Abstract
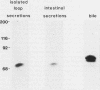
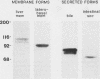
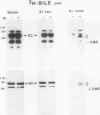
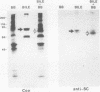
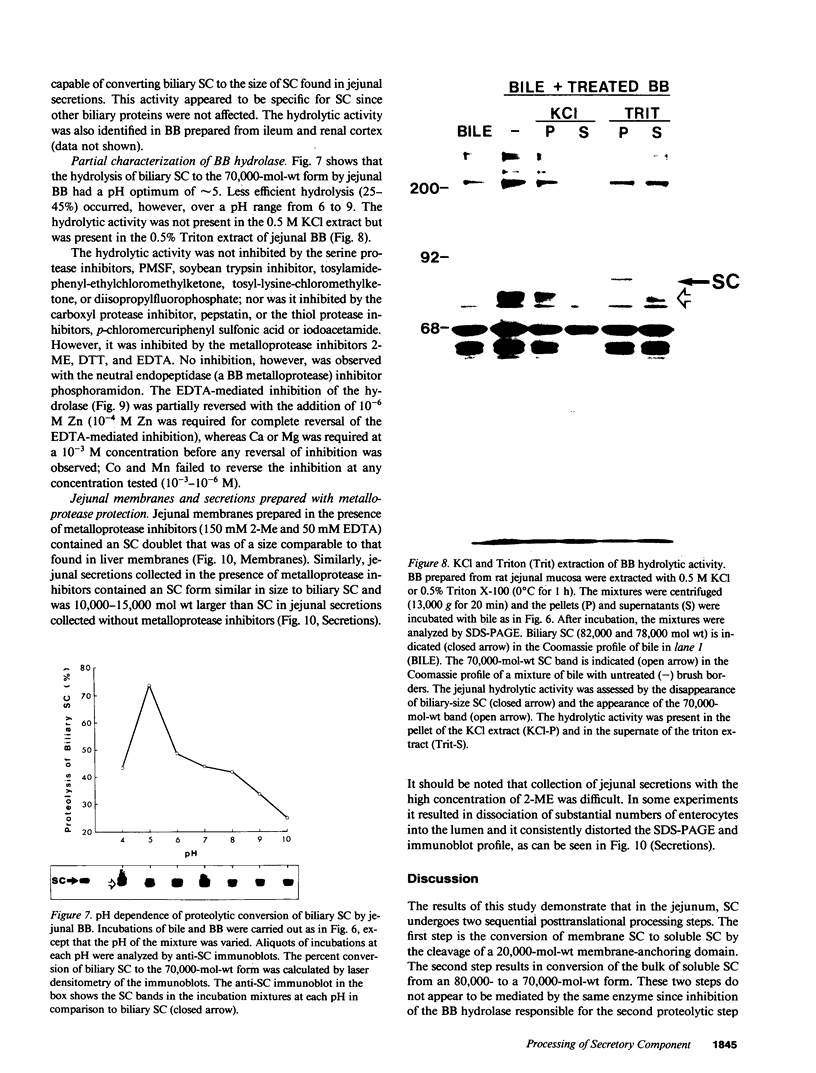
Secretory component (SC) is a glycoprotein that mediates the transcellular transport of polymeric immunoglobulins into external secretions. SC is synthesized and inserted into the plasma membrane of epithelial cells and hepatocytes as a transmembrane protein, where it serves as a receptor for polymeric immunoglobulins. SC is posttranslationally cleaved to a soluble protein before secretion into external fluids. In the rat jejunum, we observed that the molecular weights of both the major membrane and soluble forms of SC were 10,000-20,000 smaller than the comparable hepatic forms of the glycoprotein. We therefore set out to determine the reason for the differences in size of SC between these two tissues. The smaller size of jejunal SC was not due to the action of pancreatic proteases or differential glycosylation but was due to proteolysis by a jejunal brush border protease. The protease was characterized as a metalloprotease, with a pH optimum of approximately 5. It is present in jejunal, ileal, and renal tubular brush borders as an integral membrane constituent. When the protease was inhibited in vivo, conversion of jejunal secretory component to the smaller size was partially prevented. Thus, in the rat jejunum, SC undergoes two posttranslational proteolytic events: conversion of membrane secretory component to the soluble form and conversion of soluble SC to a smaller size by a previously undescribed brush border protease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Mircheff A. K., Santiago N. A., Yoshioka C., Gray G. M. Intestinal surface aminooligopeptidase. Distinct molecular forms during assembly on intracellular membranes in vivo. J Biol Chem. 1983 May 10;258(9):5960–5966. [PubMed] [Google Scholar]
- Ahnen D. J., Santiago N. A., Cezard J. P., Gray G. M. Intestinal aminooligopeptidase. In vivo synthesis on intracellular membranes of rat jejunum. J Biol Chem. 1982 Oct 25;257(20):12129–12135. [PubMed] [Google Scholar]
- Brown W. R., Isobe Y., Nakane P. K. Studies on translocation of immunoglobulins across intestinal epithelium. II. Immunoelectron-microscopic localization of immunoglobulins and secretory component in human intestinal mucosa. Gastroenterology. 1976 Dec;71(6):985–995. [PubMed] [Google Scholar]
- Desnuelle P. The Tenth Sir Hans Krebs Lecture. Intestinal and renal aminopeptidase: a model of a transmembrane protein. Eur J Biochem. 1979 Nov 1;101(1):1–11. doi: 10.1111/j.1432-1033.1979.tb04209.x. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The molecular weight and properties of a neutral metallo-endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):489–495. doi: 10.1042/bj1370489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppel T. M., Brown W. R. Rat liver membrane secretory component is larger than free secretory component in bile: evidence for proteolytic conversion of membrane form to free form. J Cell Biochem. 1984;24(4):307–317. doi: 10.1002/jcb.240240402. [DOI] [PubMed] [Google Scholar]
- Knight K. L., Rosenzweig M., Lichter E. A., Hanly W. C. Rabbit secretory IgA: identification and genetic control of two allotypes of secretory component. J Immunol. 1974 Mar;112(3):877–882. [PubMed] [Google Scholar]
- Kühn L. C., Kocher H. P., Hanly W. C., Cook L., Jaton J. C., Kraehenbuhl J. P. Structural and genetic heterogeneity of the receptor mediating translocation of immunoglobulin A dimer antibodies across epithelia in the rabbit. J Biol Chem. 1983 May 25;258(10):6653–6659. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meier P. J., Sztul E. S., Reuben A., Boyer J. L. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J Cell Biol. 1984 Mar;98(3):991–1000. doi: 10.1083/jcb.98.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A., Mizuochi T., Kobata A. Structures of the carbohydrate moieties of secretory component purified from human milk. J Biol Chem. 1982 Aug 25;257(16):9612–9621. [PubMed] [Google Scholar]
- Mostov K. E., Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem. 1982 Oct 10;257(19):11816–11821. [PubMed] [Google Scholar]
- Mostov K. E., Kraehenbuhl J. P., Blobel G. Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7257–7261. doi: 10.1073/pnas.77.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari R., Kraehenbuhl J. P. Biosynthesis of the IgA antibody receptor: a model for the transepithelial sorting of a membrane glycoprotein. Cell. 1984 Jan;36(1):61–71. doi: 10.1016/0092-8674(84)90074-6. [DOI] [PubMed] [Google Scholar]
- Stewart J. R., Chaplin M. F., Kenny A. J. Deglycosylation by trifluoromethanesulphonic acid of endopeptidase-24.11 purified from pig kidney and intestine. Biochem J. 1984 Aug 1;221(3):919–922. doi: 10.1042/bj2210919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. I. Kinetic studies of its intracellular forms. J Cell Biol. 1985 Apr;100(4):1248–1254. doi: 10.1083/jcb.100.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. II. Localization of its intracellular forms by cell fractionation studies. J Cell Biol. 1985 Apr;100(4):1255–1261. doi: 10.1083/jcb.100.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Intracellular and transcellular transport of secretory component and albumin in rat hepatocytes. J Cell Biol. 1983 Nov;97(5 Pt 1):1582–1591. doi: 10.1083/jcb.97.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Nakane P. K., Brown W. R. Ultrastructural events in the translocation of polymeric IgA by rat hepatocytes. J Immunol. 1982 Mar;128(3):1181–1187. [PubMed] [Google Scholar]