Abstract
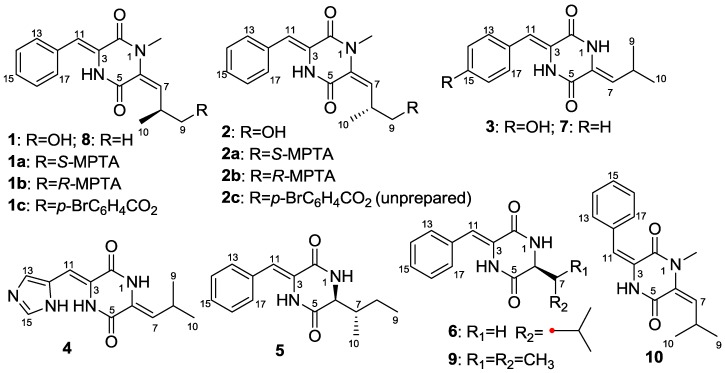
Five new diketopiperazine derivatives, (3Z,6E)-1-N-methyl-3-benzylidene-6-(2S-methyl-3-hydroxypropylidene)piperazine-2,5-dione (1), (3Z,6E)-1-N-methyl-3-benzylidene-6-(2R-methyl-3-hydroxypropylidene)piperazine-2,5-dione (2), (3Z,6Z)-3-(4-hydroxybenzylidene)-6-isobutylidenepiperazine-2,5-dione (3), (3Z,6Z)-3-((1H-imidazol-5-yl)-methylene)-6-isobutylidenepiperazine-2,5-dione (4), and (3Z,6S)-3-benzylidene-6-(2S-but-2-yl)piperazine-2,5-dione (5), were isolated from the marine-derived actinomycete Streptomyces sp. FXJ7.328. The structures of 1–5 were determined by spectroscopic analysis, CD exciton chirality, the modified Mosher’s, Marfey’s and the C3 Marfey’s methods. Compound 3 showed modest antivirus activity against influenza A (H1N1) virus with an IC50 value of 41.5 ± 4.5 μM. In addition, compound 6 and 7 displayed potent anti-H1N1 activity with IC50 value of 28.9 ± 2.2 and 6.8 ± 1.5 μM, respectively. Due to the lack of corresponding data in the literature, the 13C NMR data of (3Z,6S)-3-benzylidene-6-isobutylpiperazine-2,5-dione (6) were also reported here for the first time.
Keywords: Streptomyces, diketopiperazine derivatives, antivirus activity, H1N1
1. Introduction
Marine actinomycetes are a rich source of bioactive compounds with new structures [1], e.g., the cytotoxic thiocoraline from Micromonospora sp. L-13-ACM2-092 [2], and the anti-inflammatory cyclomarins A–C from Streptomyces sp. CNB-382 [3]. As one of the most important classes of bioactive compounds, alkaloids have received much attention. As part of our ongoing research on bioactive alkaloids with new structures from marine-derived actinomycetes [4,5,6,7], Streptomyces sp. FXJ7.328 was isolated from marine sediment, and was found to produce alkaloids by TLC visualizing with Dragendorff’s reagent in a saline culture. The ethyl acetate extract of fermentation broth exhibited antivirus activity against H1N1 influenza virus at 100 μg/mL and displayed a series of peaks with UV absorptions at 220 and 340 nm similar to those of diketopiperazine derivatives such as albonoursin and (3Z,6S)-3-benzylidene-6-isobutylpiperazine-2,5-dione by HPLC-UV analysis [8]. Chemical investigation of the extract resulted in the isolation and identification of five new diketopiperazine derivatives (Figure 1), namely (3Z,6E)-1-N-methyl-3-benzylidene-6-(2S-methyl-3-hydroxypropylidene)-piperazine-2,5-dione (1), (3Z,6E)-1-N-methyl-3-benzylidene-6-(2R-methyl-3-hydroxypropylidene)-piperazine-2,5-dione (2), (3Z,6Z)-3-(4-hydroxybenzylidene)-6-isobutylidenepiperazine-2,5-dione (3), (3Z,6Z)-3-((1H-imidazol-5-yl)methylene)-6-isobutylidenepiperazine-2,5-dione (4), and (3Z,6S)-3-benzylidene-6-(2S-but-2-yl)piperazine-2,5-dione (5). In addition, five known analogues were also isolated and their structures were identified as (3Z,6S)-3-benzylidene-6-isobutylpiperazine-2,5-dione (6) [8], albonoursin (7) [8,9,10], (3Z,6E)-1-N-methyl-3-benzylidene-6-isobutylidenepiperazine-2,5-dione (8) [9,11], (3Z,6S)-3-benzylidene-6-isopropylpiperazine-2,5-dione (9) [12], and (3E,6E)-1-N-methyl-3-benzylidene-6-isobutylidenepiperazine-2,5-dione (10) [13], respectively, by comparing their NMR data and specific rotation (Supplementary Information) with those reported in the literatures. Compounds 3, 6 and 7 displayed activity against influenza A (H1N1) virus with the IC50 values of 41.5 ± 4.5, 28.9 ± 2.2 and 6.8 ± 1.5 μM, respectively.
Figure 1.
Chemical structures of compounds 1–10 from Streptomyces sp. FXJ7.328.
2. Results and Discussion
2.1. Structure Elucidation
The ethyl acetate extract of the fermentation broth of Streptomyces sp. FXJ7.328 was subjected to extensive chromatographic separations over silica gel, Sephadex LH-20 and by HPLC to yield the new compounds 1–5 and the known analogues 6–10.
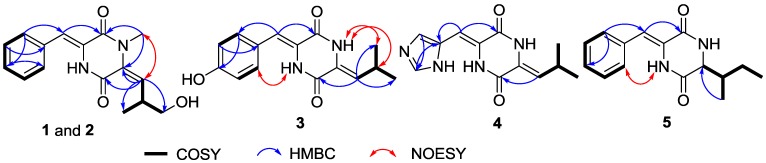
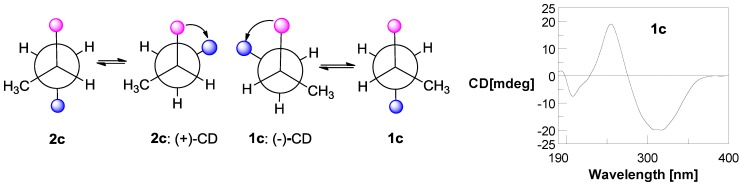
Compounds 1 and 2 were at first isolated as a racemic mixture with an excess of one enantiomer and thought to be a pure compound according to their identical NMR data and a small negative specific rotation ([α]D −5.8). When (R)-Mosher’s acyl chloride was used to determine the absolute configuration, the Mosher’s ester was found to be a separable mixture according to 1H NMR spectrum and HPLC profile. A chiral HPLC column was used to separate the enantiomeric mixture of 1 and 2, which yielded compounds 1 and 2 in an approximate ratio of 2:1 (Figure S37). The planar structures of 1 and 2 were established based on MS and NMR data of the racemic mixture. HRESIMS gave an ion peak at m/z 287.1387 [M + H]+ (calcd. for C16H19N2O3 287.1390), corresponding to the molecular formula C16H18N2O3. Analysis of the 1D NMR data (Table 1) revealed one methyl (δH/C 1.01/17.9), one N-methyl (δH/C 3.17/31.3), one sp3 oxygenated methylene (δH/C 3.36/66.7), seven sp2 methines, one sp3 methine (δH/C 3.59/34.9), and two signals (δC 158.5, 158.9) interpreted as conjugated amide carbonyls. 1H NMR signals at δH 7.52 (2H, d, J = 7.7), 7.40 (2H, t, J = 7.7) and 7.31 (1H, t, J = 7.2) revealed the presence of a monosubstituted benzene nucleus that was further identified as a dehydro-phenylalanine (deh-Phe) unit from the key HMBC correlations of H-13/17 (δH 7.52) to C-11 (δC 116.1) and C-15 (δC 128.7), and of H-11 (δH 6.75) to C-13 (δC 129.9) and C-2 (δC 158.5). 1H-1H COSY correlations of H-7/H-8/H-9 and H-8/H-10 combined with the key HMBC correlations from H-7 (δH 5.56) to C-5 (δC 158.9) and C-6 (δC 130.4), and from N-CH3 (δH 3.17) to C-6 (δC 130.4) suggested another amino acid unit, N-methyl dehydro-5-hydroxyleucine. The key HMBC correlation of N-CH3 to C-2 finally established the conjugate diketopiperazine (DKP) structure of 1 and 2 (Figure 2). The NOE correlation from N-CH3 to H-7 indicated the E-configuration of the Δ6 double bond. The Z-configuration of Δ3,11 double bond could be deduced from the relative downfield shift of H-11 because of the deshielding effect of the 2-carbonyl group, as e.g., δH-11 6.85, 6.40 and 6.74 for (3Z,6E)-1-N-methyl-3-benzylidene-6-isobutylidenepiperazine-2,5-dione [11], (3E,6E)-1-N-methyl-3-benzylidene-6-isobutylidenepiperazine-2,5-dione [13], and (3Z,6Z)-3-benzylidene-6-isobutylidenepiperazine-2,5-dione [10], respectively. The S-configuration of 1 was determined by analysis of exciton chirality CD of its p-bromobenzoate (1c) [14]. The stable conformers of the p-bromobenzoates of 1c and its enantiomer 2c (unprepared) were obtained by HyperChem Release 7.5 software [4] (Figure 3). The transition dipole orientations of two chromophores, the conjugated DKP core and the p-bromobenzoate in 1c and 2c, were oriented in counterclockwise and clockwise manners, which should result in negative and positive Cotton effects at long wavelength, respectively. The measured negative CD effect of 1c at λext 313 nm (Δε −5.2) indicates the S-configuration of 1c (Figure 3). This deduction was further validated by modified Mosher’s method for primary alcohols [15,16]. When 1 reacted with R- and S-MTPA chloride, the S- (1a) and R-MTPA esters (1b) were obtained, respectively. The chemical shift difference between two methylene protons of C-9 in S-MTPA ester 1a is larger than that in R-MTPA ester 1b (Δδ 0.08 vs. 0.01), indicating S-configuration of C-8 in 1. Thus, the structure of 1 was clearly elucidated as (3Z,6E)-1-N-methyl-3-benzylidene-6-(2S-methyl-3-hydoxypropylidene)-piperazine-2,5-dione. Compound 2 showed the opposite specific rotation and the opposite chemical shift difference between the two methylene protons of C-9 in S- and R-MTPA esters (2a and 2b), indicating R-configuration of 2. So compound 2 was identified as (3Z,6E)-1-N-methyl-3-benzylidene-6-(2R-methyl-3-hydroxypropylidene)piperazine-2,5-dione.
Table 1.
1H and 13C NMR Data for 1–5 (600 and 150 MHz, DMSO-d6, δ values).
| Position | 1 and 2 | 3 | 4 | 5 | ||||
|---|---|---|---|---|---|---|---|---|
| δC, type | δH, mult. (J in Hz) | δC, type | δH, mult. (J in Hz) | δC, type | δH, mult. (J in Hz) | δC, type | δH, mult. (J in Hz) | |
| 1 | 31.3, NCH3 | 3.17, s | 10.24, s | 8.47, s | ||||
| 2 | 158.5, qC | 158.0, qC | 157.8, qC | 161.0, qC | ||||
| 3 | 126.5, qC | 125.3, qC | 125.2, qC | 127.3, qC | ||||
| 4 | 9.82, s | 9.93, s | ||||||
| 5 | 158.9, qC | 157.4, qC | 156.8, qC | 166.8, qC | ||||
| 6 | 130.4, qC | 125.2, qC | 125.9, qC | 60.2, CH | 3.86, t, (3.3) | |||
| 7 | 129.7, CH | 5.56, d, (9.9) | 125.1, CH | 5.66, d, (10.4) | 125.5, CH | 5.68, d, (9.9) | 40.9, CH | 1.80, m |
| 8 | 34.9, CH | 3.59, m | 23.9, CH | 2.93, m | 24.4, CH | 2.95, m | 24.8, CH2 | 1.46, m; 1.18, m |
| 9 | 66.7, CH2 | 3.36 | 22.2, CH3 | 0.96, d, (6.5) | 22.8, CH3 | 0.97, d, (6.6) | 15.3, CH3 | 0.91, d, (7.1) |
| 10 | 17.9, CH3 | 1.01, d, (7.1) | 22.2, CH3 | 0.96, d, (6.5) | 22.8, CH3 | 0.97, d, (6.6) | 12.1, CH3 | 0.86, t, (7.7) |
| 11 | 116.1, CH | 6.75, s | 115.0, CH | 6.66, s | 105.1, CH | 6.60, s | 114.6, CH | 6.66, s |
| 12 | 133.9, qC | 123.9, CH | 137.0, qC | 133.9, qC | ||||
| 13/17 | 129.9, CH | 7.52, d, (7.7) | 130.9, CH | 7.36, d, (8.5) | 119.8, CH | 7.52, s | 129.3, CH | 7.45, d, (7.7) |
| 14/16 | 129.2, CH | 7.40, t, (7.7) | 115.6, CH | 6.79, d, (8.5) | 129.7, CH | 7.39, t, (7.7) | ||
| 15 | 128.7, CH | 7.31, t, (7.2) | 157.5, qC | 137.1, CH | 7.94, s | 128.5, CH | 7.29, t, (7.7) | |
Figure 2.
Selected 2D NMR correlations for 1–5.
Figure 3.
The stable conformers of 1c and 2c and the measured CD curve for p-bromobenzoate 1c.
The molecular formula of compound 3 was determined to be C15H16N2O3 based on HRESIMS with a peak at m/z 273.1231 [M + H]+ (calcd. for C15H17N2O3 273.1234). The similarity of the 1D NMR spectra to those of albonoursin (7) [8,9] indicated a conjugated DKP as well. The difference of 1H and 13C NMR data between 3 and 7 pointed to a p-hydroxy substituted phenyl system in 3 instead of the benzene ring in 7, which explains the obvious upfield shifts of H-13/17 (δH 7.36), H-14/16 (δH 6.79) and C-14/16 (δC 115.6) by the electron-donor effect of the hydroxy group. 1H-1H COSY correlations between H-13/17 and H-14/16 and the key HMBC correlations from H-11 (δH 6.66) to C-13/17 (δC 130.9) and C-2 (δC 158.0), from H-13/17 to C-15 (δC 157.5), and from H-14/16 to C-12 (δC 123.9) further supported the existence of a dehydro-tyrosine (deh-Tyr) unit. The 1H-1H COSY correlations from H-7 (δH 5.66) to H-9/10 (δH 0.96) through H-8 (δH 2.93) along with the HMBC correlations from H-7 to C-5 (δC 157.4) supported the existence of a dehydro-leucine (deh-Leu) unit. The NOE correlation between H-4 (δH 9.82) and H-13/17 indicated the Z-configuration of the Δ3,11 double bond. The NOE correlations of H-1 (δH 10.24) to H-8 and H-9/10 combined with the relative downfield shift of H-7 revealed Z-configuration of the Δ6 double bond. Therefore, compound 3 was determined to be (3Z,6Z)-3-(4-hydroxybenzylidene)-6-isobutylidenepiperazine-2,5-dione.
Compound 4 was found to have the molecular formula of C12H14N4O2 from the HRESIMS peak at m/z 247.1189 [M + H]+ (calcd. for C12H15N4O2 247.1190). 1D NMR (Table 1) and 2D NMR (Figure 2) data disclosed the same Z-deh-Leu unit as in compound 3. The remainder C6H5N3O displayed three sp2 methine signals at δH/C 6.60/105.1, 7.94/137.1 and 7.52/119.8, two sp2 quaternary carbon signals at δC 125.2 and 125.9, and one amide carbonyl signal at δC 157.8. The HMBC correlations of H-15 (δH 7.94) to C-13 (δC 119.8) and C-12 (δC 137.0), of H-13 (δH 7.52) to C-15 (δC 137.1), and of H-11 (δH 6.60) to C-2 (δC 157.8) and C-12 suggested a dehydro-histidine (deh-His) unit. The Z-configurations of both Δ3,11 and Δ6 double bonds were deduced from the relative downfield shifts of H-11 and H-7 consistent with those of (3Z,6R)-3-((1H-imidazol-5-yl)methylene)-6-isopropylpiperazine-2,5-dione (δH 6.51) [17] and compound 3, respectively. Compound 4 was therefore elucidated as (3Z,6Z)-3-((1H-imidazol-5-yl)methylene)-6-isobutylidenepiperazine-2,5-dione.
The molecular formula of C15H18N2O2 was assigned to 5 according to the HRESIMS peak at m/z 259.1439 [M + H]+ (calcd. for C15H19N2O2 259.1441), indicating an isomer of 6. The 1D NMR (Table 1) spectra were very similar to those of 6 [8] except for the leucine moiety signals, suggesting that a deh-Phe unit was also presented in the structure of 5. The NOE correlation from H-4 to H-13/17 and the relative downfield shift of H-11 accounted for the Z-configuration of the Δ3,11 double bond [8]. The main differences of the 1H NMR spectra are a methyl triplet in 5 replacing the methyl doublet in 6; further, a distinct split methylene signal in 5 substitutes the overlapped methylene proton signal in 6. These observations combined with the separate downfield and upfield shifts of methine and methylene carbon signals revealed the existence of an isoleucine moiety in 5, which was further confirmed by the 1H-1H COSY correlations of H-1/H-6/H-7/H-8/H-9 and H-7/H-10 (Figure 2) and the key HMBC correlation of H-10 to C-6. The absolute configuration of the isoleucine moiety was determined by Marfey’s method [18] combined with C3 Marfey’s method [19,20]. The 1-fluoro-2,4-dinitrophenyl-5-l-alanine amide (FDAA) derivatives of the acid hydrolysates of 5 and four authentic isoleucine samples (l-, l-allo-, d- and d-allo-) were prepared. HPLC analysis over ODS column (Figure S38) revealed that acid hydrolysates of 5 displayed the same retention time (tR 23.06 min) as the authentic l-Ile (tR 23.06 min) and l-allo-Ile (tR 23.06 min) but were different from d-Ile (tR 28.11 min) and d-allo-Ile (tR 28.11 min). The FDAA derivatives of the acid hydrolysates of 5 and the authentic l-Ile and l-allo-Ile were further analyzed by C3 HPLC column (Figure S39). The retention time of the acid hydrolysates of 5 was the same as for the authentic l-Ile (tR 38.50 min), but different from the authentic l-allo-Ile (tR 37.49 min). Thus, the isoleucine moiety in 5 was unambiguously identified as l-Ile, and the structure of compound 5 was elucidated as (3Z,6S)-3-benzylidene-6-(2S-but-2-yl)piperazine-2,5-dione.
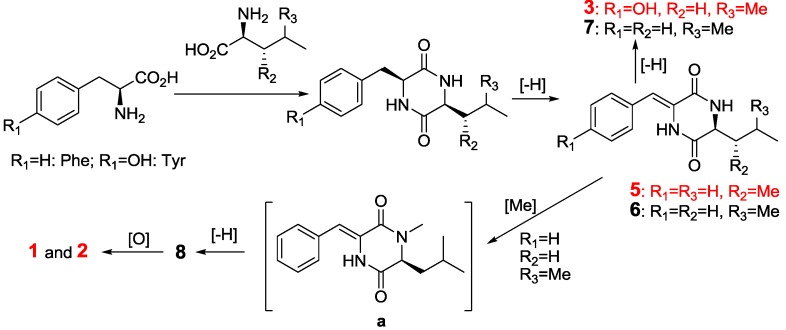
2.2. The Postulated Biosynthesis Pathway of Compounds 1–10
Compounds 1–10 were postulated to be produced biogenetically from the amino acid pathway (Figure 4). Cyclic condensation between Phe and Leu formed cyclo(Phe-Leu) that underwent dehydrogenation in Phe moiety to form compounds 6 and 7. The N-methylation of 6 produced an un-isolated intermediate (a) that further underwent another dehydrogenation in the Leu moiety to form compound 8. The oxidation of 8 at the same homoallylic positions produced compounds 1 and 2. By a similar biosynthetic pathway, compounds 3–5 and 9 were produced from the cyclic condensation and successive dehydration of Tyr with Leu, His with Leu, Phe with Ile, and Phe with Val, respectively. The dehydrogenation is favored to form Z-products because of the steric hindrance of the carbonyl oxygen, while the dehydrogenation of Leu moiety in the N-Me substituted compounds only produced the E-products due to the strong steric hindrance by N-Me. So, compound 10 could be an artifact produced from the photo-isomerization of compound 8 under light during the extraction and the subsequent isolation steps.
Figure 4.
The postulated biosynthetic pathway of 1–3 and 5–8.
2.3. The Bioactivities of Compounds 1–10 from Streptomyces sp. FXJ7.328
Compounds 1 and 3–10 were tested for antivirus effects on H1N1 by the CPE inhibition assay [21,22], separately. The IC50 values of 1, 3–10 and ribavirin (positive control) were 75.5 ± 2.2, 41.5 ± 4.5, 62.6 ± 3.9, 106.5 ± 4.2, 28.9 ± 2.2, 6.8 ± 1.5, 94.5 ± 3.0, 113.8 ± 4.9, 156.6 ± 4.0, and 38.8 ± 1.5 μM, respectively. Except for compounds 3, 6 and 7, the other compounds were inactive (IC50 > 50 μM) against H1N1 influenza virus, indicating that both (Z)-deh-Phe and Leu or (Z)-deh-Leu moieties are necessary for anti-H1N1 activity (Figure 5). The dehydrogenation of Leu increases the activity, while the hydroxylation of deh-Phe or deh-Leu, the inversion of double bonds in deh-Phe and deh-Leu, and the N1-methylation reduce the activity. In addition, the new compounds 1–5 were tested for cytotoxicity against HL-60 and K562 cell lines by MTT method [23], and A549 cell lines by SRB method [24] and for anti-inflammatory effects by inhibition of LPS-mediated NF-κB transcription activity in RAW264.7 cells [25]. The antimicrobial activities of compounds 1–10 against Escherichia coli, Enterobacter aerogenes, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus, and Candida albicans were also evaluated by 2-fold dilution method [26]. The results (Table S1, Supplementary Information) showed that new compounds 1–5 did not exhibit cytotoxicity (IC50 > 100 μM) and anti-inflammatory effects (IC50 > 10 μM); compounds 1–10 did not show antimicrobial effects as well (MIC > 100 μg/mL). Although dehydro-DKPs have been reported to display diverse bioactivities, such as inhibition of protein tyrosine kinase [27], cell cycle arrest [28], inhibition of blood platelet aggregation [29], anti-bacteria [30], antitumor [30] and anti-inflammation [31], the antivirus effect on H1N1 was reported here for the first time.
Figure 5.
The structure-activity relationship (SAR) of compounds 3–7 and 9 for anti-H1N1 viral activity.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were obtained on a JASCO P-1020 digital polarimeter. UV spectra were measured on a Beckman DU 640 spectrophotometer. IR spectra were recorded on a Nicolet Nexus 470 spectrophotometer as KBr disks. CD spectra were collected using a JASCO J-715 spectropolarimeter. NMR data of 1, 2 and 4–6 were measured on a JEOL JNM-ECP 600 spectrometer, and chemical shifts were recorded as δ values. NMR spectra of 1a, 1b, 1c, 2a, 2b, 3, and NOESY spectra of all the compounds were recorded on a Bruker Avance 600 spectrometer. HRESIMS measurements were taken on a Q-TOF ULTIMA GLOBAL GAA076 LC mass spectrometer. Semipreparative HPLC was performed using an ODS column (YMC-pak ODS-A, Kyoto, Japan, 10 × 250 mm, 5 μm, 4.0 mL/min) and chiral separation was performed on chiral column (CHIRALPAK AY-H, Kyoto, Japan, 4.6 × 150 mm, 0.5 mL/min) by HPLC. Marfey’s analysis and C3 Marfey’s analysis were implemented using ODS column (YMC-pak ODS-A, 4.6 × 250 mm, 5 μm, 1.0 mL/min) and C3 column (Agilent Zorbax StableBond C3, Palo Alto, CA, USA, 4.6 × 150 mm, 5 μm, 1.0 mL/min), respectively. TLC and column chromatography (CC, 2.5 × 103 cm) were performed on plates precoated with silica gel GF254 (10–40 μm, Qingdao Marine Chemical Factory, Qingdao, China), and over Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden), respectively. Vacuum-liquid chromatography (VLC, 7 × 40 cm) utilized silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China) and RP-18 (40–63 μm, Merck, Darmstadt, Germany). Sea salt used was made by the evaporation of seawater collected in Laizhou Bay (Weifang Haisheng Chemical Factory, Shangdong, China). Glucose (Shanghai Huixing Biochemical Reagent Co., Ltd., Shanghai, China); beef extract, yeast extract and peptone (Beinjing Shuangxuan Microbe Culture Medium Products Factory, Beijing, China); soluble starch (Beijng Aoboxing Universeen Bio-Tech Co., Ltd., Beijing, China); K2HPO4 (Tianjin Kermel Chemical Reagent Co., Ltd., Tianjin, China); MgSO4 (Shanghai Chemical Reagent Research Institute, Shanghai, China), and CaCO3 (Tianjijn Bodi Chemical Co., Ltd., Tianjin, China).
3.2. Actinomycete Material
The actinomycete strain Streptomyces sp. FXJ7.328 was isolated from coastal sediment collected at Huanghai beach (E 121.706°, N 39.007°), Dalian, China in January 2009. The sediment sample was dried at room temperature, suspended in sterile distilled water, serially diluted, heated in a water bath at 55 °C for 10 min, and spread-plated on oatmeal agar plates (ISP3, medium 3 of the International Streptomyces Project) [32]. After four weeks of incubation at 28 °C, the strain was purified on yeast extract-malt extract agar (ISP2, medium 2 of the International Streptomyces Project) [32], and was identified as a member of the genus Streptomyces on the basis of 16S rRNA gene sequence analysis. Genomic DNA isolation, PCR amplification of 16S rRNA gene and sequence alignment of the strain were performed as described previously [33]. Its 16S rRNA gene sequence (GenBank access No. JF346514) showed 99% similarity with type strains of Streptomyces albus subsp. albus (AB184781), Streptomyces almquistii (AB184258), Streptomyces flocculus (DQ442498), Streptomyces gibsonii (NR_041180) and Streptomyces rangoonensis (NR_041110). The producing strain was prepared on ISP3 medium and stored in Huang’s Lab at 4 °C.
3.3. Fermentation and Extraction
The spores of Streptomyces sp. FXJ7.328 were directly cultured in 500 mL Erlenmeyer flasks containing 150 mL fermentation media consisted of 2% glucose, 0.3% beef extract, 1% yeast extract, 1% soluble starch, 1% peptone, 0.05% K2HPO4, 0.05% MgSO4, 0.2% CaCO3, and 3.3% sea salt (pH 7.0). The cultures were incubated on a rotatory shaker at 180 rpm at 28 °C for eight days. The whole fermentation broth (120 L) was divided into three equal parts that were extracted three times with equal volumes of EtOAc separately. The EtOAc solutions were combined and evaporated under reduced pressure to give a dark brown gum (32.5 g).
3.4. Purification and Identification
The EtOAc extract (32.5 g) was subjected to SiO2 VLC eluting with CH2Cl2-petroleum ether (0%–100%), and then with MeOH-CH2Cl2 (0%–50%), to give nine fractions (Fr.1–Fr.9). Fraction 3 (3.82 g) was separated into three subfractions by gel filtration over Sephadex LH-20 with CH3OH/CH2Cl2 (1:1). Fraction 3-2 (442 mg) was further subjected to HPLC separation eluting with 70% MeOH to yield 7 (5.5 mg, tR = 7.20 min, 0.046 mg/L), 8 (20 mg, tR = 11.5 min, 0.17 mg/L), and 10 (3 mg, tR = 9.5 min, 0.025 mg/L). Fraction 4 (3.2 g) and Fraction 5 (2.78 g) were both separated into five parts (Fr.4-1–Fr.4-5 and Fr.5-1–Fr.5-5) by RP-18 column chromatography, eluting with CH3OH/H2O (5%–100%). Fraction 4-2 (512 mg) was further separated by Sephadex LH-20 with CH3OH/CH2Cl2 (1:1) to give four subfractions (Fr.4-2-1–Fr.4-2-4). Compound 3 (7 mg, 0.058 mg/L) was purified from the precipitate of Fr.4-2-2 (135 mg) after washing with MeOH, and compound 9 (6 mg, tR = 7.4 min, 0.05 mg/L) was obtained from Fr.4-2-3 (163 mg) by HPLC purification eluting with 60% MeOH. Fraction 4-4 (332.7 mg) was separated by Sephadex LH-20 with CH3OH/CH2Cl2 (1:1) to afford four subfractions (Fr.4-4-1–Fr.4-4-4). Fr.4-4-2 (94 mg) was also submitted to HPLC purification on ODS column eluting with 70% MeOH to yield a racemic mixture of 1/2 with an excess of one enantiomer (11 mg, tR = 5.59 min, 0.092 mg/L) which showed two peaks (1:2) by HPLC analysis on a chiral column (CHIRALPAK AY-H, Figure S37, Supplementary Information). Then the chiral separation by HPLC (Amylose tris(5-chloro-2-methylphenylcarbamate)) with EtOH yielded 1 (7 mg, 12.12 min, 0.058 mg/L) and 2 (3 mg, 8.82 min, 0.025 mg/L). Then Fr.4-4-3 (120 mg) was further subjected to HPLC separation eluted with 60% MeOH to yield 5 (3 mg, tR = 10.4 min, 0.025 mg/L) and 6 (4.7 mg, tR = 11.64 min, 0.039 mg/L). Fraction 5-2 (245 mg) was further purified by Sephadex LH-20 with MeOH to produce four subfractions (Fr.5-2-1–Fr.5-2-4). Compound 4 (2.5 mg, tR = 14.7 min, 0.021 mg/L) was obtained from Fr.5-2-3 (63 mg) by HPLC with 45% MeOH.
Compound 1: Yellow solid; UV (MeOH) λmax (log ε): 238 (4.10), 323 (4.51) nm; [α]25D −28 (c 0.05, CH3OH), IR (KBr) νmax 3496, 3208, 3073, 3022, 1675, 1617, 1494, 1454, 1376, 1033, 997, 763, 688 cm−1; 1H and 13C NMR data, Table 1.
Compound 2: Yellow solid; UV (MeOH) λmax (log ε): 238 (4.10), 323 (4.51) nm; [α]25D +28 (c 0.05, CH3OH), IR (KBr) νmax 3496, 3208, 3073, 3022, 1675, 1617, 1494, 1454, 1376, 1033, 997, 763, 688 cm−1; 1H and 13C NMR data, Table 1.
Compound 3: Yellow amorphous solid; UV (MeOH) λmax (log ε): 248 (3.75), 347 (4.10) nm; IR (KBr) νmax 3183, 3073, 2960, 2867, 1681, 1639, 1606, 1541, 1421, 1359, 1275, 1173, 1024, 998, 829, 761 cm−1; 1H and 13C NMR data, Table 1; HRESIMS m/z 273.1231 [M + H]+ (calcd. for C15H17N2O3 273.1234).
Compound 4: Yellow solid; UV (MeOH) λmax (log ε): 250 (3.71), 347 (4.25) nm; IR (KBr) νmax 2973, 2937, 2879, 1708, 1638, 1577, 1459, 1379, 1273, 1122, 1092, 1025, 960 cm−1; 1H and 13C NMR data, Table 1; HRESIMS m/z 247.1189 [M + H]+ (calcd. for C12H15N4O2 247.1190).
Compound 5: White solid; UV (MeOH) λmax (log ε): 229 (4.05), 302 (4.17) nm; [α]25D −36 (c 0.66, CH3OH), IR (KBr) νmax 3168, 3077, 3042, 2961, 2867, 1680, 1646, 1546, 1426, 1392, 1357, 1092, 933, 800, 760, 619 cm−1; 1H and 13C NMR data, Table 1; HRESIMS m/z 259.1439 [M + H]+ (calcd. for C15H19N2O2 259.1441).
(3Z,6S)-3-Benzylidene-6-isobutylpiperazine-2,5-dione (6): [α]25D −54 (c 0.3, DMSO); 1H NMR (600 MHz, DMSO-d6): δ 8.58 (1H, s, NH-1), 9.98 (1H, s, NH-4), 3.94 (1H, m, H-6), 1.58 (2H, m, H-7), 1.80 (1H, m, H-8), 0.89 (3H, d, J = 6.1, H-9/10), 0.88 (3H, d, J = 5.5, H-9/10), 6.67 (1H, s, H-11), 7.48 (2H, d, J = 7.7, H-13/17), 7.39 (2H, t, J = 7.7, H-14/16), 7.30 (1H, t, J = 7.7, H-15). 13C NMR (150 MHz, DMSO-d6): δ 161.0 (Cq, C-2), 127.3 (Cq, C-3), 168.1 (Cq, C-5), 54.2 (CH, C-6), 44.2 (CH2, C-7), 24.1 (CH, C-8), 23.3 (CH3, C-9), 22.6 (CH3, C-10), 114.9 (CH, C-11), 133.9 (Cq, C-12), 129.2 × 2 (CH, C-13/17), 129.8 × 2 (CH, C-14/16), 128.5 (CH, C-15). HRESIMS m/z 259.1456 [M + H]+ (calcd. for C15H19N2O2 259.1441).
3.5. Preparation of p-Bromobenzoate (1c) of Compound 1 [14]
Compound 1 (2 mg, 6.99 μmol) was dissolved in 1 mL of CH2Cl2, and triethylamine (10 μL) and p-bromobenzoyl chloride (20 mg, 92.2 μmol) were added. The mixture was stirred for 7 h at room temperature. Then 2 mL of H2O were added and the solution was extracted three times with CH2Cl2 (5 mL each). The CH2Cl2 solutions were combined and evaporated under reduced pressure to give a gum. p-Bromobenzoate 1c (2 mg, 4.27 μmol, 7.9 min, 61% yield) was obtained by HPLC purification eluting with 85% (MeOH-H2O).
p-Bromobenzoate (1c): Yellow solid; [α]25D −50 (c 0.1, CH3OH); CD (c 0.11, MeOH) λext (Δε) 313 (−5.2), 254 (+4.78), 208 (−1.73). 1H NMR (600 MHz, DMSO-d6): δ 3.18 (3H, s, 1-NCH3), 5.57 (d, J = 9.5 Hz, H-7), 4.09 (m, H-8), 4.20 (dd, J = 7.3, 10.3 Hz, H-9a), 4.28 (dd, J = 6.0, 10.4 Hz, H-9b), 1.14 (3H, d, J = 6.7 Hz, CH3-10), 6.71 (s, H-11), 7.53 (2H, d, J = 7.6 Hz, H-13/17), 7.38 (2H, t, J = 7.5 Hz, H-14/16), 7.30 (t, J = 7.4 Hz, H-15), 7.86 (2H, d, J = 8.5 Hz, H-2′/6′ in p-BrC6H4CO2-), 7.67 (2H, d, J = 8.5 Hz, H-2′/6′ in p-BrC6H4CO2-). 13C NMR (150 MHz, DMSO-d6): δ 31.3 (CH3, 1-NCH3), 158.5 (Cq, C-2), 126.0 (Cq, C-3), 158.8 (Cq, C-5), 131.1 (Cq, C-6), 130.4 (CH, C-7), 31.4 (CH, C-8), 69.3 (CH2, C-9), 17.5 (CH3, C-10), 116.6 (CH, C-11), 133.5 (Cq, C-12), 129.7 × 2 (CH, C-13/17), 129.1 × 2 (CH, C-14/16), 128.0 (CH, C-15), 165.6 (Cq, -CO2- in p-BrC6H4CO2-), 129.4 (Cq, C-1′ in p-BrC6H4CO2-), 132.3 × 2 (CH, C-2′/6′ in p-BrC6H4CO2-), 131.5 × 2 (CH, C-3′/5′ in p-BrC6H4CO2-), 127.9 (Cq, C-4′ in p-BrC6H4CO2-). ESIMS m/z 469.1 and 471.1; HRESIMS m/z 469.0755 [M + H]+ (calcd. for C23H2279BrN2O4 469.0758).
3.6. Preparation of S-MTPA and R-MTPA Esters 1a, 1b, 2a, and 2b of Compounds 1 and 2 [15,16]
Compound 1 (1 mg, 3.50 μmol) was dissolved in 500 μL of anhydrous pyridine and 4-dimethyl-aminopyridine (3 mg, 24.6 μmol) and (R)-MTPACl (10 μL) were added. The reaction was stirred for 12 h at room temperature. Then 1 mL of H2O was added, and the solution was extracted three times with CH2Cl2 (5 mL each). After removal of CH2Cl2 under reduced pressure, the residue was purified by semipreparative HPLC (70% MeOH-H2O) to yield (S)-MTPA ester 1a (1.2 mg, 2.39 μmol, tR = 27.11 min, 68% yield). By the same procedure, (R)-MTPA ester 1b (1.1 mg, 2.19 μmol, tR = 24.74 min, 63% yield), (S)-MTPA ester 2a (0.8 mg, 1.59 μmol, tR = 24.74 min, 45% yield) and (R)-MTPA ester 2b (0.9 mg, 1.79 μmol, tR = 27.11 min, 51% yield) were obtained from the reaction of 1 and 2 (1 mg, 3.50 μmol each) with (S)-MTPACl, (R)-MTPACl and (S)-MTPACl (10 μL each), respectively.
(S)-MTPA ester 1a: White solid. 1H NMR (600 MHz, DMSO-d6): δ 3.07 (3H, s, 1-NCH3), 3.99 (m, H-8), 1.06 (3H, d, J = 6.8 Hz, H-10), 5.51 (d, J = 9.6 Hz, H-7), 6.76 (s, H-11), 4.36 (dd, J = 5.6, 10.5 Hz, H-9a), 4.28 (dd, J = 7.4, 10.4 Hz, H-9b). 7.51 (2H, d, J = 7.5 Hz, H-13/17), 7.41 (2H, t, J = 7.6 Hz, H-14/16), 7.32 (t, J = 7.4 Hz, H-15). HRESIMS m/z 525.1612 [M + Na]+ (calcd. for C26H25F3N2O5Na 525.1608).
(R)-MTPA ester 1b: White solid. 1H NMR (600 MHz, DMSO-d6): δ 3.12 (3H, s, 1-NCH3), 3.99 (m, H-8), 1.07 (3H, d, J = 6.8 Hz, H-10), 5.51 (d, J = 9.6 Hz, H-7), 6.76 (s, H-11), 4.33 (2H, d, J = 6.5 Hz, H-9), 7.50 (2H, d, J = 7.6 Hz, H-13/17), 7.41 (2H, t, J = 7.6 Hz, H-14/16), 7.32 (t, J = 7.4 Hz, H-15). HRESIMS m/z 525.1613 [M + Na]+ (calcd. for C26H25F3N2O5Na 525.1608).
(S)-MTPA ester 2a: White solid. 1H NMR (600 MHz, DMSO-d6): δ 3.12 (3H, s, 1-NCH3), 3.99 (m, H-8), 1.07 (3H, d, J = 6.8 Hz, H-10), 5.51 (d, J = 9.6 Hz, H-7), 6.76 (s, H-11), 4.33 (2H, d, J = 6.5 Hz, H-9), 7.50 (2H, d, J = 7.6 Hz, H-13/17), 7.41 (2H, t, J = 7.6 Hz, H-14/16), 7.32 (t, J = 7.4 Hz, H-15). HRESIMS m/z 525.1607 [M + Na]+ (calcd. for C26H25F3N2O5Na 525.1608).
(R)-MTPA ester 2b: White solid. 1H NMR (600 MHz, DMSO-d6): δ 3.07 (3H, s, 1-NCH3), 3.98 (m, H-8), 1.06 (3H, d, J = 6.8 Hz, H-10), 5.51 (d, J = 9.6 Hz, H-7), 6.76 (s, H-11), 4.35 (dd, J = 5.6, 10.5 Hz, H-9a), 4.27 (dd, J = 7.4,10.4 Hz, H-9b), 7.50 (2H, d, J = 7.5 Hz, H-13/17), 7.41 (2H, t, J = 7.6 Hz, H-14/16), 7.32 (t, J = 7.4 Hz, H-15). HRESIMS m/z 525.1606 [M + Na]+ (calcd. for C26H25F3N2O5Na 525.1608).
3.7. Preparation of FDAA Derivatives of the Acid Hydrolysates of 5 and Four Authentic Isoleucine Samples (l-, l-allo-, d- and d-allo-) and Marfey’s Analysis [18] and C3 Marfey’s Analysis [19,20]
Compound 5 (1 mg, 3.88 μmol) was dissolved in 6 M HCl (1 mL) in a sealed tube and the mixture was heated at 105 °C for 17 h. Then the solution was cooled and evaporated to dryness. The residue, l-Ile, l-allo-Ile and d-Ile, d-allo-Ile, was dissolved in H2O (250 μL each), respectively. 50 μL of each solution was treated with 200 μL of 1% FDAA in acetone followed by 1.0 M NaHCO3 (40 μL). The reaction was maintained 1 h at 45 °C and then quenched by addition of 2.0 M HCl (10 μL). The corresponding FDAA derivatives of hydrolysate of 5, l-Ile, l-allo-Ile, d-Ile and d-allo-Ile were analyzed by ODS HPLC column maintained at 30 °C using the following programs: solvent A, H2O + 0.2% TFA; solvent B, MeCN; linear gradient, 0 min 25% B (75% A), 40 min 60% B (40% A), 45 min 100% B ; UV detection at 340 nm. The retention times for the FDAA derivatives of the hydrolysate of 5, l-Ile, l-allo-Ile, d-Ile and d-allo-Ile were 23.06, 23.06, 23.06, 28.11 and 28.11 min, respectively (Figure S38). The FDAA derivatives of the hydrolysate of 5, l-Ile, and l-allo-Ile were further analyzed by C3 HPLC column maintained at 50 oC. The column was developed with a linear gradient of 15%–60% MeOH/water (+isocratic 5% of a 1% formic acid solution in MeCN) over 55 min with UV detection at 340 nm. The retention times for the FDAA derivatives of the hydrolysate of 5, standard l-Ile, and l-allo-Ile, were 38.50, 38.50, and 37.49 min, respectively (Figure S39).
3.8. Bioassays
Cytotoxicity was assayed by the MTT [23] and SRB method [24]. In the MTT assay, HL-60 cell and K562 cell line were cultured in RPMI-1640 supplemented with 10% FBS under a humidified atmosphere of 5% CO2 and 95% air at 37 °C, 198 μL of cell suspensions with a density of 4.6 × 104 cells mL−1 was plated in 96-well microtiter plates and incubated for 24 h. Then, 2 μL of the test solutions in MeOH were added to each well and further incubated for 36 h. The MTT solution (20 μL, 5 mg/mL in IPMI-1640 medium) was then added to each well and incubated for 4 h. Old medium containing MTT (150 μL) was then gently replaced by DMSO and pipetted to dissolve any formazan crystals formed. Absorbance was then determined on a Spectra Max Plus plate reader at 570 nm. In the SRB assay, 200 μL of the A549 cell suspension was plated in 96-well plates at density of 2 × 105 cell mL−1. Then, 2 μL of the test solutions (in MeOH) were added to each well and the culture was further incubated for 24 h. The cells were fixed with 12% trichloroacetic acid and the cell layer stained with 0.4% SRB. The absorbance of the SRB solution was measured at 515 nm. Adriamycin was used as positive control (IC50 0.652 μM, 0.645 μM and 0.080 μM for HL-60, K562 and A549 cell, respectively).
The antiviral activity against H1N1 was evaluated by the CPE inhibition assay [21,22]. Confluent MDCK cell monolayers were firstly incubated with influenza virus (A/Puerto Rico/8/34 (H1N1), PR/8) at 37 °C for 1 h. After removing the virus dilution, cells were maintained in infecting media (RPMI 1640, 4 μg/mL of trypsin) containing different concentrations of test compounds at 37 °C. After 48 h incubation at 37 °C, the cells were fixed with 100 μL of 4% formaldehyde for 20 min at room temperature. After removal of the formaldehyde, the cells were stained with 0.1% crystal violet for 30 min. The plates were washed and dried, and the intensity of crystal violet staining for each well was measured in a microplate reader (Bio-Rad, Hercules, CA, USA) at 570 nm. The IC50 was calculated as the compound concentration required inhibiting influenza virus yield at 48 h post-infection by 50%. Ribavirin was used as the positive control with an IC50 value of 38.8 μM.
The anti-inflammatory effects of compounds were assayed by inhibition of lipopolysaccharide (LPS)-mediated NF-κB transcriptional activity in RAW 264.7 cells [25]. RAW 264.7 cells (2.5 × 105 cells/well) were placed in a 24-well plate. The cells were then stably transfected with pNF-κB-Luc expression plasmid (0.5 μg/well). Transfections were performed using lipofectamine 2000 in accordance with the instructions of the manufacturer (Invitrogen, Carlsbad, NM, USA). Stably transfected cells were pretreated 2 h with the test compounds and stimulated with 0.1 μg/mL of LPS for an additional 4 h. The luciferase assay was performed with the aid of a Steady-Glo Luciferase assay system in accordance with the instructions of the manufacturer (Promega, Madison, WI, USA).
The antimicrobial activities against E. coli, E. aerogenes, P. aeruginosa, B. subtilis, S. aureus, and C. albicans were evaluated by 2-fold dilution method [26]. The tested strains were cultivated on LB broth for bacteria and in YPD broth for C. albicans at 37 °C. The test compounds were dissolved in DMSO at different concentrations from 100 to 0.78 μg/mL (from 6.25 to 0.025 μg/mL for the positive controls) by the continuous 2-fold dilution methods. The minimum inhibitory concentrations (MICs) were determined in 96-well plates, and each well contains 100 μL of contents composed of 20 μL of inoculums (5 × 105 CFU/mL), test compounds and LB or YPD media. The microtiter plates were incubated at 35 °C for 24 h and were examined for microbes’ growth by turbidity in daylight. The MICs were defined as the lowest concentration at which no visible growth of microbes could be observed. Ciprofloxacin lactate and ketoconazole were used as positive controls for E. coli, E. aerogenes, P. aeruginosa, B. subtilis, S. aureus, and C. albicans with MIC values of 0.05, 0.19, 0.1, 0.39, 3.12 and 0.025 μg/mL, respectively.
4. Conclusions
Five new vinylidene substituted diketopiperazines (DKPs) 1–5 were isolated and their structures including absolute configurations were determined. The new compound 3 displayed modest activity and the known analogs 6 and 7 displayed potent activity against H1N1 virus with IC50 values of 41.5 ± 4.5, 28.9 ± 2.2 and 6.8 ± 1.5 μM, respectively. The results showed that both Z-deh-Phe and Leu or Z-deh-Leu substitutions significantly increase the anti-H1N1 activity of DKPs, while E-isomerization and hydroxylation of both deh-Phe and deh-Leu moieties, and N-methylation reduce the activity. The substitutions of both deh-Phe or deh-Tyr with deh-His and Leu or deh-Leu with iso-Leu also reduce the anti-H1N1 activity. In addition, the 13C NMR data of (3Z,6S)-3-benzylidene-6-isobutylpiperazine-2,5-dione (6) was reported here for the first time.
Acknowledgements
This work was financially supported by the grants from 973 Program of China (No. 2010CB833804), from NSFC (Nos. 21172204, 30973680 & 30670219), from 863 Program of China (Nos. 2013AA092901, 2012AA092104 and 2011AA09070106), from the Knowledge Innovation Program of Chinese Academy of Sciences (No. KSCX2-EW-J-6/KSCX2-EW-G-12B), and the Special Fund for Marine Scientific Research in the Public Interest of China (No. 2010418022-3). The cytotoxic and anti-inflammatory effects were assayed by J. Li’s group, OUC. The antiviral effect on H1N1 was assayed by Wei Wang, one of the authors.
Supplementary Files
Supplementary Information (PDF, 4268 KB)
Footnotes
Samples Availability: Available from the authors.
References
- 1.Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2011;28:196–268. doi: 10.1039/c005001f. [DOI] [PubMed] [Google Scholar]
- 2.Romero F., Espliego F., Baz J.P., Quesada T.G.D., Grávalos D., Calle F.L.D., Fernández-Puentes J.L. Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora. I. Taxonomy, fermentation, isolation, and biological activities. J. Antibiot. 1997;50:734–737. doi: 10.7164/antibiotics.50.734. [DOI] [PubMed] [Google Scholar]
- 3.Renner M.K., Shen Y.C., Cheng X.C., Jensen P.R., Frankmoelle W., Kauffman C.A., Fenical W., Lobkovsky E., Clardy J. Cyclomarins A–C, new antiinflammatory cyclic peptides produced by a marine bacterium (Streptomyces sp.) J. Am. Chem. Soc. 1999;121:11273–11276. doi: 10.1021/ja992482o. [DOI] [Google Scholar]
- 4.Fu P., Yang C.L., Wang Y., Liu P.P., Ma Y.M., Xu L., Su M.B., Hong K., Zhu W.M. Streptocarbazoles A and B, two novel indolocarbazoles from the marine-derived actinomycete strain Streptomyces sp. F MA. Org. Lett. 2012;14:2422–2425. doi: 10.1021/ol3008638. [DOI] [PubMed] [Google Scholar]
- 5.Fu P., Liu P.P., Li X., Wang Y., Wang S.X., Hong K., Zhu W.M. Cyclic bipyridine glycosides from the marine-derived actinomycete Actinoalloteichus cyanogriseus WH1-2216-6. Org. Lett. 2011;13:5948–5951. doi: 10.1021/ol202245s. [DOI] [PubMed] [Google Scholar]
- 6.Fu P., Liu P.P., Qu H.J., Wang Y., Chen D.F., Wang H., Li J., Zhu W.M. α-Pyrones and diketopiperazine derivatives from the marine-derived actinomycete Nocardiopsis dassonvillei HR10-5. J. Nat. Prod. 2011;74:2219–2223. doi: 10.1021/np200597m. [DOI] [PubMed] [Google Scholar]
- 7.Fu P., Wang S.X., Hong K., Li X., Liu P.P., Wang Y., Zhu W.M. Cytotoxic bipyridines from the marine-derived actinomycete Actinoalloteichus cyanogriseus WH1-2216-6. J. Nat. Prod. 2011;74:1751–1756. doi: 10.1021/np200258h. [DOI] [PubMed] [Google Scholar]
- 8.Kanzaki H., Yanagisawa S., Nitoda T. Biosynthetic intermediates of the tetradehydro cyclic dipeptide albonoursin produced by Streptomyces albulus KO-23. J. Antibiot. 2000;53:1257–1264. doi: 10.7164/antibiotics.53.1257. [DOI] [PubMed] [Google Scholar]
- 9.Wang H.S., Yeo S.L., Xu J., Xu X.L., He H., Ronca F., Ting A.E., Wang Y., Yu V.C., Sim M.M. Isolation of streptonigrin and its novel derivative from Micromonospora as inducing agents of p53-dependent cell apoptosis. J. Nat. Prod. 2002;65:721–724. doi: 10.1021/np0104572. [DOI] [PubMed] [Google Scholar]
- 10.Kanzaki H., Imura D., Nitoda T., Kawazu K. Enzymatic dehydrogenation of cyclo l-Phe–l-Leu to a bioactive derivative, albonoursin. J. Mol. Catal. B Enzym. 1999;6:265–270. doi: 10.1016/S1381-1177(98)00079-4. [DOI] [Google Scholar]
- 11.Gurney K.A., Mantle P.G. Biosynthesis of 1-N-methylalbonoursin by an endophytic Streptomyces sp. isolated from perennial ryegrass. J. Nat. Prod. 1993;56:1194–1198. doi: 10.1021/np50097a031. [DOI] [Google Scholar]
- 12.Davies S.G., Rodríguez-Solla H., Tamayo J.A., Cowley A.R., Concellón C., Garner A.C., Parkes A.L., Smith A.D. Asymmetric conjugate reductions with samarium diiodide: Asymmetric synthesis of (2S,3R)- and (2S,3S)-[2-2H,3-2H]-leucine-(S)-phenylalanine dipeptides and (2S,3R)-[2-2H,3-2H]-phenylalanine methyl ester. Org. Biomol. Chem. 2005;3:1435–1447. doi: 10.1039/b500566c. [DOI] [PubMed] [Google Scholar]
- 13.Tuntiwachwuttikul P., Taechowsian T., Wanbanjob A., Thadaniti S., Taylor W.C. Lansai A–D, secondary metabolites from Streptomyces sp. SUC1. Tetrahedron. 2008;64:7583–7586. doi: 10.1016/j.tet.2008.05.104. [DOI] [Google Scholar]
- 14.Gardoso C.L., Bolzani V.D.S., Silva D.H.S., Ishii H., Berova N., Nakanish K. The Absolute configuration of 1-(3′,4′-dihydroxycinnamoyl)cyclopentane-2,3-diol from the amazonian tree Chimarrhis turbinata. J. Nat. Prod. 2006;69:1046–1050. doi: 10.1021/np050522y. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda M., Toriyabe Y., Endo T., Kobayashi J. Application of modified Mosher’s method for primary alcohols with a methyl group at C2 position. Chem. Pharm. Bull. 2003;51:448–451. doi: 10.1248/cpb.51.448. [DOI] [PubMed] [Google Scholar]
- 16.Finamore E., Minale L., Riccio R., Rinaldo G., Zollo F. Novel Marine polyhydroxylated steroids from the starfish Myxoderma platyacanthum. J. Org. Chem. 1991;56:1146–1153. doi: 10.1021/jo00003a043. [DOI] [Google Scholar]
- 17.Takagi M., Motohashi K., Shin-Ya K. Isolation of 2 new metabolites, JBIR-74 and JBIR-75, from the sponge-derived Aspergillus sp. fS14. J. Antibiot. 2010;63:393–395. doi: 10.1038/ja.2010.58. [DOI] [PubMed] [Google Scholar]
- 18.Marfey P. Determination of d-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2, 4-dinitrobenzene. Carlsberg Res. Commum. 1984;49:591–596. doi: 10.1007/BF02908688. [DOI] [Google Scholar]
- 19.Ratnayake R., Fremlin L.J., Lacey E., Gill J.H., Capon R.J. Acremolides A–D, lipodepsipeptides from an Australian marine-derived fungus, Acremonium sp. J. Nat. Prod. 2008;71:403–408. doi: 10.1021/np070589g. [DOI] [PubMed] [Google Scholar]
- 20.Fremlin L.J., Piggott A.M., Ernest L., Capon R.J. Cottoquinazoline A and cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. J. Nat. Prod. 2009;72:666–670. doi: 10.1021/np800777f. [DOI] [PubMed] [Google Scholar]
- 21.Grassauer A., Weinmuellner R., Meier C., Pretsch A., Prieschl-Grassauer E., Unger H. Iota-carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008;5:107. doi: 10.1186/1743-422X-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung H.C., Tseng C.P., Yang J.M., Ju Y.W., Tseng S.N., Chen Y.F., Chao Y.S., Hsieh H.P., Shih S.R., Hsu J.T. Aurintricarboxylic acid inhibits influenza virus neuraminidase. Antivir. Res. 2009;81:123–131. doi: 10.1016/j.antiviral.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistaica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R., et al. New colorimetric cytotoxicity assay for anti-cancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 25.Aquila S., Weng Z.Y., Zeng Y.Q., Sun H.D., Rios J.L. Inhibition of NF-κB activation and iNOS induction by ent-kaurane diterpenoids in LPS-stimulated RAW264.7 murine macrophages. J. Nat. Prod. 2009;72:1269–1272. doi: 10.1021/np9001465. [DOI] [PubMed] [Google Scholar]
- 26.Fu P., Kong F.D., Wang Y.F., Wang Y., Liu P.P., Zuo G.Y., Zhu W.M. Antibiotic metabolites from the coral-associated actinomycete Streptomyces sp. OUCMDZ-1703. Chin. J. Chem. 2013;31:100–104. [Google Scholar]
- 27.Li W.R., Peng S.Z. Rational design and synthesis of unsaturated 2,5-dioxopiperazine derivatives as potential protein tyrosine kinase inhibitors. Tetrahedron Lett. 1988;39:7373–7376. [Google Scholar]
- 28.Kanzaki H., Yanagisawas S., Kanoh K., Nitoda T. A novel potent cell cycle inhibitor dehydrophenylahistin-enzymatic synthesis and inhibitory activity toward sea urchin embryo. J. Antibiot. 2002;55:1042–1047. doi: 10.7164/antibiotics.55.1042. [DOI] [PubMed] [Google Scholar]
- 29.Shigeki S. Preparation of 2,5-piperazinediones as blood platelet aggregation inhibitors. 01013074A. Jpn. Patent. 1989 Jan;
- 30.Fukushima K., Yazawa K., Arai T. Bological activities of albonoursin. J. Antibiot. 1973;26:175–176. doi: 10.7164/antibiotics.26.175. [DOI] [PubMed] [Google Scholar]
- 31.Taechowisan T., Wanbanjob A., Tuntiwachwuttikul P., Liu J.K. Anti-inflammatory activity of lansais from endophytic Streptomyces sp. SUC1 in LPS-induced RAW 264.7 cells. Food Agric. Immunol. 2009;20:67–77. doi: 10.1080/09540100902730064. [DOI] [Google Scholar]
- 32.Küster E. Outline of a comparative study of criteria used in characterization of the actinomycetes. Int. Bull. Bacteriol. Nomencl. Taxon. 1959;9:97–104. [Google Scholar]
- 33.Chun J., Goodfellow M. A phylogenetic analysis of the genus Nocardia with 16s rRNA gene sequences. Int. J. Syst. Bacteriol. 1995;2:240–245. doi: 10.1099/00207713-45-2-240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information (PDF, 4268 KB)