Abstract
Objective
To investigate whether mortality statistics show an effect of mammographic screening on population-based breast cancer mortality in England.
Design
Joinpoint regression analyses, and other analyses, of population-based mortality data.
Setting
Analysis of mortality rates in the Oxford region, UK (1979–2009) because, unlike the rest of England, all causes of death mentioned on each death certificate for its residents (not just the underlying cause) are available prior to commencement of the English National Breast Screening Programme (NHSBSP). In addition, analysis of English national breast cancer mortality rates (1971–2009).
Participants
Women who died from breast cancer in the Oxford region (1979--2009) and England (1971--2009)
Main outcome measures
Age-specific mortality rates, and age-standardized mortality rates. Joinpoint regression analysis was used to estimate years (‘joinpoints’) in which trends changed, and annual percentage change between joinpoints, with confidence intervals.
Results
In the Oxford region, trends for breast cancer mortality based on underlying cause and on mentions were very similar. For all ages combined, mortality rates peaked for both underlying cause and mentions in 1985 and then started to decline, prior to the introduction of the NHSBSP in 1988. Between 1979 and 2009, for mortality measured as underlying cause, rates declined by −2.1% (95% CI −2.7 to −1.4) per year for women aged 40–49 years (unscreened), and by the same percentage per year (−2.1% [−2.4 to −1.7]) for women aged 50–64 years (screened). In England, the first estimated changes in trend occurred prior to the introduction of screening, or before screening was likely to have had an effect (between 1982 and 1989). Thereafter, the downward trend was greatest in women aged under 40 years: −2.0% per year (−2.8 to −1.2) in 1988–2001 and −5.0% per year (−6.7 to −3.3) in 2001–2009. There was no evidence that declines in mortality rates were consistently greater in women in age groups and cohorts that had been screened at all, or screened several times, than in other (unscreened) women, in the same time periods.
Conclusions
Mortality statistics do not show an effect of mammographic screening on population-based breast cancer mortality in England.
Introduction
Population-based mortality rates for breast cancer have declined in many developed countries, including the UK, since the 1980s1–3 while incidence rates have continued to rise. The primary objective of mammographic screening for breast cancer is to reduce breast cancer mortality rates. However, in recent years, the value of screening for breast cancer has been the subject of much debate.4,5 In response to the growing controversy, the English Department of Health announced, in October 2011, that it would commission an independent review of breast cancer screening research. This review was undertaken by a panel chaired by Professor Sir Michael Marmot, and the findings were published in October 2012.6 On reviewing the evidence, the panel concluded that there was a 20% relative reduction in mortality from breast cancer in women invited to screening. However, the panel also found that ‘for each breast cancer death prevented about three overdiagnosed cases will be identified and treated’.
The publication of the Marmot report has done little to appease critics of mammography screening,7,8 and measuring the effect of mammographic screening on breast cancer mortality remains vexed.7,9 We examined the influence of multiple-cause coding of diagnoses on death certificates on the rise and fall of breast cancer mortality in England. Until the mid-1990s, only the underlying cause of death was selected and coded from each death certificate in England nationally, when more than one cause was certified. Data on the other causes were not routinely coded. The National Breast Cancer Screening Programme (NHSBSP) was introduced in 1988. It is possible that shifts in certification practice, and/or changes in the rules for selecting the underlying cause of death (which changed in England in 1984, 1993 and 2001),10–12 may have had effects on the temporal profile of breast cancer mortality. We studied a regional data-set which included all certified causes of death (conventionally termed ‘mentions’), not just the underlying cause, to study mortality trends from 1979. To the best of our knowledge, this is the longest time span of all ‘mentions’ in England at a large population-level. To provide national context, we also analyzed data on underlying cause mortality for the whole of England for a similar period. We wanted to know, in particular, whether studies of trends in mortality data for breast cancer, if based on underlying cause alone, were likely to be reliable.
We then investigated whether population-based breast cancer mortality trends, enhanced by multiple-cause coding, show an effect of mammography screening. Accordingly, we considered mortality trends before and after the introduction of the NHSBSP in 1988, comparing mortality rates for women in age groups and cohorts that had been screened at all, or screened several times, with those for unscreened women, in the same time periods.
Materials and methods
Data sources
Breast cancer mortality data for the former Oxford NHS Region (population about 2.5 million) were obtained from the database of the Oxford Record Linkage Study from 1979 to 2010, which, in turn, came from death registration data supplied by the Office for National Statistics. Because some deaths that occur in each calendar year are not registered until the next year, we used data including that for 2010 registrations, but only report on deaths by date of occurrence to the end of 2009. A total of 20,987 death certificates where female breast cancer was coded in any position (underlying cause, or mentioned elsewhere on the certificate) were included. Breast cancer incidence (1975–2008 [982,135 cases]) and mortality (1971–2009 [444,186 cases]) data for England were supplied to us in files from Cancer Research UK. These national mortality data only include cases where death from female breast cancer has been coded as underlying cause on the death certificate. Incidence and mortality records were selected using code 174 in the eighth revision of the International Classification of Diseases, 174 in the ninth, and C50 in the tenth.
Statistical analyses
Age-specific mortality rates, and age-standardized incidence and mortality rates, were calculated. Age standardization was conducted using five-year age groups, the direct method, and the age distribution of the European standard population. Trends in age-standardized incidence and mortality rates were modelled using joinpoint regression,13 which is a method for identifying when a series of annual rates changes trend, as described below.
Joinpoint regression
Joinpoint regression models,13 otherwise known as piecewise, broken line, or segmented regression models, consist of straight lines which are connected by joinpoints (also referred to as knots or change points). Each joinpoint is the estimated location of a change in the slope of the trend line. The joinpoint regression programme starts from a null hypothesis model of zero joinpoints (a straight line) and tests (by permuting the residuals) whether the alternative hypothesis model of the maximum number of joinpoints specified has a statistically significant lower sum of residual squares than the null hypothesis model, in which case, the latter is rejected. Permutation tests are applied sequentially14 until a model of best fit is reached. For example, if a maximum number of five joinpoints is specified, the final, or best fit model, will have between zero and five joinpoints. In Tables 2 and 4, we present best fit models of the data.
Table 2.
Joinpoint trends in female breast cancer mortality rates in the Oxford region, 1979–2009
| Age group (years) | Type of listing on death certificate | Period 1 | Annual percentage change (95% CI) | Period 2 | Annual percentage change (95% CI) |
|---|---|---|---|---|---|
| <40 | Mentions | 1979–2009 | −1.4* (−2.4 to −0.4) | – | – |
| Underlying cause | 1979–2009 | −1.4* (−2.4 to −0.5) | – | – | |
| 40–49 | Mentions | 1979–2009 | −2.0* (−2.7 to −1.3) | – | – |
| Underlying cause | 1979–2009 | −2.1* (−2.7 to −1.4) | – | – | |
| 50–64 | Mentions | 1979–2009 | −2.0* (−2.3 to −1.7) | – | – |
| Underlying cause | 1979–2009 | −2.1* (−2.4 to −1.7) | – | – | |
| 65–74 | Mentions | 1979–1990 | 0.8 (−0.9 to 2.6) | 1990–2009 | −2.2* (−3.0 to −1.4) |
| Underlying cause | 1979–1987 | 2.5 (−0.6 to 5.6) | 1987–2009 | −2.1* (−2.8 to −1.5) | |
| ≥75 | Mentions | 1979–1996 | 0.5 (−0.3 to 1.4) | 1996–2009 | −2.0* (−3.1 to −0.8) |
| Underlying cause | 1979–1989 | 2.7* (0.2 to 5.2) | 1989–2009 | −1.8* (−2.5 to −1.0) | |
| All age groups | Mentions | 1979–1991 | −0.1 (−0.9 to 0.8) | 1991–2009 | −2.2* (−2.6 to −1.8) |
| Underlying cause | 1979–1990 | 0.3 (−0.7 to 1.3) | 1990–2009 | −2.4* (−2.8 to −2.0) |
The annual percentage change is significantly different from zero at α = 0.05
Table 4.
Joinpoint trends in female breast cancer mortality rates in England, 1971–2009
| Age group (years) | Period 1 | Annual percentage change (95% CI) | Period 2 | Annual percentage change (95% CI) | Period 3 | Annual percentage change (95% CI) |
|---|---|---|---|---|---|---|
| <40 | 1971–1988 | −0.3 (−0.8 to 0.1) | 1988–2001 | −2.0* (−2.8 to −1.2) | 2001–2009 | −5.0* (−6.7 to −3.3) |
| 40–49 | 1971–1989 | −0.6* (−0.9 to −0.3) | 1989–2009 | −3.1* (−3.4 to −2.9) | – | – |
| 50–64 | 1971–1985 | 0.7* (0.5 to 0.9) | 1985–1990 | −1.2 (−2.5 to 0.0) | 1990–2009 | −3.0* (−3.1 to −2.8) |
| 65–74 | 1971–1989 | 1.0* (0.8 to 1.2) | 1989–2009 | −2.5* (−2.7 to −2.3) | – | – |
| ≥75 | 1971–1982 | 0.9* (0.4 to 1.4) | 1982–1985 | 5.2 (−0.9 to 11.7) | 1985–1991 | 0.6 (−0.6 to 1.8) |
| All age groups | 1971–1982 | 0.5* (0.4 to 0.7) | 1982–1986 | 1.3* (0.1 to 2.5) | 1986–1991 | −0.9* (−1.6 to −0.1) |
| Age group | Period 4 | Annual percentage change (95% CI) | Period 5 | Annual percentage change (95% CI) | Period 6 | Annual percentage change (95% CI) |
| <40 | – | – | – | – | – | – |
| 40–49 | – | – | – | – | – | – |
| 50–64 | – | – | – | – | – | – |
| 65–74 | – | – | – | – | – | – |
| ≥75 | 1991–1998 | −2.8* (−3.7 to −1.9) | 1998–2002 | 1.3 (−1.4 to 4.1) | 2002–2009 | −2.1* (−2.8 to −1.3) |
| All age groups | 1991–1998 | −3.0* (−3.4 to −2.6) | 1998–2009 | −2.2* (−2.3 to −2.0) | – | – |
The annual percentage change is significantly different from zero at α = 0.05
Joinpoints are sometimes referred to as the estimated locations of statistically significant changes in trend,15 but it is important to clarify what this means: the joinpoint represents a year in which the slope of the trend line changes, and this change is statistically significant in the sense that permutation tests show that there is a significant reduction in the total error of the trend line compared to that of a lower joinpoint model. For each line segment of the model, the programme also provides the annual percentage change (from joinpoint to joinpoint), along with the associated confidence intervals.
The log-linear model option was selected, and a maximum number of five joinpoints was allowed for each model (the default value for the number of data points considered). The overall significance level allowed for permutation tests was α = 0.05. The best fit model was compared with higher and/or lower joinpoint, non-significant models of the same data points. Separate analyses were performed for death with any mention of breast cancer on the death certificate, and for death with breast cancer coded as underlying cause.
Results
Oxford region: underlying cause as a percentage of all mentions of breast cancer
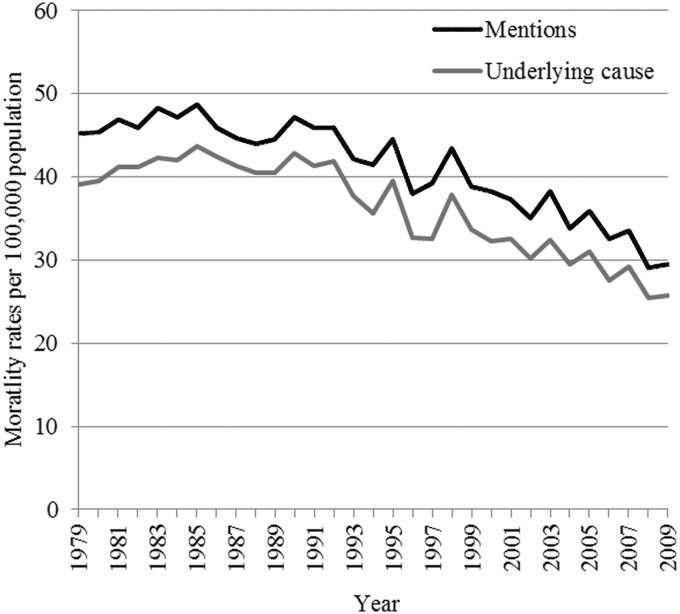
Of all women with breast cancer certified on the death certificate, breast cancer was the underlying cause of death in 96% (7266/7598) of women aged under 65 years at death, 88% (3731/4227) aged 65–74 years, 78% (3763/4841) aged 75–84 years, and 66% (2447/3701) aged 85 years and over. These percentages did not change appreciably over time and, overall, trends for mortality based on underlying cause and on mentions were similar (Figure 1).
Figure 1.
Age–standardized mortality rates for female breast cancer in Oxford, all ages, by mentions and underlying cause (1979–2009)
Oxford region: trends in mortality and their association with screening
Age-standardized mortality rates peaked for both underlying cause and mentions in 1985, prior to the introduction of the NHSBSP in England, and then started to fall. Supplementary Figures 1 and 2, showing data with logarithmic transformation, suggest that the rate of decline was greater in the age group under 40 years than in older women.
The year of peak mortality and percentage declines since then are shown by age group for mentions and for underlying cause in Table 1. The year of peak mortality for women aged under 40 years (an unscreened age group) was 2001. The peak years for women aged 40–49 years, 50–64 years, and 65–74 years were all in the 1980s, prior to the introduction of the screening programme. The peak year for women aged 75 years and over was 1992 for underlying cause and 1996 for mentions. The percentage declines since the peak year, comparing underlying cause and mentions within each age group, were very similar (Table 1). The percentage declines since 1988, when the national screening programme was introduced in England, show that the greatest declines were in women under the age of 40 years and the smallest declines were in women aged 40–49 years and 75 years and over. The percentage declines since 2000, i.e. giving data on the same time span for each age group, showed similar findings.
Table 1.
Year of peak age-specific mortality rate for female breast cancer per 100,000 population in the Oxford region, 1979–2009
| Age group (years) | Death certificate listing | Year of peak mortality | Death count (peak mortality rate) [95% CI] | Percent decline since peak | Percent decline since 1988 | Percent decline since 2000 |
|---|---|---|---|---|---|---|
| <40 | Mentions | 2001 | 36 (4.9) [3.3 to 6.5] | 72.3 | 55.5 | 52.2 |
| Underlying cause | 2001 | 34 (4.6) [3.1 to 6.1] | 70.6 | 55.5 | 49.8 | |
| 40–49 | Mentions | 1981 | 66 (50.4) [38.2 to 62.5] | 45.0 | 18.2 | 12.6 |
| Underlying cause | 1981 | 66 (50.4) [38.2 to 62.5] | 46.7 | 20.8 | 13.9 | |
| 50–64 | Mentions | 1985 | 201 (110.9) [95.5 to 126.2] | 49.0 | 40.5 | 19.7 |
| Underlying cause | 1985 | 191 (105.3) [90.4 to 120.2] | 50.7 | 41.9 | 18.1 | |
| 65–74 | Mentions | 1984 | 162 (173.3) [146.6 to 199.9] | 48.8 | 38.0 | 30.7 |
| Underlying cause | 1984 | 144 (154.0) [128.9 to 179.2] | 49.7 | 41.9 | 28.0 | |
| ≥75 | Mentions | 1996 | 350 (345.8) [309.6 to 382.1] | 29.2 | 9.0 | 14.3 |
| Underlying cause | 1992 | 256 (266.7) [234.0 to 299.3] | 30.7 | 14.8 | 6.9 | |
| All age groups | Mentions | 1992 | 734 (56.6) [52.5 to 60.7] | 26.0 | 16.9 | 15.3 |
| Underlying cause | 1992 | 649 (50.0) [46.2 to 53.9] | 29.6 | 21.9 | 11.7 |
Using the greater sophistication of joinpoint analysis, it was seen that mortality rates in the Oxford region in the age groups under 40 years, 40–49 years, and 50–64 years declined uniformly (i.e. without a detected change in trend over time) throughout the period covered by the study (Table 2). Measured as underlying cause, there was a significant change in trend (downward) in women aged 65–74 years in 1987 and in those aged 75 years and over in 1989 (i.e. both prior to any likely effect of the screening programme). In mortality measured as mentions, there were significant changes in trend (downward) in women aged 65–74 years in 1990 and in women aged 75 years and over in 1996 (Table 2).
Trends in England
Data on all mentions for England as a whole are unavailable for the 1980s and early 1990s. This section covers national analyses of mortality based on underlying cause and of incidence measured as cancer registration rates. Supplementary Figure 3 shows both the decline in age-standardized mortality rates and the rise in age-standardized breast cancer incidence rates, nationally. The year of peak mortality and percentage declines since then are shown by age group for underlying cause in Table 3. The peak year for women aged 40–49 years was 1973, and the peak years for those aged under 40, 50–64, and 65–74 years were all in the 1980s, prior to the introduction of the screening programme, or before the programme could have had a likely effect (see also Supplementary Figure 4).
Table 3.
Year of peak age-specific mortality rate for female breast cancer per 100,000 population in England, 1971–2009
| Age group (years) | Death certificate listing | Year of peak mortality | Death count (peak mortality rate) [95% CI] | Percent decline since peak | Percent decline since 1988 | Percent decline since 2000 |
|---|---|---|---|---|---|---|
| <40 | Underlying cause | 1989 | 402 (3.1) [2.8 to 3.4] | 50.9 | 48.7 | 45.5 |
| 40–49 | Underlying cause | 1973 | 1278 (46.3) [43.7 to 48.8] | 52.7 | 44.6 | 16.8 |
| 50–64 | Underlying cause | 1985 | 3827 (96.8) [93.8 to 99.9] | 45.9 | 42.5 | 19.6 |
| 65–74 | Underlying cause | 1987 | 3193 (135.5) [130.8 to 140.2] | 39.5 | 38.1 | 16.6 |
| ≥75 | Underlying cause | 1991 | 5159 (233.0) [226.6 to 239.3] | 23.5 | 19.0 | 6.5 |
| All age groups | Underlying cause | 1989 | 13110 (53.7) [52.8 to 54.6] | 30.9 | 29.7 | 12.1 |
The percentage declines since peak year of mortality, since 1988, and since 2000, show that the greatest declines were in women under the age of 40 years, and the smallest declines were in women aged 75 years and over; both are age groups in which women do not routinely receive an invitation for screening. Joinpoint analyses (Table 4) show a greater number of changes in trend in England than in Oxford, reflecting larger numbers of cases, and greater sensitivity in detecting change. The first estimated change in trend (end of period 1 in Table 4) for all age groups occurred prior to the introduction of screening, or before screening could have had any likely effect. There was a second downward shift which was more recent (2001–2009) in women aged under 40 years (i.e. an age group not routinely screened) and a downward shift in women aged 50–64 years (from 1990), but not in women aged 65–74 years. The changes in women aged 75 years and over were complicated: there was a non-significant increase in mortality between 1982 and 1985 and between 1985 and 1991, a significant decline during 1991–1998, a non-significant increase during 1998–2002, and a significant decline in the period 2002–2009. In summary, there was no consistent evidence of a decline in mortality over time that was clearly greater in screened than in unscreened age groups.
Age-specific rates for women likely and unlikely to have been screened, by time period
Supplementary Tables 1–3 show age-specific rates in greater detail. Evidence of a beneficial effect of screening on breast cancer mortality would come from data that showed that age-specific mortality rates were lower in screened groups of women than in unscreened groups; and that the lower rates were in a timeframe consistent with a likely latent period between screening and mortality-related benefit. A complication in studying this is that there are conflicting trends. Mortality rates for breast cancer rise sharply with increasing age (Supplementary Tables 1–3, columns); so, as women grow older in the years following screening, their mortality rates tend to rise. As a contrary trend, age-specific mortality rates have declined sharply over time in recent years (Supplementary Tables 1–3, rows); so, as screened cohorts grow older, they exhibit the counteracting effects of an increase in mortality associated with increasing age, but a decrease in mortality associated with calendar time.
A beneficial effect of screening might be evident in a disproportionately high decline in mortality in those age groups and time periods when women were invited for screening. In Supplementary Tables 1–3, we show percentage changes in age-specific mortality comparing rates in 1990–1994 with those in 1980–1984, i.e. before wide coverage with screening; and we compare age-specific rates in 2005–2009 with those in 1990–1994, and with those in 1995–1999, all periods when screening was well underway in the age groups 50–54, 55–59 and 60–64 years. Age groups and periods in which women would have been invited for screening are shaded. For example, in Supplementary Table 1, women aged 50–54 years in 1990–1994 would have been invited for screening in 1990–1994, invited again in 1995–1999 when they were 55–59 years, and again in 2000–2004 when they were aged 60–64 years. Age groups and periods in which women would not normally have been screened, but in which women would have been invited for screening in an earlier age and time period, are also shaded. For example, women aged 75–79 years in 2005–2009 would not normally have received an invitation for screening in recent years, but would have been invited for screening in 1990–1994 when aged 60–64 years. In all three data-sets considered, there is no evidence that declines in mortality rates were consistently greater in women in cohorts that had been screened at all, or screened several times, than in other (unscreened) women in the same time periods.
Uptake of screening invitations
Summary statistics from the NHSBSP website16 on the uptake of screening invitations over 10 years after the introduction of the programme show that around three-quarters of women aged 50–64 years who received an invitation for screening were eventually screened (2000 [75.5%], 2002 [75.6%], 2004 [75.5%], 2006 [75.4%], and 2008 [73.6%]).
Discussion
Principal findings
The analysis of multiple-cause coding shows that trends for mortality based on underlying cause and on mentions were very similar. This suggests that the decreases in mortality for breast cancer were not influenced by switches in practice between selecting breast cancer as an underlying cause of death or a contributory cause.
That the great majority of breast cancer deaths in the under 65s (96%) were certified as the underlying cause means that, in studies of mortality from breast cancer, mortality data that are confined to underlying cause are likely to be reliable, in this respect, in this age group. In older age groups, a smaller percentage of breast cancer deaths are coded as the underlying cause (for example, 78% in women aged 75–84 years), and figures on breast cancer mortality based on underlying cause alone should be treated with greater caution.
We sought evidence of a decline in population-based breast cancer mortality that could be attributed to the implementation of mammographic screening programmes. We conclude that population-based mortality statistics, at least in England, do not show a past benefit of breast cancer screening. While this does not rule out a benefit at the level of individual women, these effects are not large enough to be detected at the population-level. Our conclusions, on evidence about benefit, are unaltered by our analysis with the enhancement of using all certified causes of death.
Implications
Consideration of the value of using mortality statistics on breast cancer to assess screening programmes remains important. The Independent UK Panel on Breast Cancer Screening led by Professor Sir Michael Marmot questioned the usefulness of observational studies that compare breast cancer mortality time trends data before and after the introduction of mammographic screening programmes.6 The panel argued that the ‘extrapolation of time trends demands that decisions are made, for example, about the linearity or otherwise of the trend, the choice of time periods considered as “before” and “after” screening, and the age groups included’. This is so (although in the present study there was no extrapolation of time trends – we only modelled observations within the time periods we had data for); but evaluation of the effectiveness of mammography screening programmes remains problematic. The clinical trials that informed the Marmot report were all conducted at least two decades ago: the effect of screening on mortality needs to be considered in light of improvements in treatment,7 and technological advancements made in mammographic screening during the past 20 years. In observational studies of mortality, there are certainly secular effects, independently of screening, that can obscure screening effects, including the effects of treatment (for example, Tamoxifen17), and changes in risk factors such as childbearing patterns.18 However, as new clinical trials will probably not be conducted,19 and as opinion is divided over the value of data collected from case-control studies to study breast cancer screening,6 the use of mortality data cannot be lightly dismissed. Kirwan,9 commenting on the Marmot Report, wrote that ‘ongoing analysis of the efficacy of screening must depend on the meticulous collection of population data and subsequent modelling’.
Our study shows that age-specific mortality rates for the age groups 40–49, 50–64 and 65–74 years, both in the Oxford region and in England, peaked prior to the introduction of population-based screening in England. Percentage declines since 1988, when population-based screening was introduced in England, were greatest in women under the age of 40 years and smallest among women aged 75 years and over, both in the Oxford region and in England. Joinpoint analyses, both for the Oxford region and for England, show significant declines in mortality rates among groups of women on whom screening has mainly been focussed (i.e. those aged 50–64 years). In the Oxford region, these declines were estimated to begin in 1979, and, in England, in 1990. However, joinpoint analyses, both for the Oxford region and for England, also show significant declines in mortality rates per year among women aged under 40 years, who would not normally receive an invitation for screening.
Our findings support those in previous studies which found little to no discernible effect of mammography screening on breast cancer mortality.20–22 Our results also support findings from previous studies which show that reductions in breast cancer mortality were greatest for women in age groups that would not normally receive an invitation for screening: in a study of breast cancer mortality trends in 30 European countries, Autier et al.2 found that the greatest reductions in mortality were among women under the age of 50 years; and in an examination of breast cancer mortality data from Norway, Kalager et al.23 found that the reductions in mortality were greatest among those aged 70–84 years.
Strengths and weaknesses
A principal strength of the study is that we used a data-set that included all certified causes of death, and not just the underlying cause. Shifts in practice in the certification of death between underlying cause and contributory cause might in principle have had an effect on the temporal profile of breast cancer mortality, as they have for other diseases.24,25 For example, mortality trends for diabetes in recent years have shown conflicting trends depending on whether the trend is studied using underlying cause alone (which shows a decline in diabetes mortality) or using mentions (which shows an increase in diabetes mortality).26 For breast cancer, the trends revealed by the analysis of mentions were very similar to those shown by underlying cause. A weakness is the well-recognized fact that mortality data may be unreliable to some extent; but, in the context of studying the effects of an intervention on population-based mortality, this is an unavoidable weakness. Another limitation is that the data include cases of deaths for women who had their breast cancer diagnosed before receiving an invitation for screening. However, given that these effects are likely to diminish over time, and that we have an observation period of 39 years for England, and 31 years for Oxford, excluding these cases is unlikely to have influenced our conclusions.27
Conclusions
We conclude that decreases in mortality for breast cancer were not influenced by switches in practice between selecting breast cancer as an underlying cause of death or a contributory cause. In the great majority of deaths in women under 65 years old, with mentions of breast cancer on the death certificate, breast cancer is the underlying cause of death. In this respect, if data are only available on underlying cause, they are likely to be highly reliable. In older women, analysis of breast cancer mortality should be done, whenever possible, using mentions as well as underlying cause.
Measuring the effectiveness of mammography screening is a fundamental area of concern in countries which have established mammography screening programmes. Clinical trials have indicated that several years have to elapse between the start of screening and the emergence of a reduction in mortality, and that the benefit of screening persists many years after screening stops. We permuted the data in a number of different ways, over an observation period of 39 years, but the data show that, at least as yet, there is no evidence of an effect of mammographic screening on population-level breast cancer mortality.
DECLARATIONS
Competing interests
None declared
Funding
This work was supported by the English National Institute for Health Research (ref. RNC/035/02). The funding source had no influence over the study design, data analysis, data interpretation, report writing, nor the decision to submit the paper for publication. TKM had full access to the data for this study, and the authors had final responsibility for the decision to submit the manuscript for publication
Ethical approval
Ethical approval for the Unit of Health-Care Epidemiology's work programme, analysing routine medical data-sets, was obtained from the Central and South Bristol Multi-Centre Research Ethics Committee (04/Q2006/176)
Guarantor
TKM
Contributorship
TKM and MJG proposed and designed the study. TKM conducted statistical analyses; TKM and MJG interpreted the data. DGY extracted the data and conducted statistical analyses. TKM wrote the first draft, and all authors contributed to the final draft
Acknowledgements
We would like to thank the statistical information team at Cancer Research UK for providing English national incidence and mortality data for breast cancer
References
- 1. International Agency for Research on Cancer. Cancer Fact Sheet: Breast Cancer Incidence, Mortality and Prevalence Worldwide in 2008, Summary. See http://globocan.iarc.fr/factsheets/cancers/breast.asp (last checked 12 April 2013)
- 2.Autier P, Boniol M, La Vecchia C, et al. Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ 2010;341: c3620–c3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cancer Research UK. Cancer Stats Breast Cancer – UK. May 2009. See http://publications.cancerresearchuk.org/downloads/Product/CS_CS_BREAST.pdf (last checked 12 April 2013)
- 4.Bock K, Borisch B, Cawson J, et al. Effect of population-based screening on breast cancer mortality. Lancet 2011;378: 1775–6 [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen KJ, Keen JD, Gøtzsche PC. Is mammographic screening justifiable considering its substantial overdiagnosis rate and minor effect on mortality? Radiology 2011;260: 621–7 [DOI] [PubMed] [Google Scholar]
- 6. The Independent UK Panel on Breast Cancer Screening. The Benefits and Harms of Breast Cancer Screening: An Independent Review. See http://www.cancerresearchuk.org/prod_consump/groups/cr_common/@nre/@pol/documents/generalcontent/breast-screening-report.pdf (last checked 12 April 2013)
- 7.Baum M. Harms from breast cancer screening outweigh benefits if death caused by treatment is included. BMJ (Clin Res Ed) 2013;346: f385–f385 [DOI] [PubMed] [Google Scholar]
- 8. Gotzsche PC. The Nordic Cochrane Centre's comments on “Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012 Oct 30.” See http://www.cochrane.dk/screening/comments-on-UK-panel.htm (last checked 12 April 2013)
- 9.Kirwan CC. Breast cancer screening: what does the future hold? BMJ (Clin Res Ed) 2013;346: f87–f87 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, Vol 1, 9th revision, Geneva: WHO, 1977 [Google Scholar]
- 11.Office for National Statistics Mortality Statistics. Cause 1993 (revised) and 1994. Series DH2 (21), London: Office for National Statistics, 1996 [Google Scholar]
- 12.Office for National Statistics Mortality Statistics. Cause 2001. Series DH2 (28), London: Office for National Statistics, 2002 [Google Scholar]
- 13. National Cancer Institute. Joinpoint Regression Program, Version 3.5.2. October 2011. Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program. See https://surveillance.cancer.gov/joinpoint/download (last checked 12 April 2013)
- 14.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19: 335–51 [DOI] [PubMed] [Google Scholar]
- 15. Kim HJ, Feuer EJ, Barret M, Yu B, Fay MP, Midthune D. Is the trend changing? Joinpoint regression analysis. 2009: Presentation obtained from the lead author on December 8, 2011.
- 16. The NHS Information Centre, Workforce and Families. Breast Screening Programme, England, 2008–09. See http://www.cancerscreening.nhs.uk/breastscreen/breast-statistics-bulletin-2008-09.pdf (last checked 12 April 2013)
- 17.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 2008;99: 1763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves GK, Patterson J, Vessey MP, Yeates D, Jones L. Hormonal and other factors in relation to survival among breast cancer patients. Int J Cancer 2000;89: 293–9 [DOI] [PubMed] [Google Scholar]
- 19.Godlee F. Breast screening controversy continues. BMJ (Clin Res Ed) 2013;346: f477–f477 [Google Scholar]
- 20.Autier P, Koechlin A, Smans M, Vatten L, Boniol M. Mammography screening and breast cancer mortality in Sweden. J Natl Cancer Inst 2012;104: 1080–93 [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen KJ, Zahl PH, Gotzsche PC. Breast cancer mortality in organised mammography screening in Denmark: comparative study. BMJ (Clin Res Ed) 2010;340: c1241–c1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jørgensen KJ, Gøtzsche PC. Who evaluates public health programmes? A review of the NHS Breast Screening Programme. JRSM 2010;103: 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. New Engl J Med 2010;363: 1203–10 [DOI] [PubMed] [Google Scholar]
- 24.Goldacre MJ, Duncan ME, Cook-Mozaffari P, Griffith M. Trends in mortality rates comparing underlying-cause and multiple-cause coding in an English population 1979–1998. J Publ Health Med 2003;25: 249–53 [DOI] [PubMed] [Google Scholar]
- 25.Duncan ME, Goldacre MJ. Mortality trends for benign prostatic hyperplasia and prostate cancer in English populations 1979–2006. BJU Int 2011;107: 40–5 [DOI] [PubMed] [Google Scholar]
- 26. Duncan ME, Goldacre MJ. Certification of deaths from diabetes mellitus and obesity in England: trends into the twenty-first century. J Publ Health (Oxford, England). Epub ahead of print 7 September 2012. [DOI] [PubMed]
- 27.Tabar L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260: 658–63 [DOI] [PubMed] [Google Scholar]