Abstract
Organic extracts of 20 species of French seaweed have been screened against Trypanosoma brucei rhodesiense trypomastigotes, the parasite responsible for sleeping sickness. These extracts have previously shown potent antiprotozoal activities in vitro against Plasmodium falciparum and Leishmania donovani. The selectivity of the extracts was also evaluated by testing cytotoxicity on a mammalian L6 cell line. The ethyl acetate extract of the brown seaweed, Bifurcaria bifurcata, showed strong trypanocidal activity with a mild selectivity index (IC50 = 0.53 µg/mL; selectivity index (SI) = 11.6). Bio-guided fractionation led to the isolation of eleganolone, the main diterpenoid isolated from this species. Eleganolone contributes only mildly to the trypanocidal activity of the ethyl acetate extract (IC50 = 45.0 µM, SI = 4.0). However, a selective activity against P. falciparum erythrocytic stages in vitro has been highlighted (IC50 = 7.9 µM, SI = 21.6).
Keywords: eleganolone, linear diterpene, Bifurcaria, Plasmodium falciparum, Trypanosoma
1. Introduction
Human African trypanosomiasis (sleeping sickness) is a fatal disease, if untreated. With fewer than 12,000 cases reported per year, trypanosomiasis belongs to the most neglected tropical diseases [1]. The causative agents are Trypanosoma brucei rhodesiense in East Africa and T. b. gambiense inWest and Central Africa. Therapy of sleeping sickness remains a problem. The available drugs are outdated, complicated to administer and can cause severe adverse reactions [2]. Safe and effective drugs are urgently needed.
Marine macrophytes have shown their extensive biological activity [3,4,5], including antiprotozoal [6,7,8,9,10,11], but very little is known about the compounds responsible for these activities.
As a part of our continuous search for new natural antiprotozoal secondary metabolites, 35 polar (hydroalcoholic) and apolar (ethyl acetate) extracts from 20 species of seaweed from the Normandy coast (France), which we previously reported to have other antiprotozoal activities [6], were screened against cultured trypomastigotes of Trypanosoma brucei rhodesiense, as well as for cytotoxicity on a mammalian cell line (L6). Herein, we report the bio-guided fractionation of the most active extract and structure elucidation and antiprotozoal activity of its main constituent.
2. Results and Discussion
2.1. Selected Species
The sampling resulted in the selection of 20 species of seaweed (Table 1). The samples were collected, as described previously [6].
Table 1.
Marine algal species selected for the study [6].
| Species | Family | Collection site | Collection time |
|---|---|---|---|
| Chlorophyta | |||
| Codium tomentosum Stackhouse | Codiaceae | Cap Lévy (Manche) | June 2007 |
| Ulva lactuca (Linnaeus) | Ulvaceae | Luc-sur-Mer (Calvados) | October 2006 |
| Ulva clathrata (Roth) C. Agardh | Ulvaceae | Anse St Martin (Manche) | June 2007 |
| Heterokontophyta | |||
| Bifurcaria bifurcata R. Ross | Sargassaceae | Cap Lévy (Manche) | June 2007 |
| Dictyopteris polypodioides (A.P. de Candolle) J.V. Lamouroux | Dictyotaceae | Barneville (Calvados) | October 2007 |
| Dictyota dichotoma (Hudson) J.V. Lamouroux | Dictyotaceae | Anse St Martin (Manche) | June 2007 |
| Fucus serratus (Linnaeus) | Fucaceae | Luc-sur-mer (Calvados) | November 2005 |
| Himanthalia elongata (Linnaeus) | Himanthaliaceae | Cap Lévy (Manche) | June 2006 |
| Laminaria digitata (Linnaeus) J.V. Lamouroux | Laminariaceae | Langrunes-sur-Mer (Calvados) | January 2007 |
| Pelvetia canaliculata Decaisne & Thuret | Fucaceae | Cap Lévy (Manche) | June 2006 |
| Sargassum muticum (Yendo) Fensholt | Sargassaceae | Cap Lévy (Manche) | June 2006 |
| Rhodophyta | |||
| Calliblepharis jubata (Goodenough & Woodward) Kützing | Cystocloniaceae | Cap Lévy (Manche) | June 2007 |
| Chondrus crispus Stackhouse | Gigartinaceae | Cap Lévy (Manche) | June 2007 |
| Dilsea carnosa (Schmidel) Kuntze | Dumontiaceae | Langrune-sur-Mer (Calvados) | January 2007 |
| Gelidium latifolium Bornet ex Hauck | Gelidiaceae | Cap Lévy (Manche) | June 2006 |
| Gracilaria gracilis (Stackhouse) Steentoft, L.M. Irvine & Farnham | Gracilariaceae | Anse St Martin (Manche) | June 2007 |
| Grateloupia turuturu Yamada | Halymeniaceae | St Vaast-la-Hougue (Manche) | September 2007 |
| Halurus flosculosus (J. Ellis) Maggs & Hommersand | Ceramiaceae | Anse St Martin (Manche) | June 2007 |
| Mastocarpus stellatus (Stackhouse) Guiry | Phyllophoraceae | Cap Lévy (Manche) | June 2006 |
| Palmaria palmata (Linnaeus) Kuntze | Palmariaceae | Luc-sur-Mer (Calvados) | November 2005 |
2.2. In Vitro Trypanocidal Activity of the Selected Species
The trypanocidal activity of the resultant ethyl acetate and hydroalcoholic extracts were evaluated in vitro against Trypanosoma brucei rhodesiense trypomastigotes (STIB 900 strain). Extracts were first screened at two concentrations (1.6 and 9.7 µg/mL), and parasite growth inhibition was measured. Extracts for which parasite growth inhibition was greater than 50% at the concentration of 9.7 µg/mL were subsequently assayed to determine their IC50. Cytotoxicity to primary mammalian L6 cells was also evaluated to determine the selectivity of its activity. Table 2 presents the IC50 values and selectivity indexes (SIs) of the active extracts (ratio of cytotoxic to trypanocidal activity). An SI value >10 is generally considered to indicate antiprotozoal activity not due to general cytotoxicity.
Table 2.
In vitro trypanocidal activity of the active extracts against T. brucei rhodesiense trypomastigotes (STIB 900 strain). Data shown are means of two independent assays, which varied <±50%. SI: selectivity index; ratio of cytotoxic activity on L6 cells to trypanocidal activity. E: hydroalcoholic extract, A: ethyl acetate extract.
| IC50 (µg/mL) | Selectivity index (SI) | |||
|---|---|---|---|---|
| Antitrypanosomal activity | Cytotoxic activity | |||
| Species | Extract | T. brucei rhodesiense | L6 cells | |
| B. bifurcata | E | 29.7 | 76.0 | 2.6 |
| B. bifurcata | A | 0.5 | 6.2 | 12.4 |
| C. jubata | A | 23.3 | 71.5 | 3.1 |
| C. crispus | A | 13.6 | 84.3 | 6.2 |
| D. dichotoma | A | 5.8 | 27.8 | 4.8 |
| D. carnosa | A | 15.3 | 74.0 | 4.8 |
| G. latifolium | A | 20.5 | 62.1 | 3.0 |
| G. gracilis | A | 21.5 | 71.3 | 3.3 |
| G. turuturu | A | 10.8 | 71.2 | 6.6 |
| H. flosculosus | A | 22.4 | 58.7 | 2.6 |
| H. elongata | A | 30.3 | 88.3 | 2.9 |
| M. stellatus | A | 19.5 | 69.1 | 3.5 |
| P. canaliculata | A | 7.8 | 86.7 | 11.1 |
| S. muticum | A | 5.8 | 27.8 | 4.8 |
| Standards | ||||
| Melarsoprol | 0.004 | |||
| Podophyllotoxin | 0.007 | |||
As shown previously for antiplasmodial and leishmanicidal activities [6], the active extracts were almost entirely ethyl acetate extracts (37%), while hydroalcoholic extracts were mainly inactive (2.8%).
Four ethyl acetate extracts showed activity under 10 µg/mL: B. bifurcata, D. dichotoma, P. canaliculata and S. muticum. B. Bifurcaria and P. canaliculata extracts showed selectivity indexes >10, suggesting these extracts could be selectively active against T. brucei rhodesiense. The remaining extracts were less active, with IC50 values ranging from 10.8 to 29.7 µg/mL. Interestingly, the four most active species belong to the Heterokontophyta. Very little research has been carried out on the antiprotozoal potential of brown alga, and there are only few screening papers available on this subject [7,8,9,10]. Only one of them deals with the species studied here [7]. There were good similarities in the trypanocidal effect of Turkish [9], British [7] and French D. dichotoma. Also, French B. bifurcata, P. canaliculata and S. muticum show a similar activity profile to British ones, but with activity about twice as great [7].
B. bifurcata was the most active species, with an IC50 value of 0.5 µg/mL for the ethyl acetate extract. To our knowledge, in addition to our study and that of Spavieri, there is no data available regarding the antiprotozoal properties of extracts derived from this species. In contrast, cytotoxic activities have been described for methanolic and chloroform/methanol extracts and compounds isolated from ether extracts of B. bifurcata [12,13,14].
Indeed, phytochemistry of this species has been widely described. B. bifurcata is characterized by polyphenols (phlorotannins) [15,16], fucosterol [17], but mainly by linear oxygenated diterpenes [18,19,20,21,22,23,24,25,26], eleganolone being the major one [27]. Several studies have proven the cytotoxic activities of B. bifurcata and/or its secondary metabolites. Bifurcane and analogs showed a cytotoxic effect on fertilized sea urchin eggs (ED50 = 4–12 µg/mL) [28]. Bifurcadiol exhibited cytotoxicity against cultured tumor cell lines with ED50 values of 4 to 10 µg/mL [29]. Other trihydroxylated acyclic diterpenes were proven to be active in vitro against the NSCLC-N6 cell line derived from a human non-small-cell bronchopulmonary carcinoma, with IC50 values of 9.5 to 12.3 µg/mL [20]. Eleganolone and analogs showed anti-fouling activity [30,31]. However, no study has been performed on the antiprotozoal activities of its metabolites.
As the ethyl acetate extract has shown significant activity against T. brucei rhodesiense, we subjected it to further fractionation in order to isolate and characterize the active ingredients.
2.3. Bio-Guided Fractionation of the Most Active Extract Obtained from B. bifurcata
The ethyl acetate extract of B. bifurcata was fractionated through column chromatography on silica gel using a polarity gradient to generate five main fractions. Bio-guided fractionation was followed by the isolation of the main constituent of the most active fraction. Each fraction or compound was evaluated for trypanocidal activity against T. brucei rhodesiense trypomastigotes. Fractions and purified compound were also evaluated for antiprotozoal activities against T. cruzi amastigotes and P. falciparum intraerythrocytic stages and for cytotoxic activity towards L6 mammalian cells to assess their selectivity of activity (Table 3 and Table 4).
Table 3.
In vitro antiprotozoal activity of fractions obtained from the ethyl acetate crude extract of B. bifurcata. Data shown are means of two independent assays, which varied <±50%. SI: selectivity index; ratio of cytotoxic activity on L6 cells to antiprotozoal activity measured against a T. brucei rhodesiense, b T. cruzi and c P. falciparum. * Data from [6].
| Antiprotozoal activity | Cytotoxic activity | ||||||
|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | SI | |||||
| T. brucei rhodesiense | T. cruzi | P. falciparum | L6 cells | SI a | SI b | SI c | |
| Ethyl acetate extract | 0.5 | 4.1 * | >5 * | 6.2 * | 12.4 | 1.5 * | >1 * |
| Fractions | |||||||
| Fraction 1 | 12.9 | 32.4 | 8.5 | 40.2 | 3.1 | 1.2 | 4.7 |
| Fraction 2 | 0.5 | 9.7 | 3.8 | 7.4 | 15.4 | 0.8 | 1.9 |
| Fraction 3 | 4.3 | 11.7 | 3.7 | 16.9 | 3.9 | 1.4 | 4.6 |
| Fraction 4 | 14.7 | 25.0 | 3.0 | 55.1 | 3.7 | 2.2 | 18.4 |
| Fraction 5 | 14.1 | 32.1 | 4.8 | 38.7 | 2.7 | 1.2 | 8.0 |
| Standard drugs | |||||||
| Melarsoprol | 0.004 | ||||||
| Benznidazole | 0.536 | ||||||
| Chloroquine | 0.069 | ||||||
| Artemisinin | 0.002 | ||||||
| Podophyllotoxin | 0.007 | ||||||
Table 4.
In vitro antiprotozoal activity of eleganolone, the main component of the most active fraction obtained from the crude ethyl acetate extract of B. bifurcata. Data shown are means of two independent assays, which varied <±50%. SI: selectivity index; ratio of cytotoxic activity on L6 cells to antiprotozoal activity measured against a T. brucei rhodesiense and b P. falciparum.
| Antiprotozoal activity | Cytotoxic activity | |||||
|---|---|---|---|---|---|---|
| IC50 in µM (µg/mL) | IC50 (µg/mL) | SI | ||||
| T. brucei rhodesiense | T. cruzi | P. falciparum | L6 cells | SI a | SI b | |
| Eleganolone | 45 (13.7) | 58 (17.7) | 7.9 (2.6) | 184 (56.1) | 4.0 | 21.6 |
| Standard drugs | ||||||
| Melarsoprol | 0.005 (0.004) | |||||
| Benznidazole | 1.69 (0.536) | |||||
| Chloroquine | 0.19 (0.069) | |||||
| Artemisinin | 0.007 (0.002) | |||||
| Podophyllotoxin | 0.048 (0.007) | |||||
2.3.1. Fractions
The strong trypanocidal activity detected in the crude ethyl acetate extract (IC50 = 0.5 µg/mL) was found in fraction 2 (IC50 = 0.5 µg/mL), with a little increase in selectivity (SI = 12.4 and 15.4, respectively) (Table 3). Other fractions were less active and less selective towards T. brucei rhodesiense.
2.3.2. Pure Compound
The main constituent of the fraction 2 was isolated by successive column chromatographies on silica gel and identified as eleganolone (Figure 1). Eleganolone was unambiguously identified by comparison of its spectral data with those described in the literature [21], on the basis of chemical and spectral evidence, including 1H, 13C and Distortionless Enhancement by Polarization Transfer (DEPT) 135 nuclear magnetic resonance (NMR) experiments and High Resolution Mass Spectrometry.
Figure 1.
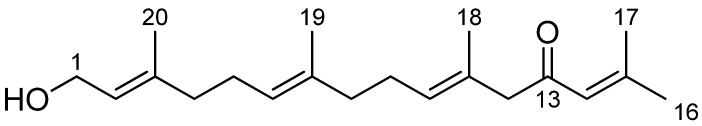
Chemical structure of eleganolone or 2,6,10,14-hexadecatetraen-4-one.
Antiprotozoal activity of eleganolone was then evaluated against the protozoa parasites to check whether or not eleganolone contribute to activity (Table 4).
Eleganolone was less active against T. brucei rhodesiense than the fraction from which it has been isolated (IC50 = 13.7 µg/mL and 0.5 µg/mL, respectively). Indeed, eleganolone showed only mild trypanocidal activity against T. brucei rhodesiense, with a poor selectivity index (SI = 4.0). On the other hand, eleganolone exhibited antiplasmodial activity with a good selectivity (SI = 21.6). The trypanocidal activity, which was lost during the fractionation, could not be attributed to eleganolone, although it is the main component of the active fraction. However, B. bifurcata contains a large variety of structurally related oxygenated diterpenoids that could contribute synergistically to the activity of the crude extract. Nevertheless, the fractionation revealed that eleganolone was selectively active against P. falciparum, compared to other protozoa or mammalian cells. To the best of our knowledge, antiplasmodial activity has not been reported yet for eleganolone.
3. Experimental Section
3.1. General Experimental Procedures
Silica gel 60 (230–400 mesh, Merck, Stockholm, Sweden) was used as the stationary phase for column chromatography. 1H-NMR and 13C-NMR spectra were recorded in deuterated chloroform (CDCl3, Eurisotop, Saint Aubin, France) on a Bruker Avance DRX-400 spectrometer at 400 MHz (1H) and 100 MHz (13C, DEPT 135). The High Resolution Electrospray Ionization Mass Spectroscopy (HREIMS) was performed on an Esquire 3000 plus ion trap mass spectrometer (Bruker Daltonics, Bremen, Germany).
3.2. Algae Collection and Identification
The 20 algae species were collected between November 2005 and September 2007 at several locations on the coast of Basse-Normandie. Table 1 reports the collection dates and sites.
Taxonomic determination was performed by Dr. A.-M. Rusig, and voucher specimens of the algae are deposited in the Herbarium of the University of Caen.
3.3. Extraction and Compound Isolation
Crude extracts were prepared as described before [6]. Briefly, freeze-dried and ground thallus of B. bifurcata (250 g) was extracted at room temperature with ethyl acetate and concentrated to dryness under vacuum. The residue (12 g) was subjected to successive flash and column chromatographies over silica gel (230–400 mesh, Merck) eluting with a cyclohexane-ethyl acetate mixture of increasing polarity to yield five main fractions, labeled 1–5. Separations of fraction 2 (2.5 g) by repeated column chromatography on silica gel eluted with a cyclohexane-ethyl acetate mixture of increasing polarity yielded eleganolone (10.3 mg).
Eleganolone: colorless oil; 1H NMR (CDCl3, 400 MHz) δ 5.36 (1H, t, J = 6.8 Hz, H-2), 5.20 (1H, t, J = 6.4 Hz, H-10), 5.08 (1H, t, J = 6.4 Hz, H-6), 4.11 (1H, d, J = 6.8 Hz, H-1), 2.99 (1H, br, s, H-12), 2.04 (2H, t, J = 8.0 Hz, H-4, H-8), 1.97 (2H, dt, J = 8.0 Hz, 6.4 Hz, H-5, H-9), 1.82 (1H, br, s, H-17), 1.62 (1H, br, s, H-18), 1.55 (3H, br, s, H-16, H-19, H-20); 13C NMR (CDCl3, 100 MHz) δ 199.5 (C, C-13), 155.8 (C, C-15), 139.8 (C, C-3), 138.8 (C, C-7), 130.9 (C, C-11), 124.7 (CH, C-14), 123.5 (CH, C-10), 123.0 (CH, C-2), 122.7 (CH, C-6), 59.0 (CH2, C-1), 55.3 (CH2, C-12), 39.8 (CH2, C-4, C-8), 26.7 (CH2, C-5), 26.4 (CH2, C-9), 25.1 (CH3, C-16), 19.9 (CH3, C-20), 17.5 (CH3 C-17), 17.2 (CH3, C-18), 16.9 (CH3, C-19); HREIMS [M + Na]+ m/z 327.23193 (calculated for C20H32O2Na, 327.22945). Spectroscopic data matched those previously published [21].
3.4. In Vitro Antiprotozoal Assays
The extracts were dissolved in dimethylsulfoxide (DMSO) to obtain a concentration of 10 mg/mL and screened for antiprotozoal activity against P. falciparum, T. cruzi and T. brucei rhodesiense and cytotoxicity against rat skeletal muscle myoblasts (L6 cells). The in vitro assays were conducted as described by Scala et al. [31]. A brief description is given below.
3.4.1. Activity against P. falciparum
In vitro activity against erythrocytic stages of P. falciparum was determined by a modified [3H]-hypoxanthine incorporation assay with the chloroquine- and pyrimethamine-resistant K1 strain [32]. Briefly, parasite cultures incubated in Roswell Park Memorial Institute (RPMI) 1640 medium with 5% Albumax (without hypoxanthine) were exposed to serial drug dilutions in microtiter plates. After 48 h of incubation at 37 °C in a reduced oxygen atmosphere, 0.5 µCi [3H]-hypoxanthine was added to each well. Cultures were incubated for a further 24 h before they were harvested onto glass-fiber filters and washed with distilled water. The radioactivity was counted with a Betaplate™ liquid scintillation counter (Wallac, Zurich, Switzerland). The results were recorded as counts per minute (CPM) per well at each drug concentration and expressed as the percentage of untreated controls. IC50 values were calculated from graphically plotted dose-response curves by linear interpolation. Chloroquine (Sigma C6628) and artemisinin (Sigma 36,159-3) were used as positive references.
3.4.2. Activity against Trypanosoma cruzi
Rat skeletal myoblasts (L6 cells) were seeded in 96-well microtiter plates at 2000 cells/well in 100 μL RPMI 1640 medium with 10% fetal bovine serum (FBS) and 2 mM L-glutamine. After 24 h, the medium was removed and replaced by 100 μL per well containing 5000 trypomastigote forms of T. cruzi Tulahuen strain C2C4 with the β-galactosidase (Lac Z) gene [33]. After 48 h, the medium was removed from the wells and replaced by 100 μL fresh medium with or without a serial drug dilution of seven 3-fold dilution steps covering a range from 90 to 0.123 μg/mL. After 96 h of incubation, the plates were inspected under an inverted microscope to assure growth of the controls and sterility. Then, the substrate CPRG/Nonidet (50 μL) was added to all wells. A color reaction developed within 2–6 h and could be read photometrically at 540 nm. The IC50 values were calculated from the sigmoidal inhibition curves with SoftMax Pro software. Benznidazole (Roche) was used as a positive reference.
3.4.3. Activity against Trypanosoma brucei rhodesiense
The assays were performed according to the procedures described by [34]. For the extracts, working stock solutions of 180 µg/mL in serum containing culture medium according to Baltz et al. [35] were prepared. One-hundred microliters of the diluted extracts were pipetted in duplicate into the first row of a 96-well microtiter plate (Costar, USA). With the complete culture medium, three-fold serial dilutions were prepared. After the addition of Trypanosoma brucei rhodesiense bloodstream form trypanosomes from axenic culture, the concentrations of the extracts ranged from 90 µg/mL to 0.13 µg/mL and from 500 to 0.07 µg/mL for pure compounds. The total number of trypanosomes in each well was 2 × 103/100 µL. The plate was then incubated for 72 h in a humidified atmosphere at 37 °C in 5% CO2. Ten microliters of resazurin solution (12.5 mg resazurin dissolved in 100 mL distilled water) were then added to each well, and incubation continued for a further 2–4 h. The plate was then read in a Spectramax Gemini XS microplate fluorometer (MolecularDevices Cooperation, Sunnyvale, CA, USA) using an excitation wavelength of 536 nm and an emission wavelength of 588 nm [34]. Fluorescence development was measured and expressed as the percentage of the control. Melarsoprol (Arsobal) was used as a positive reference.
3.5. Cytotoxicity against L6 Cells
Assays were performed in 96-well microtiter plates, each well containing 100 L of RPMI 1640 medium supplemented with 1% L-glutamine (200 mM) and 10% fetal bovine serum and 4 × 104 L6 cells (rat skeletal myoblasts). Serial drug dilutions of seven 3-fold dilution steps covering a range from 90 to 0.123 μg/mL were prepared. After 72 h of incubation, the plates were inspected under an inverted microscope to assure growth of the controls and sterile conditions. Then, 10 μL of a resazurin solution (12.5 mg resazurin dissolved in 100 mL distilled water) was added to each well and the plates incubated for another 2 h. They were then read with a Spectramax Gemini XS microplate fluorometer at an excitation wavelength of 536 nm and an emission wavelength of 588 nm. The IC50 values were calculated from the sigmoidal inhibition curves with SoftMax Pro software. Podophyllotoxin (P4405, Sigma, Saint Louis, MO, USA) was used as a positive reference.
3.6. Calculation of IC50
To measure antiplasmodial activity, the concentration of extract at which the parasite growth (=[3H]hypoxanthine uptake) was inhibited by 50% (IC50) was calculated by linear interpolation between the two concentrations above and below 50% [36]. To assess leishmanicidal, antitrypanosomal and cytotoxic activity, we transferred data into the graphic SoftMax Pro program (Molecular Devices), which calculated IC50 values from the sigmoidal inhibition curve. The values given in Table 3 and Table 4 are the means of two independent assays.
4. Conclusions
Among the marine macrophytes species tested in the current study against T. brucei rhodesiense, only B. bifurcata showed strong activity. Bio-guided fractionation led to the identification of eleganolone, the main linear diterpene described in this species. Eleganolone showed only mild trypanocidal activity, but better antiplasmodial activity, suggesting trypanocidal activity could be due to minor compounds or to the synergy of several compounds separated during fractionation. Minor compounds of the active fractions will be further characterized by LC-HRMS-Cap NMR.
Acknowledgments
We thank Monica Cal, Sibylle Sax and Christoph Stalder of the Swiss TPH for their assistance with the protozoan assays and Patrick Wehrung and Cyril Antheaume for their excellent technical assistance in the NMR and HREIMS experiments.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization. Working to Overcome the Global Impact of Neglected Tropical Diseases; First WHO Report on Neglected Tropical Diseases. WHO; Geneva, Switzerland: 2010. [Google Scholar]
- 2.Brun R., Blum J., Chappuis F., Burri C. Human African trypanosomiasis. Lancet. 2010;375:148–159. doi: 10.1016/S0140-6736(09)60829-1. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborty K., Lipton A.P., Paulraj R., Chakraborty R.D. Guaiane sesquiterpenes from seaweed Ulva fasciata Delile and their antibacterial properties. Eur. J. Med. Chem. 2010;45:2237–2244. doi: 10.1016/j.ejmech.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 4.Moo-Puc R., Robledo D., Freile-Pelegrin Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J. Ethnopharmacol. 2008;120:92–97. doi: 10.1016/j.jep.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Kubanek J., Jensen P.R., Keifer P.A., Sullards M.C., Collins D.O., Fenical W. Seaweed resistance to microbial attack: A targeted chemical defense against marine fungi. Proc. Natl. Acad. Sci. USA. 2003;100:6916–6921. doi: 10.1073/pnas.1131855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonthron-Sénécheau C., Kaiser M., Devambez I., Vastel A., Mussio I., Rusig A.M. Antiprotozoal activities of organic extracts from French marine seaweeds. Mar. Drugs. 2011;9:922–933. doi: 10.3390/md9060922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spavieri J., Allmendinger A., Kaiser M., Casey R., Hingley-Wilson S., Lalvani A., Guiry M.D., Blunden G., Tasdemir D. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from British and Irish waters. Phytother. Res. 2010;24:1724–1729. doi: 10.1002/ptr.3208. [DOI] [PubMed] [Google Scholar]
- 8.Freile-Pelegrin Y., Robledo D., Chan-Bacab M.J., Ortega-Morales B.O. Antileishmanial properties of tropical marine algae extracts. Fitoterapia. 2008;79:374–377. doi: 10.1016/j.fitote.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Orhan I., Sener B., Atici T., Brun R., Perozzo R., Tasdemir D. Turkish freshwater and marine macrophyte extracts show in vitro antiprotozoal activity and inhibit FabI, a key enzyme of Plasmodium falciparum fatty acid biosynthesis. Phytomedicine. 2006;13:388–393. doi: 10.1016/j.phymed.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Nara T., Kamei Y., Tsubouchi A., Annoura T., Hirota K., Iizumi K., Dohmoto Y., Ono T., Aoki T. Inhibitory action of marine algae extracts on the Trypanosoma cruzi dihydroorotate dehydrogenase activity and on the protozoan growth in mammalian cells. Parasitol. Int. 2005;54:59–64. doi: 10.1016/j.parint.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Spavieri J., Kaiser M., Casey R., Hingley-Wilson S., Lalvani A., Blunden G., Tasdemir D. Antiprotozoal, antimycobacterial and cytotoxic potential of some British green algae. Phytotherapy Res. 2010;24:1095–1098. doi: 10.1002/ptr.3072. [DOI] [PubMed] [Google Scholar]
- 12.Moreau D., Thomas-Guyon H., Jacquot C., Jugé M., Culioli G., Ortalo-Magné A., Piovetti L., Roussakis C. An extract from the brown alga Bifurcaria bifurcata induces irreversible arrest of cell proliferation in a non-small-cell bronchopulmonary carcinoma line. J. Appl. Phycol. 2006;18:87–93. doi: 10.1007/s10811-005-9019-1. [DOI] [Google Scholar]
- 13.Patel A.V., Wright D.C., Romero M.A., Blunden G., Guiry M.D. Molluscicidal polyphenols from species of Fucaceae. Nat. Product Commun. 2008;3:245–249. [Google Scholar]
- 14.Biard J.F., Verbist J.F., Letourneux Y., Floch R. Antimicrobially active diterpene ketols from Bifurcaria bifurcata. Planta Med. 1980;40:288–294. doi: 10.1055/s-2008-1074971. [DOI] [PubMed] [Google Scholar]
- 15.Glombitza K.W., Roesener H.U., Koch M.L. Antibiotics from algae. Part 16. Polyhydroxyoligophenyls and phenyl ether from Bifurcaria bifurcata. Phytochemistry. 1976;15:1279–1281. [Google Scholar]
- 16.Glombitza K.W., Roesener H.U. Antibiotics from algae. XI. Bifuhalol. Diphenylether from Bifurcaria bifurcata. Phytochemistry. 1974;13:1245–1247. [Google Scholar]
- 17.Valls R., Piovetti L., Deffo P. Analysis of sterols and diterpenoids of brown algae (Cystoseiraceae) Oceanis. 1991;17:305–307. [Google Scholar]
- 18.Göthel Q., Lichte E., Köck M. Further eleganolone-derived diterpenes from the brown alga Bifurcaria bifurcata. Tetrahedron Lett. 2012;53:1873–1877. doi: 10.1016/j.tetlet.2011.09.128. [DOI] [Google Scholar]
- 19.Ortalo-Magne A., Culioli G., Valls R., Pucci B., Piovetti L. Polar acyclic diterpenoids from Bifurcaria bifurcata (Fucales, Phaeophyta) Phytochemistry. 2005;66:2316–2323. doi: 10.1016/j.phytochem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Culioli G., Ortalo-Magne A., Daoudi M., Thomas-Guyon H., Valls R., Piovetti L. Trihydroxylated linear diterpenes from the brown alga Bifurcaria bifurcata. Phytochemistry. 2004;65:2063–2069. doi: 10.1016/j.phytochem.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Culioli G., Daoudi M., Ortalo-Magne A., Valls R., Piovetti L. (S)-12-Hydroxygeranyl geraniol-derived diterpenes from the brown alga Bifurcaria bifurcate. Phytochemistry. 2001;57:529–535. doi: 10.1016/s0031-9422(01)00042-5. [DOI] [PubMed] [Google Scholar]
- 22.Culioli G., Mesguiche V., Piovetti L., Valls R. Geranylgeraniol and geranylgeraniol-derived diterpenes from the brown alga Bifurcaria bifurcata (Cystoseiraceae) Biochem. Syst. Ecol. 1999;27:665–668. doi: 10.1016/S0305-1978(98)00126-4. [DOI] [Google Scholar]
- 23.Hougaard L., Anthoni U., Christophersen C., Nielsen P.H. Eleganolone-derived diterpenes from Bifurcaria bifurcata. Phytochemistry. 1991;30:3049–3051. [Google Scholar]
- 24.Hougaard L., Anthoni U., Christophersen C., Nielsen P.H. Two new diterpenoid dihydroxy-γ-butyrolactones from Bifurcaria bifurcata (Cystoseiraceae) Tetrahedron Lett. 1991;32:3577–3578. doi: 10.1016/0040-4039(91)80838-W. [DOI] [Google Scholar]
- 25.Semmak L., Zerzouf A., Valls R., Banaigs B., Jeanty G., Francisco C. Acyclic diterpenes from Bifurcaria bifurcata. Phytochemistry. 1988;27:2347–2349. [Google Scholar]
- 26.Göthel Q., Muñoz J., Köck M. Formyleleganolone and bibifuran, two metabolites from the brown alga Bifurcaria bifurcata. Phytochem. Lett. 2012;5:693–695. doi: 10.1016/j.phytol.2012.06.010. [DOI] [Google Scholar]
- 27.Biard J.F., Verbist J.F., Floch R., Letourneux Y. Epoxyeleganolone and eleganediol, two new diterpenes from Bifurcaria bifurcata Ross (Cystoseiraceae) Tetrahedron Lett. 1980;21:1849–1852. doi: 10.1016/S0040-4039(00)92796-5. [DOI] [Google Scholar]
- 28.Valls R., Piovetti L., Banaig B., Archavlis A., Pellegrini M. (S)-13-hydroxygeranyl geraniol-derived furanoditerpenes from Bifurcaria bifurcata. Phytochemistry. 1995;39:145–149. doi: 10.1016/0031-9422(94)00849-o. [DOI] [PubMed] [Google Scholar]
- 29.Zee O.P., Kim D.K., Choi S.U., Lee C.O., Lee K.R. A new cytotoxic acyclic diterpene from Carpesium divaricatum. Arch. Pharm. Res. 1999;2:225–227. doi: 10.1007/BF02976551. [DOI] [PubMed] [Google Scholar]
- 30.Hellio C., de la Broise D., Dufossé L., le Gal Y., Bourgougnon N. Inhibition of marine bacteria by extracts of macroalgae: Potential use for environmentally friendly antifouling paints. Mar. Environ. Res. 2001;52:231–247. doi: 10.1016/S0141-1136(01)00092-7. [DOI] [PubMed] [Google Scholar]
- 31.Hellio C., Thomas-Guyon H., Culioli G., Piovetti L., Bourgougnon N., le Gal Y. Marine antifoulants from Bifurcaria bifurcata (Phaeophyceae, Cystoseiraceae) and other brown macroalgae. Biofouling J. Bioadhes. Biofilm Res. 2001;17:189–201. doi: 10.1080/08927010109378478. [DOI] [Google Scholar]
- 32.Geraci S., Faimali M., Piovetti L., Cimino G. Antifouling from Nature: Laboratory Test with Balanus Amphitrite Darwin on Algae and Sponges; DSTO Technical Report No. DSTO-GD-0287. Defence Science and Technology Organisation; Melbourne, Australia: 2001. [Google Scholar]
- 33.Scala F., Fattorusso E., Menna M., Taglialatela-Scafati O., Tierney M., Kaiser M., Tasdemir D. Bromopyrrole alkaloids as lead compounds against protozoan parasites. Mar. Drugs. 2010;8:2162–2174. doi: 10.3390/md8072162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thaithong S., Beale G.H. Resistance of ten Thai isolates of Plasmodium falciparum to chloroquine and pyrimethamine by in vitro tests. Trans. R. Soc. Trop. Med. Hyg. 1981;75:271–273. doi: 10.1016/0035-9203(81)90333-3. [DOI] [PubMed] [Google Scholar]
- 35.Buckner F.S., Verlinde C.L., la Flamme A.C., van Voorhis W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freiburghaus F., Kaminsky R., Nkunya M.H.H., Brun R. Evaluation of African medicinal plants for their in vitro trypanocidal activity. J. Ethnopharmacol. 1996;55:1–11. doi: 10.1016/S0378-8741(96)01463-8. [DOI] [PubMed] [Google Scholar]


