Abstract
2,5-bis(3′-Indolyl)pyrroles, analogues of the marine alkaloid nortopsentin, were conveniently prepared through a three step procedure in good overall yields. Derivatives 1a and 1b exhibited concentration-dependent antitumor activity towards a panel of 42 human tumor cell lines with mean IC50 values of 1.54 μM and 0.67 μM, respectively. Investigating human tumor xenografts in an ex-vivo clonogenic assay revealed selective antitumor activity, whereas sensitive tumor models were scattered among various tumor histotypes.
Keywords: bis-indolyl-pyrroles, nortopsentin analogues, marine alkaloids, antitumor, ex-vivo xenografts
1. Introduction
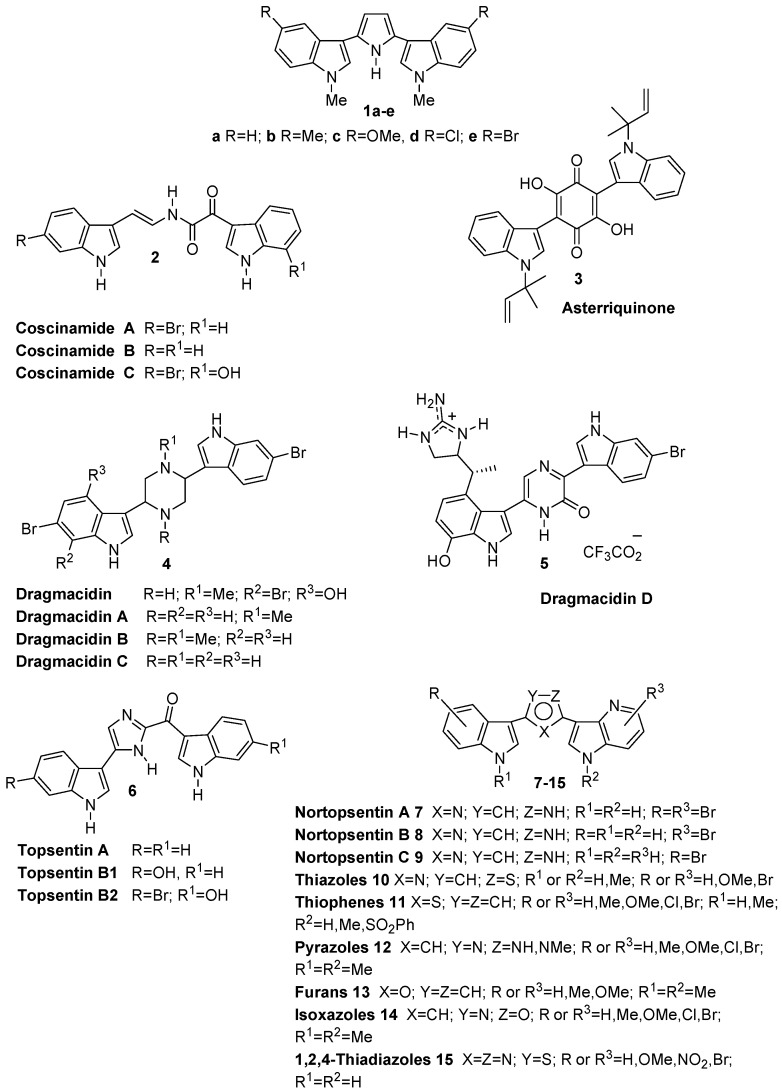
Marine organisms constitute a very important source of biologically active natural products including some of the most potent antineoplastic agents yet discovered [1,2]. In particular, bis-indole alkaloids (Figure 1), characterized by two indole units bound to a spacer through their 3 position, constitute a class of deep-sea sponge metabolites with potent biological activity such as anti-inflammatory, antimicrobial, antiviral and antitumor [3,4,5,6]. Bis-indole alkaloids can bear either an acyclic chain or a six membered carbocyclic or heterocyclic ring or a five membered heterocycle to connect the two indole units. Coscinamides A–C 2, isolated from deep marine sponge Coscinoderma sp. bearing a linear chain as a spacer, showed HIV inhibitory activity [7]. Asterriquinone 3, isolated from Aspergillus fungi and having, as a spacer, a six membered carbocyclic ring showed in vivo activity against Ehrlich carcinoma, ascites hepatoma AH13 and mouse P388 leukemia [8]. The first isolated four dragmacidins 4, containing the six membered heterocyclic link piperazine, were isolated from a large number of deep water sponges including Dragmacidon, Halicortex, Spongosorites, Hexadella and the tunicate Didemnum candidum and showed, among other biological properties, modest cytotoxic activity [9,10,11]. Successively, more complex components of this family such as dragmacidin D 5, having a pyrazinone moiety as a spacer, exhibited several biological properties such as inhibition of serine-threonine protein phosphatases, antiviral, antimicrobial and anticancer activities [12,13].
Figure 1.
bis-Indolyl alkaloids.
Topsentins 6 isolated from Mediterranean sponge Topsentia genitrix exhibited antitumor and antiviral activities [14,15].
Nortopsentins A–C 7–9, bis-indolyl alkaloids having imidazole as a five membered ring spacer, showed in vitro cytotoxicity against P388 cells (IC50 4.5–20.7 μM) and their N-methylated derivatives showed significant improvement in P388 activity compared to that of the parent compounds (IC50 0.8–2.1 μM) [16,17].
Due to the small amounts of biologically active substances extracted from natural material, several total syntheses of Nortopsentins were proposed [18,19,20,21].
Moreover, due to their interesting biological activities, marine alkaloids are considered to be important lead compounds for the discovery of new biologically active compounds. Thus, dragmacidin analogues, bearing the six membered rings pyridine, pyrimidine, pyrazine and pyrazinone as spacer were synthesized. These analogues showed strong inhibitory activity against a wide range of human tumor cell lines [22,23,24,25].
Nortopsentin analogues bearing five membered heterocycles which replaced the imidazole ring of the natural product were synthesized and exhibited remarkable antiproliferative activity, often reaching IC50 values at sub-micromolar level. Thus bis-indolyl-thiazoles 10 [22,26], thiophenes 11 [27], pyrazoles 12 [28], furans 13 [29], isoxazoles 14 [29], and 1,2,4-thiadiazoles 15, were reported [30]. Moreover, in other bis-indolyl analogues, which—apart from the heterocyclic spacer—were also modified, one or both indole units have been described. In particular, 3-indolyl-5-phenylpyridine showed antiproliferative activity in the range 5–15 μM and inhibited CDK1 at 0.3–0.7 μM level [31]; phenylthiazolyl-7-azaindoles showed antiproliferative activity against a wide range of human tumor cell lines at micromolar to nanomolar concentrations and inhibited CDK1 with IC50 values in the range 0.41–0.85 μM [32].
In this paper we report the synthesis of substituted 2,5-bis(3′-indolyl)pyrroles of type 1, nortopsentin analogues in which the imidazole ring spacer of the natural product is replaced by the pyrrole ring. Furthermore, antitumor activity of the new nortopsentin analogues was investigated by an in vitro cytotoxicity assay using human tumor cell lines and an ex-vivo clonogenic assay using human tumor xenograft.
2. Results and Discussion
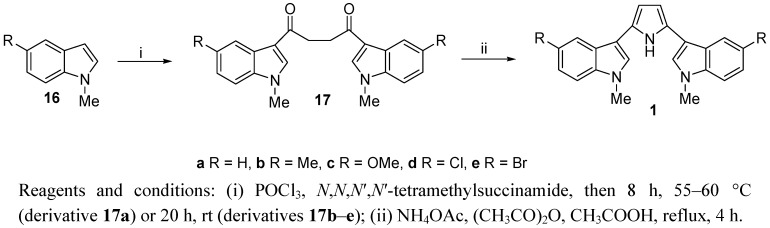
A general synthesis of 2,5-bis(3′-indolyl)pyrroles 1a–e is shown in Scheme 1. N-methyl indoles 16a–e were transformed into 1,4-butanediones 17a–e in good yields by a Vilsmeier-Haack reaction with phosphorus oxychloride and tetramethylsuccinamide [29]. 1,4-Diketones 17a–c were purified by flash chromatography whereas 1,4-diketones 17d,e resulted unstable and were used for the next step as crude products. All symmetrical 1,4-diketones 17 were converted into the corresponding 2,5-bis(3′-indolyl)pyrroles 1a–e using ammonium acetate, acetic anhydride in acetic acid under reflux [33].
Scheme 1.
Synthesis of substituted 2,5-bis(3′-indolyl)pyrroles 1a–e.
By using a monolayer cell survival and proliferation assay the five bis-indolyl-pyrroles 1a–e were screened for in vitro antitumor activity in a panel of 12 human tumor cell lines. All compounds showed cytotoxic activity in at least the highest test concentration of 100 μg/mL, exhibiting mean IC50 values in the range from 4.4 μg/mL to 0.37 μg/mL (Table 1). Adriamycin tested in parallel was used as cytotoxic positive control and showed concentration-dependent anti-cancer activity towards all cell lines.
Table 1.
In vitro activity of derivatives 1a–e towards 12 human cell lines.
| Compound | IC50 (μg/mL) | Active/Total a | Tumor Selectivity b,c | |||
|---|---|---|---|---|---|---|
| 1 (μg/mL) | 10 (μg/mL) | 100 (μg/mL) | A | B | ||
| 1a | 0.37 | 8/12 (67%) | 12/12 (100%) | 12/12 (100%) | 1/12 | + |
| 1b | 0.37 | 8/12 (67%) | 12/12 (100%) | 12/12 (100%) | 1/12 | + |
| 1c | 3.4 | 0/12 (0%) | 10/12 (83%) | 12/12 (100%) | 0/12 | − |
| 1d | 3.4 | 1/12 (8%) | 9/12 (75%) | 12/12 (100%) | 1712 | + |
| 1e | 4.4 | 0/12 (0%) | 9/12 (75%) | 12/12 (100%) | 0/12 | − |
| Adr d | 0.007 | 4/12 (33%) | 10/12 (83%) | 11/12 (92%) | 2/12 | ++ |
a Responsive (T/C < 30%)/total cell lines.
b A = selective (individual IC70 < 1/3 mean IC70)/total cell lines.
c B = rating, − (0/10 selective), + (1/10 selective), ++ (2/10 selective), +++ (≥3/10 selective).
d Adr = Adriamycin, active/total is given at 0.03, 0.3, and 3 μg/mL.
The most active candidates 1a and 1b were further profiled in monolayer cultures of 42 human tumor cell lines, reflecting 15 different solid tumor types (Table 2A).
Table 2.
In vitro and ex vivo anti-tumor activity judged by IC50 values (μM). (A) In vitro tumor cell lines (monolayer assay); (B) Ex-vivo human xenografts (clonogenic assay).
| Cell line | Tumor | |||||||
|---|---|---|---|---|---|---|---|---|
| histotype | name | 1a | 1b | histotype | name | 1a | 1b | |
| Bladder | BXF | 1218L | 0.72 | 0.32 | Bladder | BXF 1218 | 2.45 | 2.35 |
| BXF | 1352L | 0.68 | 0.41 | BXF 1228 | 2.89 | 2.86 | ||
| BXF | T24 | 1.72 | 0.58 | Colon | CXF 1103 | 4.47 | 18.36 | |
| Colon | CXF | 269L | 1.39 | 0.56 | CXF 1729 | 3.49 | 7.77 | |
| CXF | HCT116 | 3.24 | 1.64 | CXF 1783 | 37.57 | 40.70 | ||
| CXF | HT29 | 5.20 | 2.65 | CXF 280 | 26.18 | 35.70 | ||
| CXF | RKO | 1.50 | 0.63 | CXF 975 | 6.93 | 6.03 | ||
| Gastric | GXA | MKN45 | 0.84 | 0.53 | Gastric | GXF 1172 | 6.27 | >100 |
| GXF | 251L | 1.56 | 0.65 | GXF 251 | 7.10 | 25.50 | ||
| Head&Neck | HNXF | CAL27 | 0.81 | 0.50 | GXF 97 | 2.72 | 3.74 | |
| Liver | LIXF | 575L | 1.84 | 0.61 | Head&Neck | HNXF 536 | 2.45 | 2.67 |
| Lung | LXFA | 289L | 6.34 | 2.39 | HNXF 908 | 2.57 | 4.00 | |
| LXFA | 526L | 1.57 | 0.67 | Lung | LXFA 1012 | 4.76 | 27.34 | |
| LXFA | 629L | 2.28 | 1.36 | LXFA 1584 | 2.07 | 2.73 | ||
| LXFL | 1121L | 0.67 | 0.38 | LXFA 297 | 54.90 | >100 | ||
| LXFL | 529L | 1.85 | 0.67 | LXFA 526 | 2.96 | 3.15 | ||
| LXFL | H460 | 2.08 | 0.89 | LXFA 629 | 2.23 | 3.63 | ||
| Mammary | MAXF | 401NL | 1.24 | 0.64 | LXFA 677 | 23.19 | 27.02 | |
| MAXF | MCF7 | 2.44 | 0.98 | LXFA 923 | 19.50 | 25.68 | ||
| MAXF | MDA231 | 1.18 | 0.49 | LXFE 1422 | 5.90 | 1.72 | ||
| Melanoma | MEXF | 1341L | 0.52 | 0.19 | LXFL 1072 | 3.44 | 3.42 | |
| MEXF | 276L | 0.22 | 0.11 | LXFL 529 | 5.07 | 4.46 | ||
| MEXF | 462NL | 1.31 | 0.55 | LXFL 625 | 24.04 | 38.21 | ||
| Ovarian | OVXF | OVCAR3 | 1.46 | 0.58 | Mammary | MAXF 1322 | 16.80 | 9.49 |
| OVXF | 899L | 5.40 | 2.03 | MAXF 1384 | 34.48 | 33.28 | ||
| Pancreatic | PAXF | PANC1 | 0.74 | 0.41 | MAXF 401 | 5.90 | 11.32 | |
| PAXF | 1657L | 2.68 | 1.02 | Melanoma | MEXF 1539 | 19.90 | 15.31 | |
| PAXF | 546L | 2.70 | 1.16 | MEXF 276 | 1.44 | 1.75 | ||
| Prostate | PRXF | 22RV1 | 1.46 | 0.63 | MEXF 462 | 3.24 | 4.10 | |
| PRXF | DU145 | 4.45 | 1.96 | MEXF 989 | 1.18 | 0.58 | ||
| PRXF | LNCAP | 2.10 | 0.68 | Ovarian | OVXF 1353 | 20.96 | 22.90 | |
| PRXF | PC3M | 0.85 | 0.32 | OVXF 899 | 3.54 | 3.93 | ||
| Plerameso- | PXF | 1118L | 2.50 | 0.88 | Pancreatic | PAXF 546 | 3.52 | 14.63 |
| thelioma | PXF | 1752L | 0.89 | 0.43 | PAXF 736 | 2.99 | 2.10 | |
| PXF | 698L | 1.86 | 0.86 | Prostate | PRXF DU145 | 28.67 | 25.98 | |
| Renal | RXF | 1183L | 1.13 | 0.58 | PRXF PC3M | 2.89 | 2.80 | |
| RXF | 1781L | 1.77 | 0.66 | Pleurameso- | PXF 1752L | 3.71 | 4.05 | |
| RXF | 393NL | 3.14 | 1.34 | thelioma | PXF 541 | 2.20 | 0.37 | |
| RXF | 486L | 3.86 | 1.60 | Renal | RXF 1220 | 6.34 | 3.16 | |
| Sarcoma | SXF | SAOS2 | 0.72 | 0.33 | RXF 486 | 2.90 | 3.90 | |
| SXF | TE671 | 1.60 | 0.53 | RXF 631 | 2.98 | 2.81 | ||
| Uterus | UXF | 1138L | 0.72 | 0.35 | Sarcoma | SXF 1186 | 5.71 | 6.17 |
| SXF 1301 | 3.54 | 23.95 | ||||||
| SXF 627 | 3.40 | 4.06 | ||||||
| geometric mean IC50 | 1.54 | 0.67 | geometric mean IC50 | 5.69 | 7.25 | |||
| Tumor selectivity 1) | 8/42 | 5/42 | Tumor selectivity 1) | 9/44 | 14/44 | |||
| (selective/total) | (19%) | (12%) | (selective/total) | (20%) | (32%) | |||
 | ||||||||
1) Number of cell lines/xenografts with IC50 < 1/2 (mean IC50)/total.
Compounds 1a and 1b effected concentration-dependent inhibition of tumor cell growth with mean IC50 values of 1.54 μM and 0.67 μM, indicating pronounced cytotoxic potency.
Regarding compound 1a, selective activity (as defined by individual IC50 value of a distinct cell line <1/2 mean IC50 value over the 42 cell lines) was detected in two out of three cell lines of bladder cancer (BXF 1218L, BXF 1352L), two out of three melanoma cell lines (MEXF 1341L; MEXF 276L), as well as in the cell lines LXFL 1121L (lung cancer), PAXF PANC-1 (pancreatic cancer), SXF SAOS-2 (sarcoma) and UXF 1138L (cancer of the uterine body). Particular less sensitive cell lines were found among colon (HCT-116, HT-29), lung (LXFA 289L), ovarian (OVXF 899L), prostate (DU145) and renal cancer (RXF 393NL, RXF 486L).
Compound 1b exhibited pronounced activity towards cell lines derived from bladder cancer (BXF 1218L), melanoma (MEXF 1341L, MEXF 276L), prostate cancer (PRXF PC3M) and sarcoma (SXF SAOS-2).
Inhibition of clonogenicity of tumor cells was evaluated in additional tumor models using an ex vivo clonogenic assay (Table 2B). The anti-proliferative activity of 1a and 1b was evaluated in cell suspensions prepared from 44 human tumor xenografts of 13 different tumor types, which were cultured as solid tumors in serial passage on immune deficient nude mice. The results confirmed the concentration-dependent activity of 1a and 1b on cell lines with mean IC50 values of 5.69 μM (1a) and 7.25 μM (1b), respectively. With regard to 1a, IC50 values ranged from 1.18 μM to 54.9 μM, corresponding to a 46-fold difference. Selective activity was found against 9 out of the 44 tumors tested, while these sensitive tumors were scattered among various tumor histotypes, like bladder, gastric, head and neck, and lung cancer, as well as melanoma and pleuramesothelioma.
Pronounced tumor selectivity was found for compound 1b, with 14 out of 44 tumors (32%) showing IC50 values <3.6 μM (=1/2 mean IC50 value). IC50 values for 1b ranged from 0.37 μM (PXF 541) to >100 μM (GXF 1172, LXFA 297), equivalent to more than 270-fold difference between resistant and sensitive tumor models. Sensitive tumors were found among bladder, head and neck, lung, pancreatic, prostate and renal cancer as well as melanoma and pleuromesothelioma.
Cells that show anchorage independent growth in semi-solid medium contain, to a certain extent, tumor stem cells which are considered to be responsible for the metastatic and infiltrative potential of a tumor [34,35,36,37]. Thus, the clonogenic assay may inter alia be used to identify candidate tumors for subsequent in vivo studies [34,35,38,39,40]. First in vivo efficacy studies, using the patient-derived melanoma explants MEXF 276 and MEXF 989, did not result in tumor growth inhibition (data not shown). Further in vivo efficacy studies of candidate tumors as selected by the results of the clonogenic assay may be warranted.
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedure
All the commercially available reagents and solvents were used without further purification. 1,2-Diaza-1,3-diene (DD) 13 was synthesized as a mixture of E/Z isomers as previously reported [15,16]. Column chromatography was performed with Merck silica gel 230–400 Mesh ASTM or with Büchi Sepacor chromatography module (prepacked cartridge system). TLC analysis was performed on pre-loaded (0.25 mm) glass supported silica gel plates (Kieselgel 60); compounds were visualized by exposure to UV light and by dipping the plates in 1% Ce(SO4)·4H2O, 2.5% (NH4)6Mo7O24·4H2O in 10% sulfuric acid followed by heating on a hot plate. 1H NMR and 13C NMR spectra were recorded in DMSO-d6 solution on 200 (Bruker AC) MHz instrument. Proton and carbon spectra were referenced internally to solvent signals, using values of δ = 2.49 ppm for proton (middle peak) and δ = 39.50 ppm for carbon (middle peak) in DMSO-d6. The following abbreviations are used to describe peak patterns where appropriate: s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet. All coupling constants (J) are given in Hz. All melting points were taken on a Büchi-Tottoli capillary apparatus and are uncorrected; IR spectra were determined in bromoform or nujol with a Jasco FT/IR 5300 spectrophotometer. Mass spectra were recorded in the EI mode (70 eV) on a Shimadzu GC-MS QP5050A spectrometer. Elemental analyses (C, H, N) were within ±0.4% of the theoretical values.
3.1.2. Synthesis of N,N,N′,N′-Tetramethylsuccinamide
5.3 mL (0.05 mol) of succinyl chloride at 0 °C was added dropwise to a solution of dimethylamine (40% in water, 2 mmol). The mixture was stirred for 30 min and then extracted with DCM, dried and evaporated to afford the pure N,N,N′,N′-tetramethylsuccinamide. Analytical and spectroscopic data are reported elsewhere [21].
3.1.3. General Procedure for the Preparation of 1,4-bis(Indol-3-yl)butane-1,4-diones (17a–e)
Phosphorus oxychloride (5.3 mL, 57 mmol) was slowly added to N,N,N′,N′-tetramethylsuccinamide (2.58 g, 15 mmol) at 10–20 °C and the mixture was stirred for 24 h. Then N-methylindoles 16a–e (30 mmol) were slowly added keeping the temperature below 45 °C. After the addition was complete the mixture was heated for 8 h to 55–60 °C (for derivative 17a) or stirred at rt for 20 h (for derivatives 17b–e). The solution was poured onto crushed ice, made basic with sodium hydroxide 10 M and filtered. The solid was washed with water, dried and purified by chromatography using (DCM/ethyl acetate 9/1) as eluent to afford the pure derivatives 17a–c; whereas derivatives 17d,e were used for next step without purification. Analytical and spectroscopic data are reported elsewhere [29].
3.1.4. General Procedure for the Preparation of Pyrroles (1a–e)
Butanediones 17a–e (2 mmol) was refluxed under N2 for 4 h with ammonium acetate (3.93 g, 51 mmol) and acetic anhydride (1.6 mL, 16.9 mmol) in acetic acid (20 mL). The solution was poured into ice water, and the solid obtained was filtered, dried and purified by chromatography using dichloromethane as eluent.
3.1.4.1. 3,3′-(1H-Pyrrole-2,5-diyl)bis(1-methyl-1H-indole) (1a)
Green solid; yield: 65%; mp: 175 °C; IR 3438 (NH) cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 3.82 (s, 3H), 6.46 (s, 1H), 7.12 (t, 1H, J = 7.2 Hz), 7.21 (t, 1H, J = 7.2 Hz), 7.45 (d, 1H, J = 7.6 Hz), 7.68 (s, 1H), 7.89 (d, 1H, J = 7.6 Hz), 10.80 (s, 1H); 13C NMR (50.3 MHz, DMSO-d6) δ: 32.5 (CH3), 105.3 (CH), 108.7 (C), 109.8 (CH), 119.2 (CH), 119.9 (CH), 121.4 (CH), 125.0 (C), 125.1 (CH), 125.8 (C), 136.8 (C). Anal. Calcd for C22H19N3: C, 81.20; H, 5.89; N, 12.91. Found: C, 80.94; H, 5.53; N, 13.22.
3.1.4.2. 3,3′-(1H-Pyrrole-2,5-diyl)bis(1,5-dimethyl-1H-indole) (1b)
Green solid; yield: 60%; mp: 177–178 °C; IR 3454 (NH) cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 2.45 (s, 3H), 3.80 (s, 3H), 6.43 (s, 1H), 7.03 (d, 1H, J = 8.4 Hz), 7.34 (d, 1H, J = 8.4 Hz), 7.61 (s, 1H), 7.67 (s, 1H), 10.80 (s, 1H); 13C NMR (50.3 MHz, DMSO-d6) δ: 21.3 (CH3), 32.5 (CH3), 105.2 (CH), 108.2 (C), 109.5 (CH), 119.5 (CH), 123.0 (CH), 125.2 (CH), 125.3 (C), 125.8 (C), 127.7 (C), 135.3 (C). Anal. Calcd for C24H23N3: C, 81.55; H, 6.56; N, 11.89. Found: C, 81.26; H, 6.28; N, 11.60.
3.1.4.3. 3,3′-(1H-Pyrrole-2,5-diyl)bis(5-methoxy-1-methyl-1H-indole) (1c)
Green solid; yield: 60%; mp: 148–150 °C; IR 3452 (NH) cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 3.80 (s, 3H), 3.83 (s, 3H), 6.41 (s, 1H), 6.86 (dd, 1H, J = 2.4, 9.0 Hz), 7.30 (d, 1H, J = 2.4 Hz), 7.37 (d, 1H, J = 9.0 Hz), 7.60 (s, 1H), 10.80 (s, 1H); 13C NMR (50.3 MHz, DMSO-d6) δ: 32.7 (CH3), 55.4 (CH3), 101.6 (CH), 105.1 (CH), 108.3 (C), 110.6 (CH), 111.4 (CH), 125.3 (C), 125.7 (C), 125.8 (CH), 132.1 (C), 153.7 (C). Anal. Calcd for C24H23N3O2: C, 74.78; H, 6.01; N, 10.90. Found: C, 74.99; H, 5.75; N, 11.14.
3.1.4.4. 3,3′-(1H-Pyrrole-2,5-diyl)bis(5-chloro-1-methyl-1H-indole) (1d)
Green solid; yield: 50%; mp: 159–160 °C; IR 3444 (NH) cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 3.84 (s, 3H), 6.42 (s, 1H), 7.21 (d, 1H, J = 8.6 Hz), 7.51 (d, 1H, J = 8.6 Hz), 7.72 (s, 1H), 7.83 (s, 1H), 10.90 (s, 1H); 13C NMR (50.3 MHz, DMSO-d6) δ: 32.7 (CH3), 105.7 (CH), 108.4 (C), 111.5 (CH), 118.9 (CH), 121.3 (CH), 124.1 (C), 125.2 (C), 125.9 (C), 127.0 (CH), 135.3 (C). Anal. Calcd for C22H17Cl2N3: C, 67.01; H, 4.35; N, 10.66. Found: C, 66.77; H, 4.08; N, 10.37.
3.1.4.5. 3,3′-(1H-Pyrrole-2,5-diyl)bis(5-bromo-1-methyl-1H-indole) (1e)
Green solid; yield: 50%; mp: 137 °C; IR 3446 (NH) cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 3.84 (s, 3H), 6.41 (s, 1H), 7.32 (dd, 1H, J = 1.9, 8.6 Hz), 7.48 (d, 1H, J = 8.6 Hz), 7.70 (s, 1H), 7.97 (d, 1H, J = 1.9 Hz), 11.00 (s, 1H); 13C NMR (50.3 MHz, DMSO-d6) δ: 32.7 (CH3), 105.8 (CH), 108.3 (C), 112.0 (CH), 112.0 (C), 121.9 (CH), 123.9 (CH), 125.2 (C), 126.6 (C), 126.9 (CH), 135.5 (C). Anal. Calcd for C22H17Br2N3: C, 54.68; H, 3.55; N, 8.70. Found: C, 54.48; H, 3.45; N, 8.33.
3.2. Biology
3.2.1. In Vitro Antitumor Activity towards Permanent Growing Human Tumor Cell Lines
Antitumor activity of the compounds was tested in a monolayer cell survival and proliferation assay using human tumor cell lines. Studies using panels of human tumor cell lines of different origin/histotype allow for analysis of potency and tumor selectivity of test compounds and to identify active compounds that qualify for further preclinical evaluation.
3.2.1.1. Cell Lines
24 out of the 42 cell lines as tested were established at Oncotest from patient-derived human tumor xenografts passaged subcutaneously in nude mice [41]. The origin of the donor xenografts was described [34,42]. The other cell lines were obtained from ATCC (Rockville, MD, USA), DSMZ (Braunschweig, Germany) were kindly provided by the National Cancer Institute (Bethesda, MA, USA). Cells were cultured in RPMI 1640 medium, supplemented with 10% fetal calf serum and 0.1 mg/mL gentamicin under standard conditions (37 °C, 5% CO2). Authenticity of all cell lines was proven by STR analysis at the DSMZ.
3.2.1.2. Cytotoxicity Assay (Monolayer Assay)
A modified propidium iodide assay was used to assess the compounds’ activity toward human tumor cell lines [29]. Briefly, cells were harvested from exponential phase cultures by trypsinization, counted and plated in 96-well flat-bottom microtiter plates at a cell density dependent on the cell line (4000–20,000 cells/well). After 24 h recovery period to allow the cells to adhere and resume exponential growth, test compounds were added at 10 concentrations in half-log increments and left for further 4 days. The inhibition of proliferation was determined by measuring the DNA content using an aqueous propidium iodide solution (7 μg/mL). Fluorescence was measured using the Cytofluor micro-plate reader (excitation λ = 530 nm, emission λ = 620 nm), providing a direct relationship to the total viable cell number. In each experiment, all data points were determined in triplicates. Relative IC50 values were determined by non-linear regression using the analysis software GraphPad Prism® (GraphPad software Inc., La Jolla, CA, USA).
3.2.2. Ex-Vivo Antitumor Activity towards Tumor Xenografts
Effects of the test compounds on clonogenicity of tumor cells were investigated in a clonogenic assay. Tumor xenografts were derived from patient tumors engrafted as a subcutaneously growing tumor in NMRI nu/nu mice obtained from Oncotest’s breeding facility [35,36]. Details of the test procedure have been described earlier [38]. Briefly, solid human tumor xenografts were removed from mice under sterile conditions, mechanically disaggregated and subsequently incubated with an enzyme cocktail consisting of collagenase type IV (41 U/mL), DNase I (125 U/mL), hyaluronidase type III (100 U/mL) and dispase II (1.0 U/mL) in RPMI 1640-Medium at 37 °C for 60 min. Cells were passed through sieves of 200 μm and 50 μm mesh size and washed twice with sterile PBS-buffer. The percentage of viable cells was determined in a Neubauer-hemocytometer using trypan blue exclusion. The assay contained 3 layers of equal volume. The bottom layer consisted of 0.2 mL/well Iscove’s Modified Dulbecco’s Medium (IMDM, Life Technologies), supplemented with 20% (v/v) fetal calf serum (Sigma), 0.01% (w/v) gentamicin (Life Technologies) and 0.75% (w/v) agar (BD Biosciences). 1.5 × 104 to 4 × 104 cells were added to 0.2 mL of the same culture medium supplemented with 0.4% (w/v) agar and plated in 24-multiwell dishes onto the bottom layer. The test compounds were applied by continuous exposure (drug overlay) in 0.2 mL of culture medium. Every dish included six untreated control wells and drug-treated groups in triplicate at 6 concentrations. Cultures were incubated at 37 °C and 7.5% CO2 in a humidified atmosphere for 7–20 days and monitored closely for colony growth using an inverted microscope. Within this period, in vitro tumor growth led to the formation of colonies with a diameter of >50 μm. At the time of maximum colony formation, counts were performed with an automatic image analysis system (Bioreader 5000-Wα, Biosys GmbH). 24 h prior to evaluation, vital colonies were stained with a sterile aqueous solution of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride (1 mg/mL, 100 μL/well). Relative IC50 values were calculated as described in 3.2.1.2.
4. Conclusions
In the present study, the synthesis and characterization of five substituted 2,5-bis(3′-indolyl)pyrroles of type 1, nortopsentin analogues, in which the imidazole ring spacer of the natural product is replaced by the pyrrole ring, was described. Among them, 1a and 1b showed antitumor activity in the low micromolar or even sub-micromolar range towards a panel of human tumor cell lines in vitro. Furthermore, in the ex-vivo clonogenic assay, pronounced tumor selectivity was detected, in particular for 1b, whereas sensitive tumors were scattered among various tumor histotypes.
Acknowledgments
This work was financially supported by Ministero dell’Istruzione dell’Università e della Ricerca (MIUR).
References
- 1.Faulkner D.J. Marine natural products. Nat. Prod. Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- 2.Norcross R.D., Paterson I. Total synthesis of bioactive marine macrolidest. Chem. Rev. 1995;95:2041–2114. doi: 10.1021/cr00038a012. [DOI] [Google Scholar]
- 3.Shin J., Seo Y., Cho K.W., Rho J.R., Sim C.J. New bis(indole)alkaloids of the topsentin class from the sponge Spongosorites genitrix. J. Nat. Prod. 1999;62:647–649. doi: 10.1021/np980507b. [DOI] [PubMed] [Google Scholar]
- 4.Casapullo A., Bifulco G., Bruno I., Riccio R. Hamacanthin classes from the Mediterranean marine sponge Raphisia lacazei. J. Nat. Prod. 2000;63:447–451. doi: 10.1021/np9903292. [DOI] [PubMed] [Google Scholar]
- 5.Bao B., Sun Q., Yao X., Hong J., Lee C., Sim C.J., Jung J.H. Cytotoxic bisindole alkaloids from a marine sponge Spongosorites sp. J. Nat. Prod. 2005;68:711–715. doi: 10.1021/np049577a. [DOI] [PubMed] [Google Scholar]
- 6.Gul W., Hamann M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005;78:442–453. doi: 10.1016/j.lfs.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bokesch H.R., Pannell L.K., McKee T.C., Boyd M.R. Coscinamides A, B, and C, three new bis indole alkaloids from the marine sponge Coscinoderma sp. Tetrahedron Lett. 2000;41:6305–6308. [Google Scholar]
- 8.Shimizu S., Yamamoto Y., Inagaki L., Koshimura S. Antitumor effect and structure-activity relationship of asterriquinone analogs. Gann. 1982;73:642–648. [PubMed] [Google Scholar]
- 9.Kohmoto S., Kashman Y., McConnell O.J., Rinehart K.L., Jr., Wright A., Koehn F. The first total synthesis of Dragmacidin D. J. Org. Chem. 1988;53:3116–3118. [Google Scholar]
- 10.Morris S.A., Andersen R.J. Brominated bis(indole)alkaloids from the marine sponge Hexadella sp. Tetrahedron. 1990;46:715–720. doi: 10.1016/S0040-4020(01)81355-7. [DOI] [Google Scholar]
- 11.Fahy E., Potts B.C.M., Faulkner D.J., Smith K. 6-Bromotryptamine derivatives from the gulf of California Tunicate Didemnum candidum. J. Nat. Prod. 1991;54:564–569. doi: 10.1021/np50074a032. [DOI] [Google Scholar]
- 12.Wright A.E., Pomponi S.A., Cross S.S., McCarthy P. A new bis-(indole)alkaloids from a deep-water marine sponge of the genus Spongosorites. J. Org. Chem. 1992;57:4772–4775. doi: 10.1021/jo00043a045. [DOI] [Google Scholar]
- 13.Capon R.J., Rooney F., Murray L.M., Collins E., Sim A.T.R., Rostas J.A.P., Butler M.S., Carroll A.R. Dragmacidins: New protein phosphatase inhibitors from a Southern Australian deep-water Marine Sponge Spongosorites sp. J. Nat. Prod. 1998;61:660–662. doi: 10.1021/np970483t. [DOI] [PubMed] [Google Scholar]
- 14.Bartik K., Braekman J.-C., Daloze D., Stoller C. Topsentin, new toxic bis-indole alkaloids from the marine sponge Topsentia genitrix. Can. J. Chem. 1987;65:2118–2121. doi: 10.1139/v87-352. [DOI] [Google Scholar]
- 15.Gunasekera S.P., Kashman Y., Cross S.S., Lui M.S., Pomponi S.A., Diaz M.C. Topsentin, bromotopsentin, and dihydrodeoxybromotopsentin: Antiviral and antitumor bis(indolyl)imidazoles from Caribbean deep-sea sponge of the family Halichondriidae. Structural and synthetic studies. J. Org. Chem. 1988;53:5446–5453. doi: 10.1021/jo00258a009. [DOI] [Google Scholar]
- 16.Alvarez M., Salas M. Marine, nitrogen-containing heterocyclic natural products-structures and syntheses of compounds containing indole units. Heterocycles. 1991;32:1391–1429. doi: 10.3987/REV-91-429. [DOI] [Google Scholar]
- 17.Sakem S., Sun H.H. Nortopsentins A, B and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991;56:4304–4307. [Google Scholar]
- 18.Kawasaki I., Yamashita M., Ohta S. Total Synthesis of Nortopsentins A–D, marine alkaloids. Chem. Pharm. Bull. 1996;44:1831–1839. doi: 10.1248/cpb.44.1831. [DOI] [Google Scholar]
- 19.Moody C.J., Roffey J.R.A. Synthesis of N-protected Nortopsentins B and D. Arkivoc. 2000;1:393–401. [Google Scholar]
- 20.Miyake F.Y., Yakushijin K., Horne D.A. A concise synthesis of Topsentin A and Nortopsentin B and D. Org. Lett. 2000;2:2121–2123. doi: 10.1021/ol000124g. [DOI] [PubMed] [Google Scholar]
- 21.Fresneda P.M., Molina P., Sanz M.A. Microwave-assisted regioselective synthesis of 2,4-disubstituted imidazoles: Nortopsentin D synthesized by minimal effort. Synlett. 2001;2:218–221. [Google Scholar]
- 22.Jiang B., Gu X.-H. Syntheses and cytotoxicity evaluation of bis(indolyl)thiazole, bis(indolyl)pyrazinone and bis(indolyl)pyrazine: Analogues of cytotoxic marine bis(indole) alkaloid. Bioorg. Med. Chem. 2000;8:363–371. doi: 10.1016/S0968-0896(99)00290-4. [DOI] [PubMed] [Google Scholar]
- 23.Jiang B., Yang C.G., Xiong C., Wang J. Synthesis and cytotoxicity evaluation of novel indolylpyrimidines and indolylpyrazines as potential antitumor agents. Bioorg. Med. Chem. 2001;9:1149–1154. doi: 10.1016/S0968-0896(00)00337-0. [DOI] [PubMed] [Google Scholar]
- 24.Jiang B., Xiong W.-N., Yang C.-G. Synthesis and antitumor evaluation of novel monoindolyl-4-trifluoromethylpyridines and bisindolyl-4-trifluoromethylpyridines. Bioorg. Med. Chem. Lett. 2001;11:475–477. doi: 10.1016/S0960-894X(00)00704-6. [DOI] [PubMed] [Google Scholar]
- 25.Xiong W.-N., Yang C.-G., Jiang B. Synthesis of novel analogues of marine indole alkaloids: Mono(indolyl)-4-trifluoromethylpyridines and bis(indolyl)-4-trifluoromethylpyridines as potential anticancer agents. Bioorg. Med. Chem. 2001;9:1773–1780. doi: 10.1016/S0968-0896(01)00070-0. [DOI] [PubMed] [Google Scholar]
- 26.Gu X.-H., Wan X.-Z., Jiang B. Syntheses and biological activities of bis(3-indolyl)thiazoles, analogues of marine bis(indole)alkaloid nortopsentin. Bioorg. Med. Chem. Lett. 1999;9:569–572. doi: 10.1016/S0960-894X(99)00037-2. [DOI] [PubMed] [Google Scholar]
- 27.Diana P., Carbone A., Barraja P., Montalbano A., Martorana A., Dattolo G., Gia O., Dalla Via L., Cirrincione G. Synthesis and antitumor properties of 2,5-bis(3′-indolyl)thiophenes: Analogues of marine alkaloid nortopsentin. Bioorg. Med. Chem. Lett. 2007;17:2342–2346. doi: 10.1016/j.bmcl.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 28.Diana P., Carbone A., Barraja P., Martorana A., Gia O., Dalla Via L., Cirrincione G. 3,5-Bis(3′-indolyl)pyrazoles, analogues of marine alkaloid nortopsentin: Synthesis and antitumor properties. Bioorg. Med. Chem. Lett. 2007;17:6134–6137. doi: 10.1016/j.bmcl.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 29.Diana P., Carbone A., Barraja P., Kelter H.H., Fiebig G., Cirrincione G. Synthesis and antitumor activity of 2,5-bis(3′-indolyl)-furans and 3,5-bis(3′-indolyl)-isoxazoles, nortopsentin analogues. Bioorg. Med. Chem. 2010;18:4524–4529. doi: 10.1016/j.bmc.2010.04.061. [DOI] [PubMed] [Google Scholar]
- 30.Kumar D., Kumar N.M., Chang K.-H., Gupta R., Shah K. Synthesis and in-vitro anticancer activity of 3,5-bis(indolyl)-1,2,4-thiadiazoles. Bioorg. Med. Chem. Lett. 2011;21:5897–5900. doi: 10.1016/j.bmcl.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 31.Jacquemard U., Dias N., Lansiaux A., Bailly C., Logé C., Robert J.M., Lozach O., Meijer L., Merour J.Y., Routier S. Synthesis of 3,5-bis(2-indolyl)pyridine and 3-[(2-indolyl)-5-phenyl]pyridine derivatives as CDK inhibitors and cytotoxic agents. Bioorg. Med. Chem. 2008;16:4932–4953. doi: 10.1016/j.bmc.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Diana P., Carbone A., Barraja P., Montalbano A., Parrino B., Lopergolo A., Pennati M., Zaffaroni N., Cirrincione G. Synthesis and antitumor activity of 3-(2-phenyl-1,3-thiazol-4-yl)-1H-indoles and 3-(2-phenyl-1,3-thiazol-4-yl)-1H-7-azaindoles. ChemMedChem. 2011;6:1300–1309. doi: 10.1002/cmdc.201100078. [DOI] [PubMed] [Google Scholar]
- 33.Johnson M.R. Synthesis of Bronzaphyrin NS. J. Org. Chem. 1997;62:1168–1172. doi: 10.1021/jo961849u. [DOI] [Google Scholar]
- 34.Park C.H., Bergsagel D.E., McCulloch E.A. Mouse myeloma tumor stem cells: A primary cell culture assay. J. Natl. Cancer Inst. 1971;46:411–422. [PubMed] [Google Scholar]
- 35.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 36.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beachy P.A., Karhadkar S.S., Berman D.M. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 38.Fiebig H.H., Berger D.P., Dengler W.A., Wallbrecher E., Winterhalter B.R. Combined in vitro/in vivo test procedure with human tumor xenografts. In: Fiebig H.H., Berger D.P., editors. Immunodeficient Mice in Oncology. Karger; Basel, Switzerland: 1992. pp. 321–351. (Contributions to Oncology Volume 42). [Google Scholar]
- 39.Dengler W.A., Schulte J., Berger D.P., Mertelsmann R., Fiebig H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anti-Cancer Drugs. 1995;6:522–532. doi: 10.1097/00001813-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Fiebig H.H., Maier A., Burger A.M. Clonogenic assay with established human tumour xenografts: Correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur. J. Cancer. 2004;40:802–820. doi: 10.1016/j.ejca.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Roth T., Burger A.M., Dengler W., Willmann H., Fiebig H.H. Human tumor cell lines demonstrating the characteristics of the patient tumors as useful models for anticancer drug screening. In: Fiebig H.H., Burger A.M., editors. Relevance of Tumor Models for Anticancer Drug Development. Karger; Basel, Switzerland: 1999. pp. 145–156. (Contributions to Oncology Volume 54). [Google Scholar]
- 42.Fiebig H.H., Dengler W.A., Roth T. Human Tumor Xenografts: Predictivity, Characterization, and Discovery of New Anticancer Agents. In: Fiebig H.H., Burger A.M., editors. Relevance of Tumor Models for Anticancer Drug Development. Karger; Basel, Switzerland: 1999. pp. 29–50. (Contributions to Oncology Volume 54). [Google Scholar]