Abstract
[18F]fallypride is a high-affinity dopamine D2/3 receptor tracer with the ability to reliably quantify D2/3 receptor sites in both striatal and corticolimbic regions. The translational potential of [18F]fallypride imaging is, however, limited by the lengthy scanning sessions (60–80 minutes duration over a total of 3–4 hours) required by current protocols. The aims of our study were to adapt [18F]fallypride imaging for use in clinical populations with neurological and neuropsychiatric disorders, by reducing the duration of individual scanning sessions; and to establish the reproducibility and reliability of our adapted protocol in healthy older people. Eight participants (five male and three female; mean age=75.87±4.39 years) were scanned twice, 4–6 weeks apart. [18F]fallypride binding potential was determined from image data collected during three sampling times: 0–30; 60–90; and 210–240 minutes post injection. High reproducibility and reliability (test–retest variability <8% intraclass correlation coefficient >0.8) were observed in all but the prefrontal regions, and remained so when sampling times were reduced to 20 minutes (0–20; 70–90; 220–240 minutes). The adapted protocol is feasible for use across neuropsychiatric disorders in which dopamine has been implicated and is sufficiently sensitive to detect within-subject changes between 2.7% and 5.5% in striatal and limbic regions.
Keywords: dopamine, [18F]fallypride, imaging, receptors, reliability, test–retest
Introduction
Since dopamine D2/3 receptors were first visualized in vivo in humans,1 positron emission tomography (PET) tracers that target dopamine D2/3 receptors have provided crucial insights into the pathophysiology and treatment of psychiatric and neurological disorders.2, 3 Such tracers have been instrumental in guiding treatment strategies in schizophrenia, by establishing a ‘therapeutic window' of striatal D2/3 receptor occupancy by antipsychotic drugs.4, 5 More recently, the development of high-affinity D2/3 receptor tracers such as [18F]fallypride6 and [11C]FLB4577 has shifted the focus of D2/3 receptor imaging toward extrastriatal regions where receptor density is 10–100 times lower than the striatum.8 This has allowed the clinical relevance of corticolimbic D2/3 receptor occupancy to be explored, has raised important questions regarding the contribution of temporal cortical occupancy to therapeutic response,9, 10 and remains a focus of investigation across a range of atypical antipsychotic drugs.
Another major development in imaging technology has been the use D2/3 PET tracers to image endogenous neurotransmitter release. The observation that D2/3 tracers compete with endogenous dopamine for receptor sites has been used in imaging paradigms to investigate the sensitivity of the dynamic system after pharmacological or behavioral challenge.11 High-affinity D2/3 receptor tracers are increasingly used in this respect as they offer the opportunity to explore the role of corticolimbic dopamine release in human behavior12, 13 and in a range of neurological and psychiatric illnesses including schizophrenia-spectrum disorders14 and Parkinson's Disease.15
[18F]fallypride is unique among D2/3 receptor tracers, as it can provide stable estimates of both striatal and extrastriatal receptor availability within the same scanning session.6 However, techniques currently used to quantify [18F]fallypride binding involve multiple sampling periods (each lasting 60–80 minutes) over a total scan duration of 3-4 hours, to allow tracer uptake to achieve a plateau within the striatum where receptor sites are more densely concentrated.6, 16 These imaging protocols are not feasible for use in many clinical populations, particularly older, cognitively impaired individuals or those with movement disorders. Adapting [18F]fallypride imaging for use in clinical populations who are unable to tolerate lengthy scanning sessions would widen its potential for use in understanding disease mechanisms, drug occupancy, and dopamine release in response to pharmacological and behavioral challenge.
The aims of the study were as follows:
To adapt [18F]fallypride imaging by reducing the length of individual scanning sessions to 30 minutes.
To establish the test–retest reliability of the adapted protocol in healthy older people.
To investigate whether sampling times could be further reduced to 20-minute sessions without reducing reliability.
Materials and methods
Sample
Eight healthy older adults (five male and three female; mean age=75.87±4.39 years) were recruited to the study. Participants were ‘healthy controls' identified from the Dementia Case Register database within the South London and Maudsley (SLaM) NHS Trust, funded by the National Institute of Health Research (NIHR Biomedical Research Centre). All participants gave written informed consent. The study was approved by the Joint South London and Maudsley and the Institute of Psychiatry NHS Research Ethics Committee. Permission to administer [18F]fallypride was given by the Administration of Radioactive Substances Advisory Committee. Before their involvement, a full medical and psychiatric history was taken from participants, including dementia screening using the Mini-Mental State Examination.17 Exclusion criteria included (i) current or past neurological or psychiatric illness, including drug and substance abuse; (ii) Mini-Mental State Examination score <26; (iii) history of any cerebrovascular event; (iv) use of estrogen replacement therapy or other medications, which may have affected brain dopamine activity; (v) needle phobia; and (vi) other medical conditions that might affect the ability to tolerate a brain scan, such as significant cardiorespiratory disease or severe kyphosis. Structural imaging (T1-weighted magnetic resonance imaging (MRI)) was carried out at the Centre for Neuroimaging Sciences to exclude intracranial abnormalities.
PET Imaging Procedure
Participants were scanned twice at rest, 4–6 weeks apart, on a GE (GE Healthcare, Hatfield, UK) VCT Discovery PET-CT camera (FWHM 5 mm), at St Thomas' PET Centre. A molded head rest and straps were used to minimize head movement and an external webcam was used to detect significant head movements that could degrade the quality of image data. [18F]fallypride was administered via a single bolus intravenous injection of 250 MBq. Each scanning session consisted of three dynamic scans in three-dimensional (3D) mode, each lasting 30 minutes, and preceded by a low-dose computed tomography scan for attenuation correction. Image data were collected during three scanning sessions, determined from previous [18F]fallypride studies in young adults;16, 18, 19 0–30 minutes to provide an input function to model a reference region approach; 60–90 minutes to capture peak tracer binding within extrastriatal regions; and 210–240 minutes to ensure that tracer binding had achieved a plateau in the striatum of all participants (in a small proportion of people, the tracer may take up to 210 minutes to achieve equilibrium)20 and to achieve a good model fit. The initial 3 minutes of the first scanning session acquired frames with short duration (1 × 10 seconds, 10 × 5 seconds, 6 × 10 seconds, 3 × 20 seconds). The remaining 87 minutes of scanning were acquired using frame lengths of 1 minute.
Image Analysis
Data were analyzed using a simplified reference tissue model21 and the cerebellum as a reference region. This approach has been previously validated for the quantification of [18F]fallypride BPND22 and found to have good reproducibility and reliability.23 Preprocessing was performed using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8/) and all other analyses using Matlab (www.mathworks.com). [18F]fallypride images were processed using SPM8 in Matlab. Non-attenuation corrected, fully 3D iteratively reconstructed PET scans (GE ‘VuePoint' reconstruction algorithm, 4 ierations, 28 subsets, 4.8 mm Hanning 3D-filter) were used for frame-by-frame realignment. These transformations were then applied to attenuation-corrected (AC) filtered back projected (Fourier-rebinned 2D reconstruction, with geometric, deadtime, scatter, and random correction, 4.8 Hanning Transaxial filter, GE Scanner) PET images, which were used for quantification, and AC-VuePoint PET images (4 iterations, 28 subsets, 4.8 mm Hanning 3D-filter) used for warping atlases. All images were reconstructed to 128 × 128 × 47 voxels with dimensions 2 × 2 × 3.27 mm3.
Method 1
Image data collected over the three sampling times (0–30; 60–90; and 210–240 minutes) were used to quantify binding of [18F]fallypride to dopamine D2/3 receptors, expressed as BPND.24 The cerebellar reference region was defined using the Automated Anatomical Labeling atlas25 and included all cerebellar regions apart from the vermis. Regions of interest (ROI) in areas with specific binding were defined using the Tzioritzi atlas26 and included the caudate, putamen, medial and inferior temporal gyri, thalamus, amygdala, hippocampus, orbitofrontal cortex, and anterior cingulate gyrus. In addition to the Tzioritzi atlas, we used a pre-existing template that defines the striatum in terms of its functional connections, and which has been previously used to quantify [11C] Raclopride BPND in people with Alzheimer's disease.27 The striatal subdivisions were defined in standard space (Montreal Neurological Institute, MNI), using the parameters described by Martinez et al.28 The boundary between the ventral (limbic) striatum (inferiorly) and dorsal caudate and dorsal putamen (superiorly) was defined using the anterior commissure–posterior commissure transaxial plane. The ventral (limbic) striatum was sampled from the anterior boundary of the striatum to the level of the anterior commissure coronal plane. The transaxial plane was used to subdivide the dorsal caudate and putamen into associative (caudate and putamen rostral to the anterior commissure and the caudate caudal to the anterior commissure) and sensorimotor (putamen caudal to the anterior commissure) striatum.
We chose not to coregister MRI with PET data, as our specific aim was to establish the most widely applicable analysis method that would be suitable for use in older, cognitively impaired individuals, in whom MRI may be contraindicated or difficult to tolerate. As an alternative, atlases (and the striatal functional subdivisions template) were warped to subject space via a PET [18F]fallypride template in standard (MNI) space. The [18F]fallypride template was created from six healthy young subjects,29 using the following method:
Structural (MRI) data were spatially normalized to MNI space using the unified segmentation algorithm in SPM.
These transforms were applied to each of the coregistered summed (3–30 min) AC-VuePoint PET images.
A mean [18F]fallypride template was calculated by scaling each transformed PET image by the subject global mean, and then taking the mean of the six PET images.
Method 2
Sampling times were reduced from 30 to 20-minute blocks (0–20; 70–90; and 220–240 minutes). All other aspects of the image analysis were identical to Method 1.
Statistical Analysis
All statistical analysis was carried out using SPSS 19 (www.spss.com). A paired t-test was used to compare the administered dose of [18F]fallypride between test (scan 1) and retest (scan 2) scans. The reliability of the test–retest values was determined by calculating an intraclass correlation coefficient (ICC)30 for each region sampled. The absolute variability of test–retest reproducibility was calculated as follows: (2|scan1−scan2)|/(scan 1+scan 2)] × 100. Percentage variability in mean BPND between Methods 1 and 2 was determined by: (|Method1_scan1–Method2_scan1|/Method1_Scan1) × 100 and expressed as the mean and s.d. across subjects. Power calculations were performed using ‘PS' software.31 The regional percentage change in [18F]fallypride BPND detectable in a typical within-subjects comparison (sample size=15; paired t-test) was calculated using a probability (power) of 0.8, and an associated type I error probability (α) of 0.05. Percentage variability in mean BPND was used as an estimate of within-subject s.d.
Results
Administered dose of [18F]fallypride was 244.1±7.3 MBq. There were no significant differences in administered dose between scan 1 and scan 2 (mean difference=0.5±10.8 MBq, P=0.9).
Reliability Analysis
Method 1
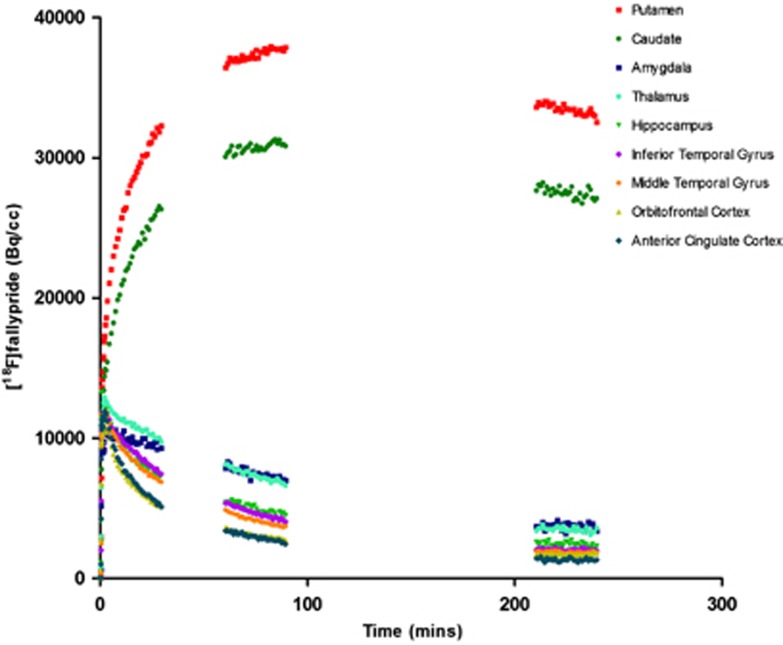
Time–activity curves for Method 1 (30-minute sampling times) are shown in Figure 1 (all ROI defined using the Tziorotzi atlas), Figure 2 (extrastriatal ROI, defined using the Tzioritzi atlas), and Figure 3 (striatal functional subdivisions), and represent the AC filtered back projected data on which the kinetic analysis was carried out. Mean percentage test–retest differences (% variability) and reliability (ICC) of regional [18F]fallypride binding for Method 1 are presented in Table 1. Reproducibility was excellent within the caudate, putamen, amygdala, and inferior temporal gyrus, where <5% variability was observed. All of the other regions examined showed high reproducibility (<8% variability), with the exception of the orbitofrontal cortex (15.04% variability) and anterior cingulate gyrus (28.84% variability). Reliability (ICC) ranged from 0.804 (limbic striatum) to 0.988 (inferior and middle temporal gyri).
Figure 1.
Time–activity curves (Method 1) are shown for regions of interest defined using the Tziortzi atlas and represent [18F]fallypride uptake in a single participant.
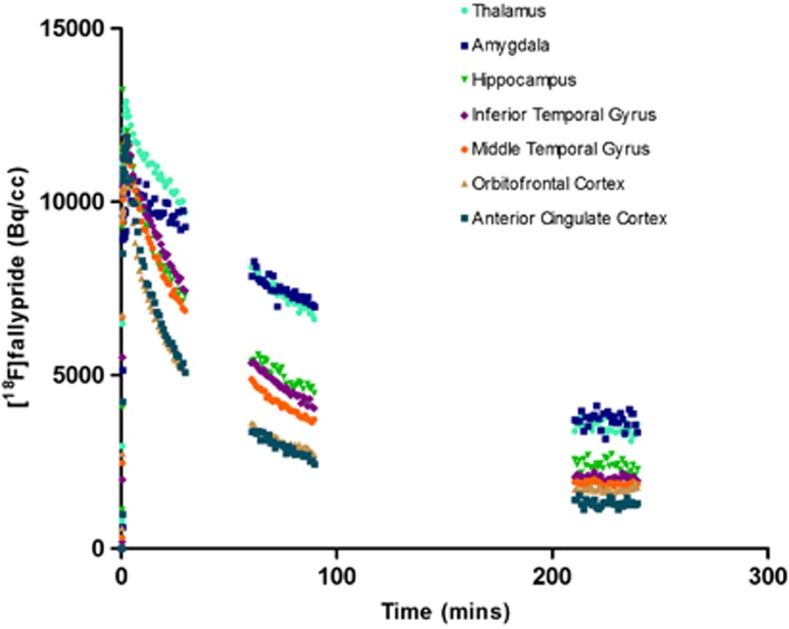
Figure 2.
Time–activity curves (Method 1) are shown for extrastriatal regions of interest and represent [18F]fallypride uptake in a single participant.
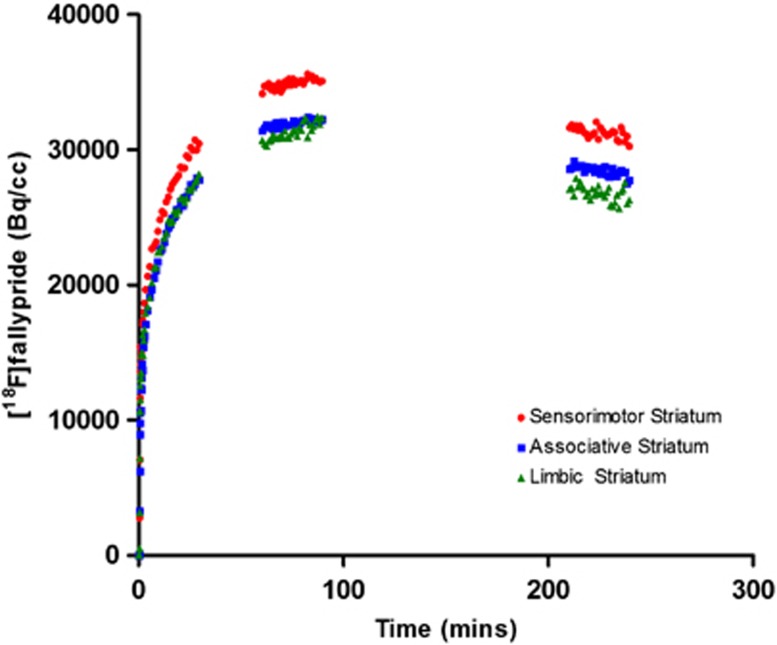
Figure 3.
Time–activity curves (Method 1) are shown for the striatal functional subdivisions and represent [18F]fallypride uptake in a single participant.
Table 1. Test–retest reproducibility of regional [18F]fallypride binding using method 1 (3 × 30 minutes).
| Region | Testa | Retesta | Variability %b | ICC |
|---|---|---|---|---|
| Sensorimotor striatum | 19.73 (2.66) | 19.70 (2.06) | 5.56 (2.69) | 0.864 |
| Associative striatum | 17.50 (2.39) | 17.58 (1.77) | 5.05 (5.33) | 0.845 |
| Limbic striatum | 16.90 (2.83) | 17.04 (2.00) | 7.12 (7.37) | 0.804 |
| Putamen | 21.19 (2.82) | 21.50 (2.15) | 4.99 (3.76) | 0.879 |
| Caudate | 16.57 (2.77) | 16.71 (2.29) | 3.44 (3.87) | 0.961 |
| Amygdala | 1.77 (0.41) | 1.76 (0.38) | 4.08 (4.64) | 0.976 |
| Thalamus | 1.48 (0.28) | 1.47 (0.24) | 6.10 (6.70) | 0.903 |
| Hippocampus | 0.82 (0.21) | 0.79 (0.19) | 7.35 (6.40) | 0.950 |
| Inferior temporal gyrus | 0.57 (0.21) | 0.58 (0.20) | 4.83 (7.61) | 0.988 |
| Middle temporal gyrus | 0.39 (0.17) | 0.39 (0.17) | 6.40 (9.45) | 0.988 |
| Orbitofrontal cortex | 0.21 (0.10) | 0.23 (0.11) | 15.04 (15.68) | 0.923 |
| Anterior cingulate gyrus | 0.18 (0.10) | 0.18 (0.09) | 28.84 (39.59) | 0.822 |
Abbreviation: ICC, intraclass correlation coefficient.
BPND given as mean across subjects (s.d.).
Absolute variance given as mean across subjects (s.d.)=(|scan 1−scan 2|/(scan 1+scan 2)/2)*100.
Method 2
Mean percentage test–retest differences (% variability) and reliability (ICC) of regional [18F]fallypride binding for Method 2 (20-minute sampling times) are presented in Table 2. Reproducibility remained excellent (<5% variability) in the caudate and amygdala, and high (<8% variability) in all but the limbic striatum (8.25% variability) and prefrontal regions (orbitofrontal cortex=17.45% anterior cingulate gyrus=37.35%). Intraclass correlation coefficient values ranged from 0.794 (anterior cingulate gyrus) to 0.986 (inferior and middle temporal gyri). When regional ‘test' BPND values were compared across the two methods (shown in Table 2) mean variability was 3.19% or less in all regions sampled apart from the anterior cingulate gyrus (14.47%) (shown in Table 2).
Table 2. Test–retest reproducibility of regional [18F]fallypride binding using Method 2 (3 × 20 minutes).
| Region | Testa | Retesta | Variability %b | ICC | Method 1 versus method 2d |
|---|---|---|---|---|---|
| Sensorimotor striatum | 19.82 (3.04) | 19.70 (2.06) | 6.37 (3.70) | 0.839 | 1.77 (1.13) |
| Associative striatum | 17.55 (2.64) | 17.56 (1.84) | 5.80 (5.91) | 0.837 | 1.66 (0.93) |
| Limbic striatum | 16.94 (3.06) | 16.99 (2.06) | 8.25 (6.97) | 0.802 | 1.56 (0.89) |
| Putamen | 21.27 (3.19) | 21.49 (2.21) | 5.68 (4.86) | 0.857 | 1.69 (0.98) |
| Caudate | 16.62 (3.06) | 16.68 (2.35) | 4.43 (4.43) | 0.946 | 1.72 (0.90) |
| Amygdala | 1.76 (0.42) | 1.75 (0.38) | 4.70 (5.30) | 0.967 | 1.02 (0.74) |
| Thalamus | 1.47 (0.29) | 1.46 (0.23) | 6.60 (7.25) | 0.890 | 1.13 (0.74) |
| Hippocampus | 0.81 (0.21) | 0.79 (0.18) | 7.67 (7.84) | 0.935 | 1.38 (1.11) |
| Inferior temporal gyrus | 0.56 (0.21) | 0.57 (0.20) | 5.28 (9.96) | 0.986 | 2.89 (2.60) |
| Middle temporal gyrus | 0.38 (0.18) | 0.38 (0.17) | 7.94 (10.47) | 0.986 | 3.19 (2.76) |
| Orbitofrontal cortex | 0.21 (0.10) | 0.22 (0.11) | 17.45 (14.23) | 0.915 | 2.70 (2.41) |
| Anterior cingulate gyrus | 0.17 (0.11) | 0.17 (0.09) | 37.35 (54.16) | 0.794 | 14.47 (20.50) |
Abbreviation: ICC, intraclass correlation coefficient.
BPND given as mean across subjects (s.d.).
Absolute variance given as mean across subjects (s.d.)=(|test−retest|/(test+retest)/2)*100.
Given as mean percentage difference between Method 1 and Method 2 test BPND (s.d.)=(|Method1_test−Method2_test|/Method1_test) × *100.
Power Analysis
The results of a power analysis for a within-subject study design and a sample size of 15 (paired t-test; α0.05, power=0.8) are shown in Table 3. This indicates that method 1 would be sufficiently sensitive to detect changes of 5% or less in all regions apart from the limbic striatum (5.5%), hippocampus (5.7%), orbitofrontal cortex (11.7%), and anterior cingulate gyrus (22.4%). Method 2 could detect changes of 5% or less across the caudate, putamen, dorsal striatal subdivisions, inferior temporal gyrus, and amygdala; and larger changes in the other regions.
Table 3. Regional detectable within-subject (%) change in [18F]fallypride bindinga.
| Region | Method 1 | Method 2 |
|---|---|---|
| Sensorimotor striatum | 4.3 | 5.0 |
| Associative striatum | 3.9 | 4.5 |
| Limbic striatum | 5.5 | 6.4 |
| Putamen | 3.9 | 4.4 |
| Caudate | 2.7 | 3.4 |
| Amygdala | 3.2 | 3.7 |
| Thalamus | 4.7 | 5.1 |
| Hippocampus | 5.7 | 6.0 |
| Inferior temporal gyrus | 3.8 | 4.1 |
| Middle temporal gyrus | 5.0 | 6.2 |
| Orbitofrontal cortex | 11.7 | 13.6 |
| Anterior cingulate gyrus | 22.4 | 29.1 |
n=15; power=0.8; α=0.05.
Discussion
This study has shown high reproducibility and reliability of an adapted [18F]fallypride protocol across the striatum, consistent with previous test–retest studies on [18F]fallypride in young adults, which have used full modeling with arterial sampling6 or a simplified reference tissue approach23 to image analysis. [18F]fallypride BPND was also highly reproducible in the amygdala, temporal cortex, thalamus, and hippocampus, comparable with previous data on [18F]fallypride.6, 23 In contrast, reproducibility in prefrontal regions was poor, particularly in the anterior cingulate cortex, where a higher mean and wider range of variability was observed compared with the other regions sampled. These findings are consistent but more marked than those of Cropley et al,23 who similarly reported a high variability in the anterior cingulate (21.8±3.8%) in healthy young adults. This is not unexpected, given the relatively low signal to noise ratio of [18F]fallypride in this region and the additional impact of age upon D2/3 receptor availability in our sample,6, 32 which is more pronounced in the anterior cingulate than other cortical regions.32 Reducing individual sampling times to 20 minutes produced the same pattern of reproducibility across striatal and extrastriatal regions and increased test–retest variability minimally in all but the prefrontal regions.
A [18F]fallypride imaging technique that requires only brief periods in the scanner could significantly enhance the translational potential of [18F]fallypride imaging across a range of neuropsychiatric disorders in which dopamine has been implicated, including patients with dementia, Parkinson's disease, movement disorders, and disorders of impulsivity. Our primary interest in adapting [18F]fallypride imaging was to develop a technique suitable for use in D2/3 receptor occupancy studies in older and/or cognitively impaired patients. Older people are extremely susceptible to antipsychotic side effects, including extrapyramidal symptoms, falls, sedation, and increased cerebrovascular mortality.33, 34 The potential mechanisms underpinning this heightened sensitivity are poorly understood, although several theories have been proposed, including alterations in central pharmacokinetics and reduced D2/3 receptor reserve in the ageing brain.35 It has been suggested that the ‘therapeutic window' of striatal D2/3 receptor occupancy may be lower in older people,36 but this has not been tested robustly using a within-subject approach, nor has it been extended to extrastriatal regions, such as the temporal cortex, that have been implicated in treatment response in young psychotic adults.9
Our adapted protocol is sufficiently sensitive to detect small percentage changes in all but the prefrontal regions, and is therefore a suitable approach to establish the relative contribution of limbic versus striatal occupancy in elderly patients during antipsychotic treatment. This includes patients with schizophrenia-like illness, who are prescribed very low doses (1/10th of the dose used in young adults) of antipsychotic medication33 and in whom a relatively low regional receptor D2/3 receptor occupancy might be anticipated. The fact that the length of individual scanning times can be reduced to 20 minutes without significantly compromising reliability increases its feasibility for use in patients with dementia. It could be argued that patients with cognitive or neurological impairment are likely to have more variable data than healthy older participants and that longer scanning sessions may be necessary to obtain data of sufficient quality. However, we anticipate that shorter sessions will maximize patient cooperation and reduce head movement confounds. The next stage of our research will be to pilot the protocol as a pretreatment (baseline) protocol in people with dementia who are about to begin antipsychotic medication, both to assess tolerability and the quality of the data collected over 30- versus 20-minute sampling times. We also aim to adapt and optimize an interrupted scanning protocol, which will be suitable for use post antipsychotic treatment, as occupancy by antipsychotic medication will reduce the number of available D2/3 receptor sites and shorten the time taken to achieve equilibrium, particularly within striatal regions.16, 18 Similar considerations will need to be taken into account when adapting the protocol for other types of pharmacological interventions, which may alter tracer kinetics and affect times of peak uptake and washout.
The decision not to use MRI data for coregistration with PET images was based on our previous experience of imaging older participants, particularly those with cognitive impairment,37 in whom a multiple scan approach is more challenging and tolerance for confined spaces is generally poor. As an alternative, regions that were predefined on an atlas were warped to participants' PET images using a [18F]fallypride template and an automated procedure. This method is similar to the approach previously used to quantify [11C] Raclopride BPND in people with Alzheimer's disease27 and has the advantage of removing any subjectivity in the placing of ROIs. However, the fact that the templates originated from structural and functional imaging data on healthy young adults could have impacted upon the warping process. Although high tracer signal forms the key component of the process, the accuracy of warping, particularly in smaller, noisier regions, may have been reduced. Partial volume effects, particularly relevant in an older sample,38 are also likely to have affected the accuracy of tracer quantification. These factors are likely to have contributed to the high test–retest variability in the anterior cingulate gyrus. However, the method of image analysis does not appear to have affected the pattern of reproducibility across other brain regions, as our findings are consistent with previously conducted test–retest studies that used the participant's MRI23 or PET-MR coregistered images6 to define ROI. A further consideration is the fact that the sampling times used to quantify cortical and striatal BPND were based upon [18F]fallypride studies carried out in young adults. Given that time to achieve peak tracer binding is dependent upon receptor density, and may be affected by age-related factors including peripheral clearance, regional cerebral blood flow, and blood brain barrier transport, it remains possible that the adapted protocol did not optimally capture the peak in all participants. These methodological limitations are less important for within-subject study designs, but will need to be addressed to maximize the potential of the protocol for use in between-subject comparisons, or to correlate regional BPND with behavioral measures.
In addition to receptor occupancy, we wanted to establish the sensitivity of our protocol to detect changes in endogenous dopamine after pharmacological or behavioral intervention. Amphetamine displacement studies carried out in young adults have found that [18F]fallypride is less sensitive to changes in cortical dopamine levels than other high-affinity tracers such as [11C]FLB457, because of its relatively low signal to noise ratio in these regions.39 A sufficiently robust effect has, however, been demonstrated across striatal and limbic (hippocampal and amygdala) regions.12, 19, 23 A power calculation, using a standard sample size of 15 (previous studies have included 10–25 subjects),12, 13, 15, 19, 39 indicates that our adapted protocol will be sufficiently sensitive to detect within-subject changes between 2.7% and 5.5% within striatal and limbic regions using method 1, and between 3.4% and 6.4% using method 2. As discussed earlier, we have not yet established whether these findings will generalize to cognitively or neurologically impaired populations, and this issue is particularly relevant for studies that aim to quantify endogenous dopamine release, where small changes in BPND are anticipated. Given the fact that sensitivity to detect within-subject change is crucial for studies of this type, it may be advisable to collect data over the longer (30 minute) sampling times used in method 1 to image endogenous neurotransmission.
We have successfully adapted [18F]fallypride imaging, using an interrupted scanning protocol that considerably shortens scanning times. The high reproducibility and reliability of this protocol means that it could be applied not only to D2/3 receptor occupancy studies but also to image endogenous neurotransmission in striatal and limbic regions. Future work will aim to pilot the protocol in clinically relevant populations and to refine the image analysis method to maximize its potential for use in between-subject study designs.
The authors declare no conflict of interest.
Footnotes
The project was funded by Guy's and St Thomas' Charity, the National Institute for Health Research (NIHR), and the Medical Research Council (MRC). This research was supported by the NIHR Biomedical Research Centre (BRC) at Guy's and St Thomas' and South London and Maudsley (SLaM) NHS Foundation Trusts and King's College London. The views expressed are those of the authors(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
References
- Farde L, Hall H, Ehrin E, Sedvall G. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science. 1986;231:258–261. doi: 10.1126/science.2867601. [DOI] [PubMed] [Google Scholar]
- Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15:2550–2559. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoessl AJ, Martin WW, McKeown MJ, Sossi V. Advances in imaging in Parkinson's disease. Lancet Neurol. 2011;10:987–1001. doi: 10.1016/S1474-4422(11)70214-9. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G, Zipursky RB, Wilson AA, Houle S. The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Sci. 1995;57:PL103–PL107. doi: 10.1016/0024-3205(95)02037-j. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Whetsell WO, Ansari MS, Votaw JR, de Paulis T, Clanton JA, et al. Identification of extrastriatal dopamine D2 receptors in post mortem human brain with [125I]epidepride. Brain Res. 1993;609:237–243. doi: 10.1016/0006-8993(93)90878-q. [DOI] [PubMed] [Google Scholar]
- Stone JM, Davis JM, Leucht S, Pilowsky LS. Cortical dopamine D2/D3 receptors are a common site of action for antipsychotic drugs—an original patient data meta-analysis of the SPECT and PET in vivo receptor imaging literature. Schizophr Bull. 2009;35:789–797. doi: 10.1093/schbul/sbn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord M, Farde L. Antipsychotic occupancy of dopamine receptors in schizophrenia. CNS Neurosci Ther. 2011;17:97–103. doi: 10.1111/j.1755-5949.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ, et al. The dopaminergic basis of human behaviors: a review of molecular imaging studies. Neurosci Biobehav Rev. 2009;33:1109–1132. doi: 10.1016/j.neubiorev.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacol. 2006;31:1016–1026. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Park S, Anderson S, Doop M, Ansari MS, Schmidt D, et al. Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [(1)(8)F]fallypride. Synapse. 2011;65:99–102. doi: 10.1002/syn.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Cowan RL, Park S, Ansari MS, Baldwin RM, Li R, et al. Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am J Psychiatry. 2011;168:418–426. doi: 10.1176/appi.ajp.2010.10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson's patients with medication-induced pathological gambling: A [11C] FLB-457 and PET study. Neurobiol Dis. 2012;48:519–525. doi: 10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Slifstein M, Frankle WG, Xu X, Hackett E, Bae SA, et al. Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F]fallypride. Neuropsychopharmacol. 2008;33:3111–3125. doi: 10.1038/npp.2008.33. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B, et al. Occupancy of striatal and extrastriatal dopamine D2 receptors by clozapine and quetiapine. Neuropsychopharmacol. 2006;31:1991–2001. doi: 10.1038/sj.npp.1301108. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010;64:350–362. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernaleken I, Janouschek H, Raptis M, Hellmann S, Veselinovic T, Brocheler A, et al. Dopamine D2/3 receptor occupancy by quetiapine in striatal and extrastriatal areas. Int J Neuropsychopharmacol. 2010;13:951–960. doi: 10.1017/S1461145710000374. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Siessmeier T, Zhou Y, Buchholz HG, Landvogt C, Vernaleken I, Piel M, et al. Parametric mapping of binding in human brain of D2 receptor ligands of different affinities. J Nucl Med. 2005;46:964–972. [PubMed] [Google Scholar]
- Cropley VL, Innis RB, Nathan PJ, Brown AK, Sangare JL, Lerner A, et al. Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse. 2008;62:399–408. doi: 10.1002/syn.20506. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Reeves S, Brown R, Howard R, Grasby P. Increased striatal dopamine (D2/D3) receptor availability and delusions in Alzheimer disease. Neurology. 2009;72:528–534. doi: 10.1212/01.wnl.0000341932.21961.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Dunn JT, Baker SX, Stone J, Cleij M, Marsden P, O'Doherty M, et al. Differential occupancy of striatal versus extrastriatal dopamine D2/D3 receptors by the typical antipsychotic haloperidol in man measured using [18F]fallypridePET. J Psychopharmacol. 2010;24 (suppl 3:A33. [Google Scholar]
- Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD. The test-retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. Neuroimage. 2010;50:524–531. doi: 10.1016/j.neuroimage.2009.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont WD, Plummer WD., Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Howard R, Rabins PV, Seeman MV, Jeste DV. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000;157:172–178. doi: 10.1176/appi.ajp.157.2.172. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacol. 2008;33:957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Mamo DC, Mulsant BH, Pollock BG, Kapur S. Increased antipsychotic sensitivity in elderly patients: evidence and mechanisms. J Clin Psychiatry. 2009;70:397–405. doi: 10.4088/jcp.08r04171. [DOI] [PubMed] [Google Scholar]
- Uchida H, Kapur S, Mulsant BH, Graff-Guerrero A, Pollock BG, Mamo DC. Sensitivity of older patients to antipsychotic motor side effects: a PET study examining potential mechanisms. Am J Geriatr Psychiatry. 2009;17:255–263. doi: 10.1097/JGP.0b013e318198776d. [DOI] [PubMed] [Google Scholar]
- Reeves S, Brown R, Matthews D, Howard R, Grasby P. No effect of donepezil on striatal dopamine release in mild to moderate Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2010;81:119–121. doi: 10.1136/jnnp.2009.172510. [DOI] [PubMed] [Google Scholar]
- Morris ED, Chefer SI, Lane MA, Muzic RF, Jr., Wong DF, Dannals RF, et al. Loss of D2 receptor binding with age in rhesus monkeys: importance of correction for differences in striatal size. J Cereb Blood Flow Metab. 1999;19:218–229. doi: 10.1097/00004647-199902000-00013. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63:447–461. doi: 10.1002/syn.20628. [DOI] [PubMed] [Google Scholar]