | 1.33 | (0.72–2.48) | 0.36 | 5.23 | (3.87–7.07) | < 0.001 |
| Prior coronary heart disease | 2.00 | (1.42–2.80) | < 0.001 | 3.36 | (2.58–4.37) | < 0.001 |
| Cerebrovascular disease | 1.25 | (0.69–2.25) | 0.46 | 2.74 | (1.89–3.98) | < 0.001 |
| Diabetes mellitus | 3.31 | (2.38–4.61) | < 0.001 | 3.87 | (2.96–5.07) | < 0.001 |
| Body-mass index, per 4.7 kg/m2 | 1.44 | (1.26–1.64) | < 0.001 | 1.26 | (1.11–1.43) | < 0.001 |
| Current smoker | 1.75 | (1.20–2.55) | 0.004 | 1.40 | (1.00–1.96) | 0.05 |
| Valvular disease | 4.08 | (2.56–6.52) | < 0.001 | 2.46 | (1.50–4.04) | < 0.001 |
| HDL cholesterol, per 16 mg/dl | 0.73 | (0.62–0.86) | < 0.001 | 0.67 | (0.57–0.78) | < 0.001 |
| Total cholesterol, per 40 mg/dl | 0.92 | (0.79–1.07) | 0.30 | 0.96 | (0.84–1.09) | 0.50 |
| ECG criteria | ||||||
| Atrial fibrillation | 2.76 | (1.75–4.34) | < 0.001 | 2.04 | (1.35–3.09) | < 0.001 |
| Left ventricular hypertrophy | 1.84 | (1.27–2.66) | 0.001 | 3.04 | (2.30–4.02) | < 0.001 |
| Left bundle branch block | 2.15 | (0.80–5.80) | 0.13 | 4.03 | (2.14–7.60) | < 0.001 |
| Right bundle branch block | 2.01 | (1.24–3.25) | 0.005 | 1.01 | (0.60–1.71) | 0.97 |
Abbreviations: CI, confidence interval; HDL, high-density lipoprotein; HFPEF, heart failure with preserved ejection fraction; HFREF, heart failure with reduced ejection fraction; HR, hazard ratio;
Multivariable-adjusted models for HFPEF and HFREF are shown in Table 4. Age, diabetes mellitus, and valvular disease were significant predictors of both types of new-onset HF. The remaining risk factors were uniquely significant for either HFPEF or HFREF. Specifically, male sex, hypertension, higher heart rate, prior cardiovascular disease, higher cholesterol level, left ventricular hypertrophy, and left bundle branch block were associated with higher risk of HFREF. In contrast, higher BMI, smoking, and a history of atrial fibrillation increased the risk of HFPEF. Of note, hypertension and left ventricular hypertrophy predicted HFPEF in age- and sex-adjusted analyses, however they did not enter the multivariable model (at a p-value threshold of 0.0025). Results were not materially different when we introduced systolic BP and anti-hypertensive therapy in place of hypertension. In exploratory analyses, at a less conservative p-value threshold of 0.05, left ventricular hypertrophy (but not hypertension) predicted HFPEF in multivariable analyses (Supplemental Table III).
Table 4.
Multivariable-adjusted risk factors of HFPEF and HFREF
| HFPEF (196 events/12,631 examinations) | HFREF (261 events/12,631 examinations) | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Demographics | ||||||
| Age, per year | 1.13 | (1.11–1.14) | < 0.001 | 1.08 | (1.06–1.09) | < 0.001 |
| Female | 1.14 | (0.85–1.53) | 0.4 | 0.48 | (0.37–0.63) | < 0.001 |
| Clinical | ||||||
| Hypertension | 1.76 | (1.28–2.41) | < 0.001 | |||
| Heart rate, per 12 bpm | 1.32 | (1.19–1.48) | < 0.001 | |||
| Prior myocardial infarction | 3.49 | (2.48–4.90) | < 0.001 | |||
| Prior coronary heart disease | 1.73 | (1.27–2.34) | < 0.001 | |||
| Cerebrovascular disease | 1.96 | (1.34–2.86) | < 0.001 | |||
| Diabetes mellitus | 2.88 | (2.05–4.05) | < 0.001 | 2.91 | (2.21–3.85) | < 0.001 |
| Body-mass index, per 4.7 kg/m2 | 1.41 | (1.23–1.61) | < 0.001 | |||
| Current smoker | 2.04 | (1.39–2.99) | < 0.001 | |||
| Valvular disease | 4.88 | (3.05–7.82) | < 0.001 | 2.44 | (1.48–4.04) | < 0.001 |
| ECG criteria | ||||||
| Atrial fibrillation | 2.47 | (1.57–3.89) | < 0.001 | |||
| Left ventricular hypertrophy | 2.73 | (2.04–3.65) | < 0.001 | |||
| Left bundle branch block | 3.41 | (1.78–6.52) | < 0.001 | |||
Abbreviations: CI, confidence interval; HDL, high-density lipoprotein; HFPEF, heart failure with preserved ejection fraction; HFREF, heart failure with reduced ejection fraction; HR, hazard ratio;
All covariates listed in Table 1 were considered in a stepwise selection model with retention of covariates at p < 0.0025.
In secondary analyses, HFPEF and HFREF were re-categorized based on an LVEF cut-off of > or ≤ 50 %, respectively. Results remained similar, with the exception of HDL cholesterol, which was a predictor of HFREF (Supplemental Table IV).
Differential effects of predictors of HFPEF versus HFREF
Despite different sets of significant variables predicting HFPEF and HFREF, only a few had differential effects on the risk of HFPEF versus HFREF (i.e. acted as true effect modifiers) (Table 5). Increasing age was associated with a higher risk of HFPEF versus HFREF (p = 0.0075), whereas male sex (p = 0.0003) and prior MI (p = 0.0003) were associated with increased hazard for HFREF versus HFPEF. Of note, there was no evidence of a differential effect of hypertension or left ventricular hypertrophy on HFPEF versus HFREF, although these covariates entered the multivariable model only for HFREF.
Table 5.
Comparison of predictors of HFPEF versus HFREF
| HFPEF | HFREF | ||||
|---|---|---|---|---|---|
| log(HR) | s.e. | log(HR) | s.e. | p value | |
| Demographics | |||||
| Age, per year increase | 0.08 | 0.01 | 0.05 | 0.01 | 0.008 |
| Female | 0.26 | 0.16 | −0.53 | 0.15 | < 0.001 |
| Clinical | |||||
| Hypertension | 0.49 | 0.18 | 0.63 | 0.16 | 0.58 |
| Heart rate, per 12 bpm increase | 0.26 | 0.07 | 0.37 | 0.06 | 0.23 |
| Prior myocardial infarction | −0.32 | 0.33 | 1.05 | 0.17 | < 0.001 |
| Prior coronary heart disease | 0.53 | 0.19 | 0.64 | 0.16 | 0.66 |
| Cerebrovascular disease | −0.24 | 0.31 | 0.38 | 0.20 | 0.09 |
| Diabetes mellitus | 0.61 | 0.18 | 0.74 | 0.14 | 0.58 |
| BMI, per 4.7 kg/m2 increase | 0.20 | 0.07 | 0.01 | 0.07 | 0.06 |
| Current smoker | 0.73 | 0.20 | 0.32 | 0.18 | 0.13 |
| Valvular disease | 1.16 | 0.25 | 0.64 | 0.26 | 0.15 |
| ECG criteria | |||||
| Atrial fibrillation | 0.66 | 0.24 | 0.16 | 0.22 | 0.12 |
| Left ventricular hypertrophy | 0.23 | 0.20 | 0.70 | 0.15 | 0.05 |
| Left bundle branch block | 1.28 | 0.52 | 2.23 | 0.34 | 0.12 |
Abbreviations: BMI, body-mass index; HFPEF, heart failure with preserved ejection fraction; HFREF, heart failure with reduced ejection fraction; log(HR), estimate of the regression parameter; s.e., standard error;
Figure 1 displays the cumulative incidence of HFPEF versus HFREF by sex. Notably, the cumulative incidence of HFPEF in men and of HFPEF and HFREF in women was similar; in contrast, the cumulative incidence of HFREF in men was markedly higher. Figure 2 displays cumulative incidence by history of prior MI, and shows that MI increased the risk only for HFREF.
Figure 1.
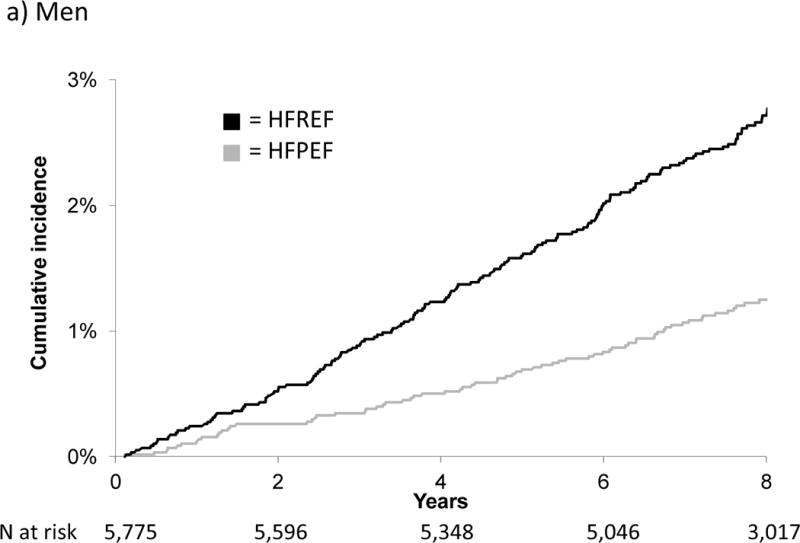
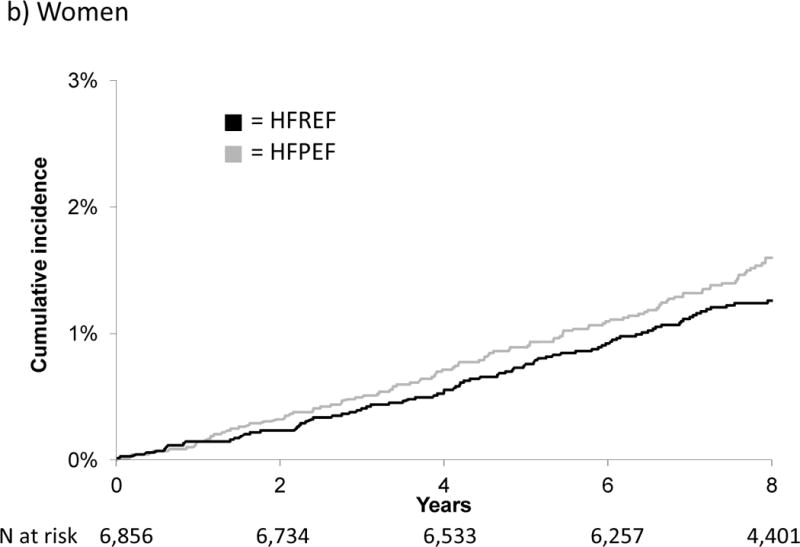
Cumulative incidence of HFPEF versus HFREF in men and women
Figure 2.
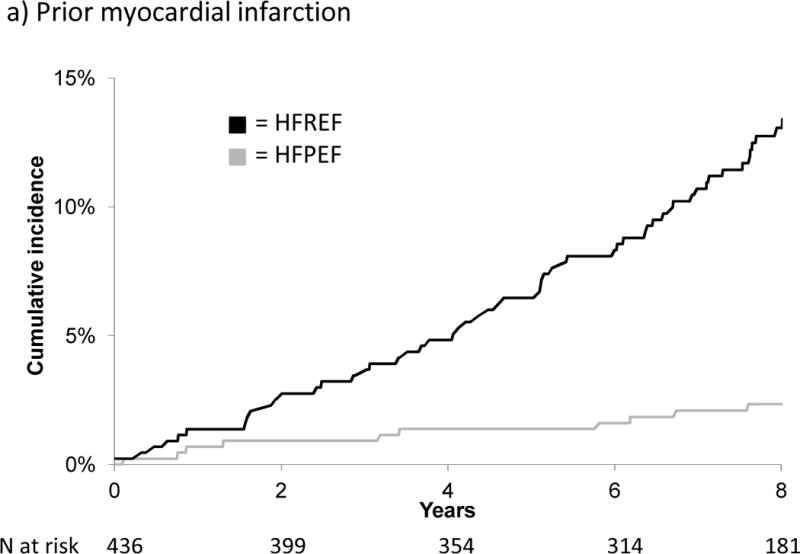

Cumulative incidence of HFPEF versus HFREF by history of prior myocardial infarction
Discussion
In a large community-based cohort, fourteen risk factors were associated with overall incident HF. Notably, distinct clusters of antecedent risk factors can be used to predict new onset HFPEF versus HFREF. Age, diabetes mellitus, and a history of valvular disease were significant predictors of both types of new-onset HF. In contrast, BMI, smoking, and atrial fibrillation were predictors of HFPEF, whereas male sex, total cholesterol, heart rate, hypertension, history of cardiovascular disease, left ventricular hypertrophy, and left bundle branch block all increased the risk for HFREF. When examined in aggregate, we demonstrated differential effects of age, sex, and prior MI on the risk of HFPEF versus HFREF. In addition, our data suggest that the incidence of HFPEF is similar in men and women, that women are at similar risk of HFPEF and HFREF, and that men by contrast are at increased risk of HFREF. The findings that distinct clusters of risk factors determine risk for either HFPEF or HFREF, highlight differences in the pathogenesis of the two subtypes of HF; this knowledge can be used in the design of clinical trials of HF prevention strategies targeting either HFREF or HFPEF.
We previously demonstrated that a constellation of 9 clinical factors (including age, heart rate, systolic BP, CHD, valvular heart disease, diabetes, cardiomegaly, left ventricular hypertrophy, and vital capacity) predicted the 4-year risk of overall HF in participants with a history of CHD, hypertension, or valvular heart disease at baseline.19 The present analysis extends these findings to a more contemporary and comprehensive cohort. Our group has also shown differences in clinical risk factors in HFPEF versus HFREF cross-sectionally at the time of HF presentation.16 The current study applies this same concept to a longitudinal study design, showing that different risk factors precede and predispose to new-onset HFPEF versus HFREF. Prior longitudinal studies examining risk factors for incident HF were similarly limited by not differentiating HFPEF from HFREF.19–24 Other studies comparing HFPEF versus HFREF have examined clinical characteristics cross-sectionally at the time of HF presentation, but not risk factors antedating the development of symptomatic HF.5, 25–29 The present analysis is unique in directly comparing and contrasting antecedent risk factor profiles for incident HFPEF versus HFREF. The findings highlight important differences in risk factors routinely ascertained prior to the onset of each type of HF, such that a given risk factor may contribute differently to risk of HFPEF versus HFREF.
These findings are particularly relevant to individuals without known structural heart disease but at risk for HF (i.e. ACC/AHA stage A HF).11 To our knowledge, no previous prospective study has examined differences in modifiable risk factors between HFPEF and HFREF, nor has any determined whether the risk factor profile for new-onset HFPEF is distinct from that of HFREF. Our findings indicate that in the preclinical stages of HF distinct clusters of risk factors precede HFPEF and HFREF. As such, our findings may have important implications for targeted strategies for HF prevention. For example, angiotensin-converting enzyme inhibitors have been shown to reduce mortality in individuals with asymptomatic LV dysfunction,30 however effective strategies to identify to detect these individuals in the community have been limited.31 The identification of specific risk factors that precede the development of HFREF could help tailor population screening strategies (e.g. blood biomarkers or cardiac imaging) to identify at-risk individuals who may benefit from specific therapies.
Interestingly, previous studies have shown that women are more likely to present with HFPEF than HFREF.3, 25–29, 32 However, when we examined the cumulative incidence of HFPEF and HFREF, the rates were similar in women, and approximate that of HFPEF in men. The significant sex-specific difference was thus driven predominantly by an increased HFREF risk in men. This difference persisted despite adjustment for CHD history and age. Previous data from FHS participants without HF show that isolated systolic hypertension leads to concentric left ventricular hypertrophy in women, whereas a pattern of eccentric hypertrophy is generally seen in men,33 a sex difference that also was seen in experimental models.34 This and other sex-specific responses to risk factors may contribute to the disparity in risk of HFPEF versus HFREF in men and women.
Increased BMI has previously been associated with higher risk of HF in the FHS.35 Our data now suggest that BMI appears to be a stronger predictor of HFPEF compared with HFREF. This is supported by the observation that participants with HPFEF appear to have higher BMI’s compared with HFREF in other population-based studies,3, 4 as well as the finding that visceral adiposity is associated with LV diastolic dysfunction specifically in women.36 The mechanism underlying obesity and HFPEF is unclear, but are likely complex, given recent data showing a greater rate of adverse events in the lowest and highest BMI categories.37
Previous studies have clearly shown that hypertension is a risk factor for HFPEF.38 This is supported in our age- and sex-adjusted analyses, where hypertension and left ventricular hypertrophy predict HFPEF. It is notable that these risk factors did not enter the multivariable model for HFPEF when a strict Bonferroni correction was used. At a less conservative threshold, left ventricular hypertrophy did predict HFPEF in multivariable analyses. However, when formally tested for effect modification, there were no differential effects of hypertension and left ventricular hypertrophy on HF subtype. Several other limitations of our study deserve mention. The diagnosis of HF was based on HF hospitalization as the primary outcome, and this may have led to underestimation of the true incidence of HF in general and HFPEF in particular. However, because this endpoint was highly specific, bias of our effect estimates due to misclassification was likely minimized, despite sensitivity that may have been low.39 Incident HFPEF versus HFREF by definition included only individuals who underwent assessment of LVEF around the time of HF diagnosis. Although the inclusion of participants with HF and unknown LVEF in the group without HF would likely have biased results toward the null, differential misclassification and bias cannot be ruled out. Of all incident HF cases, only 11% had no assessment of LV function, compared with a substantially lower proportion of patients without LV functional assessment at HF diagnosis in other studies.3, 25 LV function was assessed at the time of HF presentation, and more extensive echocardiographic data, including longitudinal changes in LVEF or diastolic function parameters were not taken into account, potentially leading to misclassification bias. Lastly, the study sample is predominantly white, and generalizability to different ethnic backgrounds may be limited, and validation in other populations is warranted. However, the clear strengths of the study were prospective ascertainment of incident HF in a large community-based sample, and the direct comparison of risk factor profiles by subtype of HF.
In conclusion, our results indicate that HF is predicted by fourteen easily obtained clinical risk factors and that HFPEF and HFREF are preceded by different constellations of antecedent risk factors. These results may be particularly important with regard to further characterizing individuals at increased risk for HF. Our results indicate that the risk profiles for HFPEF and HFREF are distinct from each other years prior to the development of symptomatic HF. Further validation of our findings in other population-based studies is warranted. In the future, the identification of individuals at high risk for the development specific types of HF could be used to design clinical trials of targeted prevention and treatment strategies aimed at differences in underlying risk profiles.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (Drs Ho and Levy, contract No. N01-HC-25195). The EFFECT study was supported by a Canadian Institutes of Health Research team grant in cardiovascular outcomes research and a grant from the Heart and Stroke Foundation of Canada. Dr. Ho is supported by an American Heart Association Clinical Research Program award. Dr. Lee is supported by a clinician-scientist award from the Canadian Institutes of Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 3.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 4.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, Fabsitz RR, Howard BV. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: The Strong Heart Study. Am J Cardiol. 2000;86:1090–1096. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 5.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 7.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 8.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–332. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 9.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 10.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 11.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 12.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: The Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 14.Cupples LA, D’Agostino RB, Kannel WB, Wolf P, Garrison RJ, editors. Framingham heart study, 30 year follow-up. Publication pb87-177499. Bethesda: National Institutes of Health; 1988. The Framingham Study: An epidemiological investigation of cardiovascular disease. section 34: Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements. [Google Scholar]
- 15.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 16.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaynor JJ, Feuer EJ, Tan CC, Wu DH, Little CR, Straus DJ, Clarkson BD, Brennan MF. On the use of cause-specific failure and conditional failure probabilities. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 18.Lunn M, McNeil D. Applying cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 19.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 20.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PWF, Kritchevsky SB, Study HA. Incident heart failure prediction in the elderly: The Health ABC heart failure score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PWF, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: The Health, Aging, And Body Composition Study. Arch Intern Med. 2009;169:708–715. doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalogeropoulos A, Psaty BM, Vasan RS, Georgiopoulou V, Smith AL, Smith NL, Kritchevsky SB, Wilson PWF, Newman AB, Harris TB, Butler J, Study CH. Validation of the health abc heart failure model for incident heart failure risk prediction: The Cardiovascular Health Study. Circ Heart Fail. 2010;3:495–502. doi: 10.1161/CIRCHEARTFAILURE.109.904300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal A, Norton CR, Thomas TN, Davis RL, Butler J, Ashok V, Zhao L, Vaccarino V, Wilson PWF. Predictors of incident heart failure in a large insured population: A one million person-year follow-up study. Circ Heart Fail. 2010;3:698–705. doi: 10.1161/CIRCHEARTFAILURE.110.938175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera M, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: The ARIC study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 26.Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J, Komajda M. Differences between patients with a preserved and a depressed left ventricular function: A report from the Euroheart Failure Survey. Eur Heart J. 2004;25:1214–1220. doi: 10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Philbin EF, Rocco TA, Lindenmuth NW, Ulrich K, Jenkins PL. Systolic versus diastolic heart failure in community practice: Clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605–613. doi: 10.1016/s0002-9343(00)00601-x. [DOI] [PubMed] [Google Scholar]
- 28.McDermott MM, Feinglass J, Sy J, Gheorghiade M. Hospitalized congestive heart failure patients with preserved versus abnormal left ventricular systolic function: Clinical characteristics and drug therapy. Am J Med. 1995;99:629–635. doi: 10.1016/s0002-9343(99)80250-2. [DOI] [PubMed] [Google Scholar]
- 29.Pernenkil R, Vinson JM, Shah AS, Beckham V, Wittenberg C, Rich MW. Course and prognosis in patients > or = 70 years of age with congestive heart failure and normal versus abnormal left ventricular ejection fraction. Am J Cardiol. 1997;79:216–219. doi: 10.1016/s0002-9149(96)00719-9. [DOI] [PubMed] [Google Scholar]
- 30.Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: A follow-up study. Lancet. 2003;361:1843–1848. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: Implications for screening. Ann Intern Med. 2003;138:907–916. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 32.Ghali JK, Kadakia S, Cooper RS, Liao YL. Bedside diagnosis of preserved versus impaired left ventricular systolic function in heart failure. Am J Cardiol. 1991;67:1002–1006. doi: 10.1016/0002-9149(91)90174-j. [DOI] [PubMed] [Google Scholar]
- 33.Krumholz HM, Larson MG, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 34.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: Gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32:1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 35.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 36.Canepa M, Strait JB, Abramov D, Milaneschi Y, Al Ghatrif M, Moni M, Ramachandran R, Najjar SS, Brunelli C, Abraham TP, Lakatta EG, Ferrucci L. Contribution of central adiposity to left ventricular diastolic function. Am J Cardiol. 2012;109:1171–1178. doi: 10.1016/j.amjcard.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the irbesartan in heart failure with preserved ejection fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topol EJ, Traill TA, Fortuin NJ. Hypertensive hypertrophic cardiomyopathy of the elderly. N Engl J Med. 1985;312:277–283. doi: 10.1056/NEJM198501313120504. [DOI] [PubMed] [Google Scholar]
- 39.Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


