Abstract
Seven new polyoxygenated steroids (1–7) were isolated together with seven known analogues (8–14) from the South China Sea soft coral, Sarcophyton sp. The structures of the new compounds were identified on the basis of extensive spectroscopic analysis and comparison with reported data. All the steroids are characterized with 3β,5α,6β-hydroxy moiety, displaying carbon skeletons of cholestane, ergostane, gorgostane and 23,24-dimethyl cholestane. In the in vitro bioassay, metabolites exhibited different levels of antimicrobial activity against bacterial species Escherichia coli and Bacillus megaterium, and fungal species Microbotryum violaceum and Septoria tritici. No inhibition was detected towards microalga Chlorella fusca. Preliminary structure-activity analysis suggests that the 11α-acetoxy group may increase both antibacterial and antifungal activities. The terminal-double bond and the cyclopropane moiety at the side chain may also contribute to the bioactivity.
Keywords: Sarcophyton sp., steroid, bioactivity, antibacterial, antifungal
1. Introduction
Soft corals are thought to produce various bioactive metabolites that chemically defend themselves from attack [1,2,3]. The soft coral Sarcophyton species (order Alcyonacea, family Alcyoniidae) are prolific in the South China Sea and are dominant in many coral reef areas [4]. Chemical research on the animals of this genus has established that it is a rich resource of steroids, diterpenes and tetraterpenes [5,6].
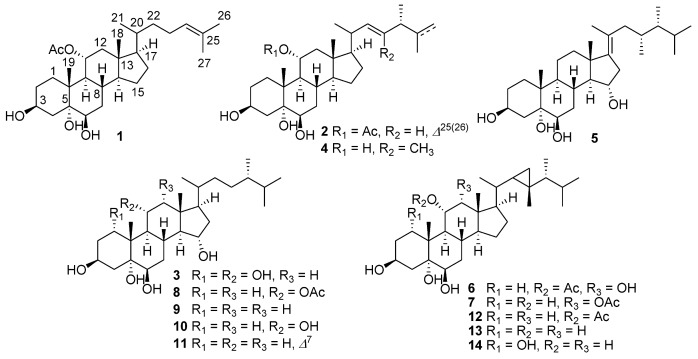
In the course of our ongoing screening for bioactive secondary metabolites from marine sources [7,8,9,10,11], we have made a collection of Sarcophyton sp. off Weizhou Island, Guangxi Province, China. Chemical investigation on the Et2O-soluble fraction of the acetone extract from Sarcophyton sp. resulted in the isolation of fourteen steroids (1–14, Figure 1) with 3β,5β,6β-hydroxy moiety. The sterols can be re-sorted into four clusters, due to their different carbon skeletons, namely cholesterol-, ergosterol-, gorgosterol- and 23,24-dimethyl cholesterol-types, displaying an excellent example of chemical diversity. The isolates were tested for in vitro antimicrobial activity against bacteria, fungi and a microalga. Here, we describe the isolation, structural elucidation and bioactivity of these new metabolites.
Figure 1.
The chemical structures of sterols 1–14.
2. Results and Discussion
Freshly collected specimens of Sarcophyton sp. were kept at −20 °C before extraction. The acetone extract of the soft coral was partitioned between Et2O and H2O. Purification of the Et2O extract, by repeated column chromatography on silica and Sephadex LH-20 and followed by reversed-phase semi-preparative HPLC, yielded the pure compounds shown in 1–14 (Figure 1).
On the basis of detailed spectroscopic analysis and comparison with reported data, the structures of the known compounds were readily determined as (24S)-11α-acetoxy-ergostane-3β,5α,6β-triol (8, sarcoaldosterol A) [12,13,14], (24S)-ergostane-3β,5α,6β-triol (9) [15], (24S)-ergostane-3β,5α,6β,11α-tetraol (10) [16], (24S)-ergostane-7-en-3β,5α,6β-triol (11) [16], 11α-acetoxy-gorgostane-3β,5α,6β-triol (12) [17], gorgostane-3β,5α,6β,11α-tetraol (13, sarcoaldosterol B) [13,18] and gorgostane-1α,3β,5α,6β,11α-pentaol (14) [19,20,21]. Sarcoaldosterols A (8)and B (13) were first isolated from the Okinawan soft coral Sarcophyton sp. [13]. Later on, sarcoaldosterol A was obtained from the soft corals of genus Heteroxenia collected from the Philippines [12] and the Red Sea [14], while sarcoaldosterol B was re-obtained from the Vietnamese sea soft coral, S. mililatensis [18]. Compound 9 was reported from the gorgonian Isis hippuris [15], a known source of gorgosteroids. Ergosteroids 10 and 11 were first isolated together from the Palauan soft coral, Lobophytum cf. pauciflorum [16], and then compound 12 was re-isolated from soft coral S. crassocaule [21], gorgonian Plexaurella grisea [20] and Junceella juncea [19]. The gorgosteroid 12 was recently reported from Egyptian Red Sea soft coral Heteroxenia ghardaqensis [17], while 14 was repeatedly reported from the scallop Patinopecten yessoensis [22] and the sponge Spongionella gracilis [23] and Dysidea fragilis [24].
Compound 1 was isolated as an optically active, white amorphous solid. The molecular formula was established as C29H48O5 by high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) from the pseudo-molecular ion at m/z 499.3397 [M + Na]+, indicating six degrees of unsaturation. The infrared (IR) absorption at 3408 cm−1 and 1712 cm−1 showed the presence of hydroxyl and carbonyl groups in the molecule. This observation was in agreement with the signals in the 13C nuclear magnetic resonance (NMR) and distortionless enhancement by polarization transfer (DEPT0 spectra (Table 1) for 3 sp2 carbon atoms (1 × OC=O, CH=C) at a lower field and 26 sp3 carbon atoms at a higher field (1 × OC, 3 × OCH, 2 × C, 5 × CH, 9 × CH2, 6 × CH3), accounting for two degrees of unsaturation. The remaining degrees of unsaturation were attributed to the presence of four rings in the molecule.
Table 1.
The 13C nuclear magnetic resonance (NMR) data of sterols 1–7 (100 a or 125 b MHz in CDCl3).
| Carbon | 1 a | 2 a | 3 b | 4 a | 5 a | 6 a | 7 a |
|---|---|---|---|---|---|---|---|
| 1 | 33.5 t | 33.5 t | 76.8 d | 34.4 t | 32.3 t | 33.7 t | 33.9 t |
| 2 | 31.3 t | 31.3 t | 37.2 t | 31.2 t | 30.9 t | 31.3 t | 31.2 t |
| 3 | 67.2 d | 67.2 d | 64.2 d | 67.4 d | 67.6 d | 67.1 d | 67.5 d |
| 4 | 41.3 t | 41.3 t | 41.3 t | 41.0 t | 40.8 t | 41.3 t | 41.3 t |
| 5 | 76.6 s | 76.6 s | 76.8 s | 76.7 s | 76.7 s | 76.6 s | 76.7 s |
| 6 | 76.1 d | 76.1 d | 75.2 d | 76.1 d | 76.1 d | 76.0 d | 76.0 d |
| 7 | 34.8 t | 34.9 t | 35.2 t | 34.5 t | 34.8 t | 34.4 t | 34.0 t |
| 8 | 29.4 d | 29.4 d | 29.4 d | 29.1 d | 28.9 d | 29.4 d | 28.9 d |
| 9 | 48.6 d | 48.7 d | 46.6 d | 53.0 d | 45.9 d | 42.7 d | 46.3 d |
| 10 | 39.9 s | 39.9 s | 42.1 s | 40.0 s | 38.4 s | 39.6 s | 39.6 s |
| 11 | 71.1 d | 71.1 d | 68.0 d | 68.7 d | 21.5 t | 73.4 d | 70.7 d |
| 12 | 46.4 t | 46.3 t | 50.9 t | 51.8 t | 37.9 t | 75.0 d | 80.5 d |
| 13 | 42.9 s | 42.7 s | 43.2 s | 43.1 s | 44.7 s | 46.7 s | 46.0 s |
| 14 | 54.6 d | 54.7 d | 54.7 d | 55.1 d | 52.2 d | 45.3 d | 47.4 d |
| 15 | 24.2 t | 24.2 t | 24.2 t | 24.2 t | 72.2 d | 23.9 t | 23.8 t |
| 16 | 28.2 t | 28.5 t | 28.2 t | 28.0 t | 34.4 t | 27.5 t | 27.6 t |
| 17 | 56.0 d | 55.9 d | 55.8 d | 56.7 d | 149.0 s | 49.2 d | 50.1 d |
| 18 | 12.7 q | 12.9 q | 12.8 q | 13.4 q | 17.3 q | 11.8 q | 12.7 q |
| 19 | 16.8 q | 16.8 q | 16.3 q | 17.0 q | 16.8 q | 16.7 q | 16.7 q |
| 20 | 35.4 d | 39.8 d | 36.1 d | 34.5 d | 133.0 s | 35.1 d | 34.6 d |
| 21 | 18.6 q | 20.7 q | 18.9 q | 20.6 q | 17.1 q | 20.1 q | 20.6 q |
| 22 | 35.9 t | 135.5 d | 33.6 t | 131.3 d | 42.0 t | 31.9 d | 31.9 d |
| 23 | 24.7 t | 131.8 d | 30.6 t | 135.7 s | 32.7 d | 25.9 s | 25.9 s |
| 24 | 125.1 d | 43.6 d | 39.1 d | 50.2 d | 44.2 d | 50.8 d | 50.8 d |
| 25 | 131.1 s | 149.8 s | 31.5 d | 30.8 d | 30.1 d | 32.0 d | 32.0 d |
| 26 | 17.6 q | 108.8 t | 20.5 q | 21.7 q | 21.6 q | 21.5 q | 21.5 q |
| 27 | 25.7 q | 20.6 q | 17.6 q | 20.1 q | 19.1 q | 22.2 q | 21.3 q |
| 28 | 18.9 q | 15.5 q | 17.0 q | 11.5 q | 15.4 q | 15.5 q | |
| 29 | 13.3 q | 13.8 q | 14.3 q | 14.3 q | |||
| 30 | 21.3 t | 21.3 t | |||||
| COOCH3 | 22.1 q | 22.1 q | 22.1 q | 22.2 q | |||
| COOCH3 | 170.4 s | 170.4 s | 170.0 s | 170.4 s |
s = singlet; d = doublet, t = triplet; q = quartet.
The NMR spectra of compound 1 were closely related to those of co-occurring (24S)-11α-acetoxy-ergostane-3β,5α,6β-triol (8), displaying identical signals for rings A, B, C and D. A difference was observed in the nature of the side chain. The methyls of the isopropyl group (δH 0.84, 3H, d, J = 6.8 Hz, H3-27; 0.76, 3H, d, J = 6.8 Hz, H3-27) in 8 were replaced by two vinyl methyls (δ 1.59 and 1.67, each 3H, s) in 1 (Table 2). In correspondence, the doublet methyl signal of H3-28 (δ 0.76, 3H, d, J = 6.7 Hz) in 8 was replaced by a trisubstituted double bond linked to a methylene (δ 5.07, 1H, br t, J = 7.2 Hz) in 1, indicating a ∆24 cholestane side chain instead of an ergostane side chain in the molecule. These data led 1 to the structure of 11α-acetoxy-cholesta-24-en-3β,5α,6β-triol. The 1H NMR and 13C NMR assignments of the side chain were identical to those of reported data [25,26,27] and were further confirmed by the proton sequence from H3-21 to H-24, as established by the 1H-1H correlation spectroscopy (COSY) experiment and the heteronuclear multiple bond (HMBC) correlation from H2-26 and H3-27 to both C-25 and C-24.
Table 2.
The 1H NMR data of sterols 1–4 (400 a or 500 b MHz in CDCl3; J in Hz).
| Position | 1 a | 2 a | 3 b | 4 a |
|---|---|---|---|---|
| 1 | 1.26 (m), 1.81( m) | 1.22 (m), 1.76 (m) | 4.11 (br s) | 1.50 (m), 1.71 (m) |
| 2 | 1.47 (m), 1.82 (m) | 1.53 (m), 1.77 (m) | 1.86 (m), 2.18 (m) | 1.56 (m), 1.86 (m) |
| 3 | 4.05 (m) | 4.05 (m) | 4.40 (m) | 4.07 (m) |
| 4 | 1.62 (ov), 2.07 (t, 12.2) | 1.58 (ov), 2.08 (d, 11.8) | 1.77 (ov), 2.08 (ov) | 1.67 (ov), 2.11 (ov) |
| 6 | 3.52 (br s) | 3.52 (br s) | 3.51 (br s) | 3.53 (br s) |
| 7 | 1.61 (m), 1.76 (m) | 1.58 (m), 1.74 (m) | 1.52 (m), 1.88 (m) | 1.73 (m), 2.08 (m) |
| 8 | 1.87 (m) | 1.76 (m) | 1.88 (m) | 1.76 (m) |
| 9 | 1.74 (m) | 1.82 (m) | 1.99 (m) | 1.34 (m) |
| 11 | 5.14 (dd, 10.3, 5.4) | 5.14 (dd, 10.5, 5.4) | 4.01 (11.0, 5.5) | 3.94 (9.7, 4.9) |
| 12 | 1.16 (ov) | 1.18 (ov) | 1.26 (ov) | 1.19 (ov) |
| 2.31 (dd, 12.2, 5.4) | 2.30 (dd, 12.1, 5.4) | 2.33 (dd, 11.5, 5.5) | 2.34 (11.5, 4.9) | |
| 14 | 1.28 (m) | 1.18 (m) | 1.19 (m) | 1.16 (m) |
| 15 | 1.06 (m), 1.56 (m) | 1.07 (m), 1.79 (m) | 1.09 (m), 1.60 (m) | 1.03 (m), 1.59 (m) |
| 16 | 1.20 (m), 1.88 (m) | 1.26 (m), 1.50 (m) | 1.26 (m), 1.90 (m) | 1.19 (m), 1.78 (m) |
| 17 | 1.15 (m) | 1.18 (m) | 1.19 (m) | 1.19 (m) |
| 18 | 0.73 (s) | 0.75 (s) | 0.66 (s) | 0.73 (s) |
| 19 | 1.28 (s) | 1.28 (s) | 1.21 (s) | 1.32 (s) |
| 20 | 1.38 (m) | 2.00 (m) | 1.37 (m) | 2.30 (m) |
| 21 | 0.90 (d, 6.5) | 0.98 (d, 6.6) | 0.94 (d, 6.5) | 0.92 (d, 6.9) |
| 22 | 1.02 (m), 1.39 (m) | 5.26 (dd, 15.4, 6.2) | 0.89 (m), 1.39 (m) | 4.87 (d, 9.5) |
| 23 | 1.87 (m), 1.99 (m) | 5.20 (dd, 15.4, 7.3) | 0.89 (m), 1.38 (m) | |
| 24 | 5.07 (t, 7.2) | 2.69 (m) | 1.19 (m) | 1.67 (m) |
| 25 | 1.60 (m) | 1.53 (m) | ||
| 26 | 1.59 (s) | 4.70 (br s) | 0.86 (d, 7.0) | 0.84 (d, 6.5) |
| 27 | 1.67 (s) | 1.67 (s) | 0.79 (d, 7.0) | 0.78 (d, 6.5) |
| 28 | 1.07 (d, 6.9) | 0.78 (d, 7.0) | 0.93 (d, 7.1) | |
| 29 | 1.50 (s) | |||
| COO CH3 | 1.98 (s) | 1.98 (s) |
s = singlet; d = doublet; t = triplet; m = multiplet; br s = broad singlet; dd = doublet of doublets; ov = overlapped signals.
Compound 2 was obtained as an optically active, amorphous powder. Its formula was established as C30H48O5 by HR-ESI-MS. The NMR data of 2 also resembled those of 8, with a recognized difference in the signals assigned to the side chains. The methyls of the isopropyl group (δH 0.84, 3H, d, J = 6.8 Hz, H3-27; 0.76, 3H, d, J = 6.8 Hz, H3-27) in 8 were replaced by a vinyl methyl (δ 1.67, 3H, s) and a terminal double bond (δ 4.70, 2H, br s) in 2 (Table 2), leading to the assignment of the double bond as ∆25. In addition, a pair of signals for a disubstituted double bond were observed (δ 5.26, 1H, dd, J = 15.4, 6.2 Hz; δ 5.20, 1H, dd, J = 15.4, 7.3 Hz). The large coupling constant between the olefinic protons indicated an E geometry of the double bond, which was consequently assigned as ∆22. The marked-down field shift of H3-21 and H3-28 from δ 0.87 and 0.76 in 8 to δ 0.98 and 1.07 in 2 supported the above conclusion. Further structural evidence came from the proton sequence from H3-21 to H3-27, as established by the 1H-1H COSY experiment and the HMBC correlation from H3-28 to C-23, C-24 and C-25, H2-26 and H3-27 to both C-25 and C-24. The 1H NMR assignments of the side chain were identical to those of (22E,24S)-ergostane-5,22,25-trien-3β-ol, isolated from the plant, Clerodendrum fragrans (Verbenaceae family) [28], which further confirmed the established structure and suggested an S configuration at C-24. These lines of evidence led to the structure (22E,24S)-11α-acetoxy-ergostane-22,25-dien-3β,5α,6β-triol for compound 2.
Compound 3 was also obtained as an optically active, amorphous powder. The HR-ESI-MS of compound 3 exhibited a pseudo-molecular ion peak at m/z 489.4582 [M + Na]+, leading to the determination of the molecular formula as C28H50O5. Analysis of the NMR spectra of 3 (Table 1 and Table 2) revealed a remarkable similarity to those of the co-occurring sterol 10, except for the replacement of a methylene group (δH 1.49, 1H, m, H-1α 1.73, 1H, m, H-1β; δC 34.4, t) in 10 by a secondary hydroxyl group (δH 4.11, 1H, br s; δC 76.8, d) in 3. This hydroxyl group was assigned at C-1, due to the proton connectivity from H-1 to H2-4, as established by the 1H-1H COSY spectrum and the obvious HMBC correlations from H3-19 to C-1, C-5, C-9 and C-10. A β configuration of H-1 was deduced from its coupling constant pattern (br s) and supported by the observation of NOE interactions between H3-19 and H-1. A comparison of the NMR data for the core structure of 3 with those of co-isolated gorgostane-1α,3β,5α,6β,11α-pentaol (14) [22,23,24] further confirmed the established structure. The absolute configuration at C-24 was determined as S, due to the 1H NMR shift values of H3-21 (δ 0.936, s), H3-27 (δ 0.790, s) and H3-26 (δ 0.856, s), similar to those reported for 8–11 [13,16,18,19,20,21], instead of those for the C-24 epimer (δ 0.925, s, H3-21; 0.805, s, H3-27; 0.853, s, H3-26) [23]. The structure of 3 was evidently established as (24S)-ergostane-1α,3β,5α,6β,11α-pentaol.
Furthermore, compound 4 was also obtained as an optically active, amorphous powder. Its formula C29H50O4 was deduced from the molecular ion at m/z 485.3532 [M + Na]+ in the HR-ESI-MS spectrum. A comparison of the 1H and 13C NMR spectra of 4 (Table 1 and Table 2) and the co-isolated 10 revealed a great similarity with the exception of an appearance of a trisubstituted double bond (δH 4.87, d, J = 9.5 Hz; δC 131.3, d, 135.7, s) and an additional vinylic methyl group (δH 1.50, s) in the side chain. The double bond was placed at C-22, due to the proton spin system of H3-21/H-20/H-22 and the long-range correlations of H3-29/C-22, C-23 and C-24, as deduced from the 1H-1H COSY and the HMBC spectra, respectively. The downfield shift of H3-28 from δ 0.77 in 10 to δ 0.93 in 4 supported the above conclusion. The 1H NMR and 13C NMR assignments of the side chain were identical to those of model compounds [29,30,31,32,33,34]. An S absolute stereochemistry at C-24 in 4 was proposed by careful comparison of 1H NMR data with those of in dinosterol and its C-24 epimer [31], where the 1H signals for H3-21 (δ 0.921, d, J = 6.9 Hz)), H3-27 (δ 0.779, d, J = 6.8 Hz) and H3-26(δ 0.836, d, J = 6.5 Hz) were close to those of dinosterol (δ 0.919, d, J = 6.5 Hz, H3-21; 0.778, d, J = 6.5 Hz, H3-26; 0.837, d, J = 6.5 Hz, H3-27) rather than its C-24 epimer (δ 0.910, d, J = 6.8 Hz, H3-21; 0.772, d, J = 6.8 Hz, H3-26; 0.848, d, J = 6.5 Hz, H3-27). Therefore, the structure of 4 is (24S)-23,24-dimethylcholesta-22-en-3β,5α,6β,11α-tetraol.
Compound 5, an optically active, amorphous powder, had the same molecular formula (C29H50O4) as that of 4, as deduced by HR-ESI-MS. Its NMR spectra also showed the characteristic signals for the 3β,5α,6β-trihydroxyl moiety. However, the trisubstituted double bond (δH 4.87, d, J = 9.5 Hz; δC 131.3, d, 135.7, s) in 4 was replaced by a tetrasubstituted double bond (δ 149.0, s; 133.0, s) in 5 (Table 1 and Table 3). The double bond was readily assigned as ∆17(20), due to the observation of the vinyl methyl singulate for H3-21 (δ 1.71, s) and its HMBC correlation with C-17, C-20 and C-22, and the HMBC correlation from H3-18 to C-17 provided further evidence. Meanwhile, the secondary alcohol at C-11 had to be assigned to C-15, due to the proton sequence from H-9 to H2-12 and from H-14 to H2-16, as established by the 1H-1H COSY experiment. The location of 15-OH was confirmed by the HMBC correlation from H-15 to both C-13 and C-17. The diagnostic interactions between H3-18 and H-15 in the NOESY spectrum indicated a β configuration of the proton. The R configuration at both C-23 and C-24 were suggested to be the same, biogenetically, as those in sarcosterol, 25-hydroxy sarcosterol and peridinosterol, produced by the soft corals, S. glaucum [35] and Sinularia mayi [36] and the symbiotic dinoflagellate [37], respectively. Biosynthetic studies suggested the same C-23 stereochemistry in sarcosterol and peridinosterol, due to the symbiotic relationship of soft coral and dinoflagellate [36]. The structure of compound 5 was tentatively determined to be (23R,24R)-23,24-dimethylcholesta-17(20)-en-3β,5α,6β-triol.
Table 3.
The 1H NMR data of sterols 5–8 (400 a or 500 b Hz in CDCl3; J values are in Hz).
| Position | 5 a | 6 a | 7 a |
|---|---|---|---|
| 1 | 1.43 (m), 1.58 (m) | 1.27 (m), 1.80 (m) | 1.63 (m), 2.16 (m) |
| 2 | 1.55 (m), 1.82 (m) | 1.52 (m), 1.81 (m) | 1.47 (m), 1.85 (m) |
| 3 | 4.11 (m) | 4.06 (m) | 4.05 (m) |
| 4 | 1.63 (ov), 2.09 (ov) | 1.61 (ov), 2.10 (ov) | 1.63 (ov), 2.15 (ov) |
| 6 | 3.59 (br s) | 3.52 (br s) | 3.54 (br s) |
| 7 | 1.50 (m), 1.69 (m) | 1.53 (m), 1.76 (m) | 1.60 (m), 1.74 (m) |
| 8 | 1.72 (m) | 1.84 (m) | 1.85 (m) |
| 9 | 1.40 (m) | 2.08 (m) | 1.62 (m) |
| 11 | 1.40 (m), 1.49 (m) | 5.27 (dd, 11.4, 3.0) | 4.12 (dd, 10.2, 3.0) |
| 12 | 1.51 (m), 2.35 (m) | 3.95 (d, 3.0) | 5.27 (d, 3.0) |
| 14 | 1.74 (m) | 1.88 (m) | 1.72 (m) |
| 15 | 4.64 (d, 5.0) | 1.08 (m), 1.61 (m) | 1.12 (m), 1.63 (m) |
| 16 | 1.65 (m), 1.78 (m) | 1.31 (m), 2.05 (m) | 1.39 (m), 2.00 (m) |
| 17 | 1.96 (m) | 1.68 (m) | |
| 18 | 0.90 (s) | 0.76 (s) | 0.79 (s) |
| 19 | 1.20 (s) | 1.27 (s) | 1.29 (s) |
| 20 | 1.02 (m) | 1.01 (m) | |
| 21 | 1.71 (s) | 1.01 (br s) | 0.93 (d, 6.5) |
| 22 | 1.95(dd, 13.3, 4.3), | 0.23 (ov) | 0.13 (ddd, 9.3, 6.1, 5.7) |
| 2.25 (dd, 12.7, 9.5) | |||
| 23 | 1.80 (m) | ||
| 24 | 1.05 (m) | 0.23 (ov) | 0.24 (m) |
| 25 | 1.62 (m) | 1.50 (m) | 1.63 (m) |
| 26 | 0.83 (d, 6.7) | 0.85 (d, 6.5) | 0.85 (d, 6.9) |
| 27 | 0.89 (d, 8.1) | 0.94 (d, 6.5) | 0.95 (d, 6.5) |
| 28 | 0.79 (d, 6.9) | 0.93 (d, 6.7) | 0.94 (d, 6.4) |
| 29 | 0.70 (d, 6.8) | 0.89 (s) | 0.89 (s) |
| 30 | 0.46 (dd, 8.8, 4.2), | 0.46 (dd, 8.7, 4.4), | |
| −0.14 (d, 5.0) | −0.13 (d, 5.0) | ||
| COO CH3 | 2.08 (s) | 2.17 (s) |
s = singlet; d = doublet; t = triplet; m = multiplet; br s = broad singlet; dd = doublet of doublets; ov = overlapped signals.
Compounds 6, 7 and 12–14 belong to the cluster of gorgosteroids, which are characterized as having a cyclopropane ring in the side chain. Compound 6 was isolated as an optically active, white amorphous solid. The molecular formula was established as C32H54O6 by HR-ESI-MS. The NMR data of 6 were almost identical to those of 12, displaying characteristic proton signals for the cyclopropane ring at the very high field (δH −0.13~0.46) (Table 3) in the 1H NMR spectrum. One methylene group (δH 2.33, dd, J = 12.2, 5.3 Hz, H-12β and 1.17, m, H-12α; δC 46.5) in 12 was replaced by a oxygenated methine group (δC 75.0, CH; δH 3.95, d, J = 3.0 Hz, 1H) in 6. This hydroxyl group was assigned at C-12, due to HMBC correlation from H3-18 to H-12 and the proton connectivity of H-9/H-11/H-12. The β configuration of H-12 was deduced from its small coupling constant with H-11 and its nuclear Overhauser effect (NOE) effect with H3-18. The structure of 6 was thus elucidated as 11α-acetoxy-gorgostane-3β,5α,6β,12α-tetraol.
Compound 7 was isolated as an optically active, white amorphous solid. The compound had the same formula (C32H54O6) as 6 on the basis of HR-ESI-MS. Inspection of NMR spectra for 7 revealed great similarity with those of 6 concerning the signals in rings A, C, D and the side chain. Analysis of the 1H-1H COSY spectrum for the proton sequence H-9/H-11/H-12 and the HMBC correlation from H3-18 to C-12 led the assignment of 12-OAc and 11-OH in 7 instead of 11-OAc and 12-OH in 6. The configuration at chiral centers remains intact due to the analysis of related proton coupling constants and the NOE effects, establishing the structure of 7 as 12α-acetoxy-gorgostane-3β,5α,6β,11α-tetraol.
The isolation of an array of sterols demonstrates an excellent example of chemical diversity, displaying carbon skeletons of cholestane, ergostane, gorgostane and 23,24-dimethyl cholestane. The co-occurrence of these metabolites supported the biosynthesis proposal of side chains in dinosterol, peridinosterol and gorgosterol [32]. The methylation of the side chain of 1 may yield the side chain of 2, which further would be methylated to the side chain of the dinosterol derivative 4 and the peridinosterol analogues, 5 and 6. The side chain of 4 was then methylated to the side chain of sterols belonging to the gorgosterol family (6, 7, 12–14).
All isolated compounds, except 5, were tested in an agar diffusion assay for their antibacterial, antifungal and algicidal properties (Table 4). All the metabolites exhibited antibacterial activity against the Gram-negative bacterium, Escherichia coli, the Gram-positive bacterium, Bacillus megaterium, and antifungal activity against the fungi, Microbotryum violaceum and Septoria tritici. However, 1, 2, 6, 7 and 8 exhibited considerable growth inhibitory activity with regard to bacteria and/or fungi species. This suggests that the 11α-acetoxy group may increase both antibacterial and antifungal activities. The terminal-double bond and the cyclopropane side chain seem to also contribute to this bioactivity. None of the sterols showed inhibition of green alga Chlorella fusca.
Table 4.
Agar diffusion assays for antibacterial, antifungal and antialgal activities a,b.
| Compound | Escherichia coli | Bacillus megaterium | Microbotryum violaceum | Septoria tritici | Botrytis cinerea | Chlorella fusca |
|---|---|---|---|---|---|---|
| 1 | 12.0 | 10.0 | 6.5 | 10.5 | 10.5 | 0 |
| 2 | 14.5 | 12.0 | 10.0 | 7.5 | 5.5 | 0 |
| 3 | 7.5 | 4.5 | 7.0 | 10.5 | 5.5 | 0 |
| 4 | 6.0 | 7.0 | 7.5 | 9.0 | 0 | 0 |
| 6 | 9.5 | 10.0 | 8.5 | 10.0 | 8.0 | 0 |
| 7 | 8.0 | 10.0 | 7.0 | 4.5 | 12.0 | 0 |
| 8 | 7.0 | 7.5 | 6.0 | 6.5 | 0 | 0 |
| 9 | 4.5 | 4.5 | 7.0 | 6.5 | 0 | 0 |
| 1 0 | 6.0 | 8.5 | 8.5 | 4.5 | 0 | 0 |
| 1 1 | 6.0 | 7.0 | 6.0 | 6.0 | 0 | 0 |
| 1 2 | 7.0 | 8.0 | 9.5 | 10.0 | 5.0 | 0 |
| 1 3 | 10.0 | 6.5 | 11.5 | 7.5 | 0 | 0 |
| 1 4 | 5.0 | 4.0 | 8.5 | 7.0 | 0 | 0 |
| Penicillin | 17.5 | 22.5 | 11.5 | 9.0 | 0 | 0 |
| Strepolin | 8.0 | 7.5 | 6.5 | 9.0 | 0 | 0 |
| Ketoconazole | 10.0 | 10.0 | 20.5 | 20.0 | 26.5 | 0 |
| Acetone | 0 | 0 | 0 | 0 | 0 | 0 |
a 0.05 mg of the test or control substances dissolved in acetone were applied to a filter disc and sprayed with the respective test organism at a concentration of 105 cells/mL. b Radii of the zones of inhibition are given in mm (gi = growth inhibition); that is, some growth within the zone of inhibition. Otherwise, the inhibition zone was clear.
3. Experimental Section
3.1. General Experimental Procedures
Commercial silica gel (200–300 mesh, 10–40 mm; Yantai, China) and Sephadex LH-20 (Pharmacia) were used for column chromatography. Precoated silica gel plates (Yantai, GF254 plate, 10–40 mm) were used for analytical thin-layer chromatography (TLC). Spots were visualized by heating Si gel plates sprayed with 10% H2SO4 in EtOH. Semipreparative HPLC was carried out on an Agilent 1100 liquid chromatography equipped with a refractive index detector using a Zorbax 300 SB-C18 column (25 cm × 9.4 mm i.d.). Melting points were determined on an XT5-XMT apparatus. Optical rotations were measured with a Perkin-Elmer 341 polarimeter. NMR spectra were recorded in CHCl3 on a Bruker DRX 400 or 500 spectrometer, and the 2D NMR spectra were obtained using standard pulse sequences. Chemical shifts are reported in parts per million (δ), with use of the residual CHCl3 signal (δH 7.26 ppm) as an internal standard for 1H NMR and CDCl3 (δC 77.0 ppm) for 13C NMR; Coupling constants (J) are reported in Hz. 1H NMR and 13C NMR assignments were complemented by heteronuclear single quantum correlation (HSQC), HMBC, 1H-1H COSY and nuclear Overhauser effect spectroscopy (NOESY) experiments. The following abbreviations were used to describe spin multiplicity: s = singlet; d = doublet; t = triplet; q = quartet; m = multiplet; br s = broad singlet; dd = doublet of doublets; ov = overlapped signals. HR-ESI-MS were recorded on a Micromass Quattro mass spectrometer. Optical rotations were measured in CHCl3 with an Autopol IV polarimeter at the sodium D line (590 nm). Infrared spectra were recorded in thin polymer films on a Nexus 470 FTIR spectrophotometer (Nicolet, USA); peaks are reported in cm−1.
3.2. Material and Methods
The soft coral Sarcophyton sp. was collected by hand using SCUBA off the coast of Weizhou Island, Guangxi Province of China, in October 2008, at a depth of 20 m and identified by Dr. Xiu-Bao Li, South China Sea Institute of Oceanology, Chinese Academy of Sciences. A voucher specimen (No. S-7-1) was deposited at the Research Center for Marine Drugs, School of Pharmacy, Second Military Medical University, Shanghai, China.
3.3. Extraction and Isolation
The frozen bodies of Sarcophyton sp. (1642 g, wet weight) were cut into small pieces, exhaustively extracted with acetone (5 L × 5) and then evaporated under reduced pressure. The acetone extract was resuspended in H2O and partitioned with Et2O 5 times. The concentrated Et2O extract (21.6 g) was subjected to column chromatography (CC) on a Si gel using petroleum ether/acetone (99:1–1:10) as eluent to yield seven fractions (Fr. A–G) on the basis of thin layer chromatography (TLC) analysis. Fractions D–F were gel-filtered on Sephadex LH-20 (n-hexane/CH2Cl2/MeOH, 2:1:1), followed by CC on Si gel eluting with gradient CHCl3/MeOH (50:1, 30:1, 20:1, 10:1) and further purification by semipreparative HPLC to yield 1 (2.3 mg, 28.4 min, 95% MeOH, 1.0 mL/min), 2 (3.3 mg, 29.6 min, 95% MeOH, 1.0 mL/min), 8 (33.7 mg, 33.9 min, 95% MeOH, 1.0 mL/min), 9 (25.5 mg, 31.2 min, 96% MeOH, 1.5 mL/min), 11 (1.2 mg, 25.1 min, 96% MeOH, 1.5 mL/min) and 12 (5.4 mg, 38.8 min, 95% MeOH, 1.0 mL/min) from fraction D, 3 (3.4 mg, 16.2 min, 95% MeOH, 1.0 mL/min), 6 (3.4 mg, 15.3 min, 95% MeOH, 1.0 mL/min) and 14 (0.5mg, 16.7 min, 95% MeOH, 1.0 mL/min) from fraction E and 4 (1.4 mg, 17.4 min), 5 (13.2 mg, 20.1 min), 7 (9.5 mg, 18.7 min), 10 (11.4 mg, 18.0 min) and 13 (4.8 mg, 20.8 min) from fraction F (94% MeOH, 1.5 mL/min).
Compound 1: white amorphous solid; melting point (m.p.) 108–110 °C; [α]D20 −34.5 (c 0.165, CHCl3); IR (film) νmax 3408 (board), 2927, 2869, 1712, 1376, 1263, 1027, 757; 1H and 13C-NMR data, see Table 1 and Table 2; HRESI-MS m/z: 499.3397 [M + Na]+ (calculated (calcd.) C29H48O5Na, 499.3399).
Compound 2: white amorphous solid; m.p. 173–175 °C; [α]D20 −44.3 (c 0.115, CHCl3); IR (film) νmax 3401 (board), 2961, 2927, 2869, 1715, 1375, 1216, 1026, 801; 1H and 13C-NMR data, see Table 1 and Table 2; HRESI-MS m/z: 511.3403 [M + Na]+ (calcd. C30H48O5Na, 511.3399).
Compound 3: white amorphous solid; m.p. 264–265 °C; [α]D20 −35.3 (c 0.085, CHCl3); IR (film) νmax 3362 (board), 2956, 2927, 2870, 1464, 1378, 1067, 1042; 1H and 13C-NMR data, see Table 1 and Table 2; HRESI-MS m/z: 467.3731 [M + H]+ (calcd. C28H50O5Na, 467.3737).
Compound 4: white amorphous solid; m.p. 245–247 °C; [α]D20 −38.9 (c 0.095, CHCl3); IR (film) νmax 3412 (board), 2960, 2926, 2869, 1462, 1261, 1096, 801; 1H and 13C-NMR data, see Table 1 and Table 2; HRESI-MS m/z: 485.3606 [M + Na]+ (calcd. C29H50O4Na, 485.3707).
Compound 5: white amorphous solid; m.p. 150–152 °C; [α]D20 −12.1 (c 0.165, CHCl3); 1H and 13C-NMR data, see Table 1 and Table 3; HRESI-MS m/z: 485.3609 [M + Na]+ (calcd. C29H50O4Na, 485.3607).
Compound 6: white amorphous solid; m.p. 170–172 °C; [α]D20 −20.0 (c 0.17, CHCl3); IR (film) νmax 3434 (board), 2957, 2931, 2874, 1716, 1460, 1372, 1255; 1H and 13C-NMR data, see Table 1 and Table 3; HRESI-MS m/z: 557.3819 [M + Na]+ (calcd. C32H54O6Na, 557.3818).
Compound 7: white amorphous solid; m.p. 150–152 °C; [α]D20 −9.8 (c 0.395, CHCl3); IR (film) νmax 3422, 2956, 2926, 2872, 1719, 1459, 1375, 1254; 1H and 13C-NMR data, see Table 1 and Table 3; HRESI-MS m/z: 557.3829 [M + Na]+ (calcd. C32H54O6Na, 557.3818).
3.4. Agar Diffusion Test for Biological Activity
An agar diffusion test was applied to determine the biological activities, as previously reported [38]. Briefly, compounds were dissolved in acetone at 1.0 mg/mL (2.1 mM for 1, 2.0 mM for 2, 2.3 mM for 3, 2.2 mM for 4 and 5, 1.9 mM for 6 and 7, 2.0 mM for 8, 2.3 mM for 9, 2.2 mM for 10, 2.3 mM for 11, 1.9 mM for 12, 2.1 mM for 13 and 2.0 mM for 14); 50 μL of the solution (0.05 mg) were pipetted onto a sterile filter disc (Schleicher & Schuell, 9 mm), which was placed onto an appropriate agar growth medium for the respective test organism and subsequently sprayed with a suspension of the test organism at a concentration of 105 cells/mL. The test organisms were bacteria, the Gram-negative bacterium, Escherichia coli, and the Gram-positive bacterium, Bacillus megaterium (both grown on NB medium), and fungi, Microbotryum violaceum, Septoria tritici and Botrytis cinerea (all grown on MPY medium), and the microalga, Chlorella fusca (grown on CP medium). Penicillin (1.0 mg/mL, 0.05 mg), strepolin (1.0 mg/mL, 0.05 mg) and ketoconazole (1.0 mg/mL, 0.05 mg) were used as positive controls. These microorganisms were chosen because (a) they are non-pathogenic and (b) had in the past proved to be accurate as initial test organisms for antibacterial and antifungal activities [38,39]. Commencing at the outer edge of the filter disc, the radius of the zone of inhibition was measured in mm.
4. Conclusions
Chemical investigation of the soft coral Sarcophyton sp. from the South China Sea led to the isolation and structural elucidation of fourteen polyoxygenated steroids (1–14) with 3β,5α,6β-hydroxy moiety, demonstrating an excellent example of chemical diversity. These compounds displayed different levels of antibacterial and antifungal bioactivities in the in vitro bioassay. Preliminary structure-activity analysis suggests that the 11α-acetoxy group may increase both antibacterial and antifungal activities. The terminal-double bond and the cyclopropane moiety at the side chain may also contribute to the bioactivity. The interesting discovery may encourage further investigations on the sterols, the antibacterial and antifungal activity, and the structure-activity relationship.
Acknowledgments
The research work was financially supported by the Natural Science Foundation of China (No. 41076082, 81172979) and the National Marine “863” Project (No. 2013AA092902). The authors are grateful to Daniel Parker (The Pennsylvania State University) for the English.
Supplementary Files
Supplementary Materials (PDF, 2362 KB)
References
- 1.Coll J.C., Labarre S., Sammarco P.W., Williams W.T., Bakus G.J. Chemical defenses in soft corals (Coelenterata, Octocorallia) of the great barrier reef: A study of comparative toxicities. Mar. Ecol. Prog. Ser. 1982;8:271–278. doi: 10.3354/meps008271. [DOI] [Google Scholar]
- 2.Slattery M., McClintock J.B., Heine J.N. Chemical defenses in antarctic soft corals: Evidence for antifouling compounds. J. Exp. Mar. Biol. Ecol. 1995;190:61–77. doi: 10.1016/0022-0981(95)00032-M. [DOI] [Google Scholar]
- 3.Fleury B.G., Lages B.G., Barbosa J.P., Kaiser C.R., Pinto A.C. New hemiketal steroid from the introduced soft coral Chromonephthea braziliensis is a chemical defense against predatory fishes. J. Chem. Ecol. 2008;34:987–993. doi: 10.1007/s10886-008-9499-y. [DOI] [PubMed] [Google Scholar]
- 4.Aratake S., Tomura T., Saitoh S., Yokokura R., Kawanishi Y., Shinjo R., Reimer J.D., Tanaka J., Maekawa H. Soft coral Sarcophyton (Cnidaria: Anthozoa: Octocorallia) species diversity and chemotypes. PLoS One. 2012;7:e30410. doi: 10.1371/journal.pone.0030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anjaneyulu A.S.R., Rao G.V. Chemical constituents of the soft coral species of Sarcophyton genus: A review. J. Indian Chem. Soc. 1997;74:272–278. [Google Scholar]
- 6.Zhang W., Guo Y.-W., Gu Y.-C. Secondary metabolites from the South China Sea invertebrates: Chemistry and biological activity. Curr. Med. Chem. 2006;13:2041–2090. doi: 10.2174/092986706777584960. [DOI] [PubMed] [Google Scholar]
- 7.Li C., La M.-P., Li L., Li X.-B., Tang H., Liu B.-S., Krohn K., Sun P., Yi Y.-H., Zhang W. Bioactive 11,20-epoxy-3,5(16)-diene briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. J. Nat. Prod. 2011;74:1658–1662. doi: 10.1021/np200330c. [DOI] [PubMed] [Google Scholar]
- 8.Li C., La M.-P., Sun P., Kurtan T., Mandi A., Tang H., Liu B.-S., Yi Y.-H., Li L., Zhang W. Bioactive (3Z,5E)-11,20-epoxybriara-3,5-dien-7,18-olide diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Mar. Drugs. 2011;9:1403–1418. doi: 10.3390/md9081403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C., La M.-P., Tang H., Pan W.-H., Sun P., Krohn K., Yi Y.-H., Li L., Zhang W. Bioactive briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Bioorg. Med. Chem. Lett. 2012;22:4368–4372. doi: 10.1016/j.bmcl.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Geng W.-L., Wang X.-Y., Kurtán T., Mándi A., Tang H., Schulz B., Sun P., Zhang W. Herbarone, a rearranged heptaketide derivative from the sea hare associated fungus Torula herbarum. J. Nat. Prod. 2012;75:1828–1832. doi: 10.1021/np300642t. [DOI] [PubMed] [Google Scholar]
- 11.Sun P., Meng L.-Y., Tang H., Liu B.-S., Li L., Yi Y.-H., Zhang W. Sinularosides A and B, bioactive 9,11-secosteroidal glycosides from the South China Sea soft coral Sinularia humilis Ofwegen. J. Nat. Prod. 2012;75:1656–1659. doi: 10.1021/np300475d. [DOI] [PubMed] [Google Scholar]
- 12.Edrada R.A., Wray V., Witte L., van Ofwegen L.P., Proksch P. Bioactive terpenes from the soft coral Heteroxenia sp. from Mindoro, Philippines. Z. Naturforsch. C. 2000;55:82–86. doi: 10.1515/znc-2000-1-216. [DOI] [PubMed] [Google Scholar]
- 13.Umeyama A., Shoji N., Ozeki M., Arihara S. Sarcoaldesterols A and B, two new polyhydroxylated sterols from the soft coral Sarcophyton sp. J. Nat. Prod. 1996;59:894–895. doi: 10.1021/np960255j. [DOI] [Google Scholar]
- 14.Mohammed R., Seliem M.A.E., Mohammed T.A.A., Abed-El Fatah A., Abo-Youssef A.M., Thabet M.M. Bioactive secondary metabolites from the Red Sea soft coral Heteroxenia fuscescens. Int. J. Appl. Res. Nat. Prod. 2012;4:15–27. [Google Scholar]
- 15.Rao C.B., Ramana K.V., Rao D.V., Fahy E., Faulkner D.J. Metabolites of the gorgonian Isis hippuris from India. J. Nat. Prod. 1988;51:954–958. doi: 10.1021/np50059a023. [DOI] [Google Scholar]
- 16.Lu Q., Faulkner D.J. Two 11α-acetoxysterols from the Palauan soft coral Lobophytum cf. pauciflorum. Nat. Prod. Lett. 1997;10:231–237. doi: 10.1080/10575639708041200. [DOI] [Google Scholar]
- 17.Elshamy A.I., Abdel-Razik A.F., Nassar M.I., Mohamed T.K., Ibrahim M.A., El-Kousy S.M. A new gorgostane derivative from the Egyptian Red Sea soft coral Heteroxenia ghardaqensis. Nat. Prod. Res. 2012 doi: 10.1080/14786419.2012.724417. [DOI] [PubMed] [Google Scholar]
- 18.Van Minh C., Cuong N.X., Tuan T.A., Choi E.M., Kim Y.H., van Kiem P. A new 9,11-secosterol from the Vietnamese sea soft coral, Sarcophyton mililatensis, increases the function of osteoblastic MC3T3-E1 cells. Nat. Prod. Commun. 2007;2:1095–1100. [Google Scholar]
- 19.Qi S.-H., Zhang S., Xiao Z.-H., Huang J.-S., Wu J., Li Q.-X. Study on the chemical constituents of the South China Sea gorgonian Junceella juncea. Chem. Pharm. Bull. 2004;52:1476–1478. doi: 10.1248/cpb.52.1476. [DOI] [PubMed] [Google Scholar]
- 20.Rueda A., Zubia E., Ortega M.J., Salva J. Structure and cytotoxicity of new polyhydroxylated sterols from the Caribbean gorgonian Plexaurella grisea. Steroids. 2001;66:897–904. doi: 10.1016/S0039-128X(01)00122-2. [DOI] [PubMed] [Google Scholar]
- 21.Duh C.Y., Wang S.-K., Chung S.G., Chou G.-C., Dai C.-F. Cytotoxic cembrenolides and steroids from the formosan soft coral Sarcophyton crassocaule. J. Nat. Prod. 2000;63:1634–1637. doi: 10.1021/np0002381. [DOI] [PubMed] [Google Scholar]
- 22.Iorizzi M., Minale L., Riccio R., Lee J.S., Yasumoto T. Polar steroids from the marine scallop Patinopecten yessoensis. J. Nat. Prod. 1988;51:1098–1103. doi: 10.1021/np50060a008. [DOI] [Google Scholar]
- 23.Madaio A., Piccialli V., Sica D., Corriero G. New polyhydroxysterols from the dictyoceratid sponges Hippospongia-communis, Spongia-officinalis, Ircinia-variabilis, and Spongionella-gracilis. J. Nat. Prod. 1989;52:952–961. doi: 10.1021/np50065a007. [DOI] [Google Scholar]
- 24.Aiello A., Fattorusso E., Menna M., Carnuccio R., Iuvone T. New cytotoxic steroids from the marine sponge Dysidea fragilis coming from the Lagoon of Venice. Steroids. 1995;60:666–673. doi: 10.1016/0039-128X(95)00055-U. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W., Guo Y.W., Mollo E., Fontana A., Cimino G. Acanthovagasteroids A-D, four new 19-hydroxylated steroids from the South China Sea Gorgonian Acanthogorgia vagae aurivillius. J. Nat. Prod. 2004;67:2083–2085. doi: 10.1021/np049859a. [DOI] [PubMed] [Google Scholar]
- 26.Piccialli V., Sica D. New dihydroxylated sterols from the marine sponge Spongionella gracilis. J. Nat. Prod. 1986;49:779–783. doi: 10.1021/np50047a002. [DOI] [Google Scholar]
- 27.Nakatsu T., Walker R.P., Thompson J.E., Faulkner D.J. Biologically-active sterol sulfates from the marine sponge Toxadocia zumi. Experientia. 1983;39:759–761. doi: 10.1007/BF01990312. [DOI] [Google Scholar]
- 28.Akihisa T., Ghosh P., Thakur S., Oshikiri S., Tamura T., Matsumoto T. 24-β-methyl cholesta-5,22E,25-trien-3-β-ol and 24-α-ethyl-5-α-cholest-22E-en-3-β-ol from Clerodendrum fragrans. Phytochemistry. 1988;27:241–244. [Google Scholar]
- 29.Shimizu Y., Alam M., Kobayashi A. Letter: Dinosterol, the major sterol with a unique side chain ion the toxic dinoflagellate, Gonyaulax tamarensis. J. Am. Chem. Soc. 1976;98:1059–1060. doi: 10.1021/ja00420a054. [DOI] [PubMed] [Google Scholar]
- 30.Finer J., Clardy J., Kobayashi A., Alam M., Shimizu Y. Identity of stereochemistry of dinosterol and gorgosterol side chain. J. Org. Chem. 1978;43:1990–1992. doi: 10.1021/jo00404a031. [DOI] [Google Scholar]
- 31.Shu A.Y.L., Djerassi C. Stereospecific synthesis of dinosterol. Tetrahedron Lett. 1981;22:4627–4630. doi: 10.1016/S0040-4039(01)82998-1. [DOI] [Google Scholar]
- 32.Giner J.L., Djerassi C. Biosynthetic studies of marine lipids. 33. Biosynthesis of dinosterol, peridinosterol, and gorgosterol: Unusual patterns of bioalkylation in dinoflagellate sterols. J. Org. Chem. 1991;56:2357–2363. doi: 10.1021/jo00007a021. [DOI] [Google Scholar]
- 33.Rao M.R., Venkatesham U., Reddy M.V.R., Venkateswarlu Y. An unusual novel C-29 steroid from the soft coral Lobophytum crassum. J. Nat. Prod. 1999;62:785–786. doi: 10.1021/np980500u. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez A.D., Rivera J., Boulanger A. New polyhydroxydinostane sterols from the Caribbean gorgonian octocoral Pseudopterogorgia americana. Tetrahedron Lett. 1998;39:7645–7648. doi: 10.1016/S0040-4039(98)01695-5. [DOI] [Google Scholar]
- 35.Kobayashi M., Tomioka A., Mitsuhashi H. Marine sterols. 8. Isolation and structure of sarcosterol, a new sterol with a ∆17(20) double bond from the soft coral sarcophyton glaucum. Steroids. 1979;34:273–284. doi: 10.1016/0039-128X(79)90079-5. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi M. Marine sterols. 27. 25-hydroxy derivative of sarcosterol, a novel marine sterol with a 23-methyl and a 17(20)E-double bond, from the soft coral Sinularia mayi. Steroids. 1994;59:27–29. doi: 10.1016/0039-128X(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 37.Swenson W., Tagle B., Clardy J., Withers N.W., Kokke W., Fenical W., Djerassi C. Peridinosterol—a new ∆17 unsaturated sterol from two cultured marine algae. Tetrahedron Lett. 1980;21:4663–4666. doi: 10.1016/0040-4039(80)88088-9. [DOI] [Google Scholar]
- 38.Zhang W., Krohn K., Ullah Z., Florke U., Pescitelli G., Di Bari L., Antus S., Kurtan T., Rheinheimer J., Draeger S., Schulz B. New mono- and dimeric members of the secalonic acid family: Blennolides A–G isolated from the fungus Blennoria sp. Chem. Eur. J. 2008;14:4913–4923. doi: 10.1002/chem.200800035. [DOI] [PubMed] [Google Scholar]
- 39.Ulrich Höller U., Wright A.D., Matthee G.F., Konig G.M., Draeger S., Aust H.J., Schulz B. Fungi from marine sponges: Diversity, biological activity and secondary metabolites. Mycol. Res. 2000;104:1354–1365. doi: 10.1017/S0953756200003117. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials (PDF, 2362 KB)