Abstract
White matter ischemia is difficult to quantify histologically. Myelin-associated glycoprotein (MAG) is highly susceptible to ischemia, being expressed only adaxonally, far from the oligodendrocyte cell body. Myelin-basic protein (MBP) and proteolipid protein (PLP) are expressed throughout the myelin sheath. We compared MAG, MBP, and PLP levels in parietal white matter homogenates from 17 vascular dementia (VaD), 49 Alzheimer's disease (AD), and 33 control brains, after assessing the post-mortem stability of these proteins. Small vessel disease (SVD) and cerebral amyloid angiopathy (CAA) severity had been assessed in paraffin sections. The concentration of MAG remained stable post-mortem, declined with increasing SVD, and was significantly lower in VaD than controls. The concentration of MBP fell progressively post-mortem, limiting its diagnostic utility in this context. Proteolipid protein was stable post-mortem and increased significantly with SVD severity. The MAG/PLP ratio declined significantly with SVD and CAA severity. The MAG and PLP levels and MAG/PLP did not differ significantly between AD and control brains. We validated the utility of MAG and MAG/PLP measurements on analysis of 74 frontal white matter samples from an Oxford cohort in which SVD had previously been scored. MAG concentration and the MAG/PLP ratio are useful post-mortem measures of ante-mortem white matter ischemia.
Keywords: amyloid angiopathy, brain ischemia, cerebrovascular disease, dementia, white matter
Introduction
Cerebral ischemia is the defining pathologic mechanism underlying most forms of vascular dementia (VaD) and is also thought to contribute to Alzheimer's disease (AD). The majority of AD cases show some degree of cerebrovascular pathology: cerebral amyloid angiopathy (CAA) is present in most patients and periventricular white matter Ischemic lesions are reported to be common.1 There is a growing body of evidence showing that risk factors for cerebrovascular disease and VaD are also risk factors for AD.2, 3, 4, 5 The mechanisms underlying alterations in the white matter in AD, and their relationship to other aspects of AD pathology, are unclear. There is a possible interdependence between ischemia and amyloid-β (Aβ) in AD: ischemia has been shown to increase Aβ production by upregulation of BACE1,6, 7 and by reduced vascular clearance and enzymatic degradation,8 whereas increased Aβ may enhance ischemia by vasoconstriction, impairment of cerebral autoregulation, and functional hyperemia,9, 10, 11, 12 and through deposition in the walls of blood vessels to cause CAA.13, 14
Current methods for post-mortem assessment of ante-mortem ischemic change in the white matter are varied and subjective. The degeneration of the myelin sheath that occurs as a result of reduced blood flow is thought to occur primarily by a mechanism known as ‘dying back oligodendrogliopathy' in which injury begins in the most distal, adaxonal part of the myelin sheath, furthest from the oligodendrocyte cell body.15 Myelin-associated glycoprotein (MAG) is produced in the cell body, and then transported to and expressed only in the adaxonal myelin loop.16, 17 Myelin-associated glycoprotein is therefore likely to be more susceptible to ischemic damage than proteins such as myelin basic protein (MBP) and proteolipid protein (PLP), which are expressed abundantly throughout the myelin sheath.18, 19 Indeed, a previous study showed preferential loss of MAG in response to hypoxia-like white matter injury during brain inflammation20 and several studies of white matter lesions in multiple sclerosis (MS) have shown loss of MAG whereas MBP and PLP remained highly conserved.21, 22
We have performed double immunofluorescent staining of MAG and PLP in human parietal white matter sections to confirm the localization of the proteins and identify any obvious differences in distribution in cases with differing levels of small vessel disease (SVD). We have measured the levels of the myelin proteins MAG, MBP, and PLP in a cohort of AD, VaD, and control brains from the South West Dementia Brain Bank (SWDBB) and looked at the relationships between these proteins and SVD to find out whether the level of MAG in relation to that of MBP or PLP would be a good measure of white matter damage. We have also performed a validation study on a series of frontal white matter samples from the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort, University of Oxford. Last, we have examined the relationships between the myelin proteins and the severity of CAA, Aβ plaque load, and APOE genotype in the SWDBB cohort to further elucidate the mechanisms of white matter injury in AD.
Materials and methods
Case Selection
This study had local Research Ethics Committee approval. We initially used tissue from the Human Tissue Authority licensed South West Dementia Brain Bank, University of Bristol. For the double immunofluorescent staining we used paraffin sections from right parietal lobe from six cases: three with low SVD score and three with high SVD score. For the MAG and PLP protein measurements, we used parietal deep white matter tissue dissected from 17 cases of VaD (ages 67 to 97 years, mean 83.4, s.d. 7.7; post-mortem delays of 20 to 70 hours, mean 43.7, s.d. 16.5), 49 cases of AD (ages 57 to 92 years, mean 77.5, s.d. 8.2; post-mortem delays of 4 to 72 hours, mean 31.4, s.d. 19.3), 25 with absent to mild CAA and 24 with moderate to severe CAA, and 33 control brains (ages 58 to 94 years, mean 79.6, s.d. 8.9; post-mortem delays of 3 to 67 hours, mean 34.0, s.d. 15.9). The demographic data are summarized in Supplementary Table 1. For the MBP protein measurements, a smaller subset of these cases was used: 12 with VaD (ages 67 to 89 years, mean 80.9, s.d. 6.7; post-mortem delays of 20 to 70 hours, mean 43.1, s.d. 17.5), 28 with AD (ages 63 to 89 years, mean 76.0, s.d. 7.9; post-mortem delays of 4 to 67 hours, mean 31.2, s.d. 20.0) of which 14 had absent to mild CAA and 14 moderate to severe CAA, and 13 control brains (ages 72 to 86 years, mean 76.3, s.d. 3.9; post-mortem delays of 12 to 59 hours, mean 36.5, s.d. 13.9). The demographic data for this cohort are summarized in Supplementary Table 2.
The AD cases were selected on the basis of a diagnosis according to CERAD23 of ‘definite AD' and a Braak tangle stage of V to VI (i.e., according the NIA-Reagan criteria24 there was a high likelihood that their dementia was caused by Alzheimer's disease pathology). The VaD cases had a clinical history of dementia, no more than occasional neuritic plaques, a Braak stage of III or less, histopathological evidence of multiple infarcts/ischemic lesions, moderate to severe atheroma and/or arteriosclerosis, and an absence of histopathological evidence of other disease likely to cause dementia. The normal controls had no history of dementia, few or no neuritic plaques, and no other neuropathological abnormalities.
To validate our initial findings we went on to analyze samples of frontal white matter in 74 cases from the OPTIMA cohort, from the Thomas Willis Brain Bank, University of Oxford.
Brain Tissue
The tissue was dissected from brains that had been removed from patients within 72 hours of death. The left cerebral hemisphere had been sliced and frozen at −80°C. The right cerebral hemisphere had been fixed in 10% formalin for ∼3 weeks before tissue was taken, processed, and paraffin sections cut for neuropathological assessment and diagnosis. SVD in paraffin sections of right parietal lobe had been previously scored on a 4-point semiquantitative scale according to the extent of thickening of the arteriolar walls and associated narrowing of vessel lumens (see Supplementary Figure 1): 0=normal vessel wall thickness, 1=slightly increased thickness, 2=moderately increased thickness, and 3=markedly increased thickness such that for many arterioles the diameter of the lumen was <50% of the outer diameter of the blood vessel. Similarly, CAA in the right parietal lobe had been previously graded using a method based on that of Olichney et al,25 in which a score of 0 corresponded to vessels devoid of amyloid, a score of 1 to scattered deposition of amyloid in a few leptomeningeal or cortical blood vessels, a score of 2 to more widespread deposition of amyloid in vessels, and a score of 3 to severe and widespread amyloid deposition.26 The APOE genotypes and the area fraction of cerebral cortex immunopositive for Aβ (the Aβ load) had been determined previously.26
Small vessel disease had been previously independently assessed in the OPTIMA cohort using a 12-point semiquantitative global scale based on loss of myelin, loosening of the parenchymal tissue, and dilatation of perivascular spaces in both deep grey matter and white matter structures.27 Because of differences in the scoring systems of the two cohorts, SVD in the OPTIMA cohort was also assessed using the 4-point semiquantitative scale used for the SWDBB cohort, as detailed above, by a neuropathologist (SL) who was masked to the previous scores and biochemical data. However, it should be noted that many of the paraffin sections of frontal lobe available for analysis of white matter SVD in the OPTIMA cohort did not include deep white matter (within which the severity of SVD tends to be greatest).
All tissue samples (200 mg) were homogenized in 1 ml 1% sodium dodecyl sulfate lysis buffer in a Precellys homogeniser (Stretton Scientific, Derbyshire, UK), and then aliquoted and stored at −80°C until required. All of the assays on myelin proteins were performed without prior clinical and pathologic information (including the SVD scores) relating to the individual cases.
Double immunofluorescent staining of myelin-associated glycoprotein and proteolipid protein
Parietal lobe sections, 7 μm thick, were collected on 3-amino-propyl-triethoxy silane-coated slides. Sections were incubated overnight at 60°C, dewaxed, dehydrated, and incubated in 3% hydrogen peroxide in methanol for 30 minutes to block endogenous peroxidase. Sections were then boiled in 10 mmol/L trisodium citrate (pH 6) in a microwave for 5 minutes, left for 5 minutes, again boiled for 5 minutes before being left to cool for 15 minutes, and then washed in cold running water for 10 minutes. Nonspecific antibody binding was blocked by incubation with 10% normal horse serum (Vector Labs, Burlingame, CA, USA) for 20 minutes at room temperature. Sections were washed twice in phosphate-buffered saline (PBS), and the rabbit polyclonal anti-PLP (Abcam, Cambridge, UK) and mouse monoclonal anti-MAG (Abcam) antibodies were both diluted 1:400 in PBS and applied overnight at room temperature. Sections were again washed twice in PBS, and Alexafluor 555 anti-rabbit and Alexafluor 488 anti-mouse antibodies (Invitrogen, Carlsbad, CA, USA) were both diluted 1:1,000 and applied to the sections for 30 minutes in the dark at room temperature. The sections were washed in distilled water for 5 minutes in the dark and then mounted in aqueous medium (Vector Labs).
Measurement of Myelin-Associated Glycoprotein by Direct Enzyme-Linked Immunosorbent assay
Brain homogenates diluted 1:10 in PBS and blanks of PBS were incubated (in duplicate) in a clear 96-well microplate (Fisher Scientific, Loughborough, UK) for 2 hours at room temperature with agitation. The plate also included serial dilutions of recombinant MAG (Abnova, Taipei City, Taiwan) to generate a standard curve (7 twofold dilutions, concentration range 400 to 6.25 ng/mL). The plate was washed 5 times with 0.05% phosphate buffered saline-Tween-20 (PBST), tapped dry on the final wash, and incubated for 2 hours at room temperature with the mouse monoclonal anti-MAG antibody (Abcam) diluted 1:1,000 in PBS. After 5 washes in PBST, the plate was incubated with the biotin-conjugated anti-mouse secondary antibody (Vector Labs) diluted 1:500 in PBS for 20 minutes at room temperature, in the dark. The plate was again washed 5 times with PBST, tapped dry on the final wash, then incubated with peroxidase-conjugated streptavidin diluted 1:500 in PBS for 20 minutes at room temperature in the dark. The plate was washed 5 times with PBST, tapped dry on the final wash, and then 100 μL of peroxidase substrate (R&D Systems, Minneapolis, MN, USA) was added to all wells for 10 minutes, followed by 50 μL Stop solution. Absorbance was measured at 450 nm in a multidetection microplate reader (FLUOstar OPTIMA, BMG Labtech, Aylesbury, UK). Absolute MAG levels were interpolated from the standard curve.
Measurement of Myelin-Basic Protein by Sandwich Enzyme-Linked Immunosorbent Assay
Clear, 96-well microplates (Fisher Scientific) were coated with capture rabbit polyclonal anti-MBP antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:4,000 in coating buffer at 4°C overnight. After 5 washes in PBST, the plate was tapped dry and incubated with 1% bovine serum albumin (Sigma-Aldrich, Gillingham, UK) for 90 minutes at room temperature with agitation. The plate was washed 5 times, tapped dry, and the samples incubated for 2 hours at room temperature with agitation. Seven twofold serial dilutions of recombinant MBP (Abnova) diluted in PBS (concentration range 20 to 0.31 μg/mL), brain homogenates diluted 1:2,000 in PBS, and blanks of PBS were all loaded, in duplicate, onto the plate. Additional controls where either capture antibody, sample, or detection antibody were omitted were also included. After 5 washes, the plate was tapped dry and incubated with the detection rat monoclonal anti-MBP antibody (Millipore, Billerica, MA, USA) diluted 1:5,000 in PBS for 2 hours at room temperature with agitation. The plate was again washed 5 times, tapped dry, and biotin-conjugated anti-rat (Vector Labs) diluted 1:1,000 was applied for 20 minutes in the dark at room temperature. The plate was again washed 5 times and then incubated with peroxidase-conjugated streptavidin diluted 1:1,000 for 20 minutes in the dark. After 5 further washes, the plate was tapped dry and 100 μL peroxidase substrate (R&D Systems) was added for 15 minutes, after which 50 μL Stop solution was added and the absorbance measured in a multidetection microplate reader (BMG Labtech) at 450 nm. Absolute protein levels were interpolated from the standard curve.
Measurement of Proteolipid Protein by Direct Enzyme-Linked Immunosorbent Assay
Seven serial dilutions of a reference brain homogenate were used to generate a standard curve (total protein range 48 to 0.75 μg/mL) and were, together with the brain homogenates (diluted 1:1,000 in PBS) and blanks of PBS, incubated (in duplicate) in a clear 96-well microplate (Fisher Scientific) for 2 hours at room temperature with agitation. The plate was washed 5 times with 0.05% PBST, tapped dry on the final wash, and incubated for 2 hours at room temperature with the rabbit polyclonal anti-PLP antibody (Abcam) diluted 1:10,000 in PBS. After 5 washes in PBST, the plate was incubated with the biotin-conjugated anti-rabbit secondary antibody (Vector Labs) diluted 1:500 in PBS for 20 minutes at room temperature, in the dark. The plate was washed 5 times with PBST, tapped dry on the final wash, and then incubated with peroxidase-conjugated streptavidin for 20 minutes in the dark. After 5 washes with PBST, the plate was tapped dry and 100 μL of peroxidase substrate (R&D Systems) was added to all wells for 3 minutes, followed by 50 μL Stop solution. Absorbance was measured at 450 nm in a multidetection microplate reader (BMG Labtech). Relative PLP levels were interpolated from the standard curve.
Measurement of Post-Mortem Stability of Myelin-Associated Glycoprotein, Myelin-Basic Protein, and Proteolipid Protein
Deep white matter tissue from the parietal lobe of 2 AD brains both removed 4 hours after death and 2 control brains removed 3 and 6 hours after death was dissected and divided into 10 aliquots. These were incubated for 0, 6, 12, 18, 24, 48, or 72 hours at room temperature or 24, 48, or 72 hours at 4°C. The aliquots were homogenized in 1 mL 1% SDS lysis buffer and MAG, MBP, and PLP protein measurements made as described above.
Statistical Analysis
Associations between MAG, MBP, PLP, the MAG/PLP ratio, and SVD severity were assessed by Kruskal–Wallis test and Dunn's post test, and correlations were assessed by Spearman's test. In the previous study on the Oxford cohort,27 SVD had been scored on a 12-point scale. To facilitate comparison with the data from the SWDBB cohort, the SVD scores on the Oxford brains were grouped for analysis into four categories: 1 to 3, 4 to 6, 7 to 9, and 10 to 12. The effect of increased incubation time on the levels of MAG, MBP, and PLP at room temperature and 4°C in the post-mortem delay simulation was assessed using Pearson's correlation. Correlations between the myelin proteins and Aβ load in the AD cohort were assessed by Spearman's test. Associations between the myelin protein concentrations and the presence of 0, 1, or 2 APOE ɛ4 alleles were assessed by Kruskal–Wallis test and Dunn's post test. Association between the myelin proteins and gender were assessed using the Mann–Whitney test. The P values of <0.05 were considered significant.
Results
Post-Mortem Stability of The Myelin Proteins
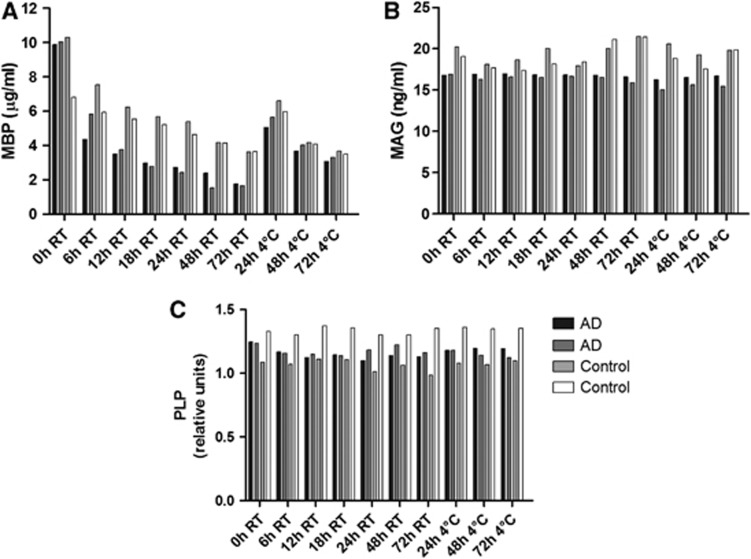
The protein levels of MAG and PLP did not alter significantly when unfixed brain tissue was incubated for up to 72 hours at room temperature or 4°C. However, the concentration of MBP fell significantly with increasing incubation time at room temperature (r=−0.775, P=0.04) and at 4°C (r=−0.951, P=0.0495, Figure 1). These findings suggest that MBP may undergo post-mortem degradation; hence, this protein was measured in only a limited number of further samples. However, MAG and PLP protein measurements are unlikely to have been affected by variations in post-mortem delay between different cases and disease groups.
Figure 1.
Changes in myelin protein levels with post-mortem delay. Post-mortem delay was simulated by incubation of homogenates of adjacent samples of cortex at room temperature (RT) and 4°C for varying lengths of time. The protein levels of myelin-associated glycoprotein (MAG), myelin-basic protein (MBP), and proteolipid protein (PLP) were measured by enzyme-linked immunosorbent assay (ELISA). (A) The protein levels of MBP fell progressively with increasing incubation time, both at room temperature (r=−0.775, P=0.0408) and at 4°C (r=−0.951, P=0.0495). (B) The protein levels of MAG and (C) PLP did not alter significantly over 72 hours.
The Myelin Proteins and Small Vessel Disease Severity
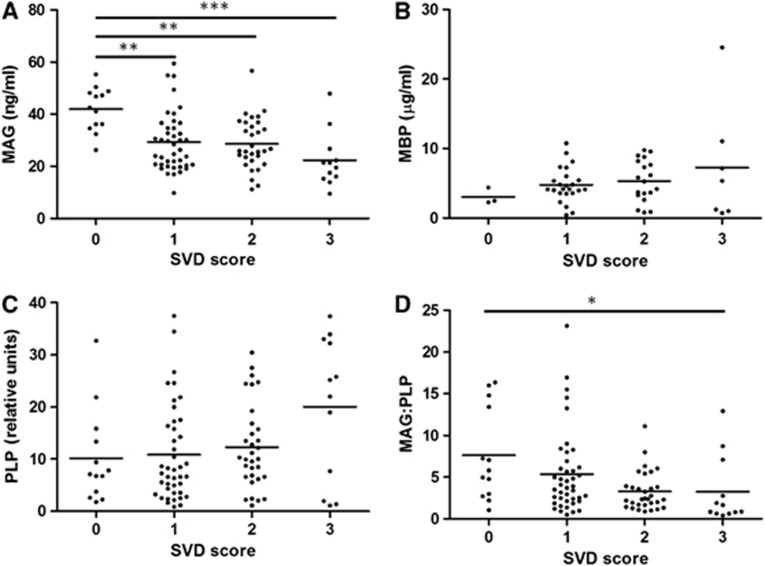
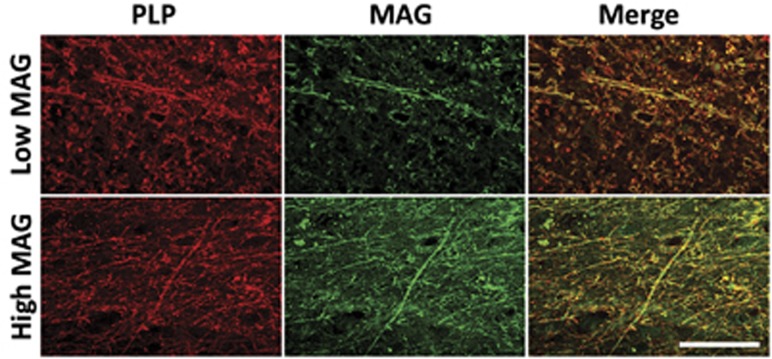
Both MAG concentration and the MAG/PLP ratio were significantly negatively correlated with SVD score (r=−0.384, P<0.0001 and r=−0.368, P=0.0002 respectively; Figure 2). There was significant variation in MAG between the SVD groups (P=0.0001) and post-testing revealed significant reduction in MAG in cases with SVD severity of 1 or 2 compared with those of 0 (P<0.01) and a highly significant reduction in MAG in cases with SVD severity of 3 compared with those with 0 (P<0.001; Figure 2A). There was also significant variation in the MAG/PLP ratio between the SVD groups (P=0.0052), with post-tests showing a significant reduction in this ratio in cases with SVD severity of 3 compared with those with 0 (P<0.05; Figure 2D). In contrast, the level of PLP increased slightly with increasing SVD severity; the two measures were significantly correlated (r=0.305, P=0.0009), although the mean PLP level did not differ significantly between the four SVD severity groups (Figure 2C). Myelin-basic protein showed a nonsignificant increase with SVD (Figure 2B). The reduction in MAG and preservation of PLP in cases of increased SVD severity along with preservation of both myelin proteins in cases of lower SVD severity could also be seen, to some extent, on double immunofluorescent labeling of the paraffin sections (Figure 3).
Figure 2.
The relationship of (A) myelin-associated glycoprotein (MAG), (B) myelin-basic protein (MBP), (C) proteolipid protein (PLP), and the (D) MAG/PLP ratio to small vessel disease (SVD) in the deep parietal white matter in the SWDBB cohort. Both MAG and the MAG/PLP ratio correlated negatively with SVD severity (r=−0.384, P=0.0001 and r=−0.368, P=0.0002, respectively), whereas PLP correlated positively with SVD (r=0.305, P=0.0009). Myelin-associated glycoprotein was significantly reduced in cases with SVD severity of 1, 2 (**P<0.01), and 3 (***P<0.001) compared with those with no SVD. The MAG/PLP ratio was significantly reduced in cases with SVD severity of 3 compared with those with a score of 0 (*P<0.05). Myelin-basic protein did not vary significantly between the different SVD groups.
Figure 3.
Double-immunofluorescent labeling of myelin-associated glycoprotein (MAG) and proteolipid protein (PLP) in the parietal white matter. Comparison between a case of high small vessel disease (SVD) severity (top panel) and one of low SVD severity (bottom panel) shows little difference in the labeling intensity of PLP but an obvious reduction in the labeling of MAG in severe SVD. Scale bar=100 μm.
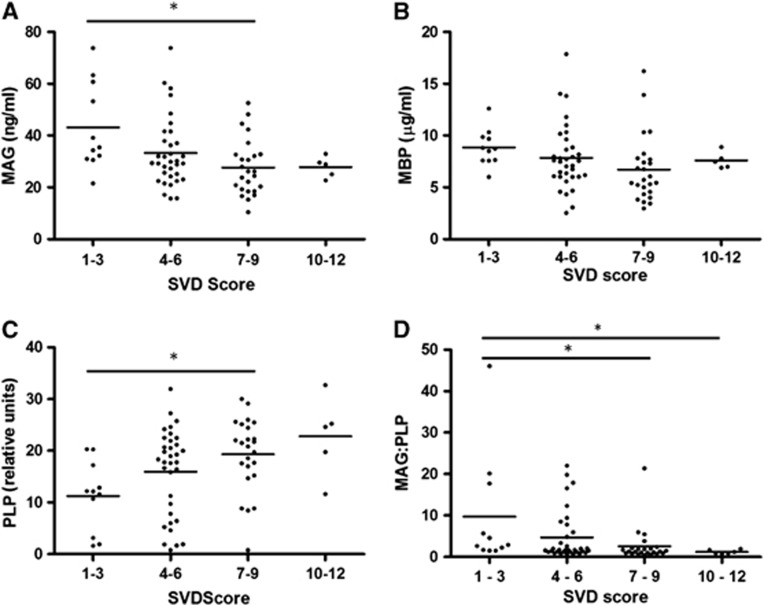
These initial findings were confirmed in the OPTIMA cohort (Figure 4). When the 12-point global SVD severity scale was used, MAG and the ratio of MAG to PLP were again significantly negatively correlated with SVD score (r=−0.311, P=0.0066 and r=−0.349, P=0.0023, respectively). There was significant variation in MAG concentration between the different SVD groups (P=0.0194) with significant reduction in MAG in cases with SVD severity of 7 to 9 compared with those with SVD severity of 1 to 3 (P<0.05; Figure 4A). The MAG/PLP ratio was also significantly altered across the different SVD severity groups (P=0.006) with reduced MAG/PLP in cases of SVD severity 7 to 9 and 10 to 12 compared with cases with SVD severity of 1 to 3 (P<0.05; Figure 4D). Again, PLP correlated positively with SVD severity (r=0.352, P=0.0021). There was significant variation in PLP between the different SVD severity groups (P=0.014) and post-tests showed significantly higher PLP level in cases of SVD severity of 7 to 9 compared with those with SVD severity of 1 to 3 (P<0.05; Figure 4C). Myelin-basic protein did not vary significantly across the SVD severity groups (Figure 4B). When SVD in the frontal white matter was assessed in the available sections (i.e., largely in the superficial rather than the deep white matter) by use of our own 4-point scale, MAG was again significantly negatively correlated with SVD (r=−0.283, P=0.0121), although differences in PLP, MBP, and the MAG/PLP ratio were not significant.
Figure 4.
The relationship of (A) myelin-associated glycoprotein (MAG), (B) myelin-basic protein (MBP), (C) proteolipid protein (PLP), and the (D) MAG/PLP ratio to small vessel disease (SVD) in the frontal white matter in the OPTIMA cohort. As in the SWDBB cohort, MAG and the MAG/PLP ratio declined significantly with increasing SVD score (r=−0.311, P=0.0066 and r=−0.349, P=0.0023, respectively) whereas PLP correlated positively with SVD severity (r=0.352, P=0.0021). Myelin-associated glycoprotein was significantly lower in cases with SVD severity of 7 to 9 than in those with severity of 1 to 3 (*P<0.05). The MAG/PLP ratio was significantly lower in cases with SVD severity of 7 to 9 and 10–12 than in those with severity of 1 to 3 (*P<0.05). Proteolipid protein was significantly higher in cases with SVD severity of 7 to 9 than those with severity of 1 to 3 (*P<0.05). Myelin-basic protein did not differ significantly between the SVD groups.
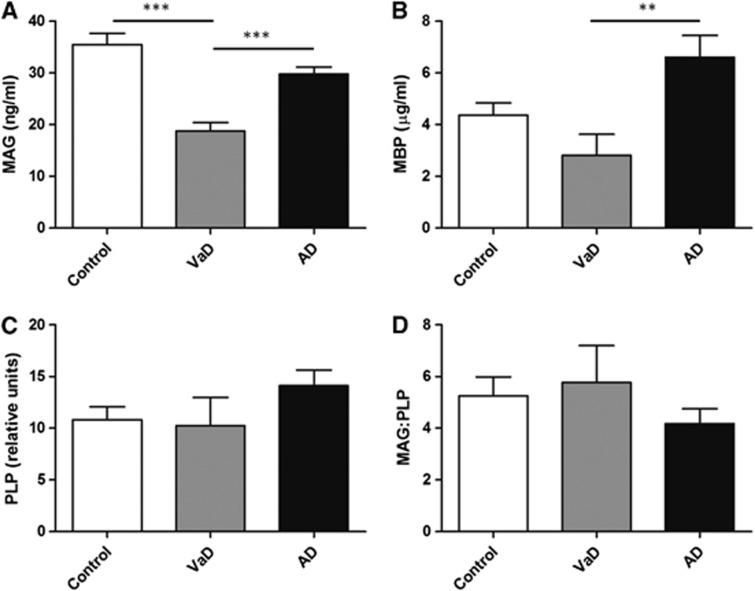
The Myelin Proteins in Vascular Dementia and Alzheimer's Disease
The level of MAG was significantly reduced in VaD compared with both controls and AD (P<0.0001) and reduced in AD compared with controls, but not significantly so (Figure 5A). The mean values for MAG concentration±s.e.m. were: controls 35.51±2.143 ng/mL, VaD 18.77±1.658 ng/mL, and AD 29.81±1.320 ng/mL. The MBP protein was significantly reduced in VaD compared with AD (P<0.01) but was not significantly changed in either VaD or AD compared with controls (Figure 5B). The mean values for MBP concentration±s.e.m. were: controls 4.36 ±0.486 μg/mL, VaD 2.819±0.814 μg/mL, and AD 6.61±0.84 μg/mL. The level of PLP was slightly reduced in VaD and slightly increased in AD compared with controls, but neither of these changes was significant (Figure 5C). The mean values for PLP level±s.e.m. were: controls 10.81±1.265 relative units, VaD 10.25±2.706 relative units, and AD 14.11±1.517 relative units. The ratio of MAG/PLP was also not significantly altered in VaD or AD compared with controls (Figure 5D). The mean values for MAG/PLP ratio±s.e.m. were: controls 5.248±0.732, VaD 5.769±1.428, and AD 4.174±0.579.
Figure 5.
Levels of (A) myelin-associated glycoprotein (MAG), (B) myelin-basic protein (MBP), (C) proteolipid protein (PLP), and (D) the MAG/PLP ratio in parietal deep white matter from the SWDBB cohort in control, vascular dementia (VaD), and Alzheimer's disease (AD) brains. Myelin-associated glycoprotein concentration was significantly lower in VaD compared with controls and AD (***P<0.0001). Levels of MBP were reduced in VaD compared with AD (**P<0.01). PLP and the MAG/PLP ratio were not significantly different between the three groups.
The Myelin Proteins and Cerebral Amyloid Angiopathy, Aβ Load, and APOE Genotype
The protein concentrations of MAG, MBP, and PLP were not significantly associated with CAA severity (Figures 6A to 6C). Although the MAG/PLP ratio did not vary significantly between the CAA severity groups (Figure 6D), there was a significant negative correlation between the MAG/PLP ratio and CAA severity (r=−0.187, P=0.045).
Figure 6.
Levels of (A) myelin-associated glycoprotein (MAG), (B) myelin-basic protein (MBP), (C) proteolipid protein (PLP), and (D) the MAG/:PLP ratio in parietal deep white matter from the SWDBB cohort, grouped according to cerebral amyloid angiopathy (CAA) severity. The levels of MAG, MBP, and PLP did not vary significantly with CAA. There was no significant difference in the ratio of MAG to PLP between the groups. However, MAG/PLP correlated negatively with the CAA severity score (r=−0.187, P=0.045).
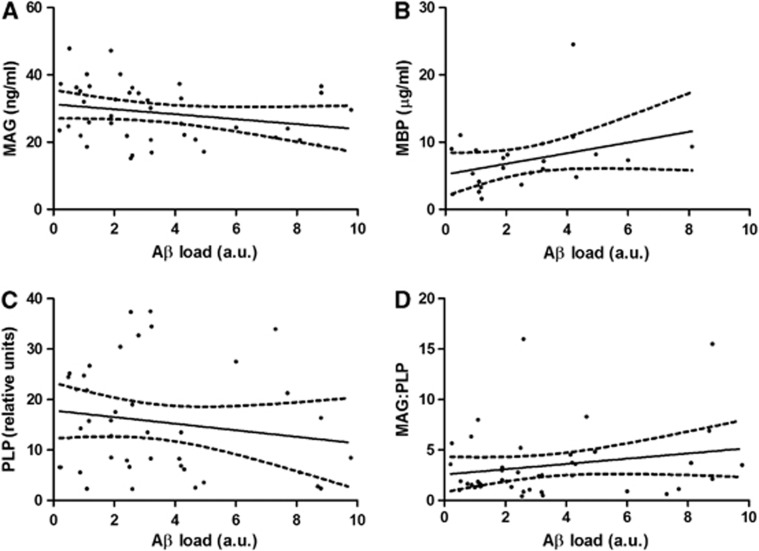
Myelin-associated glycoprotein concentration declined significantly with increasing gray matter Aβ load (r=−0.318, P=0.0429; Figure 7A), whereas MBP and PLP levels and the MAG/PLP ratio remained unchanged (Figures 7B to 7D). The myelin protein levels and the MAG/PLP ratio showed no association with APOE genotype (Supplementary Figure 2).
Figure 7.
The relationship of (A) myelin-associated glycoprotein (MAG), (B) myelin-basic protein (MBP), (C) proteolipid protein (PLP), and (D) the MAG/PLP ratio to parietal cortical amyloid-β (Aβ) plaque load in the Alzheimer's disease (AD) cohort. Levels of MAG were significantly negatively correlated with Aβ load (r=−0.318, P=0.0429) whereas MBP, PLP, and the MAG/PLP ratio were not significantly altered. The solid lines represent the best-fit linear regression lines, and the dashed lines the 95% confidence intervals. AU, arbitrary unit.
Age and Gender
Myelin-associated glycoprotein, MBP, and PLP showed no relationship to age and did not vary significantly with gender, either in the individual cohorts or when the cohorts were combined.
Discussion
Ischemic white matter injury has previously been assessed in post-mortem brain tissue using various scoring systems such as those of Kövari et al28 and Smallwood et al.27 The assessment procedures and histologic variables vary between research groups and are subjective. A reliable quantitative biochemical measure of white matter ischemic change would make such assessment more reproducible and potentially more sensitive, and should facilitate the pooling or comparison of data between different centers and cohorts. This would, in turn, help in the investigation of the contribution and pathogenesis of white matter ischemia to different types of dementia, thereby furthering our understanding of the causes and options for treatment of these diseases.
Proteins expressed in the myelin sheath appear to be affected by ischemia to differing extents depending on where along the myelin sheath they are expressed.20 We hypothesized that comparison of the level of a myelin protein that is particularly susceptible to ischemia with one relatively resistant to ischemia might be a good way to measure ischemic injury. We have shown a significant reduction in MAG level with increasing SVD severity, whereas PLP level was not reduced. In fact, PLP increased slightly with increasing SVD severity, perhaps reflecting axonal atrophy in ischemic white matter, with a relative increase in the concentration of PLP. The MAG/PLP ratio declined significantly with increasing SVD severity, confirming that the reduction in MAG was not simply a result of fiber degeneration and myelin loss.
To evaluate the general applicability of our methodological approach, we analyzed a separate cohort of white matter samples, from a different region (frontal rather than parietal) and in relation to SVD severity that had been determined independently by a different pathologist (MME rather than SL), using different criteria, as part of a previously published study.27 These results confirmed our initial findings: MAG levels and the MAG/PLP ratio were significantly reduced with increasing SVD severity whereas PLP concentration was significantly positively correlated with SVD. We also assessed SVD in the frontal white matter in these cases using our own scoring system, and although paraffin sections from many of the cases included only superficial rather than deep white matter, there was nonetheless a significant negative correlation between MAG concentration and SVD severity. This is encouraging evidence that measurement of these myelin proteins is a robust way to assess white matter ischemic alterations in post-mortem brain tissue. In future, it may prove useful to compare the levels of these myelin proteins with other ischemia sensitive markers such as hypoxia-inducible factor-1α (HIF1α), vascular endothelial growth factor, and neuroglobin.
Our findings indicate the importance of assessing the post-mortem stability of proteins for studies on autopsy tissue. We have found PLP to be a suitable myelin protein for comparison with MAG: relatively resistant to ischemia and (like MAG) well preserved post-mortem. In contrast, MBP shows poor post-mortem stability, making it unsuitable for use in studies on autopsy tissue with quite wide variation in post-mortem delay.
Although the MAG/PLP ratio declined significantly with increasing SVD severity, we did not find significant reduction in the MAG/PLP ratio in the VaD group compared with the controls, which might have been expected. Our results may reflect the extent of degeneration of nerve fibers rather than selective damage to myelin in severe end-stage VaD, resulting in a reduction of all myelin-expressed proteins. Indeed, PLP levels were slightly reduced in the VaD group compared with controls. These results may also reflect the relatively high prevalence of white matter SVD in the control cohort, reducing the overall differences between the dementia groups and the control group.
Many AD patients have evidence of cerebrovascular disease, although the extent of this varies considerably from case to case.1 The relative contributions of ischemic gray and white matter injury to the development and progression of AD are still unclear. Understanding the extent to which vascular factors contribute to AD is important in our overall understanding of the pathogenesis of this disease, and has implications for therapy. We found a reduction in the MAG/PLP ratio in AD compared with controls; however, this was not significant. Our results suggest that white matter ischemic changes (at least those in the parietal region) may contribute less to this type of dementia than has been suggested.
Cerebral amyloid angiopathy is present to varying extent in most AD patients and in ∼30% of the normal elderly.29 It causes thickening of the vessel wall, narrowing of the lumen, and reduced vessel elasticity. Cerebral amyloid angiopathy in the arterioles supplying the deep white matter is likely to cause a reduction in cerebral perfusion and may thereby contribute to ischemic injury. It can cause ischemic damage in the cerebral cortex,14, 30 and CAA has also been implicated in the development of white matter lesions.31, 32 A previous study of deep white matter lesions in the elderly revealed that CAA in the cortex was associated with increased expression of HIF1α, a marker of hypoxia, in the underlying white matter33 and a separate study showed a significant association between the level of white matter damage and both the concentration of Aβ40 in the plasma and the extent of CAA.34 The prevalence of CAA in our AD cohort was lower than might be expected because the cases of absent to mild CAA were matched with cases of moderate to severe CAA to allow comparison of the two groups. We found a reduction in the MAG/PLP ratio with increasing CAA severity, which is in keeping with other evidence that CAA may contribute to white matter ischemic injury in some patients with AD.
The Aβ accumulation in the cerebral cortex may contribute to white matter injury in the underlying tissue. Our results showed a weak but significant negative correlation between MAG concentration and Aβ load in the parietal cortex. This adds to our previous evidence that cortical Aβ deposition may contribute to cerebral hypoperfusion in AD, through enhanced vasoconstriction and reduced blood flow: we previously showed that Aβ42 increases neuronal angiotensin-converting enzyme (ACE) activity35 and that Aβ42 and Aβ40 increase neuronal and endothelial endothelin-1 (ET-1) production (via increased ECE-2 expression and ECE-1 activity)12 in vitro.
Much remains to be determined as to the mechanisms of white matter damage in aging and dementia. Disturbances of cerebral vasoregulation and angiogenesis may contribute, as may systemic factors such as atrial fibrillation, orthostatic hypotension, and diabetes. We suggest that the biochemical approaches that we have described may facilitate the study of these processes in autopsy human brain tissue.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was funded by the Medical Research Council, BRACE (Bristol Research into Alzheimer's and Care of the Elderly), and ABBUK (Alzheimer's Brain Bank UK, supporting Brains for Dementia Research).
Supplementary Material
References
- Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Rauch GM, Rauch RA, Haque A, Crawford K. Cardiovascular and other risk factors for Alzheimer's disease and vascular dementia. Ann NY Acad Sci. 2000;903:411–423. doi: 10.1111/j.1749-6632.2000.tb06393.x. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I, Nilsson L, Persson G, Lernfelt B, Landahl S, Palmertz B, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Aβ generation by altering β- and γ-cleavage of APP. Neurobiol Aging. 2009;30:1091–1098. doi: 10.1016/j.neurobiolaging.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, et al. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaevaa NN, Fisk L, Kochkina EG, Plesneva SA, Zhuravin IA, Babusikova EVA, et al. Effect of hypoxia/ischemia and hypoxic preconditioning/reperfusion on expression of some amyloid-degrading enzymes. Ann NY Acad Sci. 2004;1035:21–33. doi: 10.1196/annals.1332.002. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- Niwa K, Porter VA, Kazama K, Cornfield D, Carlson GA, Iadecola C. Aβ-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol. 2001;281:H2417–H2424. doi: 10.1152/ajpheart.2001.281.6.H2417. [DOI] [PubMed] [Google Scholar]
- Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, et al. Aβ1–40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci USA. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JC, Barker R, Kehoe PG, Love S. Endothelin-1 is elevated in Alzheimer's disease and upregulated by amyloid-beta. J Alzheimers Dis. 2012;29:853–861. doi: 10.3233/JAD-2012-111760. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: the CERAD experience, part XV. Neurology. 1996;46:1592–1596. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Hansen LA, Hofstetter CR, Grundman M, Katzman R, Thal LJ. Cerebral infarction in Alzheimer's disease is associated with severe amyloid angiopathy and hypertension. Arch Neurol. 1995;52:702–708. doi: 10.1001/archneur.1995.00540310076019. [DOI] [PubMed] [Google Scholar]
- Johnson ES, Ludwin SK. Evidence for a ‘dying-back' gliopathy in demyelinating disease. Ann Neurol. 1981;9:301–305. doi: 10.1002/ana.410090316. [DOI] [PubMed] [Google Scholar]
- Sternberger NH, Quarles RH, Itoyama Y, Webster HD. Myelin-associated glycoprotein demonstrated immunocytochemically in myelin and myelin-forming cells of developing rat. Proc Natl Acad Sci USA. 1979;76:1510–1514. doi: 10.1073/pnas.76.3.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Andrews SB, Cootauco C, Quarles R. The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. J Cell Biol. 1989;109:2417–2426. doi: 10.1083/jcb.109.5.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlin FX, Webster HD, Palkovits CG, Cohen SR. Immunocytochemical localization of basic protein in major dense line regions of central and peripheral myelin. J Cell Biol. 1982;95:242–248. doi: 10.1083/jcb.95.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LD, Friedrich VL, Behar T, Dubois-Dalcq M, Lazzarini RA. The initial events in myelin synthesis: orientation of proteolipid protein in the plasma membrane of cultured oligodendrocytes. J Cell Biol. 1989;109:717–727. doi: 10.1083/jcb.109.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Bruck W, et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- Itoyama Y, Sterhberger NH, Webster HD, Quarles RH, Cohen SR, Richardson EP. Immunocytochemical observations on the distribution of myelin-associated glycoprotein and myelin basic protein in multiple sclerosis lesions. Ann Neurol. 1980;7:167–177. doi: 10.1002/ana.410070212. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- The National Institute on Aging Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, et al. The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer's disease and Lewy body variant. Neurology. 1996;47:190–196. doi: 10.1212/wnl.47.1.190. [DOI] [PubMed] [Google Scholar]
- Chalmers K, Wilcock GK, Love S. APOE epsilon 4 influences the pathological phenotype of Alzheimer's disease by favouring cerebrovascular over parenchymal accumulation of A beta protein. Neuropathol Appl Neurobiol. 2003;29:231–238. doi: 10.1046/j.1365-2990.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- Smallwood A, Oulha A, Joachim C, Christie S, Sloan C, Smith AD, et al. Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the OPTIMA cohort. Neuropathol Appl Neurobiol. 2011;38:337–343. doi: 10.1111/j.1365-2990.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- Kövari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel J-P, et al. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- Love S. Contribution of cerebral amyloid angiopathy to Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:1–4. [PMC free article] [PubMed] [Google Scholar]
- Cadavid D, Mena H, Koeller K, Frommelt RA. Cerebral beta amyloid angiopathy is a risk factor for cerebral ischemic infarction. A case control study in human brain biopsies. J Neuropathol Exp Neurol. 2000;59:768–773. doi: 10.1093/jnen/59.9.768. [DOI] [PubMed] [Google Scholar]
- Gray F, Dubas F, Roullet E, Escourolle R. Leukoencephalopathy in diffuse hemorrhagic cerebral amyloid angiopathy. Ann Neurol. 1985;18:54–59. doi: 10.1002/ana.410180110. [DOI] [PubMed] [Google Scholar]
- Hollander D, Strich SJ.Atypical Alzheimer's disease with congophilic angiopathy presenting with dementia of acute onsetIn: Wolstenholme GEW, O'Connor M (eds). Ciba Foundation Symposium—Alzheimer's Disease and Related Conditions John Wiley & Sons: Chichester, UK; 1970105–135. [Google Scholar]
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, et al. White matter lesions in an unselected cohort of the elderly. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma β-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- Miners S, Ashby E, Baig S, Harrison R, Tayler H, Speedy E, et al. Angiotensin-converting enzyme levels and activity in Alzheimer's disease: differences in brain and CSF ACE and association with ACE1 genotypes. Am J Transl Res. 2009;1:163–177. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.