Abstract
Pyrexia soon after stroke is associated with severe stroke and poor functional outcome. Few studies have assessed brain temperature after stroke in patients, so little is known of its associations with body temperature, stroke severity, or outcome. We measured temperatures in ischemic and normal-appearing brain using 1H-magnetic resonance spectroscopy and its correlations with body (tympanic) temperature measured four-hourly, infarct growth by 5 days, early neurologic (National Institute of Health Stroke Scale, NIHSS) and late functional outcome (death or dependency). Among 40 patients (mean age 73 years, median NIHSS 7, imaged at median 17 hours), temperature in ischemic brain was higher than in normal-appearing brain on admission (38.6°C-core, 37.9°C-contralateral hemisphere, P=0.03) but both were equally elevated by 5 days; both were higher than tympanic temperature. Ischemic lesion temperature was not associated with NIHSS or 3-month functional outcome; in contrast, higher contralateral normal-appearing brain temperature was associated with worse NIHSS, infarct expansion and poor functional outcome, similar to associations for tympanic temperature. We conclude that brain temperature is higher than body temperature; that elevated temperature in ischemic brain reflects a local tissue response to ischemia, whereas pyrexia reflects the systemic response to stroke, occurs later, and is associated with adverse outcomes.
Keywords: brain temperature, ischemic stroke, lesion growth, magnetic resonance spectroscopy, outcome
Introduction
Pyrexia occurs commonly in the first few days after acute ischemic stroke1, 2, 3, 4 and is associated with severe stroke, large infarcts, neurologic deterioration, and poor functional outcome.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 It is unclear whether pyrexia is associated with poor functional outcome through its association with severe stroke (i.e., simply because pyrexia is a consequence of severe stroke) or if pyrexia accelerates ischemic brain damage and hence worsens functional outcome independently, or both. There is little information on brain temperature after ischemic stroke in patients or how temperature in the ischemic or normal brain relates to pyrexia. Nonetheless, there is increasing interest in the potential of hypothermia to reduce brain damage and improve stroke outcome.2, 12, 13
Brain temperature is difficult to measure. Invasive probes only sample a restricted local volume of tissue so cannot assess temperature distribution between abnormal and normal tissue, is only possible in patients with severe stroke who justify invasive monitoring, and opening the skull may itself alter temperature dynamics. Brain temperature can be measured noninvasively using magnetic resonance (MR) spectroscopy of the hydrogen atom (1H MR spectroscopy (MRS))14 which, when combined with spectroscopic MR imaging (MRI), provides data on temperature distribution across the brain, including the ischemic and normal tissue, with sufficient reliability for group comparisons.15, 16
Using 1HMRS and diffusion-weighted imaging (DWI),17, 18 we found previously in 40 patients with acute ischemic stroke that temperature in the DWI-defined ischemic tissue was elevated, particularly in the potential penumbral tissue, within 24 hours of stroke. However, we found no clear association between early temperature elevation in ischemic brain and stroke severity17 although ischemic lesion temperature was higher in larger lesions.18 We only had body temperature measured at the time of MRI: at that time point, brain temperature was higher than body temperature, and only one of the 40 patients was pyrexial within 24 hours of admission.18
The few other studies of brain temperature in acute stroke in patients used invasive brain temperature probes inserted at craniotomy: these studies found higher brain lesion than tympanic temperature,19 consistent with our findings using MRS, but were only able to include small numbers of patients who all had severe stroke and were unable to sample a wide range of brain tissues. No other data on temperature in normal and abnormal brain soon after ischemic stroke or at later times have been published since our original observations.17, 18
Our previous study17, 18 lacked detailed information on body temperature so was unable to provide reliable information on associations with temperature in ischemic or normal brain or with outcome. We recently reported detailed body temperature measurements in a new cohort of 44 patients up to 7 days after stroke,11 showing that it was peak rather than admission temperature, occurring at 1.5 to 2 days after stroke, that was related to stroke severity and more closely associated with poor functional outcome. We hypothesized that the temporal profile and associations with clinical variables would be different for temperature in ischemic brain and in the body after stroke and that temperature in contralateral normal (CNL) brain would have similar profile and associations with body temperature. Therefore, we compared brain temperature measured twice in the first week after stroke with serial tympanic temperature measurements to determine the relationship between temperature in ischemic and CNL brain, body temperature profile and early and late clinical (neurologic, functional) and imaging-derived outcomes.
Materials and methods
Patient Recruitment
We prospectively recruited patients with acute ischemic stroke admitted to our hospital acute stroke service. The study was approved by the Scotland A Multicentre Research Ethics Committee (06/MRE00/119) and written informed consent was obtained from the patient or assent from their relatives. Patients who had metal inserts or were MR incompatible for other medical reasons, or who received thrombolytic treatment, or who turned out to have intracerebral hemorrhage or a stroke mimic, were excluded.
On admission, a trained stroke physician measured stroke severity using National Institutes of Health Stroke Scale (NIHSS) and determined stroke subtype by the Oxfordshire Community Stroke Project classification.20 The stroke onset was defined as the time when symptoms or signs were first noticed by the patient or an observer. National Institutes of Health Stroke Scale was assessed again at 120 hours after stroke and modified Rankin Score (mRS) was assessed at 3 months, blinded to imaging and temperature measurements.
Body Temperature Measurement
We recorded body temperature from the tympanic membrane using a First Temp Genius digital aural thermometer (Pearson Surgical, Sylmar, CA, USA), taken four hourly from arrival at hospital, additionally at the time of the first 1HMRS scan, up to 120 hours or the second brain 1HMRS scan whichever came later (see below). We recorded from both ears in 11 patients to test side-to-side variation and found none. Thereafter, we recorded from the uppermost ear.21 Tympanic thermometry is considered as reliable for serial readings22 shows the least variation with age compared with axillary, rectal and oral thermometry23 and is widely used in clinical practice. We defined pyrexia as tympanic temperature ⩾37.5°C as in previous studies.24 We searched for causes of pyrexia including any evidence of infection, deep vein thrombosis or surgical intervention as well as recording paracetamol and antibiotic use. Details of tympanic temperature measurements, profiles, proportions becoming, and clinical factors associated with pyrexia in these patients were published recently.11
MR Diffusion Imaging and Spectroscopy Techniques
Patients underwent MRI as soon as possible after stroke but within a maximum of 26 hours from onset. This time window allowed for inclusion of patients who were admitted overnight or late the preceding afternoon, as the research MR scanner only operated during normal daytime working hours. Follow-up MRI was performed between 3 and 7 days after stroke (subacute). We obtained axial T2-weighted fast spin-echo and fluid attenuated inversion recovery imaging, axial diffusion tensor imaging (DTI), point resolved spectroscopy (PRESS)-localized 1HMRS on admission and subacutely. All MR data were obtained on a GE Signa HDX 1.5T (General Electric, Milwaukee, WI, USA) scanner with self-shielding gradients (33 mT/m maximum) and a ‘birdcage' quadrature head coil on the same scanner as described previously.17, 18, 25 Diffusion tensor imaging was performed with field-of-view 240 × 240 mm, 15 axial slices of thickness 5 mm, slice gap 1 mm, acquisition matrix 128 × 128, echo time 97.4 ms, repetition time 10 seconds and diffusion sensitizing gradients with scalar b-values of 1,000 s/mm2 applied in six noncollinear directions. The PRESS-localized 1HMRS was performed with field-of-view 320 × 320 mm, slice thickness 10 mm, acquisition matrix 24 × 24, echo time 145 ms and repetition time 1,000 ms. We used three-pulse chemical shift selective water suppression and shimming, optimized on the slice of interest. Additional saturation bands were placed around the PRESS box to minimize lipid contamination. The 1HMRS voxel grid was carefully centered on the slice showing the maximum ischemic lesion extent on DTI and placed within brain to avoid contamination of the spectra by lipid signal from bone marrow or subcutaneous tissue, but to include as much of the lesion and ipsilateral and contralateral brain as possible. Each 1HMRS data set took ∼9 minutes to acquire, the data being effectively ‘averaged' over this period.
1HMRS Brain Thermometry
The methodology of 1HMRS brain thermometry in stroke patients and its validity have been described and discussed in detail previously.15, 17, 18 Briefly, cerebral temperature for each voxel was calculated from the relative chemical shifts of water and N-acetyl aspartate. Temperature-dependent changes in hydrogen bonding cause the water chemical shift to vary linearly with temperature at 0.01 p.p.m. per degree,14, 16 while the chemical shift of N-acetyl aspartate is independent of temperature. Both chemical shifts are essentially independent of pH.26 N-acetyl aspartate and water peaks were identified by their characteristic appearances at echo time 145 ms. The metabolite concentration quantification accounts for coil loading (using the scanner's radiofrequency transmitter gain) and receiver gain thus enabling intersubject and intrasubject comparison of individual metabolite concentrations and temperatures. We previously found that coil uniformity was very good across an axial slice near the center of the coil (unpublished). We quantified metabolites in ‘institutional units' (i.u.).
Tissue Classification and Estimation of Tissue Metabolite Concentrations
We processed image and temperature data as previously. We coregistered 1HMRS and DTI data using a 3D affine transformation and visualized the multivoxel MR spectroscopy grid superimposed onto the corresponding admission DTI using in-house software. Spectroscopic images were interpolated to a 32 × 32 matrix yielding 1,000 mm3 voxels and all processing was performed on a voxel-by-voxel basis after setting the residual water signal in each voxel to a standard chemical shift of 4.70 p.p.m. All spectroscopic data were modeled in the time domain by five Gaussian components (corresponding to choline, creatine, N-acetyl aspartate containing compounds and the two components of lactate) using the AMARES (Advanced Method for Accurate Robust and Efficient Spectral Fitting) algorithm within the MRUI (Magnetic Resonance User Interface) package (http://www.mrui.uab.es/mrui). We transformed the data to spectra for display and visual quality control. We discarded spectra with fitted line widths <1 Hz or >10 Hz, metabolite peaks >0.1 p.p.m. offset from their expected values, from voxels lying on the edges of the PRESS excitation region or from voxels containing >20% cerebrospinal fluid. We perform weekly spectroscopy quality assurance on our scanner with appropriate phantoms to maintain scanner stability.
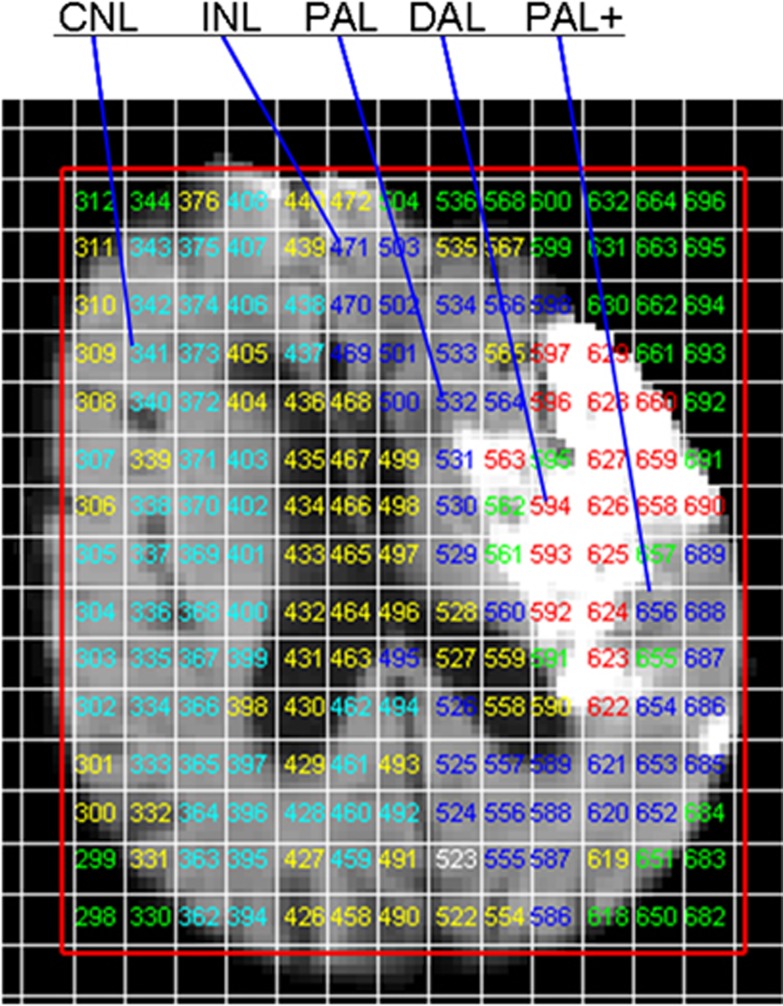
Maps of the average DWI and apparent diffusion coefficient signal were obtained from the six DWI images acquired for each slice. We superimposed the 1HMRS acquisition grid onto the DWI image to classify each grid voxel on the admission and follow-up DTI image as previously described,17, 18 blind to temperature, clinical data, and baseline/follow-up imaging as appropriate using software written in-house.27 Based on the DWI appearance, each grid voxel was classified as: definitely abnormal (DAL) corresponding to clearly hyperintense tissue on DTI representing ischemic lesion core; possibly abnormal (PAL) corresponding to minimally hyperintense tissue on DTI at the edge of the ischemic lesion; a one voxel-thick rim of DTI-healthy looking tissue located immediately outside the lesion (PAL+); ipsilateral normal (INL) corresponding to INL tissue on DTI located outside PAL+ voxels; and CNL corresponding to normal tissue (Figure 1) in the opposite hemisphere. We considered the PAL and PAL+ voxels to correspond to the ‘potential penumbral tissues' as previously.18
Figure 1.
Example of voxel grid categorization according to tissue appearance on diffusion tensor imaging (DTI). CNL, contralateral normal brain; DAL, definitely abnormal tissue; INL, ipsilateral normal brain; PAL, possible abnormal tissue; PAL+, tissue one voxel thick immediately outside the lesion.
To quantify ischemic lesion growth, we classified the DTI lesion on admission and at 3 to 7 days after stroke using a validated qualitative stroke lesion classification that distinguishes changes in lesion size due to an increase in lesion extent (scale of 1 to 8 in the middle cerebral artery territory) from changes due to lesion swelling (scale of 1 to 6).28 It also classifies lesions in the other arterial territories on a similar basis. This rating method has high observer reliability, and excellent discrimination of lesion extent.29 A trained observer rated the lesion extent and swelling on the DTI and fluid attenuated inversion recovery images at admission and 3 to 7 days, independently and blind to clinical, outcome, and temperature data. Change in lesion extent was indicated by a change in one point or more on the rating scale and by change in DTI lesion volume. The DTI lesion volume was measured by a trained observer manually outlining the hyperintense tissue on each slice on which it was visible in Analyze (Mayo Clinic, Rochester, MN, USA).
Statistical Analysis
All statistical comparisons were performed with nonparametric tests unless there were 20 or more observations and the variances were equal (variances were compared using Leven's test). We compared temperatures between five brain tissue subregions (DAL, PAL, PAL+, INL, and CNL) and between brain regions and body temperature using Kruskal–Wallis and Mann–Whitney U-tests. Not all patients contributed all tissue subregions but all available data were used (e.g., in a patient with a large lesion the MRS grid might not include INL brain although CNL brain was included). The presence or absence of lesion growth was compared with brain temperatures on admission by brain subregion (Wilcoxon test). We compared patients with good versus poor outcome at 3 months by dichotomizing the mRS score into 0-2, ‘independent', versus 3-6, ‘dependent or dead' with their admission subregional brain temperatures (Mann–Whitney U-test). The correlation between temperatures of brain subregions and NIHSS at admission was tested with the Spearman method. Where data were missing, we performed the analysis including all patients and separately with just those patients contributing data to all components of the analysis to test if there was any difference resulting from missing data. Finally, we performed multiple logistic regression including age, NIHSS, and CNL temperature (as the tissue class with the most available data) on 3-month mRS.
Results
We initiated recruitment on 48 patients but 2 with an intracerebral hemorrhage, 1 with complex migraine and 1 with functional limb weakness were excluded, leaving 44 patients with ischemic stroke. Four patients were unable to tolerate the admission MRI and produced no images, leaving 40 patients with initial imaging and body temperature data for analysis. No patients received licensed alteplase treatment (within 3 hours of stroke at that time); three patients were randomized to the control group in a trial of alteplase within 6 hours of ischemic stroke.
The mean age was 72 years, range 37 to 88, including 19 males and 21 females. There were 12 total anterior circulation, 17 partial anterior circulation, 5 posterior circulation, and 6 lacunar syndromes on the Oxfordshire Community Stroke Project classification.20 The mean NIHSS on admission was 10, median 7, range 1 to 28. The median DTI ischemic lesion volume was 15.3 mL (min 0.6, max 489 mL) at baseline and 23.3 mL (min 1.4, max 621 mL) on follow-up scanning at median 5 days after stroke. At 3 months, 17 patients were alive and independent (mRS 0-2) and 23 patients were dependent or dead (mRS 3-6).
Body Temperature Profiles
All patients were normothermic on admission, the median admission tympanic temperature being 36.4°C, (min 36°C, max 36.7°C) measured at median 4.5 hours (min 1, max 19 hours) after stroke. The median peak tympanic temperature was 37.3°C (SD 0.55; min 36°C, max 38.4°C) and was recorded at median 36 hours (1.5 days) after stroke (min 2, max 110 hours).11
Twelve patients became pyrexial during the recording period, at a median time of 29 hours (1.2 days, range 9 to 71 hours) after stroke (details provided in Karaszewski et al.11). Patients who became pyrexial had more severe stroke on admission (NIHSS 12 versus nonpyrexial 6.5, P=0.04), a larger DWI lesion volume on admission (58.8 versus apyrexial 6.5 mL, P=0.008) and reached a higher peak body temperature (38.8°C versus apyrexial 36.8°C, P<0.001) than patients who remained apyrexial, but there was no difference in age (71.9 versus 71.9) or time to peak temperature (40.2 versus apyrexial 49.8 hours, Table 1). Note that in some patients, the admission temperature was also the peak temperature or the peak temperature occurred soon after admission.
Table 1. Characteristics of patients who became pyrexial and who remained apyrexial.
| No pyrexia (n=28) | Pyrexia (n=12) | ||
|---|---|---|---|
| Sex | |||
| Male | 15 | 4 | |
| Female | 13 | 8 | |
| Mean age (years) | 71.9 (11.7) | 71.9 (11.3) | P=0.990 |
| Stroke severity | |||
| Admission NIHSS (median, IQR) | 6.5 (3–10) | 12 (5–18) | P=0.038 |
| DWI lesion volume (median) | 6,521 | 58,052 | P=0.008 (MWU) |
| Body temperature | |||
| Peak (mean °C, SD) | 36.8 (0.4) | 37.8 (0.3) | P<0.001 |
| Time to peak (mean hours after onset, SD) | 49.8 (24.5) | 40.2 (25.5) | P=0.262 |
| Outcome | |||
| mRS ⩽2 | 15 | 2 | χ2=3.5 |
| mRS ⩾3 | 13 | 10 | P=0.061 |
DWI, diffusion-weighted imaging; IQR, interquartile range; mRS, modified Rankin Scale; MWU, Mann–Whitney U-test; NIHSS, National Institute of Health Stroke Scale; SD, standard deviation.
Pyrexia was any body (aural) temperature measurement ⩾37.5°C during the recording period.
Brain Temperatures
The median time from stroke onset to admission MRI was 17 hours (range 3 to 26 hours): nine patients were imaged <6, three between 6 and 12, 14 between 12 and 18 hours, and 14 between 18 and 26 hours after stroke. Data available for some of the analyses of brain temperature were further reduced due to impaired spectra (patient movement or poor shimming) or no follow-up imaging (early patient discharge or too ill/died), but all available data were used in each analysis. Therefore, brain temperature data were available for a total of 35 patients at each time point of whom 30 individual patients provided brain temperature measures at both time points and 10 provided brain temperature measures at one or other time point but not both.
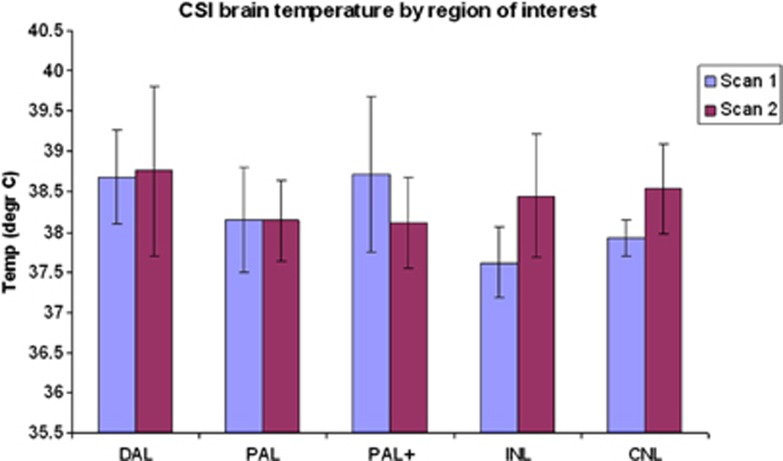
At the time of the admission MRI, the median temperatures of lesion core (DAL, 38.6°C) and potential penumbra (PAL, 38.3°C, PAL+, 38.7°C) were higher than temperatures of INL (37.6°C) and CNL brain (37.9°C: abnormal versus normal tissues, P=0.03; CNL versus INL, P<0.05; Figure 2). Thirty-seven patients underwent follow-up MRI, performed at median 5 days (range 3 to 7 days) after stroke, of whom 35 contributed brain temperature data. The temperature in DAL (38.8°C), PAL (38.1°C), and PAL+ (38.1°C) tissue and in DWI-normal tissues (ipsilateral 38.4°C, contralateral 38.5°C) were all similar (P=0.3; Figure 2).
Figure 2.
Temperatures (mean of patients' means shown) (°C) of ischemic and normal-appearing brain at admission to hospital and follow-up at around 5 days after stroke. CNL, contralateral normal brain; CSI, chemical shift imaging; DAL, definitely abnormal tissue; INL, ipsilateral normal brain; PAL, possible abnormal tissue; PAL+, tissue one voxel thick immediately outside the lesion.
Brain and Body Temperature Comparison
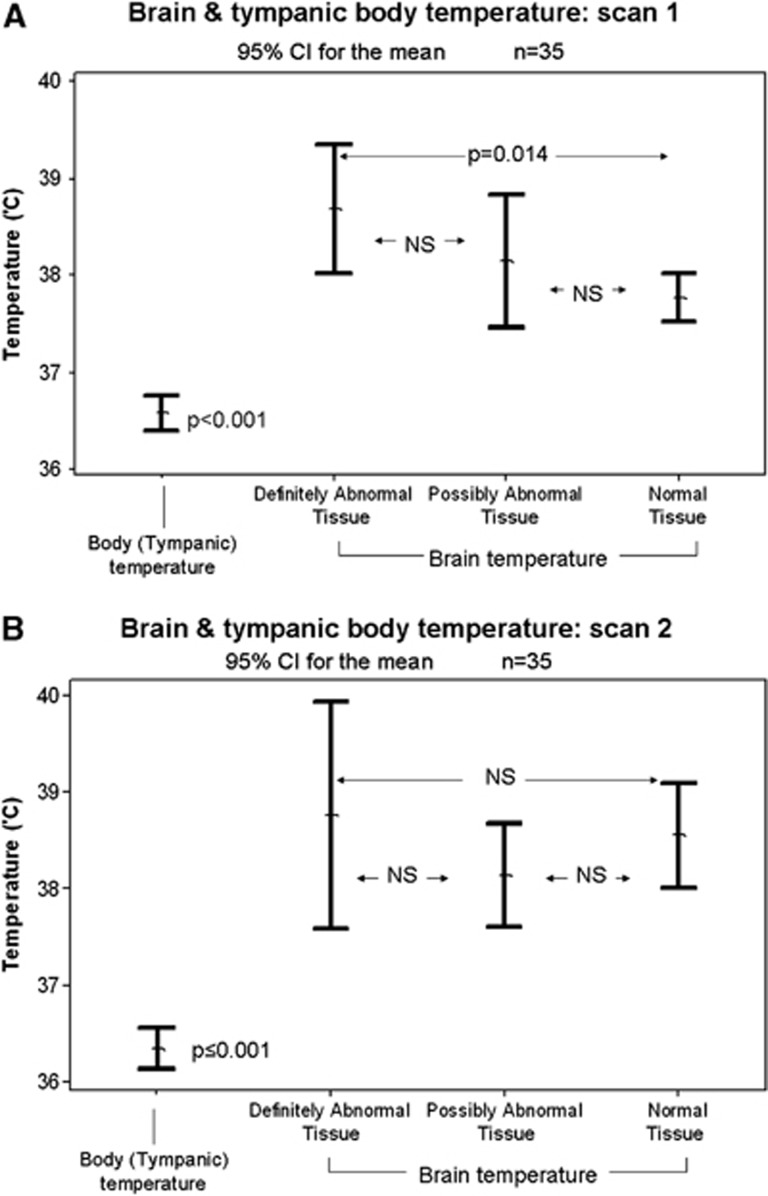
On admission MRI, all brain regions, whether DWI-abnormal or normal appearing, were hotter than concurrently measured tympanic temperatures (mean 36.6°C, P<0.001, 0.032 and 0.001, respectively) (Figure 3A). On follow-up MRI, all brain regional temperatures remained hotter than tympanic temperature measured concurrently (mean 36.3°C, P<0.001 all comparisons, Figure 3B).
Figure 3.
Brain and body temperature (A) on admission and (B) at median 5 days after stroke. CI, confidence interval.
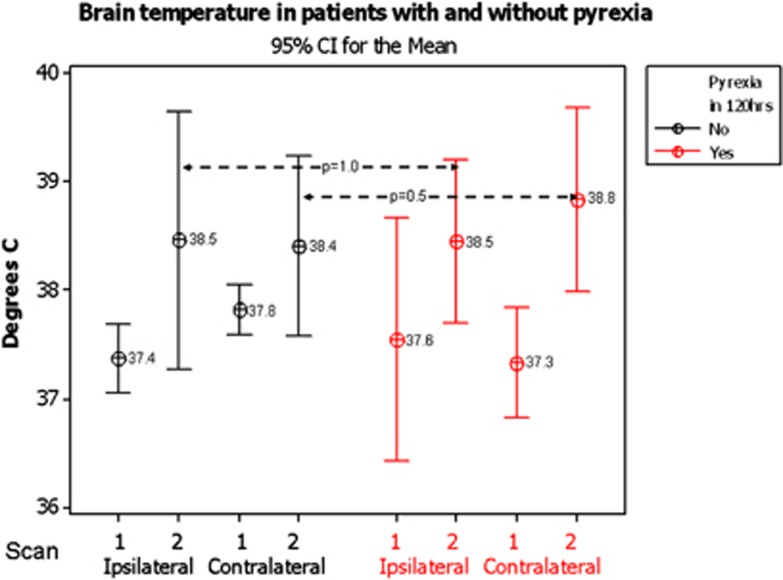
On admission MRI, the temperature in the INL or CNL-appearing brain did not differ in the 12 patients who became pyrexial from that in 28 who remained apyrexial, either on the baseline (ipsilateral, pyrexial 37.6°C versus apyrexial 37.4°C, P=0.9; contralateral, pyrexial 37.3°C versus apyrexial 37.8°C, P=0.4) or the follow-up scan (ipsilateral, pyrexial 38.4°C versus apyrexial 38.4°C, P=0.9; contralateral, pyrexial 38.8°C versus apyrexial 38.4°C, P=0.5), Figure 4.
Figure 4.
Tympanic temperature and temperature in ipsilateral and contralateral normal-appearing brain tissue in patients who became pyrexial at any time within the first 7 days after admission and those who did not. CI, confidence interval.
Admission brain temperature, early and late imaging, and clinical outcomes
Between admission and follow-up imaging, 11 patients showed lesion expansion and 27 patients had either no change or a reduction in DWI lesion size (2 patients did not have a follow-up scan). Mean temperature of CNL tissue on admission was higher in patients whose lesion grew (38.3°C) than in those whose lesion did not expand (37.7°C, P=0.014) at follow-up. There was no difference in temperatures between the two groups in the remaining brain subregions (Table 2).
Table 2. Comparison between brain temperature (°C) on admission and ischemic lesion expansion by median 5 days after stroke, change in NIHSS from admission to 5 days after stroke, and functional outcome at 3 months.
| Admission brain temperatures (°C) | DAL | PAL | PAL+ | INL | CNL |
|---|---|---|---|---|---|
| Ischemic lesion expansion by 3–5 days | |||||
| Lesion expansion | 38.8 (3) | 37.9 (7) | 38.0 (3) | 37.1 (6) | 38.3 (10) |
| No lesion expansion | 38.7 (8) | 38.0 (16) | 38.8 (13) | 37.7 (21) | 37.7 (23) |
| P value | 0.54 | 0.32 | 0.45 | 0.13 | 0.01 |
| Change in NIHSS from admission to 120 hours after stroke | |||||
| No improvement in NIHSS | 39.3 (6) | 38.8 (11) | 38.4 (9) | 38.0 (15) | 38.1 (18) |
| Improvement in NIHSS | 37.7 (4) | 37.5 (12) | 39.1 (8) | 37.3 (13) | 37.9 (15) |
| P value | 0.01 | 0.04 | 0.37 | 0.04 | 0.18 |
| Functional outcome at 3 months | |||||
| Poor outcome mRS 3-6 | 38.7 (10) | 38.6 (13) | 38.9 (8) | 37.6 (13) | 38.2 (19) |
| Good outcome mRS 0-2 | 38.8 (1) | 37.7 (11) | 38.6 (9) | 37.6 (16) | 37.6 (16) |
| P value | 0.91 | 0.31 | 0.96 | 0.59 | 0.002 |
CNL, contralateral normal brain; DAL, definitely abnormal tissue; INL, ipsilateral normal brain; PAL, possible abnormal tissue; PAL+, tissue one voxel thick immediately outside the lesion; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale.
Number in brackets is number of patients in each category. Temperatures are °C.Bold values highlight differences that are significant.
National Institutes of Health Stroke Scale on admission was associated with contralateral hemisphere brain temperature (r=0.42, P=0.01) but there were no associations with temperatures in any other brain regions (Table 2). Between admission and follow-up imaging at median 5 days, the NIHSS improved by four or more points or reached zero in 14 patients, did not change by >3 points in 17 patients, or worsened by 4 or more points in 4 patients. On admission, mean temperatures of DAL, PAL, and INL tissue were higher in patients whose NIHSS worsened or did not change by 5 days than in those whose NIHSS improved (P<0.05) but temperature in CNL tissue on admission was not associated with change in NIHSS by 5 days.
At 3 months, 17 patients achieved good (mRS 0-2) and 23 patients achieved poor (mRS 3-6) functional outcomes. Higher admission temperatures in CNL tissue were associated with poor 3-month functional outcome (P<0.05) but there were no associations between temperature in the ischemic lesion (DAL, PAL, or PAL+) and 3-month functional outcome. However after correcting for age and NIHSS on multivariable logistic regression, CNL temperature was no longer associated significantly with mRS 3-6.
Discussion
Soon after acute ischemic stroke, higher temperatures in contralateral DWI-normal appearing brain, but not in ischemic brain, are associated with worse admission NIHSS, lesion expansion by 5 days and poor 3-month functional outcome. This confirms our hypothesis, based on earlier work in 40 patients18 but now including data on 80 patients, that soon after ischemic stroke, temperatures in ischemic lesion core and potential penumbral tissues are hotter than ipsilateral or contralateral DWI-normal-appearing brain. Neither study found associations between ischemic brain temperature and admission stroke severity. The associations between CNL temperature and clinical findings parallel those found for tympanic temperature in the same patients11 and that others have found for body temperature in other studies1, 2, 3, 4, 5, 6, 7, 8, 9, 10 summarized in Karaszewski et al.11 The difference in the pattern of brain temperature elevation in the ischemic lesion, combined with the different associations with clinical and imaging features, suggests that the mechanism(s) for early temperature elevation in ischemic brain are different to those which elevate temperature in normal-appearing brain and cause pyrexia. This leads to several important conclusions:
The elevated temperature in ischemic tissue is not directly related to body temperature and possibly not to progression of brain injury, and appears to have a different mechanism to that of body and normal-appearing brain temperature;
that body temperature elevation after ischemic stroke occurs secondary to the systemic (presumably inflammatory) response to brain injury and is reflected in temperature in normal-appearing brain. Hence, body temperature takes time to rise, peaks at around 1 to 2 days after stroke onset, and (as summarized previously11) patients are usually apyrexial within the first few hours of stroke. None of the present study patients was pyrexial on admission; 12 became pyrexial up to 5 days after stroke. This interpretation is also consistent with the observed relationship between plasma inflammatory markers and temperature profiles in normal-appearing but not in ischemic brain, reported previously30;
and that brain temperature is consistently higher than body temperature, perhaps due to the heat byproducts of the metabolically very active brain.
The early temperature elevation in ischemic brain has several explanations. It may reflect continued cell metabolism generating heat but inadequate blood flow to remove the heat and cool the tissue, or activation of mechanisms intended to counteract ischemic damage by dissipating energy, that cannot be used by ischemic cells, as heat. For example, genes that control mitochondrial oxidative activity like uncoupling proteins mediate tolerance to ischemic insults in experimental models31 and uncoupling proteins single-nucleotide polymorphisms are associated with risk of cardiovascular disease in humans.32
This study has limitations. The relatively small sample size (40) reflects the difficulty in undertaking complex MRI studies in patients with moderate to severe acute stroke. The absence of other studies of brain temperature after stroke and the very few using spectroscopy is testament to this difficulty. Nonetheless, we have doubled the existing literature: data on 80 patients in two independent studies show the same brain temperature pattern after ischemic stroke. In both current and previous studies,17, 18 temperatures across the brain were measured at a relatively wide range of times (baseline up to 26 hours after stroke), many within the first 12 hours. However, within the first 24 hours, many pathologic processes occur in ischemic brain33, 34 that are likely to influence local heat production and dissipation. However, the comparison of ischemic with nonischemic brain and body temperature is valid as each patient acted as their own control. None of the patients received alteplase: therefore, these results reflect the ‘natural history' of nonthrombolysis-treated ischemic stroke. It is possible that alteplase, by restoring tissue perfusion, will alter ischemic tissue temperature profiles, or by reducing stroke severity and infarct extent will alter body temperature profiles and reduce pyrexia. The work should be repeated in thrombolysis-treated patients. We only performed limited multivariate statistical modeling as the sample size precluded reliable results.
Methodological limitations related to 1HMRS thermometry, discussed previously,15, 17, 18 are worth reiterating. Calibration, in this and our previous work, currently assumes that brain temperature in healthy subjects is 37°C. However, some data suggest that normal brain temperature may be slightly higher; in volunteer studies, we find temperatures of 38°C even with this calibration (unpublished data). Unfortunately, there is no reference standard. True temperatures may be slightly different to the values given by 1HMRS thermometry, but within an individual they are comparable. More studies are required to examine important factors like temperature variation in gray and white matter, effect of tissue water content, and stage in infarction.
This study has strengths. MR thermometry enables simultaneous measurement of tissues across the ischemic lesion to normal brain. Other studies have measured brain temperatures using invasive probes, but this restricts measurement to a small volume of brain, of uncertain location, affects brain temperature through breaching the skull and can only be used when invasive monitoring is justified clinically, i.e., in the severe strokes. We performed all assessments in standardized ways, blinded all analyses. We used operational definitions of ischemic lesion core and potential penumbral tissues.18 We used validated software to assign tissue classifications, with good rater reliability and biologic relevance.27 Our sample, though small, was representative of hospital admitted patients.
Why should body temperature be related to initial stroke severity, and why should body temperature elevation adversely influence lesion growth and functional outcome? Experimental data suggest that higher temperatures not only accelerate ischemic damage but also accelerate opening of the blood–brain barrier.35 If these observations also apply in humans,35 then higher temperatures in contralateral brain could influence lesion progression and worsen outcome by promoting blood–brain barrier opening in the ischemic tissue, exacerbating tissue swelling, worsening tissue capillary blood flow, leading to recruitment of more tissue into the ischemic lesion, lesion expansion and worse functional outcome.36
If the foregoing interpretation is correct, then therapeutic hypothermia might work by cooling nonischemic tissue, preventing blood–brain barrier opening, reducing peri-infarct damage, and preventing infarct growth, but might not have much influence on the primary ischemic core tissue. Hence, the combination of thrombolysis to salvage ischemic but viable cells,37 plus hypothermia to reduce blood–brain barrier opening, tissue edema, and recruitment of peri-infarct tissue into the infarct, might be doubly effective in improving functional outcome. Thrombolysis might also enhance hypothermia because the act of restoring blood flow to the ischemic cells could also help to remove the excess heat generated. Thus, therapeutic hypothermia might be beneficial even if started >6 hours after stroke and even modest reductions of only 1°C or 2°C might protect at risk tissue from progression to permanent damage or recruitment of initially not at risk tissue into the infarct. Further trials of hypothermia38 should be encouraged to test these hypotheses. If our observations are correct, then body temperature is likely to be the more relevant measure for clinical outcomes.
Acknowledgments
The imaging was performed in the Brain Research Imaging Centre (www.bric.ed.ac.uk), University of Edinburgh, UK, a center in the SINAPSE Collaboration (Scottish Imaging Network, a Platform for Scientific Excellence, www.sinapse.ac.uk). We thank the radiographic staff of the Brain Research Imaging Centre and Scottish Stroke Research Network for nursing support. Dr Kamil Chwojnicki is acknowledged for his assistance with statistical calculations.
The authors declare no conflict of interest
Footnotes
This work was supported by the UK Stroke Association (TSA 2006/11), Foundation for Polish Science, International Brain Research Organization (IBRO) and Polish Ministry of Science and Higher Education to Dr Karaszewski, The Row Fogo Charitable Trust to Dr Armitage; the Cohen Charitable Trust to Dr Carpenter; and The Scottish Funding Council through the SINAPSE (Scottish Imaging Network – A Platform for Scientific Excellence, www.sinapse.ac.uk) Collaboration to Professor Wardlaw and Dr Lymer.
References
- Castillo J, Davalos A, Marrugat J, Noya M. Timing for fever-related brain damage in acute ischemic stroke. Stroke. 1998;29:2455–2460. doi: 10.1161/01.str.29.12.2455. [DOI] [PubMed] [Google Scholar]
- Olsen TS, Weber UJ, Kammersgaard LP. Therapeutic hypothermia for acute stroke. Lancet Neurol. 2003;2:410–416. doi: 10.1016/s1474-4422(03)00436-8. [DOI] [PubMed] [Google Scholar]
- Reith J, Jorgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347:422–425. doi: 10.1016/s0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- Kallmunzer B, Krause C, Pauli E, Beck A, Breuer L, Kohrmann M, et al. Standardized antipyretic treatment in stroke: a pilot study. Cerebrovasc Dis. 2011;31:382–389. doi: 10.1159/000321733. [DOI] [PubMed] [Google Scholar]
- Wass CT, Lanier WL, Hofer RE, Scheithauer BW, Andrews AG. Temperature changes of > or=1 degree C alter functional neurologic outcome and histopathology in a canine model of complete cerebral ischemia. Anesthesiology. 1995;83:325–335. doi: 10.1097/00000542-199508000-00013. [DOI] [PubMed] [Google Scholar]
- den Hertog HM, van der Worp HB, van Gemert HM, Algra A, Kappelle LJ, van Gijn J, et al. An early rise in body temperature is related to unfavorable outcome after stroke: data from the PAIS study. J Neurol. 2011;258:302–307. doi: 10.1007/s00415-010-5756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammersgaard LP, Jorgensen HS, Rungby JA, Reith J, Nakayama H, Weber UJ, et al. Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke. 2002;33:1759–1762. doi: 10.1161/01.str.0000019910.90280.f1. [DOI] [PubMed] [Google Scholar]
- Kyobutungi C, Grau A, Stieglbauer G, Becher H. Absolute temperature, temperature changes and stroke risk: a case-crossover study. Eur J Epidemiol. 2005;20:693–698. doi: 10.1007/s10654-005-0703-x. [DOI] [PubMed] [Google Scholar]
- Millan M, Grau L, Castellanos M, Rodriguez-Yanez M, Arenillas JF, Nombela F, et al. Body temperature and response to thrombolytic therapy in acute ischaemic stroke. Eur J Neurol. 2008;15:1384–1389. doi: 10.1111/j.1468-1331.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lim LL, Levi C, Heller RF, Fisher J. Influence of admission body temperature on stroke mortality. Stroke. 2000;31:404–409. doi: 10.1161/01.str.31.2.404. [DOI] [PubMed] [Google Scholar]
- Karaszewski B, Thomas RG, Dennis MS, Wardlaw JM. Temporal profile of body temperature in acute ischemic stroke: relation to stroke severity and outcome. BMC Neurol. 2012;12:123. doi: 10.1186/1471-2377-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, et al. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312–317. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Petersson J, Norrving B, Hacke W, Dirnagl U, Wagner M, et al. Hypothermia for stroke: call to action 2010. Int J Stroke. 2010;5:489–492. doi: 10.1111/j.1747-4949.2010.00520.x. [DOI] [PubMed] [Google Scholar]
- Cady EB, D'Souza PC, Penrice J, Lorek A. The estimation of local brain temperature by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 1995;33:862–867. doi: 10.1002/mrm.1910330620. [DOI] [PubMed] [Google Scholar]
- Marshall I, Karaszewski B, Wardlaw J, Wartolowska K, Armitage P, Carpenter T, et al. Measurement of regional brain temperature using proton spectroscopic imaging: validation and application to acute ischemic stroke. Magn Reson Imaging. 2006;24:699–706. doi: 10.1016/j.mri.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Germain D, Chevallier P, Laurent A, Saint-Jalmes H. MR monitoring of tumour thermal therapy. MAGMA. 2001;13:47–59. doi: 10.1007/BF02668650. [DOI] [PubMed] [Google Scholar]
- Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K, Haga K, et al. Early brain temperature elevation and anaerobic metabolism in human acute ischaemic stroke. Brain. 2009;132:955–964. doi: 10.1093/brain/awp010. [DOI] [PubMed] [Google Scholar]
- Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K, Haga K, et al. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischaemic stroke. Ann Neurol. 2006;60:438–446. doi: 10.1002/ana.20957. [DOI] [PubMed] [Google Scholar]
- Schwab S, Spranger M, Aschoff A, Steiner T, Hacke W. Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology. 1997;48:762–767. doi: 10.1212/wnl.48.3.762. [DOI] [PubMed] [Google Scholar]
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- Rampen AJ, van Breda EJ, Dippel DW. Tympanic measurement of body temperature in stroke patients "turned on its ear". J Neurol Neurosurg Psychiatry. 2005;76:1041–1042. doi: 10.1136/jnnp.2004.058149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa T. Body temperature measurement. Clin Phys Physiol Meas. 1985;6:83–108. doi: 10.1088/0143-0815/6/2/001. [DOI] [PubMed] [Google Scholar]
- Lu SH, Leasure AR, Dai YT. A systematic review of body temperature variations in older people. J Clin Nurs. 2009;19:14–16. doi: 10.1111/j.1365-2702.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39:3029–3035. doi: 10.1161/STROKEAHA.108.521583. [DOI] [PubMed] [Google Scholar]
- Karaszewski B, Thomas RGR, Chappell FM, Armitage PA, Carpenter TK, Lymer GKS, et al. Brain choline concentration: early quantitative marker of ischemia and infarct expansion. Neurology. 2010;75:850–856. doi: 10.1212/WNL.0b013e3181f11bf1. [DOI] [PubMed] [Google Scholar]
- Martin M, Labouesse J, Canioni P, Merle M. N-acetyl-L-aspartate and acetate 1H NMR signal overlapping under mild acidic pH conditions. Magn Reson Med. 1993;29:692–694. doi: 10.1002/mrm.1910290518. [DOI] [PubMed] [Google Scholar]
- Armitage PA, Rivers CS, Karaszewski B, Thomas RG, Lymer GK, Morris Z, et al. A grid overlay framework for analysis of medical images and its application to the measurement of stroke lesions. Eur Radiol. 2012;22:625–632. doi: 10.1007/s00330-011-2284-2. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Sellar RJ. A simple practical classification of cerebral infarcts on CT and its interobserver reliability. AJNR Am J Neuroradiol. 1994;15:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, von Kummer R, Farrall AJ, Chappell FM, Hill M, Perry D. A large web-based observer reliability study of early ischaemic signs on computed tomography. The Acute Cerebral CT Evaluation Of Stroke Study (ACCESS) PLoS ONE. 2010;5:e15757. doi: 10.1371/journal.pone.0015757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley W, Thomas R, Lowe G, Rumley A, Karaszewski B, Armitage P, et al. Do acute phase markers explain body temperature and brain temperature after ischemic stroke. Neurology. 2012;79:152–158. doi: 10.1212/WNL.0b013e31825f04d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- Dhamrait SS, Stephens JW, Cooper JA, Acharya J, Mani AR, Moore K, et al. Cardiovascular risk in healthy men and markers of oxidative stress in diabetic men are associated with common variation in the gene for uncoupling protein 2. Eur Heart J. 2004;25:468–475. doi: 10.1016/j.ehj.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Sobrino T, Castillo J. Evolving paradigms for neuroprotection: molecular identification of ischemic penumbra. Cerebrovasc Dis. 2006;21 (Suppl 2:71–79. doi: 10.1159/000091706. [DOI] [PubMed] [Google Scholar]
- Castillo J, Rodriguez I. Biochemical changes and inflammatory response as markers for brain ischaemia: molecular markers of diagnostic utility and prognosis in human clinical practice. Cerebrovasc Dis. 2004;17 (Suppl 1:7–18. doi: 10.1159/000074791. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Sharma HS. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience. 2009;161:926–939. doi: 10.1016/j.neuroscience.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM. Neuroimaging in acute ischaemic stroke: insights into unanswered questions of pathophysiology. J Intern Med. 2010;267:172–190. doi: 10.1111/j.1365-2796.2009.02200.x. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41:2265–2270. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]