Abstract
Glial cells constitute a large percentage of cells in the nervous system. During recent years, a large number of studies have critically attributed to glia a new role which no longer reflects the long-held view that glia constitute solely a silent and passive supportive scaffolding for brain cells. Indeed, it has been hypothesized that glia, partnering neurons, have a much more actively participating role in brain function. Alteration of intraglial ionic homeostasis in response to ischemic injury has a crucial role in inducing and maintaining glial responses in the ischemic brain. Therefore, glial transporters as potential candidates in stroke intervention are becoming promising targets to enhance an effective and additional therapy for brain ischemia. In this review, we will describe in detail the role played by ionic transporters in influencing astrocyte, microglia, and oligodendrocyte activity and the implications that these transporters have in the progression of ischemic lesion.
Keywords: astrocytes, ionic transporters, microglia, oligodendrocytes, stroke
Introduction
Glial cells constitute a large percentage of cells in the nervous system. During recent years, a large number of studies have attributed to glia a new role that no longer reflects the long-held view that glia constitute solely a silent and passive supportive scaffolding for brain cells. Indeed, it has been hypothesized that glia, partnering neurons, have a much more actively participating role in brain function.
Indeed, glial cells are involved in almost every type of neurodegenerative diseases. However, conversely, alteration of intracellular ionic homeostasis in response to ischemic injury has a crucial role in inducing and maintaining glial responses in the injured brain.1, 2, 3 For instance, neuronal injury may elicit a series of glial reactions that are of critical importance for the progress of neural pathology.
Astrocytes in Brain ischemia
Astrocytes perform several functions that are essential for normal neuronal activity, including glutamate uptake, glutamate release, K+ and H+ buffering, and water transport. Accordingly, they can influence neuronal survival during ischemia. Furthermore, they also influence neurite outgrowth and other processes that contribute to brain recovery.
Astrocytes are divided into four major types.4, 5
(a) Protoplasmic astrocytes with numerous highly branched short processes.
(b) Interlaminar astrocytes extending striking long, frequently unbranched processes throughout the layers of the cortex.
(c) Polarized astrocytes residing in the deep layers of the cortex and extending one or two long unbranched processes away from the white matter.
(d) Fibrous astrocytes displaying more stellate shapes, with smooth, long less-branched processes.
Astrocytes also include other cells such as velate astrocytes of the cerebellum, tanycytes (found in the periventricular organs, the hypophysis, and the raphe part of the spinal cord), pituicytes in the neuro-hypophysis, and perivascular and marginal astrocytes.4
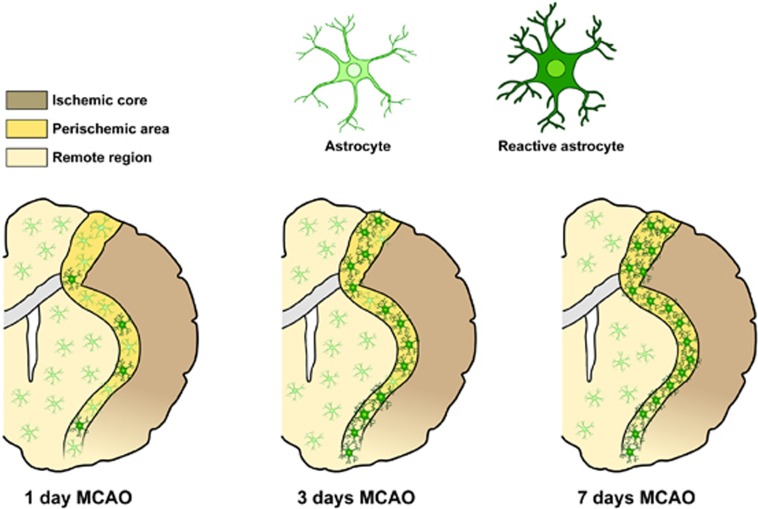
After brain ischemia, a massive and extensive astrocyte death in the core of the lesion occurs.6, 7 In the surrounding penumbra region, astrocytes become reactive and subsequently form the glial scar.6, 7 Several investigations suggest that astrocytes are better preserved than neurons in animal models of stroke inside the core.6, 7 Indeed, although in the ischemic core neuronal markers are decreased as soon as 1 hour after middle cerebral artery occlusion (MCAO), GFAP expression, a common marker of fibrous astrocytes, is preserved over the first 3 hours of reperfusion.3 At later reperfusion periods, GFAP increases in the periinfarct area that later develops into the glial scar (Figure 1). In contrast, Liu et al.8 reported the deterioration of some astrocyte markers before that of neuronal markers. Discrepancy in findings may be owing to the different methods used to detect astrocytes and to the different experimental model.
Figure 1.
Schematic anatomic diagram describing distribution of the different astrocyte phenotypes in the postischemic brain. MCAO, middle cerebral artery occlusion.
The effects of astrocytes on brain injury are divided into those (I) with immediate influence on cell survival and those (II) with long-term or delayed influence, eventually affecting later recovery. Astrocytes react to stroke by triggering the complex phenomenon known as reactive astrogliosis.7 Reactive astrocytes undergo dramatic morphological changes9 and alterations in gene expression.7 It has been long debated whether reactive astrocytes are harmful or beneficial. In the past few years, both types of effects have been observed10, 11 and raise the question of whether there might be different subtypes of reactive astrocytes, elicited depending on the nature and localization of the injury, that differ in their functions.
An expanding and intriguing research direction aimed at unraveling mechanisms by which astrocytes may be more or less vulnerable than neurons to brain ischemia is the study of ionic homeostasis in astrocytes during cerebral injury. Indeed, there is now strong evidence that astrocytes by maintaining ionic homeostasis have a critical role in the processes related to cell death and survival of other brain cells. In fact, glial cells are essential for the maintenance of (1) cellular pH in a range compatible with central nervous system (CNS) cell survival and (2) of Na+, Ca2+, and K+ homeostasis.
In gray matter, astrocytes are connected by an extensive network of gap junctions12 permeable to K+. In white matter, astrocytes are much less abundantly coupled.13 Nevertheless, ionic redistributions in interconnected cells of the CNS are of fundamental importance.
Thus, astrocytes form a syncytium for rapid redistribution of K+ from regions with high neuronal activity to perivascular areas. This astrocytic-mediated involvement in K+ redistribution is primarily mediated by inwardly rectifying K+ channels.14
Other mechanisms, such as the exchange between K+ and Na+, catalyzed by the astrocytic Na+, K+-ATPase, contribute to extracellular K+ homeostasis. Active uptake of K+ into astrocytes may also be mediated by the Na+–K+–Cl− cotransport.15 Particularly relevant is the function of all those transporters, such as Na+/H+ exchanger (NHE) and Na+–HCO3− cotransporter, that by controlling the concentration of H+ and HCO3− ions regulate cellular pH.16, 17 In addition, other transporters, including Na+/Ca2+ exchanger (NCX) and Na+–K+–Cl− cotransport, by controlling Ca2+ homeostasis influence gliotransmitter release and cerebral blood flow.18, 19 In addition to generally acknowledged Ca2+ excitability of astroglia, recent studies have demonstrated that neuronal activity triggers transient increases in the cytosolic Na+ concentration ([Na+]i) in perisynaptic astrocytes. These [Na+]i transients are controlled by multiple Na+-permeable channels and Na+-dependent transporters; spatiotemporally organized [Na+]i dynamics in turn regulate diverse astroglial homeostatic responses, such as metabolic/signaling utilization of lactate and glutamate, transmembrane transport of neurotransmitters and K+ buffering. In particular, near-membrane [Na+]i transients determine the rate and the direction of the transmembrane transport of GABA and Ca2+.20
Microglia in Brain Ischemia
Microglia are the immunocompetent cells of the CNS and account for 10% of the total glial cell population in the brain.2 During embryonic development, microglia differentiate in the bone marrow from hematopoietic stem cells to monocytes and then travel to the brain, where they settle and further differentiate into microglia.2
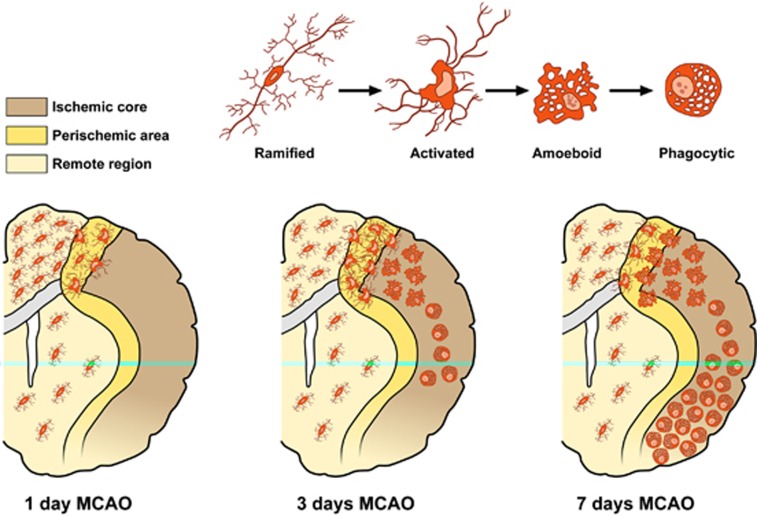
In the brain, microglial cells may appear in resting and multiple activated states, including ameboid and phagocytic. Under physiological conditions, microglial cells are in the resting state, characterized by a small cell body with fine, highly branched processes. In the developing brain, these cells support neuronal differentiation and clearance of cells deemed for elimination through programed cell death. In the mature brain, resting microglia serve the role of immune surveillance and host defence. Any disturbance or loss of brain homeostasis, as it occurs during an ischemic insult, evokes a rapid transformation of resting microglia either into an activated or into a phagocytic state. During this transition, resting microglia retract their processes, increase the size of cell body, modify the expression of enzymes, receptors, and immune response molecules. Microglial cells become motile, and using ameboid-like movements, migrate rapidly to the injury site along the chemokine gradients and also in response to chemoattractants released after the injury. However, if the damage persists and the CNS cells die, microglial cells undergo further transformation and become phagocytes. In fact, besides morphological changes and surface molecule upregulation, activated microglia secrete a wide array of soluble factors. Microglia response to pathological tissue alterations in the postischemic brain may be beneficial when neurotrophic factors, such as IGF-1, NGF, NT-3, NT-4, and BDNF, are released or become harmful when neurotoxic and inflammatory molecules such as free radicals, reactive oxygen species, nitric oxide (NO), superoxide, and fatty acid metabolites molecules are secreted. In addition, they produce a wide range of immunocompetent molecules, which include the proinflammatory cytokines interleukin (IL)1-β, IL-6; IL-3, and the tumor necrosis factor-alpha. All these molecules regulate the inflammatory processes and control the immune response.2
The signals triggering microglial activation are not completely known. It is recognized that both withdrawal of molecules normally released under physiological conditions, ‘off signals', or release or upregulation of molecules, ‘on signals', might be involved in microglial activation. Neurotransmitters such as ATP and neuropeptides are recognized as ‘on signals'. Microglia are clearly susceptible to cell death induced by severe hypoxia, particularly when combined with aglycemia.21 The temporal sequence of microglial activation during an ischemic event has been well characterized using animal models of focal cerebral ischemia.22, 23 In particular, microglia response begins in the ischemic penumbra within few hours after reperfusion. Its activation is protracted in the periinfarct zone and becomes striking by 3 days and peaks at 7 days. After 14 days, the number of activated microglia in the periischemic area is significantly lower than that after 3 and 7 days. In the ischemic core, the majority of microglial cells degenerate by 12 hours after reperfusion. Later, 24 hours after the insult, several round microglia/macrophage cells invade the ischemic core and dramatically increase within this region throughout the first week after MCAO. At this time, they can no longer be microscopically or immunohistochemically differentiated from invading blood-borne brain macrophages. At later time points, after 2 weeks, the number of round cells decreases. However, the innate microglia, rather than the blood-borne immune cells, are the predominant immunocompetent cells in the brain for the first 3–4 days after ischemia (Figure 2).24
Figure 2.
Schematic anatomic diagrams describing the distribution of the different microglia phenotypes in the postischemic brain. MCAO, middle cerebral artery occlusion.
During the last two decades, a large number of papers have been published describing both the detrimental and beneficial roles of microglia in various brain disorders, including stroke. However, the specific conditions that induce microglia to take beneficial phenotypes remain unknown. Furthermore, although in the ischemic brain microglia appears to be a major cellular contributor of postischemic inflammation, there is also growing evidence that some aspects of the inflammatory response are important for tissue repair.25 Indeed, microglia has been shown to be neurosupportive by removing cell debris, by engulfing polymorphonuclear neutrophiles,26 and by promoting adult neurogenesis.27 The benefits of microglial activation have been further demonstrated by several findings: (1) the exacerbation of neuropathology in inducible mouse models lacking microglia,28(2) the finding of protective microglia in cases of cerebral ischemia,26 and (3) the improvement, after CNS injury, of neurite growth and functional recovery after microglia transplantation.29
Oligodendrocytes in Brain Ischemia
The term oligodendroglia was introduced by Rio Hortega in 1921 to describe those neuroglial cells that show few processes, hence the prefix ‘olig'.30
In the adult CNS, oligodendrocytes (OLGs) can be classified as follows: (a) OLG precursor cells (OPCs), (b) myelin-forming cells, and (c) non-myelinating OLGs.
Oligodendrocyte Precursor cells
Oligodendrocytes arise from OPCs that colonize both the gray and white brain matter during development.31 Oligodendrocytes develop from OPCs through distinct phenotypic stages, which can be identified by the sequential expression of specific markers characteristic of progenitors, including A2B5, NG2, PDGFalpha receptor, O4, or of mature and myelinating OLGs, identified by selective markers such as CNPase, myelin-associated glycoprotein, and myelin basic protein. Although many OPCs differentiate into mature myelinating OLGs during the early and much later stages of human brain development, a considerable number of them do persist in the adult brain at the pro-OLG stage, and may provide a source of new OLGs, protoplasmic astrocytes, and neurons.32, 33 Because of their apparent stem-cell-like characteristics, adult OPCs have recently gained much attention for their potential reservoir of cells capable of self-renewal, differentiation, and remyelination after CNS injury.34
Myelin-forming Oligodendrocytes
Myelinating OLGs are classified in four categories, according to the morphology, size, and thickness of the myelin sheath they form. Type I and type II are small cells supporting the short, thin myelin sheaths of 15 to 30 small-diameter axons. Types III OLGs have larger cell bodies and myelinate up to five thick axons. Finally, type IV OLGs are the largest cells forming long, thick myelin sheaths of 1 to 3 large-diameter axons.
The main function of myelinating OLGs is the production of myelin, which insulates axons and facilitates the fast saltatory conduction of action potential. In addition to myelination, these glial cells, by producing and releasing several neurotrophins, such as NGF, BDNF, and NT-3, can provide trophic support on nearby neurons.35 Furthermore, myelinating OLGs use the lactate transporter MCT1 to provide metabolic support to neurons.36
Non-myelinating Oligodendrocytes
A population of non-myelinating OLGs attached to large neurons are present in the gray matter and are known as ‘perineuronal' or ‘satellite' OLGs. Satellite OLGs have generally received little attention and their precise function is still unknown. Previous in vitro studies suggested that they are involved in neuronal support35 and, more interestingly, may remyelinate denuded axons after injury.37 This observation, together with the recent finding showing that these cells carry transcripts for all the major myelin proteins, without translating them under physiological conditions, suggests that non-myelinating OLGs do possess a latent myelinating machinery that can be activated after a demyelinating episode.38
Among the different glial cell types, gray and white matter OPCs and OLGs are the most vulnerable to perturbations of ionic homeostasis, as it occurs in stroke.39 Their vulnerability to ischemia, comparable to that occurring in neurons, is owing to their high sensitivity to energy deprivation, oxidative stress, and hypoxia. Damage to OLGs during cerebral ischemia causes demyelination, white matter dysfunction, and axonal impairment (Wallerian degeneration). Actually, demyelination is a pathological process in which myelin sheaths are lost around axons with consequent alteration of conduction and impairment of sensation, movement, cognition, or other functions depending on the nerves involved. White matter ischemia that is usually more severe than gray matter ischemia because of the lower blood flow, and little collateral blood supply39 is a clinically important part of stroke. Indeed, white matter lesions are often observed in stroke patients and have been thought to contribute mainly to cognitive impairment.40
Glutamate-mediated excitotoxicity is implicated in the loss of myelin and OLGs occurring in stroke.41, 42 OPCs and OLGs located in the infarct core undergo anoxic depolarization rapidly. Loss of ion homeostasis triggered by ischemia causes acute axonal depolarization and elevation in axoplasmic [Na]i+; these changes result in reversal of the Na+/glutamate transporter and massive release of glutamate. Glutamate, in turn, triggers Ca2+ entry into OLGs mainly through AMPA receptors. Oligodendrocytes do not possess the GluR2 AMPA subunit rendering their AMPA receptors permeable to Ca2+ ions. Oligodendrocyte precursor cells, which express high levels of AMPA/Kainate receptors, appear to be the most vulnerable to glutamate toxicity. Depending on the intensity of the insult, Ca2+ overload in OLGs triggers oxidative stress and mitochondrial damage, with subsequent induction of necrosis or apoptosis.
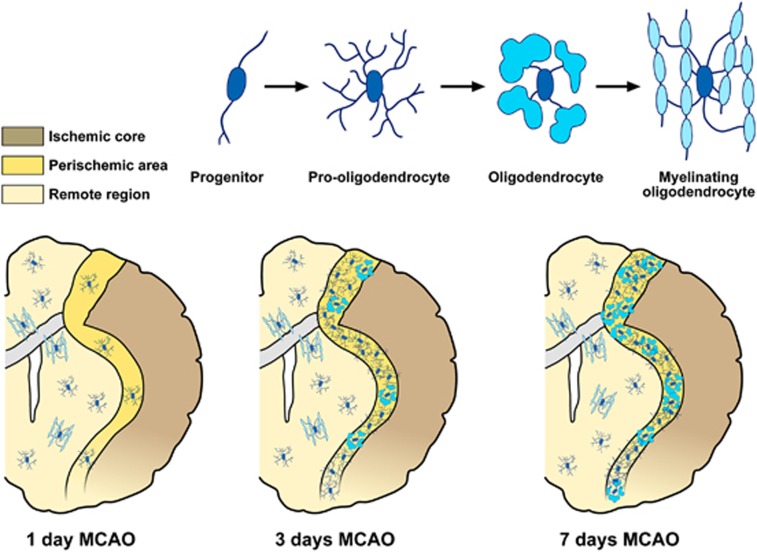
Although a rapid decrease in the number of OLGs, OPCs, and myelin density occurs in the infarct core 1–2 days after tMCAO, the periinfarct area, including white matter regions bordering the lesion, exhibits a steady increase in the number of OPCs 3 and 7 days after ischemia onset and a gradual recovery of OLGs 2 weeks after MCAO.43, 44 Periinfarct OLGs progressively increase the expression of myelin marker PLP and MBP from 24 hours to maximal levels at day 7 after tMCAO (Figure 3).45 Interestingly, in the periischemic ischemic lesion, NG2 cells display multipotent differentiation after focal ischemia.44
Figure 3.
Schematic anatomic diagrams describing the distribution of the different adult oligodendrocyte (OLG) progenitor cells (OPCs) and OLG phenotypes in the postischemic brain. MCAO, middle cerebral artery occlusion.
Alterations in ionic homeostasis may have a crucial role in OPC response during demyelination and remyelination processes. In fact, changes in ionic homeostasis, in particular the increase in [Ca2+]i levels, not only influence the developmental processes that accompany the transition of OPCs into mature myelinating OLGs, but also intervene in the initiation of myelination and remyelination processes.46 Furthermore, the increase in [Ca2+]i levels influences the developmental processes that accompany the transition of human OPCs into mature myelinating OLGs, including OPC migration, lineage progression, and differentiation.47, 48
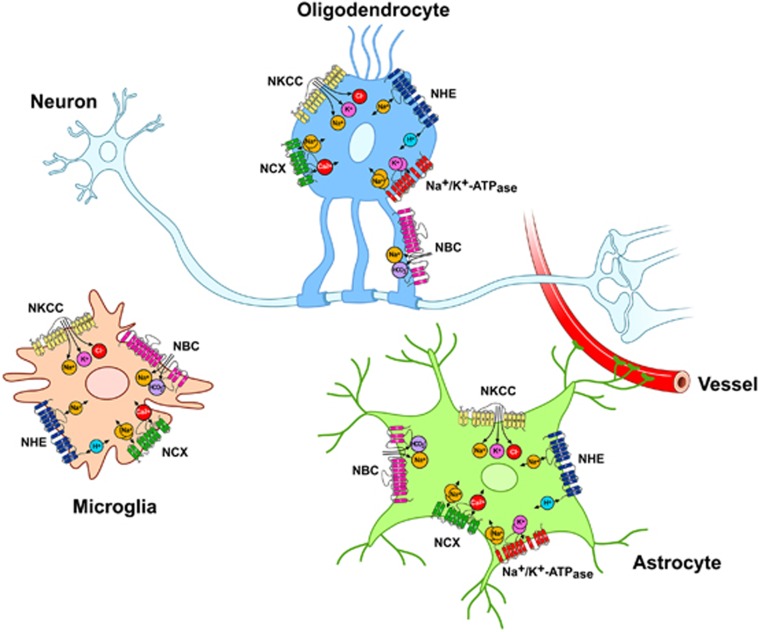
Finally, as the ionic fluxes through plasmamembrane transporters in OLGs or OPCs are involved in several functions regulating OLGs development, cell death, or repair processes, it would be valuable to further elucidate the role of selective transporters expressed on these cells (Figure 4).
Figure 4.
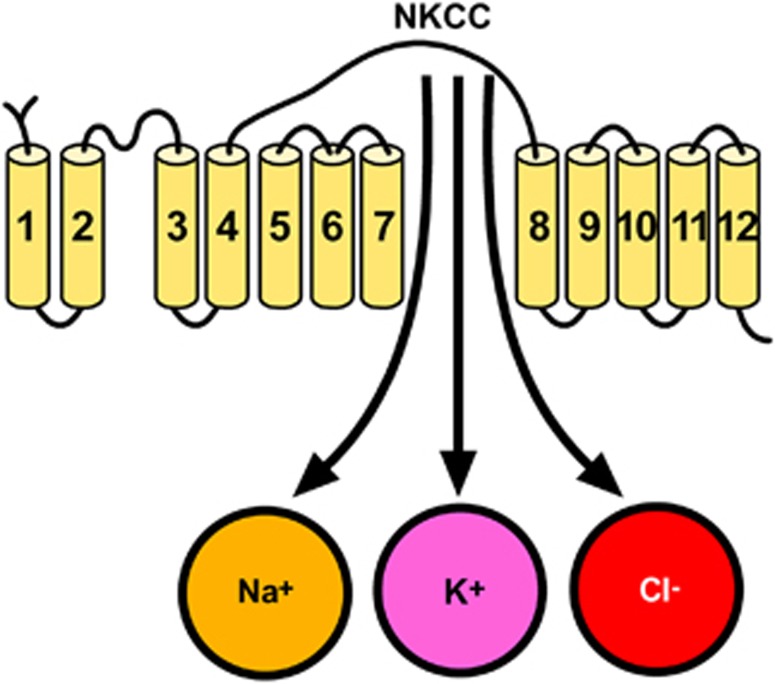
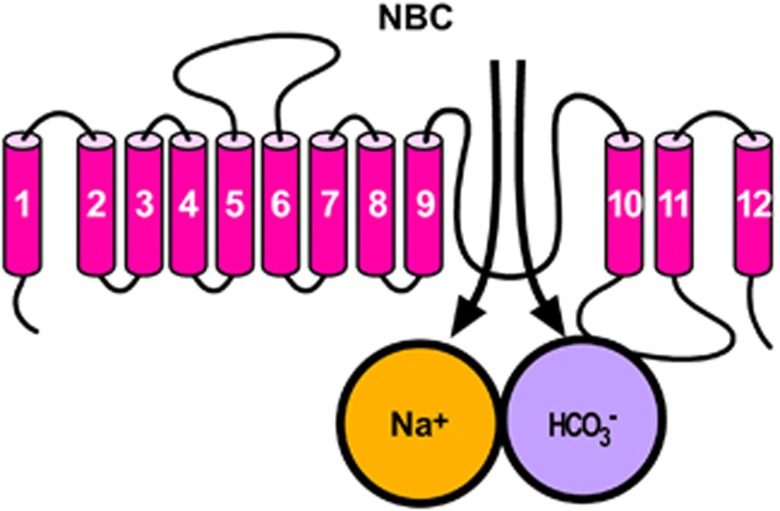
Major ionic transporters expressed in microglia, astrocytes, and oligodendrocytes (OLGs). NBC, Na+/HCO3− cotransporter; NCX, Na+/Ca2+ exchanger; NHE, Na+/H+ exchanger; NKCC, Na+–K+–Cl− cotransporter.
Expression and function of ionic transporters in glial cells during physiological conditions and in brain ischemia
Na+/Ca2+ Exchanger
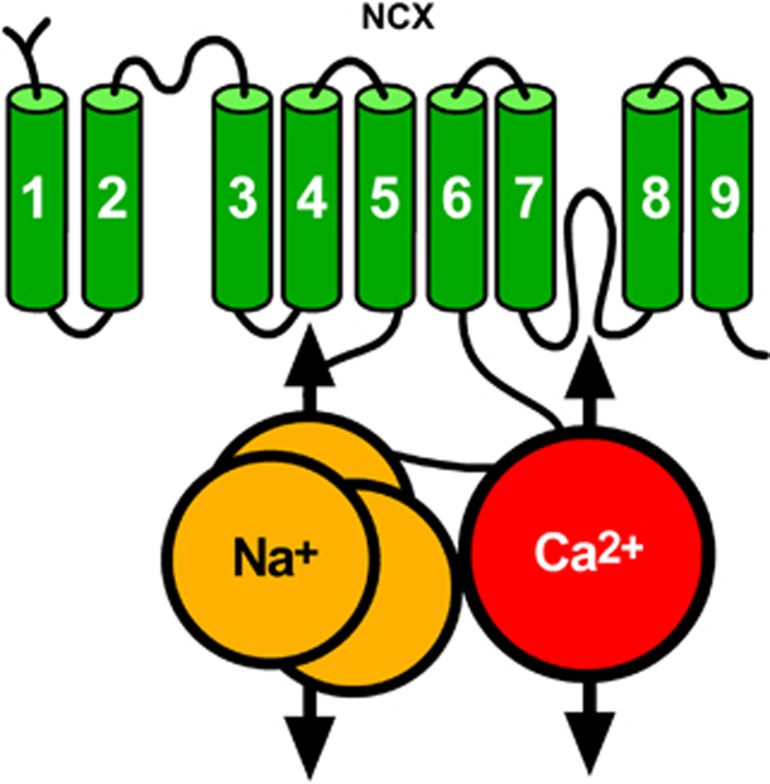
The NCX is an ionic transporter that exchanges three Na+ ions for one Ca2+ ion.49, 50 When [Ca2+]i rise, this exchanger couples the uphill extrusion of Ca2+ ions to the entry of Na+ ions into the cells. This mode of operation, defined as forward mode,keeps the 104-fold difference in [Ca2+]i across the cell membrane. In other physiological or pathophysiological conditions when the [Na+]i rise, NCX reverses its mode of operation and mediates the extrusion of Na+ and the entry of Ca2+ ions. This mode of operation is defined as reverse mode. Na+/Ca2+ exchanger is composed by nine transmembrane segments (TMSs)51 that can be divided into an N-terminal hydrophobic domain, composed of the first five TMS1-5, and into a C-terminal hydrophobic domain, composed of the last four TMS6-9, separated through a large intracellular loop named f loop (Figure 5).51 The f loop is responsible for the regulation of NCX activity elicited by several transductional mechanisms, such as H+, Ca2+, and Na+ ions, NO, phosphatidylinositol, PKC, PKA, and ATP.49
Figure 5.
Putative topology of Na+/Ca2+ exchanger (NCX).
Three genes coding for the three different NCX1-352, 53, 54 proteins have been identified. Na+/Ca2+ exchanger 1 displays an ubiquitous expression, whereas NCX2 and NCX3 are present exclusively in neuronal and skeletal muscle tissues.55 Notably, these NCX isoforms have a fundamental role in the pathophysiology of stroke damage. In particular, NCX1 and NCX3 downregulation or genetic ablation worsens ischemic damage in mice and rats,56, 57, 58 whereas its pharmacological activation determines a reduction in the brain infarct volume.59
Na+/Ca2+ Exchanger in Astrocytes
Na+/Ca2+ exchanger 1 represents the most expressed NCX isoform in astrocytes,60 where when operating in the reverse mode, it can deliver Ca2+ to the cytosol.61
The most important role played by NCX1 in astrocytes is related to the regulation of gliotransmitter release.18 Hence, mild depolarization of astrocytes forces NCX to operate in the reverse mode and generates cytosolic Ca2+ increases leading to release of gliotransmitters, such as glutamate.62, 63
Only few studies have investigated the pathophysiological role of astrocytic NCX in ischemic astrocytes. In in vitro models of ischemia, 1 hour of oxygen–glucose deprivation (OGD) does not cause astrocyte death but does cause a long-lasting decrease in mitochondrial membrane potential and a loss of mitochondrial cytochrome c. In another model of hypoxia acidosis, it has been shown that astrocyte death depends on extracellular Ca2+ and is prevented by NCX inhibition.64
Na+/Ca2+ Exchanger in Microglia
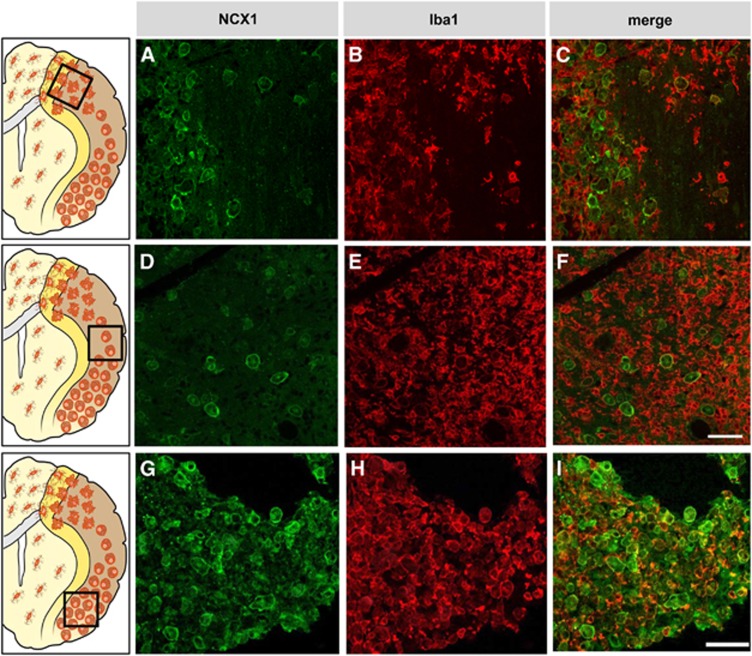
All three NCX exchangers, NCX1, NCX2, and NCX3, are expressed in microglia, but NCX1 is the most prominent.23, 65 Na+/Ca2+ exchanger activity in microglia was first recorded by Matsuda et al.66 who observed that a Na+-dependent45Ca2+ uptake was inhibited by the NCX inhibitor SEA0400. Interestingly, NCX1 in microglia has a key role in response to interferon-γ (IFN-γ) and NO,67 two cell products implicated in several CNS pathologies, including stroke. Treatment of microglia with IFN-γ causes a biphasic increase in NCX activity. The delayed increase in NCX activity is accompanied by increases in messenger RNA and protein levels of all NCX isoforms.67 Interestingly, protein kinase C and tyrosine kinase inhibitors prevent the transient increase in NCX activity induced by IFN-γ, whereas MAP kinase and ERK are involved in the delayed increase in NCX activity.65 Furthermore, Nagano et al. showed that NCX is activated by NO and is involved in NO-induced depletion of Ca2+ in the endoplasmic reticulum,thus leading to endoplasmic reticulum stress.67 The responses of NCX to IFN-γ and NO implies that Ca2+ uptake via NCX operating in reverse mode may have a role for microglial activation under pathological conditions. Recently, a prominent role of NCX in microglial migration has been suggested.68 For instance, Ca2+ influx via reverse-mode activity of NCX is a prerequisite for bradykinin and B1 receptor agonist-induced microglial motility. The knocking out of NCX1 impairs bradykinin-induced chemotaxis or migration in microglia. The relevance of NCX1 function in microglia is further supported by the findings that ncx1–/– embryos have no detectable microglia in the brain.69 The crucial role of NCX1 in activated microglia is becoming further evident. Phagocytosis and the ensuing nicotinamide adenine dinucleotide phosphate oxidase-mediated respiratory burst are important aspects of microglial activation that require calcium influx. Inhibiting the NCX reverse mode with KB-R7943 dose dependently reduces the phagocytosis-stimulated respiratory burst.70 In accordance with the role played by NCX in phagocytic microglia, 3 and 7 days after permanent MCAO (pMCAO), NCX1 signal progressively increases in the Iba1-positive microglia invading the infarct core. In these cells, NCX1 expression is limited to the round phenotype (Figure 6).23 However, it should be underlined that Iba1 does not discriminate between round phagocytic microglia and circulating blood-borne brain macrophages invading the infarct core.
Figure 6.
(A–I) Photomicrographs showing round phagocytic croglia displaying both Na+/Ca2+ exchanger 1 (NCX1) and Iba1 immunoreactivity in the inner periischemic region (A–C), in the ischemic region more distal (D–F) or more proximal (G–I) to the middle cerebral artery. The schematic anatomical diagram at the left side of each photograph set describes the ischemic region from where the images were taken. Scale bars 50 μm.
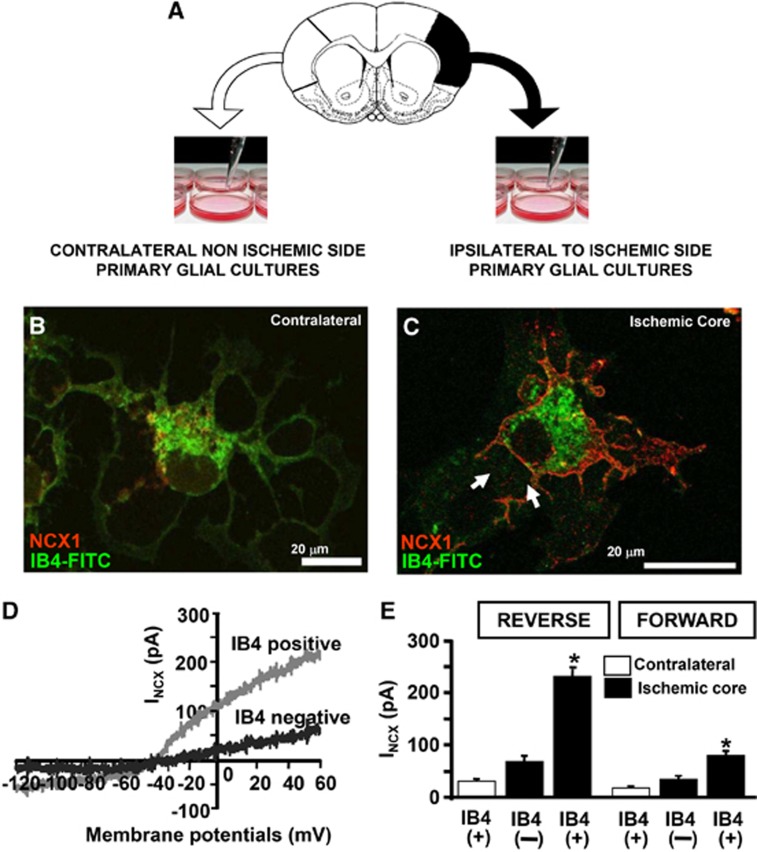
To further explore NCX1 expression and NCX activity in microglial cells of the infarct core, Boscia et al.23 used primary glial cultures dissociated ex vivo from the cortical core of ischemic rat brain and the corresponding contralateral region 7 days after pMCAO. Double-labeling experiments of NCX1 protein with the microglial marker IB4 revealed that only in microglia isolated from the core NCX1 signal was intensely detected on the plasmamembrane. Accordingly, NCX activity recorded in the reverse and forward mode of operation is significantly higher in IB4 positive cells obtained from the core than that recorded both in IB4-negative cells of the same region and IB4 positive cells of the contralateral hemisphere. Relevantly, in microglia obtained from the core, the currents carried by NCX working in the reverse mode is much higher than those recorded in the forward mode (Figure 7). Finally, the direct effects of hypoxic conditions on NCX1 expression and activity have been studied in BV2 microglial cell line exposed to OGD followed by reoxygenation. Under these experimental conditions, a significant increase in NCX1 protein expression occurs particularly 24 hours after reoxygenation; whereas a significant decrease in both NCX2 and NCX3 occurs both at earlier and later intervals of reoxygenation. In microglia exposed to OGD, INCX currents, recorded both in the forward and reverse modes of operation, are significantly higher than those detected under normoxia.23
Figure 7.
(A) Schematic diagram of primary microglial cell culture obtained from brain of rats bearing tMCAO; (B–C) Colocalization of Na+/Ca2+ exchanger 1 (NCX1) with IB4–fluorescein isothiocyanate (FITC) in microglial cells obtained from the contralateral hemisphere (B) or from the core region of the ipsilateral hemisphere (C). Scale bars: 20 μm. (D) INCX traces recorded from IB4(+) microglia and IB4 (−) cells isolated from the core. (E) INCX quantification is expressed as current densities recorded in IB4(+) and IB4(−) cells obtained from the core and in the IB4(+) cells from the corresponding contralateral cortex (n=20 per group). *P<0.05. Modified from Boscia et al 2009. License number 3110331370094.
When NCX1-silenced BV2 cells are exposed to OGD plus 6 hours of reoxygenation, the increase in [Ca2+]i is completely prevented. This finding, together with the results showing that the protein expression of the other NCX isoforms—NCX2 and NCX3—significantly decreases after OGD, demonstrates the relevant role played by the NCX1 isoform in round phagocytic microglia during hypoxic conditions.
Na+/Ca2+ Exchanger in Oligodendrocytes
All three NCX isoforms are expressed in primary cultures of OLGs and OLG precursor MO3.13 cell line.71 The NCX1 staining in the myelinated axons of the sciatic nerve, optic nerve, and spinal cord is similar being associated with axons, and with cell bodies and processes. The lack of NCX staining in some white matter regions, as NCX1 in the corpus callosum, raises the possibility that the localization of the three exchanger isoforms may be different within the white matter tracts.72 Na+/Ca2+ exchanger 1 and Na+/Ca2+ exchanger 3 isoforms are differently expressed in OPCs and are divergently modulated during differentiation of OPCs into OLGs. Indeed, whereas NCX1 isoform decreases, NCX3 isoform is strongly upregulated during OLG maturation. Expression and functional studies suggest that NCX1 represents the main contributor to NCX currents (INCX) recorded in OPCs.71 Likewise, NCX1, but not NCX2, is highly expressed in OPCs and pharmacological inhibition of NCX1 or its selective silencing with small interfering RNA largely inhibits migration and GABA-induced Ca2+ signaling in cultured OPCs. Na+/Ca2+ exchanger 3 represents the main isoform expressed in mature OLGs and, consequently, the main contributor to INCX recorded in these cells.73 The importance of calcium signaling mediated by NCX3 exchanger during OLG development and myelin formation is supported by several findings: (1) the knocking down of NCX3 expression and activity by small interfering RNA strategy in OPC cultures prevents the formation of myelin proteins; (2) NCX3 overexpression induces the upregulation of the two myelin markers CNPase and MBP; and (3) NCX3 knock-out mice exhibit hypomyelination that is accompanied by an augmented number of the OPC cells, and a reduction of spinal cord size.71
Previous studies have demonstrated a central role of NCX in anoxic and ischemic injury of both central and peripheral myelinated axons. Indeed, NCX reverse mode of operation has been implicated in axonal damage during spinal cord anoxia,74 stretch injury of axons,75 and experimental allergic encephalomyelitis.76 In OLGs, NCX1 and NCX3 are co-localized with Na+–K+–Cl− cotransporter 1 (NKCC1). The reverse-mode operation of NCX and NKCC1 has been also implicated in OLG cell death induced by AMPA-mediated excitotoxicity. Activation of AMPA receptors leads to NKCC1 phosphorylation that, in turn, enhances NKCC1-mediated Na+ influx. The latter triggers NCX in the reverse mode of operation with consequent Ca2+ overload, thereby compromising mitochondrial function and cellular viability.77
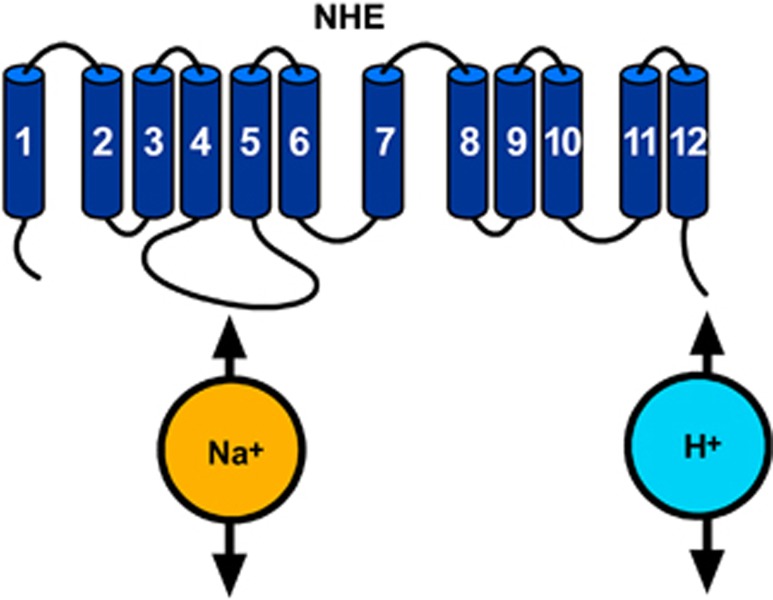
Na+/H+ Exchanger
The mammalian NHE family is a group of integral membrane transport proteins that mediates an electroneutral 1:1 exchange of intracellular H+ for extracellular Na+ and in doing so regulates pHi homeostasis and cell volume.78 All members of NHE family are composed of 12 transmembrane domains and contain two functional domains: the N-terminal transmembrane domain, which is necessary and sufficient to catalyze ion translocation, and the C-terminal cytoplasmic domain, which is crucial for modulating NHE activity (Figure 8).78 To date, nine isoforms have been cloned. These isoforms differ in their tissue expression, subcellular distribution, kinetic properties, inhibitor sensitivity, and physiological functions. Na+/H+ exchanger 1 is ubiquitously expressed and is considered to be a ‘housekeeping' isoform. Na+/H+ exchanger 2–3 are highly expressed in the apical epithelia of the kidney and intestine. Na+/H+ exchanger 4 is mainly present in the stomach and basolateral epithelia of the kidney. Na+/H+ exchanger 5 predominantly resides in the brain. Na+/H+ exchanger 6–9 are targeted to the membranes of intracellular organelles. Na+/H+ exchanger 1 is by far the most extensively studied isoform and is associated with many physiological and pathophysiological conditions.79
Figure 8.
Putative topology of Na+/H+ exchanger (NHE).
As NHE is involved in numerous essential cell functions, its activity is crucial for the correct cell functioning even under pathophysiological conditions. Indeed, the pharmacological blockade of NHE-1 activity reduces infarct volume in animal models of brain ischemia.80 In addition, ischemia/reperfusion determines an increase in NHE-1 expression/activity.78
Na+/H+ Exchanger in Astrocytes
Na+/H+ exchanger represents the most important plasmamembrane transporter involved in the astrocytic pH regulation. Indeed, the NHE blocker amiloride decreases the resting pHi by 0.10 to 0.42 pH units in rat cortical astrocytes.81 Similar decreases in the resting pHi by amiloride have been found in rat cerebellar and hippocampal astrocytes.82 Furthermore, amiloride abolishes pHi recovery in astrocytes after hypoxia-induced acid loads.82 These results are likely related to the role played by NHE in the progression of ischemic lesion. Indeed, it has been shown that inhibition of NHE-1 attenuates the detrimental consequences of ischemia and reperfusion.80 However, it is still unknown whether these protective effects are attributable to inhibition of NHE activity in neurons and/or astrocytes. However, data recently obtained in NHE-1−/− mice seem to resolve this debate. Indeed, the genetic ablation of NHE-1 reduces the OGD-mediated rise in [Na+]i and swelling in cortical astrocytes, suggesting that the loss of NHE-1 activity reduces ischemic injury mainly through astrocyte-mediated effects.80
Na+/H+ Exchanger in Microglia
Na+/H+ exchanger-1 is abundantly expressed in primary microglia and M4T.4 microglial cell line. This exchanger has a key role in maintaining resting intracellular pH and extruding H+ after acidosis in microglia.83
Na+/H+ exchanger-1 is the major protein involved in the regulation of pHi in activated microglia, whereas bicarbonate transporters (see below section Na+/HCO3− cotransporter) have only a minor role. Blockade of NHE-1 function with the more potent and selective NHE inhibitor cariporide (HOE642) significantly acidifies primary microglia and abolishes the H+ extrusion. Liu et al. demonstrated that activation of microglia with lipopolysaccharide or OGD plus reoxygenation stimulates NHE-1 activity to maintain H+ homeostasis. The elevated H+ extrusion mechanism prevents intracellular acidosis and sustains nicotinamide adenine dinucleotide phosphate oxidase-mediated respiratory burst. Overstimulation of NHE-1 activity mediates intracellular Na+ overload and consequently triggers Ca2+ influx through NCX reverse mode, which promotes the microglial respiratory burst and the production of proinflammatory cytokines.83 Interestingly, pharmacological inhibition or genetic ablation of NHE-1 gene expression not only abolishes pHi regulation but also reduces the production of the superoxide anion, proinflammatory cytokines, and inducible NO synthase under ischemic conditions, in vitro and in vivo.83
Na+/H+ Exchanger in Oligodendrocytes
Acid extrusion and cellular alkalinization in cerebellar OLGs are almost exclusively mediated by the activity of NHE. In bicarbonate-free buffer, pHi recovery after an acid load is completely dependent on the presence of sodium in the extracellular space and is inhibited by the NHE inhibitor amiloride.84 Ro and Carson,85 by using a ratiometric pH indicator, demonstrated that OLGs contain spatially restricted alkaline and acidic pH microdomains generated by differential subcellular distribution of NHE, Na+/HCO3− cotransporter (NBC), and carbonic anhydrase. Carbonic anhydrase II (CAII) is the main isozyme present in the brain, where it is concentrated in the myelin compartment of OLGs. In OLGs, CAII is colocalized with NHE in the perikaryon, and with NBC in the processes.85 Acidic regions in the cytoplasmic C-terminal region of NHE interact with CAII, creating a transport metabolon to facilitate H+ export, particularly under acidic conditions.85 The existence of pH microdomains in OLGs may serve to buffer activity-dependent fluctuations in intracellular pH and may also influence local electrical activity.
Thus, given that NHE regulates OLG pHi, it can be hypothesized that during ischemic damage the modulation of NHE in OLG lineage may give rise to a neuroprotective effect.
Potassium–chloride Cotransporters
Four distinct but related genes encoding potassium–chloride cotransporter (KCC) have been cloned, KCC1-4, all of which have 70% sequence identity to each other.86 They share the same topology with 12 putative membrane-spanning domains, a large extracellular loop between fifth and sixth transmembrane domains, a short cytoplasmic amino terminus with a long carboxy terminus.86 All KCC1-4 genes have been described in the CNS. Potassium–chloride cotransporter 2 is mainly expressed in neurons and astrocytes, KCC3 and KCC4 are predominantly found in glial cells.87 Under physiological conditions, KCC export 1K+ and 1Cl− across the membrane. Normally, transport direction is outward, but it can be reversible and dictated by the free-energy transmembrane gradients for K+ and Cl−. Potassium–chloride cotransporters are involved in intracellular chloride concentration maintenance, cell volume regulation, control of CNS excitability, and epithelial ion transport.88 They can be activated during brain ischemia as their activity is influenced by cell swelling, intracellular acidification, reactive oxygen radicals, or intracellular signaling processes, such as protein phosphorylation.88
Potassium–Chloride Cotransporter in Astrocytes
The expression pattern of KCC isoforms in astrocytes reveals strong expression levels for KCC1, KCC2, and KCC3.89, 90
Maintenance and regulation of cell volume is a major function of KCC as demonstrated for red blood cells and many other cell types.89, 90 Furthermore, a similar role for KCC has been recently reported also in astrocytes.90 As KCC is involved in brain cell volume regulation, it may also have a role during the development of ischemic or traumatic brain edema, pathological conditions where cell volume regulation is disturbed.
Potassium–Chloride Cotransporter in Microglia
All four KCC subtypes are constitutively expressed in primary microglial cells and BV2 microglia cell line.91 Potassium–chloride cotransporter cotransporters participate in cellular mechanisms that promote the transformation of microglial cells from ramified into ameboid morphology. Indeed, changes in cell phenotype after lysophosphatidylcholine-induced microglia activation are prevented by the simultaneous inhibition of cation channels and KCC with Gd3+ and the potent KCC blocker DIOA, respectively.92 More recently, the involvement of KCC in the formation of lamellipodial protrusion during microglial cell migration has been demonstrated.91 Ameboid microglia form broad lamellipodia, required for cell migration and phagocytosis of cell debris. After ischemia, the increase in extracellular K+ concentration reverses flux direction of KCC with a consequent increase in intracellular osmolarity. Such increase evokes volume changes that are essential for the sprouting of a lamellipodium. When BV-2 cells or primary microglial cells are exposed to a high KCl (70–135 mmol/L) or hypoosmotic saline solution they do not show an equal swelling in each direction but develop a delicate extension at one pole of the cell, which then proceeds rapidly into a vigorously moving lamellipodium within seconds. After the formation of a lamellipodium, the cell starts to migrate. Blockade of chloride influx with DIOA significantly prevents the formation of lamellipodia.91
Potassium–Chloride Cotransporter in Oligodendrocytes
In OLGs and Schwann cells, KCC may participate in the regulation of cell volume under physiological and pathological conditions. Potassium–chloride cotransporter 2 signal is detected within the cell bodies of OLGs, whereas KCC3 expression is abundantly observed in white matter-rich regions of the brain, including the myelinated tracts of the internal capsule, corpus callosum, and spinal cord.93, 94 Loss-of-function mutations of the kcc3 gene are responsible for agenesis of the corpus callosum-associated peripheral neuropathy (Anderman syndrome), the first hereditary sensorimotor neuropathy to be attributed to a defect in an ion transporter.95 Potassium–chloride cotransporter 3-knockout mice reproduce the peripheral neuropathy observed in human, with locomotor and sensorimotor deficits. Impairment of the cell volume regulatory mechanism in both neurons and glia may contribute to hypomyelination, decompaction of myelin, demyelination, axonal swelling, and fiber degeneration as observed in peripheral nerves and spinal cord of KCC3-knockout mice.95, 96
Oligodendrocyte damage under anoxia/ischemia may be attributable more to volume alterations in myelin sheath than to cytoskeletal dissolution as it occurs in axons.97 As a consequence, altered conduction along white matter tracts will be impaired. Potassium–chloride cotransporter may also contribute to such Cl−-dependent volume alterations under ischemic conditions. In fact, combined inhibition of Na+ influx and K+ and Cl− efflux, obtained through the blockade of KCC, protects white matter against anoxia better than Na+-channel blockers alone.98
Na+–K+–Cl− Cotransporter
The electroneutral NKCC mediates the influx of Na+, K+, and Cl− with a stoichiometry of 1Na+:1K+:2Cl−.99
To date, only two distinct isoforms, NKCC1 and NKCC2, have been identified. Na+–K+–Cl− cotransporter 1 has a broad tissue distribution, whereas NKCC2 is only found in vertebrate kidney. Na+–K+–Cl− cotransporter serves multiple functions, including ion and fluid movements in secreting or reabsorbing epithelia and cell volume regulation.99 The structure of NKCC1 consists of 12 putative transmembrane domains, flanked by large cytoplasmic amino and carboxyl termini, which contain regulatory phosphorylation sites (Figure 9).99
Figure 9.
Putative topology of Na+–K+–Cl− cotransporter (NKCC).
Na+–K+–Cl− cotransporter 1 protein is expressed in neurons throughout the brain where it contributes to the maintenance of [Cl−]i. Thus, NKCC1 may affect neuronal excitability by regulating [Cl−]i. Expression of NKCC1 protein has also been found in astrocytes and OLGs.100 The pharmacological inhibition of NKCC1 with bumetanide101 or its transgenic ablation significantly attenuates infarction and swelling after tMCAO.99 Therefore, NKCC1 represents an attractive target for novel therapeutic strategies against stroke.
Na+–K+–Cl− Cotransporter in astrocytes
Na+–K+–Cl− cotransporter 1 in astrocytes is mainly devoted to the control of K+ concentration, in the attempt to avoid excessive K+ accumulation that often occurs after cell swelling. Astrocytes undergo rapid swelling in a number of acute brain pathological states, such as ischemia and traumatic brain injury.102 Unresolved astrocyte swelling triggers a series of detrimental effects including reduction of extracellular space, decrease of normal cerebral blood flow, and accumulation of excitatory amino acids such as glutamate.103 In addition, swollen astrocytes would have a diminished capacity to perform their normal homeostatic functions. In this regards, astrocytic NKCC1 protein could function in clearing off excessive [K+]o.104, 105 Indeed, a short interval of anoxia/ischemia raises extracellular [K+] to ∼60 mmol/L106 and NKCC1 contributes to K+ influx into the cell. The activity of NKCC1 is involved not only in the control of extracellular K+ but also of extracellular Ca2+ ions. In fact, the influx of Na+ ions through NKCC1 can in turn induce NCX to work in the reverse mode, thus eliciting Ca2+ entry into the astrocytes and accumulation of cytoplasmic [Ca2+].107 In addition, the high extracellular [K+]-induced activation of NKCC1 is completely abolished by either removal of extracellular Ca2+ or by blocking the L-type voltage-dependent Ca2+ channels with nifedipine.108 In addition, intracellular Cl− accumulation increases significantly in response to ischemia and is abolished by inhibition of NKCC1.108, 109 These data suggest that in astrocytes the cotransporter activity is stimulated under high extracellular [K+] via Ca2+-mediated signal transduction pathways.
Another aspect that establishes a direct relationship between astrocytic NKCC and progression of ischemic damage is the influence that NKCC exerts on glutamate concentration. Indeed, astrocytic NKCC1 stimulation takes part to excitotoxic injury by increasing swelling-induced glutamate release or decreasing glutamate clearance from the extracellular space.104, 105, 109
Another key element promoting NKCC1-dependent cell damage in astrocytes during ischemia is represented by the loss of the plasmamembrane Na+ gradient.110 In this regards, the activation of several Na+ entry pathways, including NKCC1, also contributes to ischemia-induced loss of Na+ homeostasis. In fact, OGD in astrocytes results in both an increase in NKCC1 phosphorylation and activity.107 This stimulation of NKCC1 activity is accompanied by an accumulation of Cl− and Na+ in astrocytes.111 Na+–K+–Cl− cotransporter 1-mediated increases in [Na+]i after in vitro anoxia have also been linked to Ca2+ loading and subsequent mitochondrial damage. As a consequence of the increases in [Na+]i, NCX-mediated Ca2+ extrusion is reduced and NCX may function in the reverse mode, thus increasing [Ca2+]i. Therefore, NKCC1, working in a concerted way with NCX, may contribute to intracellular Na+ and Ca2+ overload in astrocytes after in vitro anoxia.111 In addition, Na+ and Ca2+ overload in ischemic astrocytes leads to a dissipation of the mitochondrial membrane potential and release of cytochrome C.112, 113
Na+–K+–Cl− Cotransporter in Microglia
Na+–K+–Cl− cotransporter contrasporter does not contribute to the swelling-activated Cl− current recorded in microglial cells. Indeed, cell swelling evoked by glutamate release from primary microglia is not prevented by inhibiting NKCC.70
Na+–K+–Cl− Cotransporter in Oligodendrocytes
Na+–K+–Cl− cotransporter 1 is abundantly expressed in cultured OLGs and in the white matter of the rat spinal cord. The expression of NKCC1 in developing spinal cords and OLGs parallels that of spinal cord myelination.1, 114 Na+–K+–Cl− cotransporter 1 serves to maintain [Cl−]i above electrochemical equilibrium, thereby having a pivotal role in GABAergic functions in OLGs. In fact, NKCC1 activity is required for GABA-A receptor activation and for GABAergic trophic effects. Indeed, the oligodendroglial cotransporter activity is significantly stimulated in response to activation of GABA-A receptor by muscimol and blocked by the GABA-A receptor antagonist bicuculline. Furthermore, survival of OLGs after withdrawal of growth factors requires NKCC1 activation.115 Na+–K+–Cl− cotransporter 1 may also contribute to maintenance of K+ and Na+ homeostasis. Prolonged AMPA treatment in OLGs causes a sustained intracellular calcium overload that leads to mitochondrial Ca2+ accumulation, cytochrome c release, and OLG cell death.116 This phenomenon is connected to NKCC1 activitation. Indeed, inhibition of NKCC1 significantly attenuates AMPA-induced OLG death. The protective effects may result from a reduction in intracellular Na+ overload occurring via NKCC1 and consequent Ca2+ overload mediated by the activation of NCX in the reverse mode.77
Na+/HCO3− Cotransporter
Na+/HCO3− cotransporter, 12 transmembrane domains containing an intracellular C and N terminus (Figure 10), represents, together with NHE, one of the main cellular systems involved in the regulation of ionic and pH homeostasis in the CNS117 where it can transport either two or three bicarbonate ions per one sodium ion.117 Several evidence demonstrate that exposure to acute and chronic hypoxia or ischemia produces changes in intracellular pH in neurons and glia,118 and more severe acidosis correlates with more severe injury,118 therefore NBC modulation may profoundly influence ischemic pathophysiology.
Figure 10.
Putative topology of Na+/HCO3− cotransporter (NBC).
Na+/HCO3− Cotransporter in Astrocytes
Schmitt et al.119 and Giffard et al.120 performed the early NBC localization studies on rat brain and found widespread expression of NBC1 throughout the brain in a pattern consistent with glial expression. The authors observed NBC1 messenger RNA expression in the astrocytes of the cortex, dentate gyrus, and brainstem. In double-labeling studies using antibodies to GFAP and NBC1, NBC1 expression was found in astrocytes of hippocampus and cerebellum. Among the three NBC1 splice variants (A, B, and C), NBC1-B and likely -C are the predominant variants expressed in rat brain astrocytes.118
Several evidence reported that NBC in astrocytes may modulate neuronal excitability through pH changes. In fact, when a neuron fires an action potential, there is an increase in extracellular K+ that depolarizes neighboring astrocytes. This depolarization leads to stimulation of NBC activity in astrocytes and transport of Na+, HCO3− and net-negative charge into the cells. The ensuing decrease in pHo tends to dampen further neuronal activity by inhibiting many pH-sensitive voltage- and ligand-gated channels. This negative-feedback model is predicted to be neuroprotective under pathophysiological conditions associated with ischemia.
Jung et al.121 examined the expression profiles of NBC1 in the ischemic penumbra in a rat model of focal cerebral ischemia induced by tMCAO, and observed an increased NBC1 expression in ischemic penumbra. Although the reason for this ischemia-induced NBC1 increase is unknown, the accompanying increase in NBC electrogenic activity may be associated to a reduction in pHi leading to cell death. The studies of Sohn et al.122 are in accordance with this detrimental role associated to NBC overexpression. Indeed, the absence of immunoreactivity for astrocytic NBC in the CA3 hippocampal region is associated to the known resistance of this brain region to the ischemic insult.122
Na+/HCO3− Cotransporter in Microglia
Na+/HCO3− cotransporter is expressed in microglia,123 where it contributes to cellular acid–base homeostasis in physiological and pathophysiological processes. The Cl−/HCO3− exchanger is the main acid loader in microglia.124 As concern the role of microglial NBC in brain ischemia, double immunofluorescence study, carried out in ischemic rat brain, with glial markers showed that NBC immunoreactivity is present in astrocytes and not in microglia thus suggesting that this transporter has a less important role in controlling pHi in microglia after stroke.122
Na+/HCO3− Cotransporter in Oligodendrocytes
A Na+-dependent HCO3− mechanism, which mediates the influx of HCO3− ions into the cell, is involved in pHi homeostasis of cultured OLGs from embryonic mouse spinal cord125 and adult rat cerebellum.84 In the presence of external bicarbonate/CO2, resting pHi is not modified by the removal of external chloride, or by blockers of the chloride-coupled transport Cl−/HCO3− systems. Unlike astrocytes, the pHi regulation in mature OLGs is exclusively dependent on the Na+ gradient.84 By contrast, a diisothiocyanatostilbene disulfonic acid-sensitive Cl−/HCO3− exchanger was found in OLG precursor cells.126 As reported in the NHE section (KCC), spatial non-uniformity of pH in OLGs is generated by differential subcellular distribution of NHE, NBC, and CAII, which colocalizes with either NHE or NBC. The changes in the expression of NBCs could be associated with dysregulation of pHi and/or intracellular sodium concentration and this may have a role in the pathophysiological events seen in brain ischemia.127
Na+/K+-ATPase
The Na+/K+-ATPase is an important protein complex ubiquitously expressed in the human body128, 129, 130 that mediates the active exchange of cytosolic sodium for extracellular potassium in a 3:2 ratio, a process required for the maintenance of transmembrane ionic gradients for all mammalian cells, which in turn is essential for setting the cellular resting potential, regulating osmolarity, and powering the secondary transport of other important solutes, such as calcium and protons.131, 132 As the brain is the primary consumer of ATP, the sodium–potassium pump is particularly vulnerable to ATP depletion, a condition characterizing ischemic stroke. This vulnerability suggests that pharmacological inhibition of the Na+/K+-ATPase could further compromise ATP-depleted neurons, nevertheless there is accumulating evidence that inhibiting the sodium–potassium pump can actually provide neuroprotection in the context of ischemia.131, 133 The debate on the neuroprotective or neurodetrimental role of Na+/K+-ATPase in brain ischemia is still open and several research groups are carrying out their research in the attempt to solve this question.
The Na+/K+-ATPase consists of a heterodimeric core of α and β subunits that may be accompanied by a third γ subunit.131, 132 The expression of different α and β isoforms is regulated in both a developmental and tissue-specific manner.134 The α1, α2, and α3 isoforms are expressed in the brain.135 In particular, the α2 isoform is widely expressed in neurons during development136 but becomes confined primarily to glia and few types of neurons in the adult brain;134, 137 in contrast, the α3 isoform shows neuronal expression in the adult.134, 137 Similar to the α subunit, the three β subunit isoforms are expressed to varying degrees in the brain, both in neurons and glia.134, 138, 139
Na+/K+-ATPase in Astrocytes
The α2 subunit of the Na+/K+-ATPase has been almost exclusively found to be expressed in astrocytes where it colocalizes with both glial glutamate aspartate transporter and glutamate transporter-1 on fine astrocytic processes surrounding glutamatergic synapses.140 Notably, the Na+–K+-ATPase, bearing α2 subunits, has been found to colocalize with NCX in cortical astrocytes at plasma membrane–endoplasmic reticulum junction level, where tightly regulated ‘sodium microdomains' may be present.141 Although it has been shown that after hypoxia an increase in the expression of Na+/K+-ATPase α1 and β1 subunits occurs in reoxygenated astrocytes, they may not be able to maintain the typical plasmamembrane function of the pump for the lack of ATP. In fact, immediately after ischemia induction, despite the occurrence of an increase in Na+/K+-ATPase expression,142 its activity was reported to be suppressed.143
In effect, there is a substantial heterogeneity among reactive astrocytes, with some close to the ischemic lesion showing decreased buffering capacity and those in the penumbra region showing a higher ionic buffering ability.142
Na+/K+-ATPase in Microglia
The Na+–K+-ATPase is not functionally active in microglia. However, another ATPase pump named H+–K+-ATPase has been found to have a Na+/K+-ATPase vicariate role in these glial cells.144
Na+/K+ ATPase in Oligodendrocytes
Oligodendrocytes express the alpha 1 and alpha 2 isoforms of the Na+/K+-ATPase catalytic subunit145 and represent the unique glial cells that express the β3 isoform.146 Expression of the alpha 2 isoform in mature OLGs is regulated by neuronal contact and is related to myelination.147 The properties of the Na+/K+ pump in OLGs are comparable to those typically reported for the Na+/K+ pump in many other cell types. The Na+/K+ pump is strongly activated by increasing [K+]o by two mechanisms: direct activation by [K+]o and indirect activation by [K+]o-induced OLGs cell membrane depolarization.148 Although no studies have reported the specific effect of brain ischemia on Na+/K+-ATPase in OLGs, it is possible to hypothesize that this transporter is activated after brain ischemia.
Ionic transporters as potential target to modulate the activity of astrocytes, microglia, and oligodendrocytes in brain ischemia
In the last few years, the development of neuroprotective strategies for brain ischemia has been primarily focused on targeting neuronal cell death. However, considering the role played by the different glial cells in neuronal survival, in debris removal and in functional remodeling after stroke, these cells are becoming a promising target to enhance an effective and additional therapy for brain ischemia. Thus, maintenance of supportive glia function or limitation of pathological processes needs to be considered when designing future stroke neurotherapeutics.
Although few studies have specifically targeted glial cells for their protective role in stroke, there is some evidence indicating that glial cells can actually represent a successful target to setting on effective strategies against stroke.
Indeed, recent results indicate that induction of BDNF in astrocytes by galectin-1 reduces neuronal apoptosis in ischemic boundary zone and improves functional recovery.149 In addition, enhancing astrocyte resistance to ischemic injury by overexpressing protective proteins or antioxidant enzyme results in improved survival of CA1 neurons after forebrain ischemia.150
In addition, recent work suggests that the maintenance of ionic homeostasis, intracellular pH control, and cell swelling in glial cells may contribute to the modulation of brain damage after stroke.
Concerning the modulation of ionic transporters expressed in glial cells, preliminary studies indicate NHE as a potential druggable target in stroke interventions. Indeed, the genetic ablation of NHE-1 reduces the OGD-mediated rise in [Na+]i and swelling in cortical astrocytes, suggesting that the inhibition of NHE-1 activity reduces ischemic injury through an effect mainly mediated by astrocytes.79 Another important target could be the plasmamembrane exchanger NCX; in fact, Kintner et al.113 showed a strong protective effect against astrocytic damage induced by OGD through the inhibition of NCX working in the reverse mode. Chen et al.77 supported this hypothesis and indicated that NCX colocalizes with NKCC1 in OLGs, thus suggesting a coordinate action of the two transporters that should be simultaneously modulated to observe a protective effect. Similar examples of glial transporters as potential candidate target in stroke intervention can be adducted for almost every transporter mentioned in the present review; nonetheless, a concerted view rather than a single examination of effects should be carried out to achieve translatable results. Undoubtedly, future studies devoted to unravel the importance of ionic transporters as key factors in the processes of glia survival and death will have a strong impact in setting on new therapeutic strategies.
The authors declares no conflict of interest.
Footnotes
We thank Dr. Paola Merolla for editorial revision. The present study was supported by grants from COFIN 2008, Ricerca Ordinaria 2009.
References
- Kettenmann H, Blankenfeld GV, Trotter J. Physiological properties of oligodendrocytes during development. Ann N Y Acad Sci. 1991;633:64–77. doi: 10.1111/j.1749-6632.1991.tb15596.x. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Vogel P, Fritze K, Back T, Hossmann KA, Wiessner C. Monitoring the temporal and spatial activation pattern of astrocytes in focal cerebral ischemia using in situ hybridization to GFAP mRNA: comparison with sgp-2 and hsp70 mRNA and the effect of glutamate receptor antagonists. Brain Res. 1996;735:285–297. doi: 10.1016/0006-8993(96)00578-1. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Prenatal development of fibrous (white matter), protoplasmic (gray matter), and layer I astrocytes in the human cerebral cortex: a Golgi study. J Comp Neurol. 1995;357:554–572. doi: 10.1002/cne.903570407. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PC, Lu SD, Huang YL, Sun FY. [The expression of nestin in ischemia-injured brain of adult rat] Sheng Li Xue Bao. 2002;54:294–299. [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci USA. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Kang J, Lin JH, Bueno E, Hansen TW, He L, et al. Astrocytic gap junctions remain open during ischemic conditions. J Neurosci. 1998;18:2520–2537. doi: 10.1523/JNEUROSCI.18-07-02520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SM, Bjorkman ST, Miller SM, Colditz PB, Pow DV. Morphological changes in white matter astrocytes in response to hypoxia/ischemia in the neonatal pig. Brain Res. 2010;1319:164–174. doi: 10.1016/j.brainres.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. Astrocytes, as well as neurons, express a diversity of ion channels. Can J Physiol Pharmacol. 1992;70 (Suppl:S223–S238. doi: 10.1139/y92-266. [DOI] [PubMed] [Google Scholar]
- Hertz L, Chen Y, Spatz M. Involvement of non-neuronal brain cells in AVP-mediated regulation of water space at the cellular, organ, and whole-body level. J Neurosci Res. 2000;62:480–490. doi: 10.1002/1097-4547(20001115)62:4<480::AID-JNR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bondarenko A, Svichar N, Chesler M. Role of Na+-H+ and Na+-Ca2+ exchange in hypoxia-related acute astrocyte death. Glia. 2005;49:143–152. doi: 10.1002/glia.20107. [DOI] [PubMed] [Google Scholar]
- Obara M, Szeliga M, Albrecht J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem Int. 2008;52:905–919. doi: 10.1016/j.neuint.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 2012;4:103–119. doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability. Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Lai AY, Todd KG. Hypoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia. 2006;53:809–816. doi: 10.1002/glia.20335. [DOI] [PubMed] [Google Scholar]
- Boscia F, Esposito CL, Di Crisci A, de Franciscis V, Annunziato L, Cerchia L. GDNF selectively induces microglial activation and neuronal survival in CA1/CA3 hippocampal regions exposed to NMDA insult through Ret/ERK signalling. PLoS One. 2009;4:e6486. doi: 10.1371/journal.pone.0006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscia F, Gala R, Pannaccione A, Secondo A, Scorziello A, Di Renzo G, et al. NCX1 expression and functional activity increase in microglia invading the infarct core. Stroke. 2009;40:3608–3617. doi: 10.1161/STROKEAHA.109.557439. [DOI] [PubMed] [Google Scholar]
- Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196:290–297. doi: 10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Madinier A, Bertrand N, Mossiat C, Prigent-Tessier A, Beley A, Marie C, et al. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS One. 2009;4:e8101. doi: 10.1371/journal.pone.0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Sauerzweig S, Ronicke R, Gunzer F, Dinkel K, Ullrich O, et al. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai F, Suzuki H, Oda J, Ninomiya T, Ono K, Sano H, et al. Neuroprotective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab. 2007;27:488–500. doi: 10.1038/sj.jcbfm.9600362. [DOI] [PubMed] [Google Scholar]
- Iglesias-Rozas JR, Garrosa M. The discovery of oligodendroglia cells by Rio-Hortega: his original articles. Clin Neuropathol. 2012;31:437–439. [PubMed] [Google Scholar]
- Chong SY, Chan JR. Tapping into the glial reservoir: cells committed to remaining uncommitted. J Cell Biol. 2010;188:305–312. doi: 10.1083/jcb.200905111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Kotter MR. The biology of CNS remyelination: the key to therapeutic advances. J Neurol. 2008;255 (Suppl 1:19–25. doi: 10.1007/s00415-008-1004-6. [DOI] [PubMed] [Google Scholar]
- Dai X, Lercher LD, Clinton PM, Du Y, Livingston DL, Vieira C, et al. The trophic role of oligodendrocytes in the basal forebrain. J Neurosci. 2003;23:5846–5853. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin SK, Maitland M. Long-term remyelination fails to reconstitute normal thickness of central myelin sheaths. J Neurol Sci. 1984;64:193–198. doi: 10.1016/0022-510x(84)90037-6. [DOI] [PubMed] [Google Scholar]
- Szuchet S, Nielsen JA, Lovas G, Domowicz MS, de Velasco JM, Maric D, et al. The genetic signature of perineuronal oligodendrocytes reveals their unique phenotype. Eur J Neurosci. 2011;34:1906–1922. doi: 10.1111/j.1460-9568.2011.07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Arai K, Lo EH. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp Transl Stroke Med. 2009;1:6. doi: 10.1186/2040-7378-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190–1198. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Sanchez-Gomez MV, Perez-Samartin A, Rodriguez-Antiguedad A, et al. Excitotoxic damage to white matter. J Anat. 2007;210:693–702. doi: 10.1111/j.1469-7580.2007.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus HL, Walberer M, Simard ML, Emig B, Muesken SM, Rueger MA, et al. NG2 and NG2-positive cells delineate focal cerebral infarct demarcation in rats. Neuropathology. 2012. [DOI] [PubMed]
- Honsa P, Pivonkova H, Dzamba D, Filipova M, Anderova M. Polydendrocytes display large lineage plasticity following focal cerebral ischemia. PLoS One. 2012;7:e36816. doi: 10.1371/journal.pone.0036816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen R, Christensen T, Lehrmann E, Diemer NH, Finsen B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Exp Brain Res. 2001;138:384–392. doi: 10.1007/s002210100715. [DOI] [PubMed] [Google Scholar]
- Paez PM, Fulton DJ, Spreuer V, Handley V, Campagnoni CW, Macklin WB, et al. Golli myelin basic proteins regulate oligodendroglial progenitor cell migration through voltage-gated Ca2+ influx. J Neurosci. 2009;29:6663–6676. doi: 10.1523/JNEUROSCI.5806-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliven B. Calcium signalling in cells of oligodendroglial lineage. Microsc Res Tech. 2001;52:672–679. doi: 10.1002/jemt.1051. [DOI] [PubMed] [Google Scholar]
- Kettenmann H. Electrophysiological behavior of microglia. Neuropathol Appl Neurobiol. 1994;20:177–178. [PubMed] [Google Scholar]
- Annunziato L, Pignataro G, Di Renzo GF. Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- Philipson KD, Nicoll DA. Sodium-calcium exchange: a molecular perspective. Annu Rev Physiol. 2000;62:111–133. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Ottolia M, Lu L, Lu Y, Philipson KD. A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J Biol Chem. 1999;274:910–917. doi: 10.1074/jbc.274.2.910. [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Longoni S, Philipson KD. Molecular cloning and functional expression of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. Science. 1990;250:562–565. doi: 10.1126/science.1700476. [DOI] [PubMed] [Google Scholar]
- Li Z, Matsuoka S, Hryshko LV, Nicoll DA, Bersohn MM, Burke EP, et al. Cloning of the NCX2 isoform of the plasma membrane Na(+)-Ca2+ exchanger. J Biol Chem. 1994;269:17434–17439. [PubMed] [Google Scholar]
- Nicoll DA, Quednau BD, Qui Z, Xia YR, Lusis AJ, Philipson KD. Cloning of a third mammalian Na+-Ca2+ exchanger, NCX3. J Biol Chem. 1996;271:24914–24921. doi: 10.1074/jbc.271.40.24914. [DOI] [PubMed] [Google Scholar]
- Lee SL, Yu AS, Lytton J. Tissue-specific expression of Na(+)-Ca2+ exchanger isoforms. J Biol Chem. 1994;269:14849–14852. [PubMed] [Google Scholar]
- Molinaro P, Cuomo O, Pignataro G, Boscia F, Sirabella R, Pannaccione A, et al. Targeted disruption of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J Neurosci. 2008;28:1179–1184. doi: 10.1523/JNEUROSCI.4671-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro G, Gala R, Cuomo O, Tortiglione A, Giaccio L, Castaldo P, et al. Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke. 2004;35:2566–2570. doi: 10.1161/01.STR.0000143730.29964.93. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Tortiglione A, Scorziello A, Giaccio L, Secondo A, Severino B, et al. Evidence for a protective role played by the Na+/Ca2+ exchanger in cerebral ischemia induced by middle cerebral artery occlusion in male rats. Neuropharmacology. 2004;46:439–448. doi: 10.1016/j.neuropharm.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Molinaro P, Cantile M, Cuomo O, Secondo A, Pannaccione A, Ambrosino P, et al. Neurounina-1, a novel compound that increases Na+/Ca2+ exchanger activity, effectively protects against stroke damage. Mol Pharmacol. 2012;83:142–156. doi: 10.1124/mol.112.080986. [DOI] [PubMed] [Google Scholar]
- He Z, Tong Q, Quednau BD, Philipson KD, Hilgemann DW. Cloning, expression, and characterization of the squid Na+-Ca2+ exchanger (NCX-SQ1) J Gen Physiol. 1998;111:857–873. doi: 10.1085/jgp.111.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas H, Ramos M, Benaim G, Caputo C, DiPolo R. The activity of the Na+/Ca2+ exchanger largely modulates the Ca2+i signal induced by hypo-osmotic stress in rat cerebellar astrocytes. The effect of osmolarity on exchange activity. J Physiol Sci. 2008;58:277–279. doi: 10.2170/physiolsci.RP009208. [DOI] [PubMed] [Google Scholar]
- Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, et al. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem. 2007;103:1196–1207. doi: 10.1111/j.1471-4159.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- Reyes RC, Parpura V. The trinity of Ca2+ sources for the exocytotic glutamate release from astrocytes. Neurochem Int. 2009;55:2–8. doi: 10.1016/j.neuint.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner DB, Wang Y, Sun D. Role of membrane ion transport proteins in cerebral ischemic damage. Front Biosci. 2007;12:762–770. doi: 10.2741/2099. [DOI] [PubMed] [Google Scholar]
- Nagano T, Kawasaki Y, Baba A, Takemura M, Matsuda T. Up-regulation of Na(+)-Ca2+ exchange activity by interferon-gamma in cultured rat microglia. J Neurochem. 2004;90:784–791. doi: 10.1111/j.1471-4159.2004.02511.x. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, et al. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- Nagano T, Osakada M, Ago Y, Koyama Y, Baba A, Maeda S, et al. SEA0400, a specific inhibitor of the Na+-Ca2+ exchanger, attenuates sodium nitroprusside-induced apoptosis in cultured rat microglia. Br J Pharmacol. 2005;144:669–679. doi: 10.1038/sj.bjp.0706104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, et al. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci. 2007;27:13065–13073. doi: 10.1523/JNEUROSCI.3467-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Stanley EF, Schlichter LC. Reversed Na+/Ca2+ exchange contributes to Ca2+ influx and respiratory burst in microglia. Channels (Austin) 2007;1:366–376. doi: 10.4161/chan.5391. [DOI] [PubMed] [Google Scholar]
- Boscia F, D'Avanzo C, Pannaccione A, Secondo A, Casamassa A, Formisano L, et al. Silencing or knocking out the Na(+)/Ca(2+) exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ. 2012;19:562–572. doi: 10.1038/cdd.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen I, Waxman SG, Mills L, Stys PK. Immunolocalization of the Na(+)-Ca2+ exchanger in mammalian myelinated axons. Brain Res. 1997;776:1–9. doi: 10.1016/s0006-8993(97)00868-8. [DOI] [PubMed] [Google Scholar]
- Tong XP, Li XY, Zhou B, Shen W, Zhang ZJ, Xu TL, et al. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–128. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jiang Q, Stys PK. Important role of reverse Na(+)-Ca(2+) exchange in spinal cord white matter injury at physiological temperature. J Neurophysiol. 2000;84:1116–1119. doi: 10.1152/jn.2000.84.2.1116. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain. 2004;127:294–303. doi: 10.1093/brain/awh032. [DOI] [PubMed] [Google Scholar]
- Chen H, Kintner DB, Jones M, Matsuda T, Baba A, Kiedrowski L, et al. AMPA-mediated excitotoxicity in oligodendrocytes: role for Na(+)-K(+)-Cl(-) co-transport and reversal of Na(+)/Ca(2+) exchanger. J Neurochem. 2007;102:1783–1795. doi: 10.1111/j.1471-4159.2007.04638.x. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Xue J, Chen JY, Haddad CG, Alper SL, Haddad GG. Chronic intermittent hypoxia decreases the expression of Na/H exchangers and HCO3-dependent transporters in mouse CNS. J Appl Physiol. 2003;95:292–299. doi: 10.1152/japplphysiol.01089.2002. [DOI] [PubMed] [Google Scholar]
- Luo J, Chen H, Kintner DB, Shull GE, Sun D. Decreased neuronal death in Na+/H+ exchanger isoform 1-null mice after in vitro and in vivo ischemia. J Neurosci. 2005;25:11256–11268. doi: 10.1523/JNEUROSCI.3271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama J, Kitazono T, Yao H, Ooboshi H, Takaba H, Ago T, et al. Inhibition of Na+/H+ exchanger reduces infarct volume of focal cerebral ischemia in rats. Brain Res. 2001;922:223–228. doi: 10.1016/s0006-8993(01)03175-4. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Su G, Lenart B, Ballard AJ, Meyer JW, Ng LL, et al. Increased tolerance to oxygen and glucose deprivation in astrocytes from Na(+)/H(+) exchanger isoform 1 null mice. Am J Physiol Cell Physiol. 2004;287:C12–C21. doi: 10.1152/ajpcell.00560.2003. [DOI] [PubMed] [Google Scholar]
- Pizzonia JH, Ransom BR, Pappas CA. Characterization of Na+/H+ exchange activity in cultured rat hippocampal astrocytes. J Neurosci Res. 1996;44:191–198. doi: 10.1002/(SICI)1097-4547(19960415)44:2<191::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kintner DB, Chanana V, Algharabli J, Chen X, Gao Y, et al. Activation of microglia depends on Na+/H+ exchange-mediated H+ homeostasis. J Neurosci. 2010;30:15210–15220. doi: 10.1523/JNEUROSCI.3950-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussouf A, Lambert RC, Gaillard S. Voltage-dependent Na(+)-HCO3- cotransporter and Na+/H+ exchanger are involved in intracellular pH regulation of cultured mature rat cerebellar oligodendrocytes. Glia. 1997;19:74–84. doi: 10.1002/(sici)1098-1136(199701)19:1<74::aid-glia8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Ro HA, Carson JH. pH microdomains in oligodendrocytes. J Biol Chem. 2004;279:37115–37123. doi: 10.1074/jbc.M403099200. [DOI] [PubMed] [Google Scholar]
- Lauf PK, Adragna NC. K-Cl cotransport: properties and molecular mechanism. Cell Physiol Biochem. 2000;10:341–354. doi: 10.1159/000016357. [DOI] [PubMed] [Google Scholar]
- Le Rouzic P, Ivanov TR, Stanley PJ, Baudoin FM, Chan F, Pinteaux E, et al. KCC3 and KCC4 expression in rat adult forebrain. Brain Res. 2006;1110:39–45. doi: 10.1016/j.brainres.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Adragna NC, Di Fulvio M, Lauf PK. Regulation of K-Cl cotransport: from function to genes. J Membr Biol. 2004;201:109–137. doi: 10.1007/s00232-004-0695-6. [DOI] [PubMed] [Google Scholar]
- Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW. Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol. 2005;288:C1451–C1460. doi: 10.1152/ajpcell.00503.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel F, Plesnila N. Expression and functional role of potassium-chloride cotransporters (KCC) in astrocytes and C6 glioma cells. Neurosci Lett. 2008;442:219–223. doi: 10.1016/j.neulet.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Zierler S, Frei E, Grissmer S, Kerschbaum HH. Chloride influx provokes lamellipodium formation in microglial cells. Cell Physiol Biochem. 2008;21:55–62. doi: 10.1159/000113747. [DOI] [PubMed] [Google Scholar]
- Schilling T, Stock C, Schwab A, Eder C. Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur J Neurosci. 2004;19:1469–1474. doi: 10.1111/j.1460-9568.2004.03265.x. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Plasticity of neuronal networks in the spinal cord: modifications in response to altered sensory input. Prog Brain Res. 2000;128:61–70. doi: 10.1016/S0079-6123(00)28007-2. [DOI] [PubMed] [Google Scholar]
- Pearson MM, Lu J, Mount DB, Delpire E. Localization of the K(+)-Cl(-) cotransporter, KCC3, in the central and peripheral nervous systems: expression in the choroid plexus, large neurons and white matter tracts. Neuroscience. 2001;103:481–491. doi: 10.1016/s0306-4522(00)00567-4. [DOI] [PubMed] [Google Scholar]
- Howard HC, Mount DB, Rochefort D, Byun N, Dupre N, Lu J, et al. The K-Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet. 2002;32:384–392. doi: 10.1038/ng1002. [DOI] [PubMed] [Google Scholar]
- Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, et al. Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. Embo J. 2003;22:5422–5434. doi: 10.1093/emboj/cdg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Black JA, Ransom BR, Stys PK. Anoxic injury of rat optic nerve: ultrastructural evidence for coupling between Na+ influx and Ca(2+)-mediated injury in myelinated CNS axons. Brain Res. 1994;644:197–204. doi: 10.1016/0006-8993(94)91680-2. [DOI] [PubMed] [Google Scholar]
- Malek SA, Coderre E, Stys PK. Aberrant chloride transport contributes to anoxic/ischemic white matter injury. J Neurosci. 2003;23:3826–3836. doi: 10.1523/JNEUROSCI.23-09-03826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Luo J, Kintner DB, Shull GE, Sun D. Na(+)-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:54–66. doi: 10.1038/sj.jcbfm.9600006. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Norenberg MD. The Na-K-Cl Co-transporter in astrocyte swelling. Metab Brain Dis. 2010;25:31–38. doi: 10.1007/s11011-010-9180-3. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang Z, Miyamoto Y, Reinach PS. Cell signaling pathways mediating epidermal growth factor stimulation of Na:K:2Cl cotransport activity in rabbit corneal epithelial cells. J Membr Biol. 2001;183:93–101. doi: 10.1007/s00232-001-0057-6. [DOI] [PubMed] [Google Scholar]
- Rutledge EM, Kimelberg HK. Release of [3H]-D-aspartate from primary astrocyte cultures in response to raised external potassium. J Neurosci. 1996;16:7803–7811. doi: 10.1523/JNEUROSCI.16-24-07803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int. 2000;36:291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Su G, Kintner DB, Flagella M, Shull GE, Sun D. Astrocytes from Na(+)-K(+)-Cl(-) cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am J Physiol Cell Physiol. 2002;282:C1147–C1160. doi: 10.1152/ajpcell.00538.2001. [DOI] [PubMed] [Google Scholar]
- Su G, Kintner DB, Sun D. Contribution of Na(+)-K(+)-Cl(-) cotransporter to high-[K(+)](o)- induced swelling and EAA release in astrocytes. Am J Physiol Cell Physiol. 2002;282:C1136–C1146. doi: 10.1152/ajpcell.00478.2001. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part I: pathophysiology. (1992) J Neurosurg. 2008;108:616–631. doi: 10.3171/JNS/2008/108/3/0616. [DOI] [PubMed] [Google Scholar]
- Lenart B, Kintner DB, Shull GE, Sun D. Na-K-Cl cotransporter-mediated intracellular Na+ accumulation affects Ca2+ signaling in astrocytes in an in vitro ischemic model. J Neurosci. 2004;24:9585–9597. doi: 10.1523/JNEUROSCI.2569-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Feighan D, Brown A, Ransom B. Intrinsic optical signals in the rat optic nerve: role for K(+) uptake via NKCC1 and swelling of astrocytes. Glia. 2002;37:114–123. doi: 10.1002/glia.10023. [DOI] [PubMed] [Google Scholar]
- Rothman SM. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985;5:1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Waxman SG, Ransom BR. Effects of glucose deprivation, chemical hypoxia, and simulated ischemia on Na+ homeostasis in rat spinal cord astrocytes. J Neurosci. 1998;18:3554–3562. doi: 10.1523/JNEUROSCI.18-10-03554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A, Chesler M. Rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia. 2001;34:134–142. doi: 10.1002/glia.1048. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Ritchie JM. Molecular dissection of the myelinated axon. Ann Neurol. 1993;33:121–136. doi: 10.1002/ana.410330202. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Luo J, Gerdts J, Ballard AJ, Shull GE, Sun D. Role of Na+-K+-Cl- cotransport and Na+/Ca2+ exchange in mitochondrial dysfunction in astrocytes following in vitro ischemia. Am J Physiol Cell Physiol. 2007;292:C1113–C1122. doi: 10.1152/ajpcell.00412.2006. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Wang C, Rougon G, Kiss JZ. Requirement of polysialic acid for the migration of the O-2A glial progenitor cell from neurohypophyseal explants. J Neurosci. 1994;14:4446–4457. doi: 10.1523/JNEUROSCI.14-07-04446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Burnham CE. Na+:HCO(3-) cotransporters (NBC): cloning and characterization. J Membr Biol. 2001;183:71–84. doi: 10.1007/s00232-001-0055-8. [DOI] [PubMed] [Google Scholar]
- Majumdar D, Bevensee MO. Na-coupled bicarbonate transporters of the solute carrier 4 family in the nervous system: function, localization, and relevance to neurologic function. Neuroscience. 2010;171:951–972. doi: 10.1016/j.neuroscience.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt BM, Berger UV, Douglas RM, Bevensee MO, Hediger MA, Haddad GG, et al. Na/HCO3 cotransporters in rat brain: expression in glia, neurons, and choroid plexus. J Neurosci. 2000;20:6839–6848. doi: 10.1523/JNEUROSCI.20-18-06839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard RG, Papadopoulos MC, van Hooft JA, Xu L, Giuffrida R, Monyer H. The electrogenic sodium bicarbonate cotransporter: developmental expression in rat brain and possible role in acid vulnerability. J Neurosci. 2000;20:1001–1008. doi: 10.1523/JNEUROSCI.20-03-01001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YW, Choi IJ, Kwon TH. Altered expression of sodium transporters in ischemic penumbra after focal cerebral ischemia in rats. Neurosci Res. 2007;59:152–159. doi: 10.1016/j.neures.2007.06.1470. [DOI] [PubMed] [Google Scholar]
- Sohn Y, Yoo KY, Park OK, Kwon SH, Lee CH, Choi JH, et al. Na+/HCO3- cotransporter immunoreactivity changes in neurons and expresses in astrocytes in the gerbil hippocampal CA1 region after ischemia/reperfusion. Neurochem Res. 2011;36:2459–2469. doi: 10.1007/s11064-011-0572-5. [DOI] [PubMed] [Google Scholar]
- Faff L, Ohlemeyer C, Kettenmann H. Intracellular pH regulation in cultured microglial cells from mouse brain. J Neurosci Res. 1996;46:294–304. doi: 10.1002/(SICI)1097-4547(19961101)46:3<294::AID-JNR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Rose CR. pH regulation and proton signalling by glial cells. Prog Neurobiol. 1996;48:73–103. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Schlue WR. Intracellular pH regulation in cultured mouse oligodendrocytes. J Physiol. 1988;406:147–162. doi: 10.1113/jphysiol.1988.sp017373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussouf A, Gaillard S. Intracellular pH changes during oligodendrocyte differentiation in primary culture. J Neurosci Res. 2000;59:731–739. doi: 10.1002/(SICI)1097-4547(20000315)59:6<731::AID-JNR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lascola C, Kraig RP. Astroglial acid-base dynamics in hyperglycemic and normoglycemic global ischemia. Neurosci Biobehav Rev. 1997;21:143–150. doi: 10.1016/s0149-7634(96)00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Van Hardeveld C, Everts ME. Significance of cation transport in control of energy metabolism and thermogenesis. Physiol Rev. 1991;71:733–774. doi: 10.1152/physrev.1991.71.3.733. [DOI] [PubMed] [Google Scholar]
- Friberg H, Wieloch T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2002;84:241–250. doi: 10.1016/s0300-9084(02)01381-0. [DOI] [PubMed] [Google Scholar]
- Astrup J. Energy-requiring cell functions in the ischemic brain. Their critical supply and possible inhibition in protective therapy. J Neurosurg. 1982;56:482–497. doi: 10.3171/jns.1982.56.4.0482. [DOI] [PubMed] [Google Scholar]
- Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Avila J, Cozar-Castellano I, Brownleader MD, Trevan M, Francis MJ, et al. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep. 2000;20:51–91. doi: 10.1023/a:1005580332144. [DOI] [PubMed] [Google Scholar]
- Scheiner-Bobis G. The sodium pump. Its molecular properties and mechanics of ion transport. Eur J Biochem. 2002;269:2424–2433. doi: 10.1046/j.1432-1033.2002.02909.x. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G, Emanuel JR, Levenson R. Cell-specific expression of mRNAs encoding Na+,K(+)-ATPase alpha- and beta-subunit isoforms within the rat central nervous system. Proc Natl Acad Sci USA. 1991;88:7425–7429. doi: 10.1073/pnas.88.16.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull GE, Greeb J, Lingrel JB. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986;25:8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Moseley AE, Lieske SP, Wetzel RK, James PF, He S, Shelly DA, et al. The Na,K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice. J Biol Chem. 2003;278:5317–5324. doi: 10.1074/jbc.M211315200. [DOI] [PubMed] [Google Scholar]
- McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J Neurosci. 1991;11:381–391. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R, Klein L, Shyjan AW, Rakic P, Levenson R. Neurons and astroglia express distinct subsets of Na,K-ATPase alpha and beta subunits. Brain Res Mol Brain Res. 1994;21:333–343. doi: 10.1016/0169-328x(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Lecuona E, Luquin S, Avila J, Garcia-Segura LM, Martin-Vasallo P. Expression of the beta 1 and beta 2(AMOG) subunits of the Na,K-ATPase in neural tissues: cellular and developmental distribution patterns. Brain Res Bull. 1996;40:167–174. doi: 10.1016/0361-9230(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+,K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex. 2002;12:515–525. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci. 2002;976:356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Leis JA, Bekar LK, Walz W. Potassium homeostasis in the ischemic brain. Glia. 2005;50:407–416. doi: 10.1002/glia.20145. [DOI] [PubMed] [Google Scholar]
- Peng L, Martin-Vasallo P, Sweadner KJ. Isoforms of Na,K-ATPase alpha and beta subunits in the rat cerebellum and in granule cell cultures. J Neurosci. 1997;17:3488–3502. doi: 10.1523/JNEUROSCI.17-10-03488.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirihai O, Smith P, Hammar K, Dagan D. Microglia generate external proton and potassium ion gradients utilizing a member of the H/K ATPase family. Glia. 1998;23:339–348. [PubMed] [Google Scholar]
- Fink D, Knapp PE, Mata M. Differential expression of Na,K-ATPase isoforms in oligodendrocytes and astrocytes. Dev Neurosci. 1996;18:319–326. doi: 10.1159/000111422. [DOI] [PubMed] [Google Scholar]
- Martin-Vasallo P, Wetzel RK, Garcia-Segura LM, Molina-Holgado E, Arystarkhova E, Sweadner KJ. Oligodendrocytes in brain and optic nerve express the beta3 subunit isoform of Na,K-ATPase. Glia. 2000;31:206–218. doi: 10.1002/1098-1136(200009)31:3<206::aid-glia20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Itkis OS, Mata M. Neuronal interaction determines the expression of the alpha-2 isoform of Na, K-ATPase in oligodendrocytes. Brain Res Dev Brain Res. 2000;125:89–97. doi: 10.1016/s0165-3806(00)00125-5. [DOI] [PubMed] [Google Scholar]
- Dobretsov M, Stimers JR. Characterization of the Na/K pump current in N20.1 oligodendrocytes. Brain Res. 1996;724:103–111. doi: 10.1016/0006-8993(96)00171-0. [DOI] [PubMed] [Google Scholar]
- Qu WS, Wang YH, Wang JP, Tang YX, Zhang Q, Tian DS, et al. Galectin-1 enhances astrocytic BDNF production and improves functional outcome in rats following ischemia. Neurochem Res. 2010;35:1716–1724. doi: 10.1007/s11064-010-0234-z. [DOI] [PubMed] [Google Scholar]
- Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58:1042–1049. doi: 10.1002/glia.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]