Abstract
The marine sponge, Axinyssa djiferi, collected on mangrove tree roots in Senegal, was investigated for glycolipids. A mixture containing new glycosphingolipids, named axidjiferoside-A, -B and -C, accounted for 0.07% of sponge biomass (dry weight) and for 2.16% of total lipids. It showed a significant antimalarial activity, with a 50% inhibitory concentration (IC50) of 0.53 ± 0.2 μM against a chloroquine-resistant strain of Plasmodium falciparum. They were identified as homologous β-galactopyranosylceramides composed of 2-amino-(6E)-octadec-6-en-1,3,4-triol, and the major one, axidjiferoside-A (around 60%), contained 2-hydroxytetracosanoic acid. Cytotoxicity was studied in vitro on human cancer cell lines (multiple myeloma, colorectal adenocarcinoma, glioblastoma and two lung cancer NSCLC-N6 and A549). Results of this investigation showed that axidjiferosides are of interest, because they proved a good antiplasmodial activity, with only a low cytotoxicity against various human cell lines and no significant antitrypanosomal and antileishmanial activity. Thus, it seems that galactosylceramides with a β anomeric configuration may be suitable in searching for new antimalarial drugs.
Keywords: Axinyssa, marine sponge glycolipids, glycosphingolipids, antimalarial activity, cytotoxicity
Abbreviations
- COSY
homonuclear correlation spectroscopy
- FAME
fatty acid methyl esters
- LCB
long chain base
- GC-MS
gas chromatography-mass spectrometry
- GL
glycolipid(s)
- GSL
glycosphingolipid(s)
- amu
atomic mass unit
- HMBC
heteronuclear multiple bond coherence
- HSQC
heteronuclear single quantum coherence
- IC50
50% inhibitory concentration
- ESI-MS
electrospray ionization mass spectrometry
- TLC
thin layer chromatography
- dw
dry weight
- MEC
minimum effective concentration
1. Introduction
Malaria caused by the Anopheles mosquito-transmitted parasite, Plasmodium, is one of the leading causes of mortality and morbidity in more than 100 tropical and subtropical countries of the world, P. falciparum remaining the most dangerous species and causing the most lethal form of malaria [1,2]. In spite of some important studies towards the development of an efficient vaccine, there is only a limited number of drugs in widespread use for the treatment of malaria [3,4]. In addition to this paucity of drugs is the rapid development of resistance of this parasite to standard antimalarial drugs, leading to the increasing number of deaths from malaria in sub-Saharan African countries [5,6]. Therefore, the rational development of novel pharmacophores for the purpose of malaria intervention requires the identification of new chemotherapeutic targets [7,8]. The potential of natural products is currently investigated [9]. The new breakthrough in malaria treatment could come with the development of a marine lead compound, taking into account the great potential of marine invertebrates to produce a large array of biological-active metabolites [10,11]. Interestingly, the latter reviews on marine antimalarials published in 2009 did not mention any glycolipid (GL), and it was the same from any other natural sources, including plants [9]. Nevertheless, four new ether diglycosides, named matayosides, were isolated from the Brazilian plant, Matayba guianensis, and inhibited the growth of P. falciparum in vitro [12]. The administration of the natural killer T (NKT)-cell ligand α-galactosylceramide, KRN 7000, a synthetic compound originally derived from the sponge glycosphingolipids, named agelasphins, resulted in rapid, strong antimalarial activity, inhibiting the development of the intrahepatocytic stages of P. yoelii and P. berghei [13]. It was the first GL to show the ability of potent antimalarial activity [14,15].
Glycolipids are ubiquitous cell membrane constituents in animals, which play a fundamental role in major phenomena, such as cell-cell recognition and antigenic specificity [16]. They exhibit a wide range of biological functions that might be related to the amphipathic nature of the molecule [15,16]. Several GL, mainly glycosphingolipids (GSL) in particular cerebrosides, have been isolated from a number of marine sources, mainly sponges and echinoderms, and they displayed immunomodulating and antitumor activities [15,16,17,18,19,20,21,22,23,24].
In a previous work on the Senegalese sponge, Axinyssa djiferi [25], we isolated a GSL complex mixture, which included nine principal closely related compounds, named axidjiferosides, responsible for an interesting antimalarial activity [26]. In the present work, we wished to confirm the presence of such GSL in this sponge and isolate and identify the main component responsible for the antimalarial activity. Thus, a mixture of only three homologous GSL, named axidjiferoside-A, -B and -C, was isolated from the GSL fraction and displayed an antimalarial activity. The main component, axidjiferoside-A, was characterized by the usual spectroscopic methods. Furthermore, it seemed interesting to study the cytotoxicity and the potential activity of these axidjiferosides on other parasites from the genera Leishmania and Trypanosoma.
2. Results and Discussion
2.1. Glycolipid Isolation and Structure Determination
The specimens of the Senegalese sponge, Axinyssa djiferi [25,26], were extracted with dichloromethane-methanol mixtures, and the crude lipids were fractionated on a silica gel column, eluted with chloroform, acetone (glycolipids) and, then, methanol. Several successive chromatographic steps allowed the isolation of a fraction containing the mixture of three glycosphingolipids (GSL) named axidjiferoside-A, -B and -C. This axidjiferoside mixture accounted for 0.07% of the sponge biomass (dw), 2.16% of total lipids and 17.45% of the total GL and was used for biological studies. The chemical structure and composition of axidjiferosides were obtained by electrospray ionization mass spectrometry (ESI-MS) and NMR studies of the peracetylated GL and by controlled chemical degradation. The peracetylated axidjiferosides exhibited the characteristic signals of a sphingoid base and a β-galactopyranose in the 1H-NMR spectrum (Figure 1, Table 1). The ESI-MS showed three molecular ion peaks, corresponding to galactosylceramides. Indeed, the peracetylated major component, axidjiferoside-A, displayed an adduct ion [M + Na]+ at m/z 1160.7436 (high resolution ESI-MS) in accordance with the formula C62H107NO17Na (a molecular mass of 843.7430 amu (atomic mass unit) for the intact GSL). In addition to the major GSL, two minor GSL (axidjiferoside-B and -C) displayed sodiated molecular ions at m/z 1146.7280 and 1174.7593, in accordance with a methylene less or more than the major one.
Figure 1.
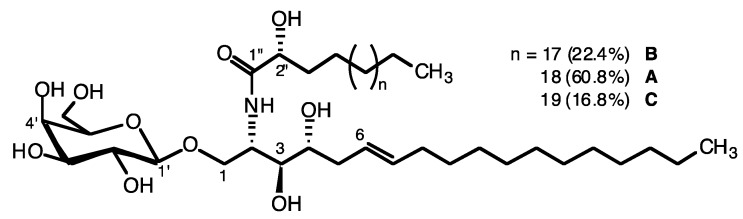
Axidjiferoside-A, B and C, glycosphingolipids from Axinyssa djiferi.
Table 1.
1H (500 MHz) and 13C (125.75 MHz) NMR data for peracetylated axidjiferosides in CDCl3.
| Position | δH ppm, mult., J in Hz | δC ppm |
|---|---|---|
| 1a | 3.88 (dd, J = 10.6/3.1) | 66.19 |
| 1b | 3.70 (dd, J = 10.6/2.7) | - |
| 2 | 4.33 (m) | 48.09 |
| 2-NH | 6.81 (d, J = 9.0) | - |
| 3 | 5.12 (m) | 71.95 |
| 4 | 4.95 (m) | 72.64 |
| 5 | 2.40 (m) | 20.75 |
| 6 | 5.50 (dt, J = 6.8/15.0) | 124.03 |
| 7 | 5.28 (dt, J = 7.0/15.0) | 134.58 |
| 8 | 1.85 (m) | 24.86 |
| terminal methyl | 0.87 (t, J = 6.8) | 19.23 |
| acetates | 2.05/2.25/2.17/2.06/2.11/1.99/2.07 (7 s) | 169.44/169.86/170.0/170.06/170.18/170.36/170.74 |
| CH2 (C9–C17) | 1.27 (m) | 29.21–30.06 |
| CH2 (C4″–C20″) | 1.32 (m) | 29.21–30.06 |
| 1′ | 4.47 (d, J = 7.8) | 100.66 |
| 2′ | 5.17 (m) | 73.88 |
| 3′ | 5.02 (dd, J = 10.4/3.3) | 70.79 |
| 4′ | 5.38 (d, J = 3.3) | 66.96 |
| 5′ | 3.95 (t, J = 6.7) | 70.79 |
| 6′ | 4.15 (d, J = 6.7) | 61.06 |
| 1″ | - | 171.08 |
| 2″ | 5.14 (t, J = 3.6) | 68.6 |
| 3″ | 1.31 (m) | 34.41 |
The structure of axidjiferoside-A, -B and -C was determined on the basis of chemical and spectroscopic evidence. Thus, they contained an unsaturated long-chain amino alcohol as a sphingoid base. All the chemical shifts of the ceramide are given in the 13C and 1H NMR spectra (Table 1). The sugar linked to the ceramide was identified as galactopyranose by NMR spectroscopy. First of all, the anomeric proton of the β-galactopyranose (δH = 4.47, d, J = 7.8 Hz) was correlated with the anomeric carbon at δC = 100.66 ppm in the heteronuclear single quantum coherence (HMQC) spectrum. Starting from this proton, all the 1H and 13C NMR signals of the sugar were assigned by using the homonuclear correlation spectroscopy (COSY), HMQC and heteronuclear multiple bond coherence (HMBC) spectra, and the vicinal proton-proton coupling constants were determined (Table 1). The galacto configuration of the sugar, as well as its β anomeric configuration, was established on the basis of the ring proton coupling constants (J1,2 = 7.8 Hz, J2,3 = 10.4 Hz, J3,4 = 3.3 and J4,5 = 6.7 Hz). The linkage of the galactopyranoside to the ceramide was confirmed by the three bond 13C–1H couplings of anomeric C-1′ with H-1a and H-1b, observed in the HMBC spectrum. The location of the double bond and its configuration in the dihydrosphingosine moiety was determined from the 1H-NMR spectrum in CDCl3 with a double triplet at 5.50 ppm (J = 15.0, 7.0 Hz) and a double triplet at 5.28 ppm (J = 15.0, 6.8 Hz), characterizing an unusual (E)-Δ6 phytosphingosine. This was supported by two carbon signals at δ 20.75/24.86 for the carbons next to the double bond in the 13C-NMR spectrum [21,24], which were assigned by means of COSY and HMQC. COSY correlations were observed between H-5 and H-6 and also H-7 and H-8. Also, the key HMBC correlations from H-5 to C-6, C-7 and C-8 confirmed the location of the double bond. In addition, NMR spectrum showed the presence of terminal methyl groups.
To confirm the structure of the fatty acid methyl esters (FAME) and the long-chain base (LCB), axidjiferosides were subjected to an acid methanolysis. GC-MS analysis of the FAME indicated that the fatty acid moiety was C22–24 methyl 2-hydroxylated (mainly C24). Indeed, the 2-hydroxy FAME produced the characteristic ions at m/z 90 (McLafferty) and m/z 103. The results were as follows: 2-OH-23:0, tR = 39.6 min (22.4%), m/z 384 (M+); 2-OH-24:0, tR = 42.0 min (60.8%), m/z 398 (M+); 2-OH-25:0, tR = 44.3 min (16.8%), m/z 412 (M+). These fatty acid structures were confirmed by GC-MS analysis of N-acyl pyrrolidides showing fragment ions at m/z 129 (McLafferty) and the expected corresponding molecular ions. The peracetylated methylglycoside from axidjiferosides analyzed by GC-MS was shown to be methyl galactopyranoside, with a retention time similar to that of an authentic sample. Other diagnostic ions were observed at m/z 331 (M–OMe)+, 303 (M–OAc)+, 243, 200, 157, 145 and 115. The sphingoid base was identified as 2-amino-1,3,4-trihydroxy-octadecene, tR = 34.12 min, m/z 482 (M+), and it produced the characteristic ions at m/z 43 (base peak) and m/z 144. The 2S,3S,4R stereochemistry was assumed by comparison of the 13C-NMR chemical shifts of C-2 and C-3 with those of particular GSL, halicerebrosides, amphiceramides and plakosides [18,21,24,27]. The absolute stereochemistry at C-2′ in the 2-hydroxy acyl chains is also assumed to be (R), as usually found for glycosylceramides from sponges [16,18,21,24].
In conclusion, axidjiferosides were identified as three homologous β-galactopyranosylceramides composed of 2-amino-(6E)-octadec-6-en-1,3,4-triol and the major one, axidjiferoside-A (around 60%), contained 2-hydroxytetracosanoic acid.
2.2. Antimalarial, Antileishmanial, Antitrypanosomal and Antiproliferative Activity
The antimalarial activity of axidjiferosides was evaluated against the chloroquine-resistant FcB1/Colombia strain of Plasmodium falciparum (50% inhibitory concentration (IC50) value for chloroquine of 0.1 μM) [28]. The antiplasmodial activity was determined according to Labaied et al. [29]. Axidjiferosides showed a significant activity against P. falciparum FcB1, with an IC50 of 0.53 ± 0.2 μM.
Axidjiferosides were evaluated in vitro for other antiparasitic activities against Leishmania donovani (donovani forms) and Trypanosoma brucei (bloodstream forms) and showed no activity against these parasites (IC50 > 100 μM and minimum effective concentration (MEC) > 100 μM, respectively). The antiproliferative activity of axidjiferosides was investigated upon the human cancer cell lines [22,30]. Cytotoxicity and antitumor activity of axidjiferosides, with IC50 > 35 μM on two human non-small cell lung cancer cell lines (NSCLC-N6 and A549) and with IC50 > 60 μM on three human cancer cell lines (KMS-11, GBM and HCT-116), were non-significant (Table 2).
Table 2.
50% inhibitory concentration (IC50) and minimum effective concentration (MEC) values (μM) for axidjiferosides.
| Plasmodium falciparum FCB1 | 0.53 ± 0.2 |
|---|---|
| Leishmania donovani | IC50 > 100 |
| NSCLC-N6 | IC50 > 35 |
| A 549 | IC50 > 35 |
| KMS-11 | IC50 > 60 |
| GBM | IC50 > 60 |
| HCT-116 | IC50 > 60 |
| Trypanosoma brucei | MEC > 100 |
It is of interest to compare the antiproliferative activity of axidjiferosides with those of several GSL having also a single sugar, which is most frequently β-d-glucopyranose, rather than β-d-galactopyranose. The biological activity of such GSL is nowadays little documented. Halicerebrosides from the sponge Haliclona sp. are glucosylceramides with a sphingoid base unsaturated at C-6/C-7, like axidjiferosides. Thus, the only notable difference in their structure is the presence of a glucose instead of a galactose. Halicerebrosides displayed a cytotoxic activity against murine leukemia cells, P388 (IC50 of 20 μg/mL) [24]. Halicylindrosides from the sponge Halichondria cylindrata are GSL containing N-acetyl-glucosamine as the sugar head. These GSL showed a cytotoxic activity on leukemia cells, P388, with IC50 of 6.8 μg/mL [31]. Furthermore, ophidiacerebrosides isolated from some starfishes showed an antiproliferative activity against murine leukemia cells, L1210, at 2 μg/mL [17]. These GSL contain a β-glucopyranoside as the sugar head and a 9-methyl-branched 4,8,10-triunsaturated long-chain amino alcohol as the sphingoid base. One of them, ophidiacerebroside-C from the starfish Narcissia canariensis, displayed an interesting cytotoxic activity on adherent human cancerous cell lines (multiple myelomas, colorectal adenocarcinoma, multiform glioblastoma) with an IC50 around 20 μM [23]. Interestingly, the IC50 values found for axidjiferosides on the same cancer cells were found to be superior, 60 μM. We could then conceive that the presence of a galactose decreases or cancels the cytotoxicity of the GSL. Unfortunately, data on the cytotoxicity of a galactosylceramide are missing to date. Plakosides A and B, from the sponge Plakortis simplex, are β-galactosylceramides possessing a prenylated sugar hydroxyl group and cyclopropane-containing alkyl chains. They are immunosuppressors with a non-cytotoxic mechanism of action [18].
Activity of axidjiferosides against P. falciparum was five-times less than those of chloroquine (IC50 = 0.10 μM), but, on the other hand, axidjiferosides had no high cytotoxicity effect. In addition, the compound exhibited a specific antimalarial activity, since no activity was observed against kinetoplatids. So, it is of interest for further research work on malaria treatment.
3. Experimental Section
3.1. General Procedures
High resolution electrospray ionization mass spectrometry (HR-ESI-MS, positive mode, ion-source acceleration 4.5 kV, ion-source temperature 200 °C, methanol as solvent) mass spectra were recorded with a Micromass Zab Spec Tof spectrometer. 1H- and 13C-NMR, as well as 2D-NMR spectra were obtained on a NMR Bruker Avance-500 spectrometer with triple Probe TBI multinuclear in CDCl3 at 500.13 MHz and 125.76 MHz, respectively, with reference to an internal standard of tetramethylsilane. Chemical shifts and coupling constants were expressed in δ (ppm) and Hz respectively. GC-MS spectra were performed on a Hewlett-Packard 5890II gas chromatograph linked to a HP 5989A spectrometer and a HP 98785A integrator, moderately polar column DB-1, 30 m length × 0.25 mm i.d. × 0.33 μm phase thickness. The column temperature was varied, after a pause of 2–4 min from the injection, from 110 to 310 °C, with a slope of 3 °C·min−1. Analytical thin layer chromatography (TLC) was performed on precoated silica gel F254 plates. After development, the dried plates were sprayed with 50% H2SO4-vanillin and orcinol reagents. High-performance liquid chromatography (HPLC) was carried out with an Intersphere 5 μm ODS1, C18, 250 × 4.6 mm, Interchim, with a 230 nm refractive or UV detector.
3.2. Animal Material
Axinyssa djiferi (Figure 2) [25,26] is found along the Senegalese coasts about 150 km south of Dakar, near the village of Djifer, on mangrove tree roots, near the surface at low tide and was collected by hand during an expedition organized by Oceanium of Dakar, on the site named Keur Bamboung (Sine-Saloum). The sponge specimens were frozen shortly after collection and then freeze-dried. A voucher specimen was deposited in the Museum National d’Histoire Naturelle MNHN, Paris, France.
Figure 2.
The sponge Axinyssa djiferi.
3.3. Lipid Extraction and Axidjiferoside Isolation
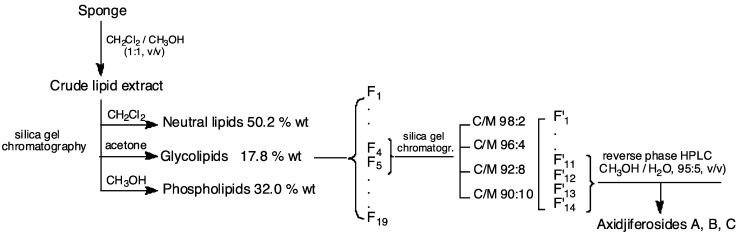
Whole bodies of the collected specimens were chopped and investigated for their GL fractions. They were twice extracted with CH2Cl2/MeOH (1:1, v/v) at room temperature. The combined extracts were concentrated in vacuo to give the crude extract, which was partitioned between H2O and CH2Cl2. The dried organic layer was concentrated in vacuo, and the residue was submitted to a column chromatography over silica gel (230–400 mesh), eluting with pure solvents of increasing polarity, to obtain the different lipid classes: dichloromethane (neutral lipids, 50.2%), acetone (GL, 17.8%) and methanol (phospholipids, 32.0%) (Figure 3).
Figure 3.
Schematic outline of the experimental protocol for isolation and purification of axidjiferosides.
The last fractions, F′11–F′14, were pooled and purified by repeated reverse phase HPLC (MeOH/H2O, 95:5, v/v) achieved on a 250 × 4.6 mm, Intersphere 5 μm ODS1, Interchim column, until obtaining the fraction containing only axidjiferoside-A, -B and -C. It presented a single spot with a similar polarity to a commercial standard (galactocerebroside with a 2-hydroxy fatty acyl chain type I) (Rf = 0.42 on silica gel thin layer chromatography, CH2Cl2/MeOH/CH3CN, 99:1:2, v/v/v).
3.4. Acid Methanolysis of Axidjiferosides
An aliquot (3 mg) of axidjiferoside mixture was heated with 1 mL of MeOH/H2O/HCl (29:4:3, vol/vol/vol) at 80 °C for 16 h. The reaction mixture was extracted with H2O/CH2Cl2 (3:9, vol/vol). Methyl glycosides were obtained from the aqueous layer, whereas the organic layer contained a mixture of fatty acid methyl esters and the sphingoid base. The FAME were transformed into N-acyl pyrrolidides by heating in a pyrrolidine/acetic acid (10:2, vol/vol, 1 mL) during 1 h at 85 °C. The peracetylated methyl glycosides from axidjiferosides (acetic anhydride/dry pyridine, room temperature) were analyzed by GC-MS [column temperature 110 °C (2 min) and then (temperature increasing at 3 °C/min until 240 °C)]; tR = 37.1 min (methyl galactopyranoside), similar to that of an authentic sample.
3.5. Antiplasmodial Activity of Axidjiferosides
According to Labaied et al. [29], extracts were prepared in DMSO at a concentration of 10 mg/mL and serially diluted with culture medium before being added to asynchronous parasite cultures (1% parasitemia and 1% final hematocrite) in 96-well microplates. Plates were maintained for 24 h at 37 °C. 0.5 μCi of [3H]hypoxanthine was then added to each well, and parasites were maintained for another 24 h. Growth inhibition was determined by comparison of the radioactivity incorporated into the treated culture with that in the control culture maintained on the same plate. Concentrations causing 50% inhibition of parasite growth (IC50) were calculated from the drug concentration-response curves. Each experiment was performed in triplicate.
3.6. Antileishmanial Activity of Axidjiferosides
Antileishmanial activity was evaluated on the Leishmania donovani (MHOM/ET/67/HU3) line, called LV9. Promastigote forms were grown in M-199 medium supplemented with 10% inactivated fetal calf serum, 40 mM HEPES, 100 μM adenosine and 0.5 mg·L−1 hemin in the presence of 50 μg·mL−1 gentamicin at 26 °C in 5% CO2. Peritoneal macrophages were harvested from female CD1 mice three days after an intraperitoneal injection of 1.5 mL of sodium thioglycolate (Biomérieux) and were dispensed into eight-well chamber slides (LabTek Ltd., Melbourn, England) at a density of 5 × 104 cells per well (400 μL per well) in RPMI 1640 medium supplemented with 10% FCS, 25 mM HEPES and 2 mM glutamine. Four hours after, wells were washed to eliminate fibroblasts. After a 24 h incubation period, the macrophages were infected with promastigotes at a stationary phase in a ratio of 10 parasites per macrophage. After 18 h, free promastigotes were eliminated, and intramacrophagic amastigotes were treated with various concentrations of compounds. Pentamidine and amphotericin B were used as reference compounds. The culture medium was renewed 48 h later, and a new culture medium containing the drug was added. The experiment was stopped after five days, and the percentages of infected macrophages were evaluated microscopically after Giemsa staining. IC50 values were determined by linear regression analysis. Each experiment was performed in triplicate.
3.7. Antitrypanosomal Activity of Axidjiferosides
Antitrypanosomal evaluation was performed as follows: the bloodstream forms of T. brucei were purified by centrifugation from the blood of an infected mouse and were maintained in vitro for 24 h at 37 °C in a 5% CO2 atmosphere in a minimum essential medium supplemented with 25 mM HEPES, Earle’s salts, 2 mM glutamine, 1 g·L−1 of glucose, minimum essential medium non-essential amino acids, 0.2 mM 2-mercaptoethanol, 2 mM sodium pyruvate, 0.1 mM hypoxanthine, 0.016 mM thymidine, 15% heat-inactivated horse serum and 50 μg·mL−1 of gentamycin. Drug evaluation was carried out in 96-well plates in a final volume of 200 μL containing 2 × 105 trypomastigotes and the compounds to be tested. Pentamidine was used as the reference compound. The minimum effective concentration (MEC) was defined as the minimum concentration at which no viable parasite was observed microscopically. This value was confirmed by injecting intraperitoneally the culture from the well corresponding to the MEC into a mouse to confirm that the non-motile parasites were really killed and not able to divide within mice.
3.8. Antiproliferative Activity of Axidjiferosides
The antiproliferative activity of axidjiferosides was investigated upon the cell lines, NSCLC-N6, (derived from a human non-small-cell bronchopulmonary carcinoma, moderately differentiated, rarely keratinized, classified as T2N0M0) [30], A549 (obtained from ATCC collection reference CCL-185) [32], GBM (astrocytoma cells obtained after tumor resection of patients with glioblastoma multiform-primary culture) [33], HCT-116 (colorectal adenocarcinoma cells derived from a patient with Lynch’s syndrome) [34] and KMS-11 (adherent plasma cells obtained from patients with multiple myeloma) [35], as previously described [22,23]. Experiments were performed at least in triplicate, 4 wells per axidjiferoside concentration being used. IC50 values were calculated from the dose-response curves.
4. Conclusions
The sponge Axinyssa djiferi, which lives in the particular biotope of the African mangrove, produced a series of glycosphingolipids, including the three new axidjiferosides isolated in this work. They contained the unusual Δ6-phytosphingosine and a not-so-common galactopyranose. Interestingly, axidjiferosides showed a promising antiplasmodial activity, with only a low cytotoxicity against various human cell lines and no significant antitrypanosomal and antileishmanial activity. If α-galactosylceramide, as a NKT cell ligand, showed a rapid and strong antimalarial activity, it seems nevertheless that a galactosylceramide, such as axidjiferoside with a β anomeric configuration, may be suitable in searching for new antimalarial drugs.
Acknowledgements
This work was supported by the Cancéropôle du Grand Ouest, “Valorisation des produits de la mer, Glycoconjugués et Cancer”, France (Head: Philippe Bougnoux, Inserm, Université de Tours, France). We wish to thank Haidar El-Hali, Oceanium, Dakar, Senegal, for help in harvesting Axinyssa djiferi. Pierre Guenot, Philippe Jehan and Fabian Lambert, Centre Régional de Mesures Physiques de l’Ouest, CNRS, and Université de Rennes-1, France, for ESI-MS experiments. The first biological evaluations were performed under the auspices of the French National Scientific Research Centre within a network called Research Group on Natural Products managed by Thierry Sévenet (GDR G1206, CNRS, ICSN, Gif s/Yvette, France). We also wish to thank Mr. Benoît Serive, University of Nantes, for his help in lipid fractionations and Vony Rabesaotra, Faculty of Pharmacy, Nantes, for GC-MS experiments.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Tuteja R. Malaria—An overview. FEBS J. 2007;274:4670–4609. doi: 10.1111/j.1742-4658.2007.05997.x. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. World Malaria Report 2011. WHO Press; Geneva, Switzerland: 2011. pp. 1–248. [Google Scholar]
- 3.Moorthy V., Smith P.G., Kieny M.P. A vaccine against malaria: A substantial step forward. Lancet. 2009;373:1411–1412. doi: 10.1016/S0140-6736(09)60802-3. [DOI] [PubMed] [Google Scholar]
- 4.Nayyar G.M.L., Breman J.G., Newton P.N. Poor-quality antimalarial drugs in southeast Asia and Africa. Lancet. 2012;12:488–496. doi: 10.1016/S1473-3099(12)70064-6. [DOI] [PubMed] [Google Scholar]
- 5.Bell D.R., Jorgensen P., Christophel E.M., Palmer K.L. Malaria risk: Estimation of the malaria burden. Nature. 2005;437:E3–E4. doi: 10.1038/nature04179. [DOI] [PubMed] [Google Scholar]
- 6.Petersen I., Eastman R., Lanzer M. Drug-resistant malaria: Molecular mechanisms and implications for public health. FEBS Lett. 2011;585:1551–1562. doi: 10.1016/j.febslet.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 7.Fidock D.A., Rosenthal P.J., Croft S.L., Brun R., Nwaka S. Antimalarial drug discovery: Efficacy models for compound screening. Nat. Rev. Drug Discov. 2004;3:509–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 8.Grellier P., Deregnaucourt C., Florent I. Advances in Antimalarial Drug Evaluation and New Targets for Antimalarials. In: Omolade O., editor. Malaria Parasites. InTech Europe; Rijeka, Croatia: 2012. pp. 321–350. [Google Scholar]
- 9.Kaur K., Jain M., Kaur T., Jain R. Antimalarials from nature. Bioorg. Med. Chem. 2009;17:3229–3256. doi: 10.1016/j.bmc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 10.Gademann K., Kobylinska J. Antimalarial natural products of marine and freshwater origin. Chem. Rec. 2009;9:187–198. doi: 10.1002/tcr.200900001. [DOI] [PubMed] [Google Scholar]
- 11.Fattorusso E., Taglialatela-Scafati O. Marine antimalarials. Mar. Drugs. 2009;7:130–152. doi: 10.3390/md7020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Mesquita M.L., Grellier P., Blond A., Brouard J.P., de Paula J.E., Espinola L.S., Mambu L. New ether diglycosides from Matayba guianensis with antiplasmodial activity. Bioorg. Med. Chem. 2005;13:4499–4503. doi: 10.1016/j.bmc.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Aseguinolaza G., de Oliveira C., Tomaska M., Hong S., Bruna Romero O., Nakayama T., Taniguchi M., Bendelac A., van Kaer L., Koezuka Y., et al. α-Galactosylceramide-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc. Natl. Acad. Sci. USA. 2000;97:8461–8466. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurent D., Pietra F. Antiplasmodial marine natural products in the perspective of current chemotherapy and prevention of malaria—A review. Mar. Biotechnol. 2006;8:433–447. doi: 10.1007/s10126-006-6100-y. [DOI] [PubMed] [Google Scholar]
- 15.Kornprobst J.M. Encyclopedia of Marine Natural Products. Vol. 2. Wiley-Blackwell; Weinheim, Germany: 2010. pp. 577–582. [Google Scholar]
- 16.Fattorusso E., Mangoni A. Marine glycolipids. Prog. Chem. Org. Nat. Prod. 1997;72:215–301. doi: 10.1007/978-3-7091-6527-0_3. [DOI] [PubMed] [Google Scholar]
- 17.Jin W., Rinehart K.L., Jares-Erijman E.A. Ophidiacerebrosides: Cytotoxic glycosphingolipids containing a novel sphingosine from a sea star. J. Org. Chem. 1994;59:144–147. doi: 10.1021/jo00080a023. [DOI] [Google Scholar]
- 18.Costantino V., Fattorusso E., Mangoni A., di Rosa M., Ianaro A. Glycolipids from sponges. 6. Plakoside A and B, two unique prenylated glycosphingolipids with immunosuppressive activity from the marine sponge Plakortis simplex. J. Am. Chem. Soc. 1997;119:12465–12470. [Google Scholar]
- 19.Costantino V., Fattorusso E., Mangoni A. Glycolipids from sponges. Part 9: Plakoside C and D, two further prenylated glycosphingolipids from the marine sponge Ectyoplasia ferox. Tetrahedron. 2000;56:5953–5957. [Google Scholar]
- 20.Costantino V., Fattorusso E., Imperatore C., Mangoni A., Freigang S., Teyton L. Corrugoside: A new immunostimulatory α-galactoglycosphingolipid from the marine sponge Axinella corrugata. Bioorg. Med. Chem. 2008;16:2077–2085. doi: 10.1016/j.bmc.2007.10.098. [DOI] [PubMed] [Google Scholar]
- 21.Costantino V., Fattorusso E., Imperatore C., Mangoni A., Teta R. Amphiceramide A and B, novel glycosphingolipids from the marine sponge Amphimedon compressa. Eur. J. Org. Chem. 2009;2009:2112–2119. doi: 10.1002/ejoc.200801230. [DOI] [Google Scholar]
- 22.Farokhi F., Wielgosz-Collin G., Robic A., Debitus C., Malleter M., Roussakis C., Kornprobst J.M., Barnathan G. Antiproliferative activity against human non-small cell lung cancer of two O-alkyl-diglycosylglycerols from the marine sponges Myrmekioderma dendyi and Trikentrion laeve. Eur. J. Med. Chem. 2012;49:406–410. doi: 10.1016/j.ejmech.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Farokhi F., Wielgosz-Collin G., Clément M., Kornprobst J.M., Barnathan G. Cytotoxicity on human cancer cells of Ophidiacerebrosides isolated from the African starfish Narcissia canariensis. Mar. Drugs. 2010;8:2988–2998. doi: 10.3390/md8122988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch S., Kashman Y. New glycosphingolipids from marine organisms. Tetrahedron. 1989;45:3897–3906. [Google Scholar]
- 25.Boury-Esnault N., Marschal C., Kornprobst J.M., Barnathan G. A new species of Axinyssa Lendenfeld, 1897 (Porifera, Demospongiae, Halichondrida) from the Senegalese coast. Zootaxa. 2002;117:1–8. [Google Scholar]
- 26.Genin E., Njinkoué J.M., Wielgosz-Collin G., Houssay C., Kornprobst J.M., Debitus C., Bonin M., Micouin L., Boury-Esnault N., Hooper J.N.A., et al. Glycolipids from marine sponges: Monoglycosylceramides and alkyldiglycosylglycerols: Isolation, characterization and biological activity. Boll. Musei Ist. Biol. Univ. Genova. 2004;68:327–334. [Google Scholar]
- 27.Kang S.S., Kim J.S., Son K.H., Kim H.P., Chang H.W. Cyclooxygenase-2 inhibitory cerebrosides from Phytolaccae radix. Chem. Pharm. Bull. 2001;49:321–323. doi: 10.1248/cpb.49.321. [DOI] [PubMed] [Google Scholar]
- 28.Frappier F., Jossang A., Soudon J., Calvo F., Rasoanaivo P., Rastimamanga-Urveg S., Saez J., Schrevel J., Grellier P. Bisbenzylisoquinolines as modulators of chloroquine resistance in Plasmodium falciparum and multidrug resistance in tumor cells. Antimicrob. Agents Chemother. 1996;6:1476–1481. doi: 10.1128/aac.40.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labaied M., Dagan A., Dellinger M., Geze M., Egee S., Thomas S.L., Wang C., Gatt S., Grellier P. Anti-Plasmodium activity of ceramide analogs. Malaria J. 2004;3:49–59. doi: 10.1186/1475-2875-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roussakis C., Gratas C., Audouin A.F., le Boterff J., Dabouis C., André M.J., Moyon E., Vo N.H., Pradal G., Verbist J.F. Study of in vitro drug sensitivity on a newly established cell line from a primary bronchial epidermoid carcinoma of human origin (NSCLCN6) Anticancer Res. 1991;11:2239–2244. [PubMed] [Google Scholar]
- 31.Li Y., Matsunaga S., Fusetani N. Halicylindrosides, antifungal and cytotoxic cerebrosides from the marine sponge Halichondria cylindrata. Tetrahedron. 1995;51:2273–2280. doi: 10.1016/0040-4020(94)01097-J. [DOI] [Google Scholar]
- 32.Giard D.J., Aaronson S.A., Todaro G.J., Arnstein P., Kersey J.H., Dosik H., Parks W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 33.Cartron J.P., Juin P., Oliver L., Martin S., Meflah K., Vallette F.M. Non-redundant role of Bax and Bak in Bid-mediated apoptosis. Mol. Cell. Biol. 2003;23:4701–4712. doi: 10.1128/MCB.23.13.4701-4712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brattain M.G., Fine W.D., Khaled F.M., Thompson J., Brattain D.E. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 1981;41:1751–1756. [PubMed] [Google Scholar]
- 35.Namba M., Ohtsuki T., Mori M., Togawa A., Wada H., Sugihara T., Yawata Y., Kimoto T. Establishment of five human myeloma cell lines. In Vitro Cell. Dev. Biol. 1989;25:723–729. doi: 10.1007/BF02623725. [DOI] [PubMed] [Google Scholar]