Abstract
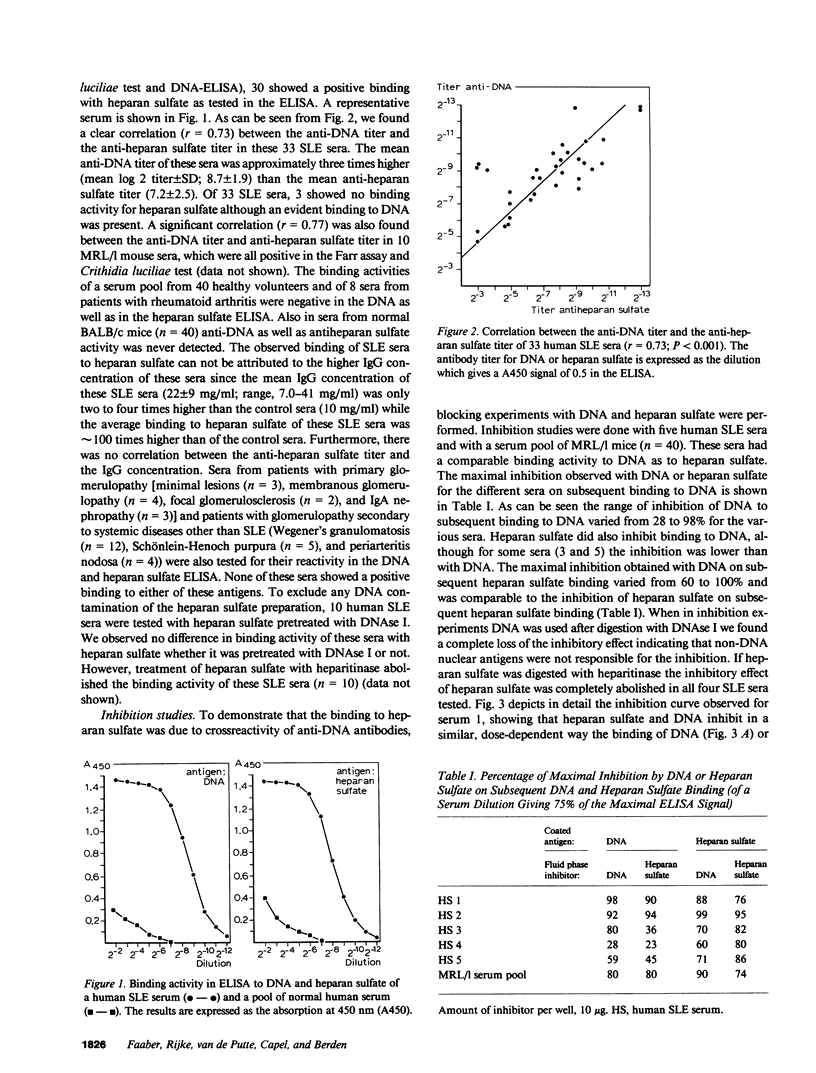
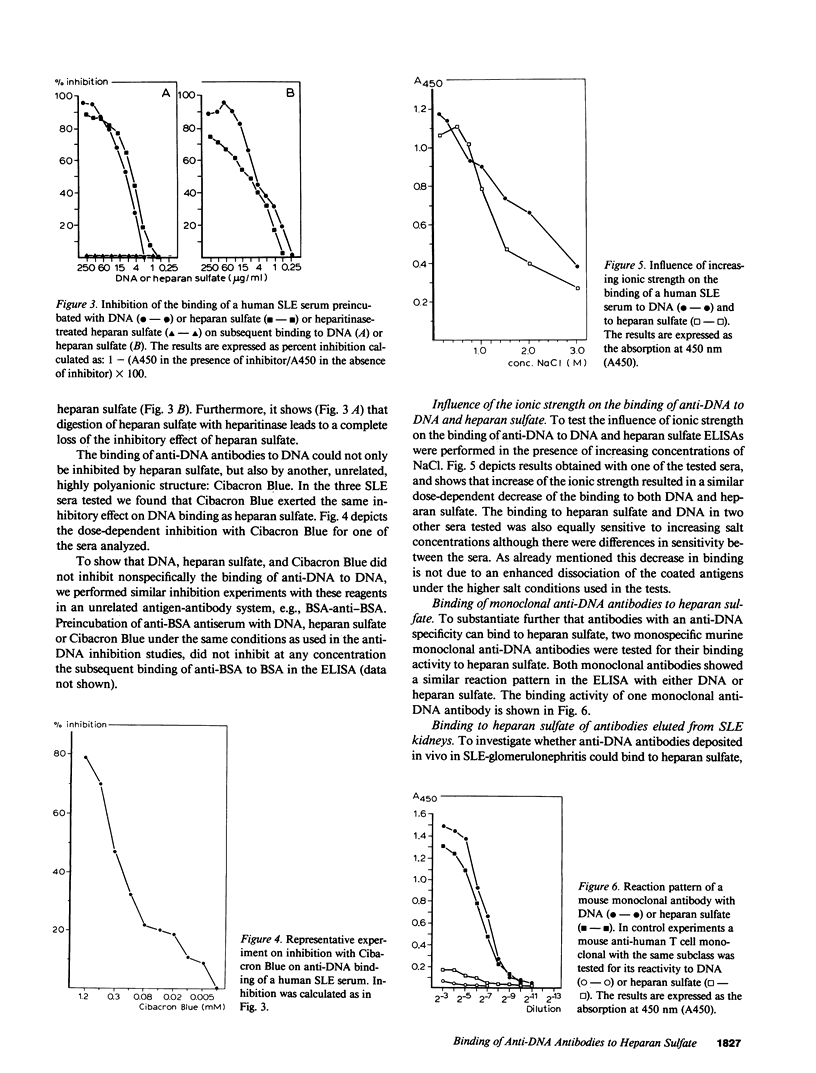
In 30 of 33 human systemic lupus erythematosus (SLE) sera and in 10 sera from MRL/l mice with spontaneous SLE, antibodies against heparan sulfate were detected. The anti-heparan sulfate titers showed a significant correlation with the anti-DNA antibody titers. By inhibition studies it was demonstrated that heparan sulfate could inhibit the binding of anti-DNA antibodies to DNA, whereas DNA could block the binding to heparan sulfate. That this reaction is due to crossreactivity of anti-DNA antibodies was further substantiated by the finding that two monoclonal anti-DNA antibodies also bound to heparan sulfate. Antibodies eluted from human and mouse kidneys with diffuse SLE glomerulonephritis showed a similar binding to DNA and heparan sulfate when these eluted antibodies were tested in vitro. Heparan sulfate is the major glycosaminoglycan constituent of the glomerular basement membrane. Our findings suggest that heparan sulfate might serve as a target antigen in vivo for cross-reactive anti-DNA antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., Lakmaker F., Feltkamp T. E. Immunology of DNA. I. The influence of reaction conditions on the Farr assay as used for the detection of anti-ds DNA. J Immunol Methods. 1976;10(1):27–37. doi: 10.1016/0022-1759(76)90004-1. [DOI] [PubMed] [Google Scholar]
- Aarden L. A., de Groot E. R., Feltkamp T. E. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann N Y Acad Sci. 1975 Jun 30;254:505–515. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André-Schwartz J., Datta S. K., Shoenfeld Y., Isenberg D. A., Stollar B. D., Schwartz R. S. Binding of cytoskeletal proteins by monoclonal anti-DNA lupus autoantibodies. Clin Immunol Immunopathol. 1984 May;31(2):261–271. doi: 10.1016/0090-1229(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Barnes J. L., Radnik R. A., Gilchrist E. P., Venkatachalam M. A. Size and charge selective permeability defects induced in glomerular basement membrane by a polycation. Kidney Int. 1984 Jan;25(1):11–19. doi: 10.1038/ki.1984.2. [DOI] [PubMed] [Google Scholar]
- Border W. A., Ward H. J., Kamil E. S., Cohen A. H. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J Clin Invest. 1982 Feb;69(2):451–461. doi: 10.1172/JCI110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen W., Burdick G. Purification of DNA antibodies using cibacron blue F3GA affinity chromatography. J Immunol Methods. 1983 Aug 26;62(2):205–215. doi: 10.1016/0022-1759(83)90248-x. [DOI] [PubMed] [Google Scholar]
- Faaber P., Capel P. J., Rijke G. P., Vierwinden G., van de Putte L. B., Koene R. A. Cross-reactivity of anti-DNA antibodies with proteoglycans. Clin Exp Immunol. 1984 Mar;55(3):502–508. [PMC free article] [PubMed] [Google Scholar]
- Faaber P., vd Broek M. F., Rijke T. P., Capel P. J., Berden J. H. Direct binding of monomeric anti-DNA antibodies to Raji cells. Scand J Immunol. 1985 Nov;22(5):539–548. doi: 10.1111/j.1365-3083.1985.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Hunsicker L. G., Shearer T. P., Shaffer S. J. Acute reversible proteinuria induced by infusion of the polycation hexadimethrine. Kidney Int. 1981 Jul;20(1):7–17. doi: 10.1038/ki.1981.98. [DOI] [PubMed] [Google Scholar]
- Jacob L., Tron F., Bach J. F., Louvard D. A monoclonal anti-DNA antibody also binds to cell-surface protein(s). Proc Natl Acad Sci U S A. 1984 Jun;81(12):3843–3845. doi: 10.1073/pnas.81.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T., Nagasawa R., Nagata N., Shirai T. Specificity of mouse hybridoma antibodies to DNA. Immunol Lett. 1982 Feb;4(2):93–97. doi: 10.1016/0165-2478(82)90006-2. [DOI] [PubMed] [Google Scholar]
- Koike T., Tomioka H., Kumagai A. Antibodies cross-reactive with DNA and cardiolipin in patients with systemic lupus erythematosus. Clin Exp Immunol. 1982 Nov;50(2):298–302. [PMC free article] [PubMed] [Google Scholar]
- Lafer E. M., Rauch J., Andrzejewski C., Jr, Mudd D., Furie B., Furie B., Schwartz R. S., Stollar B. D. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981 Apr 1;153(4):897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard K. M., Webb J. Partial purification of anti-DNA antibodies from systemic lupus erythematosus serum by dye-ligand chromatography. J Immunol Methods. 1982 Oct 15;54(1):81–94. doi: 10.1016/0022-1759(82)90116-8. [DOI] [PubMed] [Google Scholar]
- Scott P. G. Macromolecular constituents of basement membranes: a review of current knowledge on their structure and function. Can J Biochem Cell Biol. 1983 Aug;61(8):942–948. doi: 10.1139/o83-120. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Schwartz R. S. Immunologic and genetic factors in autoimmune diseases. N Engl J Med. 1984 Oct 18;311(16):1019–1029. doi: 10.1056/NEJM198410183111605. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tron F., Jacob L., Bach J. F. Binding of a murine monoclonal anti-DNA antibody to Raji cells. Implications for the interpretation of the Raji cell assay for immune complexes. Eur J Immunol. 1984 Mar;14(3):283–286. doi: 10.1002/eji.1830140316. [DOI] [PubMed] [Google Scholar]
- Vernier R. L., Klein D. J., Sisson S. P., Mahan J. D., Oegema T. R., Brown D. M. Heparan sulfate--rich anionic sites in the human glomerular basement membrane. Decreased concentration in congenital nephrotic syndrome. N Engl J Med. 1983 Oct 27;309(17):1001–1009. doi: 10.1056/NEJM198310273091701. [DOI] [PubMed] [Google Scholar]
- de Groot E. R., Lamers M. C., Aarden L. A., Smeenk R. J., van Oss C. J. Dissociation of DNA/anti-DNA complexes at high pH. Immunol Commun. 1980;9(5):515–528. doi: 10.3109/08820138009066012. [DOI] [PubMed] [Google Scholar]
- de Heer E., Daha M. R., van Es L. A. Lymph node cells from rats with Heymann's nephritis produce in vitro autoantibodies directed against purified renal tubular antigen. Immunology. 1984 Aug;52(4):743–752. [PMC free article] [PubMed] [Google Scholar]



