Abstract
Assessment of whether pesticide exposure is associated with neurodevelopmental outcomes in children can best be addressed with a systematic review of both the human and animal peer-reviewed literature. This review analyzed epidemiologic studies testing the hypothesis that exposure to pesticides during pregnancy and/or early childhood is associated with neurodevelopmental outcomes in children. Studies that directly queried pesticide exposure (e.g., via questionnaire or interview) or measured pesticide or metabolite levels in biological specimens from study participants (e.g., blood, urine, etc.) or their immediate environment (e.g., personal air monitoring, home dust samples, etc.) were eligible for inclusion. Consistency, strength of association, and dose response were key elements of the framework utilized for evaluating epidemiologic studies. As a whole, the epidemiologic studies did not strongly implicate any particular pesticide as being causally related to adverse neurodevelopmental outcomes in infants and children. A few associations were unique for a health outcome and specific pesticide, and alternative hypotheses could not be ruled out. Our survey of the in vivo peer-reviewed published mammalian literature focused on effects of the specific active ingredient of pesticides on functional neurodevelopmental endpoints (i.e., behavior, neuropharmacology and neuropathology). In most cases, effects were noted at dose levels within the same order of magnitude or higher compared to the point of departure used for chronic risk assessments in the United States. Thus, although the published animal studies may have characterized potential neurodevelopmental outcomes using endpoints not required by guideline studies, the effects were generally observed at or above effect levels measured in repeated-dose toxicology studies submitted to the U.S. Environmental Protection Agency (EPA). Suggestions for improved exposure assessment in epidemiology studies and more effective and tiered approaches in animal testing are discussed.
The potential developmental effects of environmental chemical exposures have been studied for several decades and remain a topic of considerable interest (Bjorling-Poulsen et al., 2008; Bruckner, 2000; Grandjean and Landrigan, 2006; Mendola et al., 2002; Rice, 2005; Wigle et al., 2007, 2008). In particular, the potential effects of pesticide exposures to the developing fetus and child are of interest to society and regulatory agencies. Although the neurotoxic associations of high level prenatal and early childhood exposure to certain pesticides are well established (Eaton et al., 2008), the implications of potential effects observed at low exposures are less straightforward, particularly in the absence of a clinically defined adverse outcome. Studies evaluating potential neurodevelopmental effects associated with pesticide exposure are challenging to interpret, in part because of the diversity of types and classes of chemicals, differences in exposure measures, and the wide range of instruments used to assess outcomes (Rice, 2005). Nevertheless, it is important to critically evaluate the evidence to date, as well as to identify important research gaps and methodological issues that require further attention in order to advance our understanding of observed effects.
Neurodevelopmental deficits include a broad spectrum of disorders and dysfunctions such as autism spectrum disorder, attention deficit hyperactivity disorder (ADHD), decreased intelligence, learning disabilities, developmental delays, emotional or behavioral problems, and deficits in gross or fine motor skills. The exact prevalence of these deficits is difficult to ascertain; however, it has been estimated that approximately 3 to 8% of infants and 12% of children are affected by one or more of these conditions (National Academy of Sciences, 1988). This phenomenon provides sufficient motivation in the scientific and medical communities to identify factors that may contribute to adverse events in the developing nervous system.
Findings from human and animal studies demonstrated that some environmental contaminants may be toxic to the developing neurological system (Hass, 2006), and it was suggested that approximately one quarter of developmental disorders can be attributed to environmental exposures or complex gene–environment interactions (Grandjean and Landrigan, 2006; National Academy of Sciences, 2000). However, the extent to which these exposures influence the incidence of developmental deficits and the exact mechanisms for initiation and progression are unclear (Hass, 2006). The development of the nervous system extends beyond birth into childhood and adolescence (Watson et al., 2006); however, the critical periods of development are most likely to occur in utero (Rice and Barone, 2000). Neurulation, the process by which the central nervous system develops during embryogenesis, begins in the third week of gestation in humans (DeSesso et al., 1999; Desesso, 2012). The human brain develops from a small number of cells located on the epiblast into the central nervous system that contains billions of specialized cells (Grandjean and Landrigan, 2006). Exposure to environmental contaminants, including pesticides, during specific periods of development may impair neurologic development in children. Numerous studies were conducted in humans and animals to evaluate the potential effect of myriad exposures, including environmental contaminants, on neurologic development.
Research on pesticide exposure has been increasing, particularly over the past two decades (Arcury et al., 2006). Billions of pounds of pesticides (including herbicides, insecticides, rodenticides, etc.) are used throughout the world for agricultural purposes and in residential homes and gardens for crop protection and pest management. Several studies showed that agricultural workers have substantially greater opportunity for pesticide exposure than the population at large (Curl et al., 2002; Fenske et al., 2002; McCauley et al., 2001; O'Rourke et al., 2000; Curwin et al., 2007). In addition, biomonitoring data indicate that exposures to farm spouses and children are determined largely by the degree of direct contact with the application process, and that exposure profiles varied by specific chemical for each family member (applicator, spouse, children) (Thomas et al., 2010; Curl et al., 2002; Mandel et al., 2005). In the general population, there is also evidence to suggest that contact with pesticides or their residues is widespread (Barr et al., 2005). Pregnant women and children may be vulnerable to these exposures (Berkowitz et al., 2003). Further, young children are thought to have increased opportunities for pesticide exposure because of dietary and physical behaviors (Barr et al., 2004).
There have been relatively few evaluations of both the animal and human literature on the effects of pesticides on neurodevelopment. Previous reviews focused primarily on summarizing significant adverse associations reported in the epidemiology literature or significant adverse associations on neurodevelopmental endpoints in the animal literature (Weselak et al., 2007; Bjorling-Poulsen et al., 2008; Julvez and Grandjean, 2009; Wigle et al., 2007, 2008). These reviews have not included a systematic evaluation of both the absence and presence of outcomes, or an evaluation of the evidence for and against a causal interpretation, or integrated the outcomes reported in analytic epidemiology studies with mechanisms of action determined by animal studies. In addition, for the most part, the reviews of the animal literature summarize the findings as reported by the primary authors of the original papers and do not include a discussion of how the reported effect levels compare with no-observed-adverse-effect levels (NOAELs) determined by subchronic and chronic toxicity studies that are used to derive reference doses (RfDs) and other acceptable levels of exposure for the general population.
The objective of the current review was to compile the epidemiologic studies that evaluate potential associations between exposure to specific pesticides in pregnant or nursing women or in infants or young children and neurobehavioral outcomes or head circumference in infants or young children. Further in vivo mammalian literature evaluating the effects of pesticides on functional neurodevelopmental endpoints was surveyed. The epidemiology and animal literature was systematically reviewed with respect to the following questions: (1) What is the evidence of causality between exposure to specific pesticides (or classes of pesticides) during critical periods of brain development and neurobehavioral outcomes in the epidemiologic literature? (2) What are the lowest dose levels for adverse functional neurodevelopmental effects in animals in the published literature, and how do they compare with effect levels from repeat dose toxicity studies used to derive the chronic RfD? In addition, an evaluation of the types of developmental neurotoxicity (DNT) studies that were submitted to the U.S. Environmental Protection Agency (EPA) in comparison with other studies that contribute toward defining the chronic RfD was provided based on publically available information on the U.S. EPA Office of Pesticide Program's websites or in the published literature.
APPROACH TO EVALUATION OF EPIDEMIOLOGIC STUDIES
Our review of the epidemiologic studies began with identification, documentation, and evaluation of the reported associations in the peer-reviewed literature. Distinguishing causal from noncausal effects is particularly challenging in epidemiology because of observational study designs and the inevitable role of chance, bias and confounding. These methodological challenges, which are inherent to epidemiology studies, are critical for causal interpretation. In addition, comparison of study methodology (including characteristics of the study population, timing of exposure measurement, and neurobehavioral testing) is essential in order to interpret similarities and differences in outcomes observed across studies. Careful attention to the type and specificity of exposure metrics and to the validity of methods used in measuring outcomes is also important in evaluating the evidence for and against causality.
There are several guideposts to consider when evaluating the evidence from a body of epidemiologic literature, including strength of the association (e.g., magnitude of the relative risk estimate or regression coefficient), consistency, dose response, and biological plausibility (Hill, 1965). Although these principles are not criteria per se, the U.S. EPA has recommended using them to evaluate epidemiology data in its “Draft Framework for Incorporating Human Epidemiologic & Incident Data in Health Risk Assessment” (U.S. Environmental Protection Agency, 2010a). The “strength” of association is an arbitrary term for the magnitude of a relative risk (RR) estimate (e.g., odds ratio, risk ratio, or rate ratio). The strength (magnitude) of a regression coefficient is less straightforward since the estimate is reflective of statistical transformation and the unit of measure (e.g., inches or centimeters). In addition to strength of association, the precision of the confidence interval was also considered. Poole (2001) recommended computing the confidence interval ratio to consider the stability of relative risk estimates with wide intervals, particularly for small studies. Since “precision” is no better defined than “strong,” for the purposes of this review, the confidence intervals and the resulting relative risk estimates or regression coefficients were considered to be “imprecise” if the ratio of the upper versus lower limit was greater than 5. Statistical significance of a positive or negative association was determined if the 95% confidence interval excluded the null value. Risk estimates for which the p value was less than .05 were considered statistically significant and were discussed in this context. Statistically significant risk estimates with narrow confidence intervals were given greater weight than imprecise risk estimates with large confidence intervals. The direction of nonstatistically and imprecise significant associations was not considered to be evidence of consistency. Statistical significance incorporates strength of association and sample size into its calculations, ruling out the role of chance, particularly in the direction of the association.
Consistency was viewed as observing similar exposure-outcome associations either internally within the same population or externally in independent studies. However, the observational nature of epidemiology permits pesticide exposure to be analyzed with varying levels of specificity. Therefore, consistency was evaluated on a continuum of specificity of exposure both within and across studies. Replications of associations were of particular interest when related to a specific pesticide exposure or biomarker and a specific health outcome. For example, an adverse association of an outcome such as lower motor development index and increased urinary organophosphate would be considered to be consistent with an adverse association between motor development index and urinary organophosphate in another study. However, the specific organophosphate metabolite malathion dicarboxylic acid (MDA) in one study would not provide good corroboration of specific results of sum of diethylphosphate metabolite levels (ΣDEP) in another because malathion does not metabolize to ΣDEP. Adverse health associations from different tests conducted in the same cohort were not considered to be evidence of consistency because different tests are designed to measure different domains. An example would be a longitudinal study reporting adverse associations observed with Bayley's motor development index at age 12 mo and with Bayley's mental developmental index at age 36 mo.
Further, because all observational epidemiologic studies suffer from some degree of bias (i.e., systematic error) and confounding, there was a particular emphasis on evidence of replication of exposure-outcome associations in independent studies. Several epidemiologic studies included multiple agents or outcomes; in this case, “positive” or “significant” results can be easily generated by chance only. This emphasizes the importance of consistency of results among independent studies.
Although dose response is a requirement for guideline animal studies, the observational nature of epidemiologic studies and exposure measurement limitations often precludes a quantitative assessment of dose response. Typically, “doses” are established based on the distribution of the data post hoc, for example, into tertiles or quartiles. It might be efficient to collapse the groups into high and low exposure levels; however, this essentially eliminates the ability to determine an exposure-response trend. If data are continuous, general linear regression models maintain the ability to test for a response trend. However, they cannot test for other forms of monotonic exposure-response relationships. Where available, categorical analyses with at least three exposure levels or continuous modeling were considered more robust.
Consideration of biological plausibility includes the question of whether the findings from the epidemiologic studies are consistent with data from comparable animal studies. While simple to describe, this concept is more difficult in practice. The specific endpoints do not always match, especially for neurobehavior. Internal dose for humans must be estimated for the critical periods based on available data. This was summarized as the integration of the epidemiology and animal sections. Since many of the exposure measures were collected at just one or two points in time, no evaluation of timing of exposure during pregnancy was possible.
In summary, this approach evaluated whether the epidemiologic data indicate a strong, consistent pattern of causality across studies from exposures to a specific pesticide (or class of pesticides) with neurobehavioral outcomes in infants or young children or with head circumference at birth.
SCOPE OF THE EPIDEMIOLOGY REVIEW AND INCLUSION CRITERIA
This review evaluated epidemiologic studies that reported information regarding exposure to pesticides during critical periods of brain development (i.e., in utero, infancy, or early childhood) and neurodevelopmental endpoints measured in infancy or early childhood or head circumference measured in newborns. Although outcomes in adolescents may be related to in utero exposures, the current review is limited to outcomes in newborns and early childhood. Endpoints of interest included behaviorally defined outcomes (e.g., pervasive developmental disorder or PDD as measured by the Child Behavior Checklist or CBCL) and subclinical deficits or differences in performance on neurobehavioral tests (e.g., Bayley Scales of Infant Development or BSID). All epidemiologic studies published in English and available in print or in electronic form in MEDLINE by April 30, 2011, were included. The search did not apply limitations on the geographic location of the study. Studies that ascertained pesticide exposure data by questionnaires, environmental monitoring (e.g., air, soil, dust), or biomarkers were eligible for inclusion provided that exposure to specific pesticides or classes of pesticides was measured or queried directly. Publications with inferred exposures were not included. For example, studies for which exposure data were limited to questions such as “Have you ever lived on a farm?” did not meet the inclusion criteria because determination of exposure to pesticide per se could not be identified. In contrast, exposure data from questions such as “Have you ever applied pesticides?” were considered. Studies that reported pesticide poisoning or exposures at acute or toxic levels beyond the directed or approved level of use were also excluded, as were pesticide biomonitoring studies that reported pesticide levels in biologic specimens but did not evaluate health outcomes.
Exposure Measurements: Specific and Nonspecific Biomarkers of Pesticide Exposure
Most of the epidemiologic studies included in this review were studies in which biomarkers of pesticide exposure were measured directly from maternal blood during pregnancy, maternal urine during pregnancy, cord or placental blood of the infant directly after birth, infant or child urine, or breast milk during pregnancy.
Where possible, this review summarized results for specific pesticides. Some studies reported associations between the outcomes of interest and the parent compound or a specific biomarker, while others reported biomarkers of exposure to the class of pesticide. The following broad categories were used to group pesticides and address similar mechanisms of action: organophosphate (OP), organochlorine (OC), N-methyl carbamate, pyrethroid, and other pesticides. In order to provide a summary of the existing data, results were provided and discussed for the specific pesticides, as well as broad classes where available.
A brief description of the main biomarkers for OP and OC pesticides evaluated in the studies that met the inclusion criteria follows. Limited space did not permit a description of every biomarker that was evaluated. Readers are referred to the original papers for further details and information.
Organophosphate (OP) insecticides Many of the OP insecticides metabolize to the broad class of dialkyl phosphates (DAP) that can be measured in urine, and represent the sum of diethylphosphates (ΣDEP) and dimethylphosphates (ΣDMP) (Sudakin and Stone, 2011). Urinary ΣDEP represent metabolites containing ethyl groups from 10 OP insecticides including chlorpyrifos (CPF) and diazinon. The ΣDMP are a broad class of dimethylphosphate metabolites of 17 methyl OP insecticides including malathion and chlorpyrifos methyl (a pesticide registered separately from CPF because of structural and metabolic differences from CPF). (Barr et al., 2004) In urine, 3,5,6-trichloro-2-pyridinol (TCPy) is the more specific biomarker of CPF exposure, as well as TCPy residues in the environment or diet (Barr and Angerer, 2006). Malathion exposure is estimated by the metabolite malathion dicarboxylic acid (MDA), and parathion and methylparathion are estimated by the metabolite 4-nitrophenol (PNP) (Eskenazi etal., 2004). CPF and diazinon can be measured directly in blood. In general, the biological half-life of all OP pesticides is relatively short; for example, the half-life of CPF in blood and urine ranges from 15–24 h and the half-life of malathion in blood is less than 1 h (Barr and Angerer, 2006).
Organochlorine (OC) insecticides The OC include insecticides such as aldrin, chlordane, dichlorodiphenyl-trichloroethane (DDT), and mirex. In contrast to the OP insecticides, which are rapidly cleared in hours, the OC pesticides have a half-life of several years. (Longnecker et al., 1997). The long half-life has implications for exposure measurement in epidemiologic studies. Since the chemicals are persistent in the body tissue, there is less day-to-day variability in internal exposure levels as compared to an OP that is rapidly cleared from the body. The commercial grade DDT that was once applied to crops was a mixture of p,p′–DDT (approximately 85%), o,p′-DDT (approximately 15%), and trace amounts of o,o′-DDT (Agency for Toxic Substances and Disease Registry, 2002). Dichlorodiphenyl-dichloroethylene (DDE) and dichlorodiphenyl-dichloroethane (DDD) are the metabolites and breakdown products of DDT in the environment. DDT, DDE, and DDD can be measured in fat, blood, urine, and breast milk. The half-life of DDT is about 7 yr, and that of DDE is longer (Longnecker et al., 1997). Because the relation of biological half-lives of DDT compounds is DDE > DDT > DDD, detection of higher ratios of DDD or DDT to DDE is postulated to indicate more recent exposure while lower ratios are presumed to indicate longer term exposure and storage capacity. Notably, decades after DDT use was banned in most countries, virtually all of the general population is exposed to the metabolite DDE through the diet.
Paraoxonase (PON1) Activity and Genotype
Included were studies evaluating the interaction between PON1 enzyme activity or genotype and pesticide exposure. Reports of main effects of PON1 enzyme activity or genotype were not the focus of this review and were only considered in relationship to pesticide exposure. Briefly, PON1 is an enzyme that is capable of metabolizing the active metabolites (oxons) of certain OP insecticides. Variation in PON1 polymorphism influences the speed with which individuals detoxify oxon metabolites of OP. Reduced PON1 activity may be related to higher toxicity as a result of reduced detoxification of the oxon (Furlong et al., 1988). Animal experiments indicated that PON1 exerts protection against OP toxicity, depending on the specific OP compound (Costa et al., 2012). Based on physiologically based pharmacokinetic and pharmacodynamic modeling, Timchalk et al. (2002) predicted that at low, environmentally relevant exposures to CPF other metabolic systems redundant to PON1 will compensate for slower PON1 activity. Thus, the significance of the role of PON1 status in modulating toxicity at lower levels of exposure to the parent OP insecticides is uncertain (Timchalk et al., 2002; Cole et al., 2005; Costa et al., 2012). PON1 enzyme activity is considered to be a more reliable measure of PON1 functional activity than PON1 genotype (Furlong et al., 2005; Cole et al., 2005). As will be discussed in greater detail in the results section, two cohort studies (Mt. Sinai and University of Berkeley Center for the Health Assessment of Mothers and Children of Salinas) evaluated PON 1 activity and/or polymorphisms in DNA (Berkowitz et al., 2004; Engel et al., 2007; Eskenazi et al. 2010). Both epidemiology studies focused on the PON1Q192R polymorphism in DNA, which is based on whether the amino acid present at position 192 is glutamine (Q) or arginine (R). Some studies estimate that the R form has eight- to ninefold higher catalytic activity than the Q form and hence provides more resistance to the acute toxicity of OP at higher doses (Furlong et al., 2005; Cole et al., 2005). Therefore, mothers homozygous for the Q192 alloform (QQ) would be predicted to have increased sensitivity to certain OP compared to those homozygous for the R192 alloform (RR).
Outcome Measurements
Head circumference and neurobehavioral outcomes were evaluated. All studies of head circumference measurements were based on data obtained directly after birth and were often abstracted from hospital or patient records. Head circumference was included because some investigators have related newborn head circumference with neurodevelopmental outcomes such as reduced intelligence in children (Ivanovic et al., 2004). The other outcomes included measures of psychomotor development, behavior, attention, and intelligence using standard tests and indices. Following is a brief description of the main assessment tools used in the papers in this review, presented in alphabetical order. Space does not permit a summary of every instrument used. Readers are referred to the original papers and their reference sections for additional information.
-
•
Brazelton Neonatal Behavioral Assessment Scale (BNBAS): The BNBAS groups the measurement of behavioral abilities and reflexes into the following seven domains: habituation, orientation, motor performance, range of state, regulation of state, autonomic stability, and number and type of abnormal reflexes (Brazelton and Nugent, 1995). This test is used by physicians, psychologists, and other health professionals to describe individual differences in information processing and regulation observed in newborns up to 2 mo of age. This test is also referred to as the Neonatal Behavioral Assessment Scale (NBAS).
-
•
Bayley Scales of Infant Development II (BSID). The BSID was developed to assess motor and mental development in infants, age 1 to 42 mo. The Mental Development Index (BSID:MDI) assesses general cognitive development and higher order mental processing, with 178 individual items that measure memory, habituation, generalization, classification, vocalizations, visual preference, visual acuity skills, problem solving, early number concepts, language, and social skills and development (Black and Matula, 2000; Sattler, 2001; Strauss et al., 2006). The Psychomotor Development Index (BSID:PDI) assesses overall motor development and contains 111 items that measure quality of movement, sensory integration, motor planning, fine and gross motor skills, and perceptual-motor integration (Black and Matula, 2000; Strauss et al., 2006). Standardized scores for the BSID:MDI and BSID:PDI have a mean of 100 and a standard deviation of 15, and range from 50 to 150.
-
•
Child Behavior Checklist (CBCL): The CBCL is administered to parents of children 1.5 to 5 yr of age to measure emotional and behavioral problems that have occurred in the previous two mo (Achenbach and Rescorla, 2000). The CBCL generates results for nine scales that are completed by the parent: adaptability, aggression, anxiety, attention problem, atypicality, conduct problems, depression, hyperactivity, leadership, social skills, somatization, and withdrawal. The results of the CBCL are consistent with the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DMS–IV), diagnoses (American Psychiatric Association, 2000). The CBCL uses the parent's ratings of 99 problem items as: 0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true, within the past 2 mo (Rescorla, 2005). The scores are obtained by summing the subtotal items for each child and then the total problem score is summed for all of the items combined. Each subscale may be scored continuously or categorically as normal, borderline, or clinical range (Rescorla, 2005).
-
•
Continuous Performance Test (CPT): The studies that implemented the CPTs in our review used a version of the test referred to as the Michigan Catch-the-Cat Test (version 1.2) (Jacobson et al., 1992), CPT with pictures from the Neurobehavioral Evaluation System 2 (NES2), and the Conners' Kiddie Performance Test (K-CPT) (Marks et al., 2010). These tests measure sustained attention or impulsivity to children preschool ages (4–5 yr) and older. Most of the tests involve presenting a visual stimulus on a computer screen to the child at variable intervals. The child's task is to indicate (e.g., by pressing a button) when the target stimulus (e.g., a ball) is presented. A record is kept of the number of correct responses, the number of misses, and the number of times the child responds to an incorrect stimulus. Separate scores are derived for attention, reaction time, and impulsivity. Scores may be influenced by anxiety, fatigue, boredom with the task, use of cold medication, and other problems that may interfere with concentration (Sattler and Hoge, 2006). It is recommended that CPT scores “never be used independently to make a diagnosis about ADHD” (Sattler and Hoge, 2006, p. 380).
-
•
NEPSY-A Developmental Neuropsychological Assessment; Visual Attention Subtest: The NEPSY is a neuropsychological test for children ages 3–12 yr, consisting of 27 subtests, which are divided into a core battery and a full battery. The visual attention subtest instructs children to scan an array of pictures and circle the “target” picture as quickly and accurately as possible. Speed and accuracy are measured. This subtest is part of the Attention/Executive Functions domain of the NEPSY.
-
•
Fagan Test of Infant Intelligence (FTII): The FTII is a test of visual recognition memory, utilizing a “novelty problem” paradigm (Fagan and Detterman, 1992; Benasich and Bejar, 1992). First, infants are presented with one picture or two identical pictures to study for a preset accumulated looking time. Then, the now-familiar picture is paired with a new or novel picture. A novelty preference score is computed for each test item by dividing the time spent looking at the novel picture during the test trial by the total amount of looking at both stimuli during that time. Looking times and form of novelty problems vary as a function of the infant's age. A mean novelty preference score is computed across the series of 10 problems by each age group. The mean and range of the FTII preference scores by test age (weeks) are as follows: 27 wk (59.0, 41.7–75.5), 29 wk (60.1, 44.2–74.4), 39 wk (58.3, 31.3–75.8), and 52 wk (59.9, 42.3–77.7) (Fagan, 2005).
-
•
McCarthy Scales of Children's Abilities (MSCA): The MSCA is composed of 18 subtests that yield a General Cognitive Index and scores for 5 scales: verbal, perceptual-performance, quantitative, memory, and motor (McCarthy, 1972). It is designed to be used in children 2.5 to 8.5 yr old to assess cognitive ability as well as gross and fine-motor skills. Studies suggest that the Wechsler Preschool and Primary Scale of Intelligence–Revised (WPPSI-R) yields IQs that are generally similar to those of the MSCA for children who score in the normal range of functioning (Sattler, 2001).
-
•
Wechsler Intelligence Scale for Children—4th Edition (WISC-IV). The WISC-IV was developed to assess intelligence in children age 6–16 yr. It contains 15 subtests that form four “Composites”: Verbal Comprehension (Similarities, Vocabulary, Comprehension, Information, Word Reasoning); Perceptual Reasoning (Block Design, Picture Concepts, Matrix Reasoning, Picture Completion); Working Memory (Digit Span, Letter-Number Sequencing, Arithmetic); and Processing Speed (Coding, Symbol Search, Cancellation) (Sattler, 2008b). The standard scores for the composite indexes and for Full Scale IQ are mean = 100, standard deviation = 15. Full Scale IQs vary based on several demographic factors, including race/ethnicity, parental education level, and geographic residence (Sattler, 2008b).
-
•
Wechsler Preschool and Primary Scale of Intelligence—3rd Edition (WPPSI-III). The WPPSI-III is an intelligence test developed for preschool and early primary school children, with different structures for two age groups: ages 30 mo to 3 yr 11 mo; and ages 4–7 yr. For ages 4–7 yr, the Full Scale consists of the following 7 core subtests: (a) Information, Vocabulary, and Word Reasoning (Verbal Composite); (b) Block Design, Matrix Reasoning, and Picture Concepts (Performance Composite); and (c) Coding (in neither Verbal nor Performance Composite). Five supplemental subtests are Similarities, Comprehension, Object Assembly, Picture Completion, and Symbol Search. Symbol Search may be combined with Coding to form the Processing Speed Composite (Levin et al., 2010). Like the WISC, the standard mean scores for the WPPSI Full Scale IQ, Verbal IQ, Performance IQ and Processing Speed Quotient are 100 (standard deviation = 15). Full Scale IQ scores vary by demographic variables, including gender, race/ethnicity, parental education level, and geographic region (Sattler, 2008a).
Epidemiology Literature Search
A comprehensive search of the published literature in MEDLINE was conducted. Our primary search combined broad exposure terms (i.e., “pesticide” OR “insecticide” OR “herbicide” OR “fumigant” OR “rodenticide” OR “fungicide”) with neurodevelopmental endpoints (i.e., “neuro” OR “neurotoxicity syndromes” OR “neurotoxic” OR “neurotoxins” OR “neurotoxicity” OR “neurologic” OR “neurological” OR “nervous system” OR “neurobehavior” OR “neurobehavioral” OR “behavior” OR “motor skills” OR “psychomotor” OR “cognitive” OR “cognition” OR “cognitive development” OR “impaired cognitive function” OR “motor development” OR “intelligence” OR “autism”) and population parameters (i.e., “parental” OR “parent” OR “neonate” OR “neonatal” OR “prenatal” OR “pregnancy” OR “pregnant” OR “fetus” OR “fetal” OR “maternal” OR “developmental” OR “child” OR “children” OR “teen” OR “adolescent” OR “utero”). To identify the studies that reported head circumference measures, an additional search was performed using the aforementioned exposure terms and population parameters in combination with fetal growth endpoints (i.e., “head circumference” OR “head size” OR “head” OR “birth weight” OR “birth length” OR “weight” OR “length” OR “fetal growth” OR “infant growth” OR “Ponderal Index” OR “small for gestational age” OR “ small-for-gestational-age” OR “small size”). Although the focus was on studies that reported head circumference, an exhaustive search of fetal growth parameters was conducted to confirm inclusion of all relevant literature on head circumference. To ensure the completeness of the search, additional searches using specific pesticide names or classes were performed (e.g., “organophosphate,” “organophosphorus,” “nematicide,” “carbamates,” “pyrethroid,” “pyrethrin,” “dithiocarbamate”). Reference lists in recent reviews and articles on this topic were cross-checked to identify any relevant papers that may have been missed by the electronic search.
RESULTS AND DISCUSSION OF EPIDEMIOLOGIC STUDIES
Table 1 summarizes the study characteristics of the epidemiologic studies reviewed. In total, our inclusion criteria were met by 46 publications from 16 epidemiologic studies or study populations that evaluated head circumference and/or neurobehavioral endpoints. Table 2 provides information about the exposure biomarkers used in each of the epidemiologic studies, including the detection level for each chemical or metabolite and the proportion of subjects with detectable values, when available.
TABLE 1.
Summary of epidemiologic study characteristics, in alphabetical order of Author, Year
| Author, Year | Study Name or Description (Location) | Outcome Assessment Tool(s)♦ | Chemical(s) or Metabolite(s)♦♦ | Timing of Biological Specimen Collection | Biological Specimen♦♦♦ | Other | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy in Mother | At Delivery | Post Delivery | MB | UCB | PT | BM | MU | CU | IB | Personal Air during Pregnancy | |||||
| Mother | Child | ||||||||||||||
| Barr et al., 2010 | New Jersey Study (New Jersey, USA) | HR | CPF | X | X | X | |||||||||
| Carbofuran | X | X | X | ||||||||||||
| Dacthal | X | X | X | ||||||||||||
| Dichloran | X | X | X | ||||||||||||
| Metalochlor | X | X | X | ||||||||||||
| Trifluralin | X | X | X | ||||||||||||
| Diethyltoluamide | X | X | X | ||||||||||||
| Berkowitz et al., 2004 | Mt. Sinai Children's Environmental Health Cohort Study (New York, USA) | HR | TCPy | X | X | ||||||||||
| PBA | X | X | |||||||||||||
| Bouchard et al., 2011 | The Center for Health Assessment of Mothers and Children of Salinas (CHAMACOS) Study (California, USA) | WISC-IV | DAPs | X | X | X | X | ||||||||
| DMPs | X | X | X | X | |||||||||||
| DEPs | X | X | X | X | |||||||||||
| Darvill et al., 2000 | Oswego Newborn and Infant Development Project Study (New York, USA) | FTII | DDE | X | X | ||||||||||
| Engel et al., 2007 | Mt. Sinai Children's Environmental Health Cohort Study (New York, USA) | BNBAS | DAPs | X | X | ||||||||||
| DMPs | X | X | |||||||||||||
| DEPs | X | X | |||||||||||||
| MDA | X | X | |||||||||||||
| DDE | X | X | |||||||||||||
| Engel et al., 2011 | Mt. Sinai Children's Environmental Health Cohort Study (New York, USA) | BSID-II | DAPs | X | X | ||||||||||
| WPPSI-III | DEPs | X | X | ||||||||||||
| WISC-IV | DMPs | X | X | ||||||||||||
| DDE | X | X | |||||||||||||
| Eskenazi et al., 2004 | CHAMACOS Study (California, USA) | HR | DAPs | X | X | ||||||||||
| DMPs | X | X | |||||||||||||
| DEPs | X | X | |||||||||||||
| MDA | X | X | |||||||||||||
| TCPy | X | X | |||||||||||||
| PNP | X | X | |||||||||||||
| Cholinesterase | X | X | X | X | |||||||||||
| Butyrylcholinesterase | X | X | X | X | |||||||||||
| Eskenazi et al., 2006 | CHAMACOS Study (California, USA) | BSID-II | DDT | X | X | ||||||||||
| DDE | X | X | |||||||||||||
| Eskenazi et al., 2007, 2010 | CHAMACOS Study (California, USA) | BSID-II | DAPs | X | X | X | X | ||||||||
| CBCL | DMPs | X | X | X | X | ||||||||||
| DEPS | X | X | X | X | |||||||||||
| MDA | X | X | |||||||||||||
| TCPy | X | X | |||||||||||||
| Fenster et al., 2007 | CHAMACOS Study (California, USA) | BNBAS | DDT | X | X | ||||||||||
| DDE | X | X | |||||||||||||
| Gladen et al., 1988 | The North Carolina Breast Milk and Formula Project Study (North Carolina, USA) | BSID-I | DDE | X | X | X | |||||||||
| Gladen & Rogan, 1991 | The North Carolina Breast Milk and Formula Project Study (North Carolina, USA) | MSCA Grades | DDE | X | X | X | |||||||||
| Horton et al., 2011 | CCCEH Study (New York, USA) | BSID-II | Permethrin piperonyl butoxide | X | X | X | X | X | |||||||
| X | |||||||||||||||
| Jusko et al., 2006 | Child Health and Development Study (California, USA) | HR | DDE | X | X | ||||||||||
| DDT | X | X | |||||||||||||
| Longnecker et al., 2001 | US Collaborative Perinatal Project (Maryland, USA) | HR | DDE | X | X | ||||||||||
| Lopez-Espinosa et al., 2007 | INfancia y Medio Ambiente (Environment and Childhood) Study, Granada cohort (Spain) | Not reported | DDT | X | X | ||||||||||
| DDE | X | X | |||||||||||||
| Endosulphan | X | X | |||||||||||||
| Lovasi et al., 2011 | CCCEH Study (New York, USA) | BSID-II | CPF | X | X | ||||||||||
| Marks et al., 2010 | CHAMACOS Study (California, USA) | CBCL | DAPs | X | X | X | X | ||||||||
| NEPSY-II | DMPs | X | X | X | X | ||||||||||
| K-CPT Hillside | DEPs | X | X | X | X | ||||||||||
| Morales et al., 2008 | Asthma Multicentre Infants Cohort Study—Menorca cohort (Spain) | MSCA | DDT | X | X | ||||||||||
| DDE | X | X | |||||||||||||
| Pan et al., 2009 | Pregnancy, Infection, and Nutrition Babies Study (North Carolina, USA) | Mullen MacArthur-Bates CDI | DDT | X | X | ||||||||||
| DDE | X | X | |||||||||||||
| Perera et al., 2003 | Columbia Center for Children's Environmental Health (CCCEH) Study (New York, USA) | HR | CPF | X | X | ||||||||||
| Puertas et al., 2010 | INfancia y Medio Ambiente (Environment and Childhood) Study, Granada cohort (Spain) | MSCA | Mirex | X | X | ||||||||||
| Rauh et al., 2006 | CCCEH Study (New York, USA) | BSID-II CBCL |
CPF | X | X | ||||||||||
| Rauh et al., 2011 | CCCEH Study (New York, USA) | WISC-IV | CPF | X | X | ||||||||||
| Ribas-Fito et al., 2003 | Flix Cohort Study (Spain) | BSID-II | DDE | X | X | ||||||||||
| Griffiths | HCB | X | X | ||||||||||||
| Ribas-Fito et al., 2006 | Ribera d'Ebre and Menorca Birth Cohorts (Spain) | MSCA | DDT | X | X | ||||||||||
| DDE | X | X | |||||||||||||
| Ribas-Fito et al., 2007 | Ribera d'Ebre and Menorca Birth Cohorts (Spain) | CPSCS ADHD DSM-IV MSCAa | HCB | X | X | ||||||||||
| Rogan et al., 1986 | The North Carolina Breast Milk and Formula Project Study (North Carolina, USA) | HR BNBAS | DDE | X | X | ||||||||||
| Rogan and Gladen, 1991 | The North Carolina Breast Milk and Formula Project Study (North Carolina, USA) | BSID-1 | DDE | X | X | X | |||||||||
| Sagiv et al., 2007 | Birth-cohort at PCB-Contaminated Superfund Site (Massachusetts, USA) | HR | DDE | X | X | ||||||||||
| HCB | X | X | |||||||||||||
| Sagiv et al., 2008 | Birth-cohort at PCB-Contaminated Superfund Site (Massachusetts, USA) | BNBAS | DDE | X | X | ||||||||||
| Sagiv et al., 2010 | Birth-cohort at PCB-Contaminated Superfund Site (Massachusetts, USA) | CRS-T | DDE | X | X | ||||||||||
| Stewart et al., 2000 | Oswego Newborn and Infant Development Project Study (New York, USA) | BNBAS | DDE | X | X | ||||||||||
| HCB | X | X | |||||||||||||
| Stewart et al., 2003 | Oswego Newborn and Infant Development Project Study (New York, USA) | CPT | DDE | X | X | ||||||||||
| HCB | X | X | |||||||||||||
| Stewart et al., 2005 | Oswego Newborn and Infant Development Project Study (New York, USA) | CPT | DDE | X | X | ||||||||||
| HCB | X | X | |||||||||||||
| Stewart et al., 2008 | Oswego Newborn and Infant Development Project Study (New York, USA) | WISC-III | DDE | X | X | ||||||||||
| HCB | X | X | |||||||||||||
| Mirex | X | X | |||||||||||||
| Sunyer et al., 2010 | Asthma Multicentre Infants Cohort Study—Menorca cohort (Spain) | MSCA | DDT | X | X | X | X | ||||||||
| HCB | X | X | X | X | |||||||||||
| Tan et al., 2009 | Singapore Cohort Study (Singapore) | Not reported | β-HCH | X | X | ||||||||||
| Chlordane | X | X | |||||||||||||
| DDE | X | X | |||||||||||||
| DDD | X | X | |||||||||||||
| DDT | X | X | |||||||||||||
| Torres-Sanchez et al., 2007, 2009 | State of Morelos, Mexico, Study (Mexico) | BSID-II | DDE | X | X | ||||||||||
| Whyatt et al., 2004, 2005 | CCCEH Study (New York, USA) | HR | CPF | X | X | X | |||||||||
| Diazinon | X | X | X | ||||||||||||
| Propoxur | X | ||||||||||||||
| 2-Isopropoxyphenol | X | X | |||||||||||||
| Wolff et al., 2007 | Mt. Sinai Children's Environmental Health Cohort Study (New York, USA) | HR | DDE | X | X | ||||||||||
| DAPs | X | X | |||||||||||||
| DMPs | X | X | |||||||||||||
| DEPs | X | X | |||||||||||||
| MDA | X | X | |||||||||||||
| Butyrylcholinesterase | X | X | |||||||||||||
| Young et al., 2005 | CHAMACOS Study (California, USA) | BNBAS | DAPs | X | X | ||||||||||
| DMPs | X | X | |||||||||||||
| DEPs | X | X | |||||||||||||
Outcome assessment tools: ADHD DSM-IV = Attention Deficit Hyperactivity Disorder Criteria of Diagnostic and Statistical Manual of Mental Disorders (4th edition); BNBAS = Brazelton Neonatal Behavioral Assessment Scale; BSID-I or BSID-II = Bayley Scales of Infant Development (1st or 2nd edition); CBCL = Child Behavior Checklist; CPSCS = California Preschool Social Competence Scale; CPT = Continuous Performance Test; CRS-T = Conners' Rating Scale for Teachers; FTII = Fagan Test of Infant Intelligence; Grades = Grades from report cards; Griffiths = Griffiths Mental Development Scales; Hillside = Hillside Behavior Rating Scale; HR = Hospital or patient record; K-CPT = Conners' Kiddie Continuous-Performance Test; MacArthur-Bates CDI = MacArthur-Bates Communicative Development Inventories; MSCA = McCarthy Scales of Children's Abilities; Mullen = Mullen Scales of Early Learning; NEPSY-II = A Developmental NEuroPSYchological Assessment (2nd edition); WISC-III or WISC-IV = Wechsler Intelligence Scale for Children (3rd or 4th edition); WPPSI-III = Wechsler Preschool and Primary Scale of Intelligence (3rd edition).
Chemicals/Metabolites: CPF = Chlorpyrifos; DAPs = Total dialkylphosphate metabolites, including DEPs and DMPs; DDD = Dichlorodiphenyldichloroethane; DDE = Dichlorodiphenyldichloroethylene; DDT = dichlorodiphenyltrichloroethane; DEPs = Diethylphosphate metabolites, including diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP); DMPs = Dimethylphosphate metabolites, including dimethylphosphate (DMP), dimethylthiophosphate (DMPT), and dimethyldithiophosphate (DMDPT); HCB = Hexachlorobenzene; β-HCH = Beta-hexachlorocyclohexane; MDA = Malathion dicarboxylic acid; PBA = 3-phenoxybenzoic acid; PBO = Piperonyl butoxide; PNP = 4-nitrophenol; TCPy = 3,5,6-trichloro-2-pyridinol (CPF, CPF-methyl).
Biological specimen: BM = Breast milk; CU = Child or infant urine; IB = Infant blood or serum; MB = Maternal blood or serum; MU = Maternal urine; PT = Placental tissue; UCB = Umbilical cord blood.
The study authors call it the McCarthy Scales for Infant Development.
TABLE 2.
Descriptive information for exposure biomarkers in the epidemiologic studies, by endpoint and reference year
| First Author, Year | Biological Specimen | Chemical/Metabolitea | Detection Level | % with Detectable Levels | Median | Mean or Geometric Mean |
|---|---|---|---|---|---|---|
| Newborn Head Circumference Endpoint | ||||||
| Rogan et al., 1986 | Transplacentalb | DDE | NR | NR | NR | NR |
| Longnecker et al., 2001 | Maternal blood | DDE | 0.61 μg/L | 100% | 25 μg/L | NR |
| Perera et al., 2003 | Umbilical cord blood | CPF | NR | 94% | NR | 7.5 pg/g |
| Berkowitz et al., 2004 | Maternal urinec | TCPy | 11.0 μg/L | 43% | 7.6 μg/L; 11.5 μg/g | NR |
| Maternal urinec | PBA | 15.0 μg/L | 57% | 20.0 μg/L; 19.8 μg/g | NR | |
| Eskenazi et al., 2004 | Maternal urined | DAP | 0.05–1.2 nmol/L | 99.8% | 136 nmol/L | NR |
| Maternal urined | ΣDMP | 0.08–1.2 nmol/L | 99.8% | 101 nmol/L | NR | |
| Maternal urine | ΣDEP | 0.05–0.8 nmol/Ld | 99.8% | 22 nmol/L | NR | |
| Maternal urined | MDA | 0.29 μg/L | 30.1% | 0.2 μg/L | NR | |
| Maternal urined | TCPy | 0.26 μg/L | 76.3% | 3.3 μg/L | NR | |
| Maternal urined | PNP | 0.14 μg/L | 54.4% | 0.5 μg/L | NR | |
| Umbilical cord blood | Cholinesterase | NA | NA | 3.8 μmol/min/mL | 3.8 μmol/min/mL | |
| Maternal blood (delivery) | Cholinesterase | NA | NA | 5.1 μmol/min/mL | 5.7 μmol/min/mL | |
| Maternal blood (pregnancy) | Cholinesterase | NA | NA | 5.7 μmol/min/mL | 5.2 μmol/min/mL | |
| Umbilical cord blood | Butyrylcholinesterase | NA | NA | 1.2 μmol/min/mL | e | |
| Maternal blood (delivery) | Butyrylcholinesterase | NA | NA | 1.4 μmol/min/mL | e | |
| Maternal blood (pregnancy) | Butyrylcholinesterase | NA | NA | 1.4 μmol/min/mL | e | |
| Whyatt et al., 2004 | Personal air during pregnancy | CPF | NR | NR | NR | 15.3 ng/m3 |
| Personal air during pregnancy | Diazinon | NR | NR | NR | 117.2 ng/m3 | |
| Personal air during pregnancy | Propoxur | NR | NR | NR | 53.6 ng/m3 | |
| Umbilical cord blood | CPF | NR | 69% | NR | 4.0 pg/g | |
| Umbilical cord blood | Diazinon | NR | 52% | NR | 1.1 pg/g | |
| Newborn Head Circumference Endpoint | ||||||
| Whyatt et al., 2004 | Umbilical cord blood | 2-Isopropoxyphenol | NR | 50% | NR | 3-1 pg/g |
| Whyatt et al., 2005 | Personal air during pregnancy | CPF | NR | 99.7% | NR | 14.3 ng/m3 |
| Personal air during pregnancy | Diazinon | NR | 100% | NR | 99.5 ng/m3 | |
| Personal air during pregnancy | Propoxur | NR | 100% | NR | 53.5 ng/m3 | |
| Umbilical cord blood | CPF | NR | 64% | NR | 3.7 pg/g | |
| Umbilical cord blood | Diazinon | NR | 44% | NR | 1.2 pg/g | |
| Umbilical cord blood | 2-Isopropoxyphenol | NR | 40% | NR | 3.0 pg/g | |
| Jusko et al., 2006 | Maternal blood | p,p′-DDE | NR | NR | 5.88 μg/g | 6.85 μg/g |
| Maternal blood | p,p′-DDT | NR | NR | 1.61 μg/g | 1.93 μg/g | |
| Maternal blood | o,p′-DDT | NR | NR | 0.20 μg/g | 0.27 μg/g | |
| Maternal blood | ΣDDT | NR | NR | 7.95 μg/g | 9.05 μg/g | |
| Rauh et al., 2006 | Umbilical cord blood | CPF | 0.5–1 pg/g | 65% | NR | NR |
| Lopez-Espinosa et al., 2007 | Placental tissue | p,p′-DDT | NR | 59% | 0.50 ng/g | 1.02 ng/g |
| Placental tissue | o,p′-DDT | NR | 58.94% | 0.50 ng/g | 0.60 ng/g | |
| Placental tissue | p,p′-DDE | NR | 96.03% | 1.78 ng/g | 2.37 ng/g | |
| Placental tissue | Endosulphan-I | NR | 58.95% | 0.28 ng/g | 0.67 ng/g | |
| Placental tissue | Endosulphan-II | NR | 24.50% | <LOD | 0.40 ng/g | |
| Placental tissue | Endosulphan-diol | NR | 76.86% | 4.46 ng/g | 5.11 ng/g | |
| Sagiv et al., 2007 | Umbilical cord blood | p,p′-DDE | 0.07 ng/g | 96% | 0.30 ng/g | NR |
| Umbilical cord blood | HCB | 0.02 ng/g | NR | 0.02 ng/g | NR | |
| Wolff et al., 2007 | Maternal bloodf | DDE | 0.07 μg/L; 10 ng/g lipid | 97.4%; 98.9% | 0.64 μg/L; 110 ng/g lipid | NR |
| Maternal urineg | DAP | h | 97.2% | 75.9 nm/L | NR | |
| Maternal urineg | ΣDMP | h | 92.6% | 42.2 nm/L | NR | |
| Maternal urineg | ΣDEP | h | 88.1% | 18.8 nm/L | NR | |
| Maternal urine | MDA | 0.3 μg/L | 20.5% | 0.3 μg/L | NR | |
| Maternal blood | Butyrylcholinesterase | NA | NA | NR | NR | |
| Tan et al., 2009 | Umbilical cord blood | β-HCH | NR | 54% | 3.38 ng/g | 85.4 ng/g |
| Tan et al., 2009 | Umbilical cord blood | trans-chlordane | NR | 80% | 4.44 ng/g | 4.83 ng/g |
| Umbilical cord blood | cis-chlordane | NR | 78% | 0.7 ng/g | 1.95 ng/g | |
| Umbilical cord blood | p,p′-DDE | NR | 100% | 201 ng/g | 402 ng/g | |
| Umbilical cord blood | p,p′-DDD | NR | 63% | 2.69 ng/g | 3.83 ng/g | |
| Umbilical cord blood | p,p′-DDT | NR | 88% | 21.9 ng/g | 34.5 ng/g | |
| Barr et al., 2010 | Maternal bloodi | CPF | 0.001 pg/mL | 1.4% | 0.0007 ng/g | 0.09 ng/g |
| Maternal bloodi | Carbofuran | 0.01 pg/mL | 6.5% | 0.007 ng/g | 0.61 ng/g | |
| Maternal bloodi | Dacthal | 0.01 pg/mL | 92.0% | 3.30 ng/g | 3.73 ng/g | |
| Maternal bloodi | Dichloran | 0.01 pg/mL | 34.8% | 0.007 ng/g | 1.39 ng/g | |
| Maternal bloodi | Metalochlor | 0.01 pg/mL | 5.1% | 0.007 ng/g | 0.09 ng/g | |
| Maternal bloodi | Trifluralin | 0.01 pg/mL | 31.2% | 0.00 ng/g | 0.75 ng/g | |
| Maternal bloodi | Diethyltoluamide | 0.01 pg/mL | 100% | 2.78 ng/g | 3.21 ng/g | |
| Umbilical cord bloodi | CPF | 0.001 pg/mL | 37.2% | 0.0007 ng/g | 0.55 ng/g | |
| Umbilical cord bloodi | Carbofuran | 0.01 pg/mL | 48.6% | 0.007 ng/g | 4.36 ng/g | |
| Umbilical cord bloodi | Dacthal | 0.01 pg/mL | 70.9% | 2.15 ng/g | 2.06 ng/g | |
| Umbilical cord bloodi | Dichloran | 0.01 pg/mL | 53.4% | 1.62 ng/g | 1.70 ng/g | |
| Umbilical cord bloodi | Metalochlor | 0.01 pg/mL | 43.2% | 0.007 ng/g | 0.93 ng/g | |
| Umbilical cord bloodi | Trifluralin | 0.01 pg/mL | 75.0% | 2.11 ng/g | 2.16 ng/g | |
| Umbilical cord bloodi | Diethyltoluamide | 0.01 pg/mL | 100% | 2.90 ng/g | 3.12 ng/g | |
| Neurobehavioral Endpoints | ||||||
| Rogan et al., 1986 | Transplacentalb | DDE | NR | NR | NR | NR |
| Gladen et al., 1988 | Transplacentalb | DDE | NR | NR | NR | NR |
| Breast milk | DDE | NR | NR | NR | NR | |
| Gladen et al., 1991 | Transplacentalb | DDE | NR | NR | NR | NR |
| Breast milk | DDE | NR | NR | NR | NR | |
| Rogan and Gladen, 1991 | Transplacentalb | DDE | NR | NR | NR | NR |
| Breast milk | DDE | NR | NR | NR | NR | |
| Darvill et al., 2000 | Umbilical cord blood | DDE | NR | NR | NR | NR |
| Stewart et al., 2000 | Umbilical cord blood | DDE | NR | NR | 0.10 ng/g | NR |
| Umbilical cord blood | HCB | NR | NR | 0.04 ng/g | NR | |
| Ribas-Fito et al., 2003 | Umbilical cord blood | p,p′-DDE | 0.09 ng/mL | NR | 0.85 ng/mL | NR |
| Umbilical cord blood | HCB | 0.03 ng/mL | NR | NR | NR | |
| Neurobehavioral Endpoints | ||||||
| Stewart et al., 2003 | Umbilical cord blood | DDE | NR | NR | NR | NR |
| Umbilical cord blood | HCB | NR | NR | NR | NR | |
| Stewart et al., 2005 | Umbilical cord blood | DDE | NR | NR | NR | NR |
| Umbilical cord blood | HCB | NR | NR | NR | NR | |
| Young et al., 2005 | Maternal urinejk | DAP | 0.05–1.2 nmol/L | NR | 132 nmol/L; 222 nmol/L | NR |
| Maternal urinejk | ΣDMP | 0.08–1.2 nmol/L | NR | 97 nmol/L; 160 nmol/L | NR | |
| Maternal urinejk | ΣDEP | 0.05–0.8 nmol/L | NR | 21 nmol/L; 27 nmol/L | NR | |
| Eskenazi et al., 2006 | Maternal blood | p,p′-DDT | 0.06–4.70 ng/g | 100% | NR | 22.0 ng/g |
| Maternal blood | o,p′-DDT | 0.04–0.69 ng/g | 95.8% | NR | 1.8 ng/g | |
| Maternal blood | DDE | 0.06–4.83 ng/g | 100% | NR | 1436.9 ng/g | |
| Rauh et al., 2006 | Umbilical cord blood | CPF | 0.5–1 pg/g | 65% | NR | NR |
| Ribas-Fito et al., 2006 | Umbilical cord bloodlm | p,p′-DDT | 0.02 ng/mL | 71.43%; 91.85% | 0.05 ng/mL; 0.08 ng/mL | NR |
| Umbilical cord bloodlm | p,p′-DDE | 0.02 ng/mL | 100%; 100% | 0.86 ng/mL; 1.03 ng/mL | NR | |
| Engel et al., 2007 | Maternal blood | DDE | NR | 98% | 0.6 μg/L | NR |
| Maternal urine | DAP | NR | 96.5% | 82.0 nm/L | NR | |
| Maternal urine | ΣDMP | 0.2–0.5 μg/L | 90.2% | 47.8 nm/L | NR | |
| Maternal urine | ΣDEP | 1–4 nm/L | 88.8% | 24.7 nm/L | NR | |
| Maternal urine | MDA | 0.3 μg/L | 21.6% | <0.3 μg/L | NR | |
| Eskenazi et al., 2007 | Maternal urined,k | DAP | 0.05–1.2 nmol/L | NR | NR | 114.9 nmol/L |
| Maternal urined,k | ΣDMP | 0.08–1.2 nmol/L | NR | NR | 81.5 nmol/L | |
| Maternal urined,k | ΣDEP | 0.05–0.8 nmol/L | NR | NR | 18.1 nmol/L | |
| Maternal urined,k | MDA | 0.29 μg/L | 39% | 0.82 μg/L | NR | |
| Maternal urined,k | TCPy | 0.26 μg/L | 91% | 3.54 μg/L | NR | |
| Child urinen | DAP | NR | NR | NR | 45.5 nmol/L; | |
| 59.5 nmol/L; | ||||||
| 70.9 nmol/L | ||||||
| Child urinen | ΣDMP | NR | NR | NR | 23.8 nmol/L; | |
| 32.9 nmol/L; | ||||||
| 48.6 nmol/L | ||||||
| Eskenazi et al., 2007 | Child urinen | ΣDEP | NR | NR | NR | 10.6 nmol/L; |
| 15.2 nmol/L; | ||||||
| 10.5 nmol/L | ||||||
| Fenster et al., 2007 | Maternal blood | p,p′-DDT | 0.06–4.70 ng/g | 100% | 14.1 ng/g | 23.2 ng/g |
| Maternal blood | o,p′-DDT | 0.04–6.05 ng/g | 95.4% | 1.4 ng/g | 1.8 ng/g | |
| Maternal blood | p,p′-DDE | 0.06–4.83 ng/g | 100% | 1103.7 ng/g | 1464.2 ng/g | |
| Ribas-Fito et al., 2007 | Umbilical cord bloodo | HCB | 0.02 ng/mL | 100% | 0.73 ng/mL; | NR |
| 1.13 ng/mL; | ||||||
| 0.68 ng/mL | ||||||
| Torres-Sanchez et al., 2007 | Maternal bloodp | p,p′-DDE | 0.05 ng/mL | 100% | NR | 6.4 ng/mL; |
| 6.8 ng/mL; | ||||||
| 7.8 ng/mL | ||||||
| Morales et al., 2008 | Umbilical cord blood | p,p′-DDT | 0.02 ng/mL | NR | NR | 0.17 ng/mL |
| Umbilical cord blood | p,p′-DDE | 0.02 ng/mL | NR | NR | 1.63 ng/mL | |
| Sagiv et al., 2008 | Umbilical cord blood | p,p′-DDE | 0.07 ng/g | 96% | 0.30 ng/g | 0.48 ng/g |
| Stewart et al., 2008 | Placental tissue | DDE | NR | NR | NR | NR |
| Placental tissue | HCB | NR | NR | NR | NR | |
| Placental tissue | Mirex | NR | NR | NR | NR | |
| Pan et al., 2009 | Breast milkq | p,p′-DDT | NR | 96% | 5 ng/g; 33 ng/g/mo | NR |
| Breast milkq | p,p′-DDE | NR | 100% | 121 ng/g; 871 ng/g/mo | NR | |
| Torres-Sanchez et al., 2009 | Maternal bloodp | p,p′-DDE | 0.05 ng/mL | 100% | NR | 6.3 ng/mL; |
| 6.5 ng/mL; | ||||||
| 7.9 ng/mL | ||||||
| Eskenazi et al., 2010 | Maternal urinek,r | DAP | 0.05–1.2 nmol/L | NR | NR | 110 nmol/L |
| Maternal urinek,r | ΣDMP | 0.08–1.2 nmol/L | NR | NR | 77 nmol/L | |
| Maternal urinek,r | ΣDEP | 0.05–0.8 nmol/L | NR | NR | 18 nmol/L | |
| Marks et al., 2010 | Maternal urined,k | DAP | 0.05–1.2 nmol/L | NR | NR | 109.0 nmol/L |
| Maternal urined,k | ΣDMP | 0.08–1.2 nmol/L | NR | NR | 76.8 nmol/L | |
| Maternal urined,k | ΣDEP | 0.05–0.8 nmol/L | NR | NR | 17.7 nmol/L | |
| Child urines | DAP | NR | NR | NR | 77.5 nmol/L; | |
| 92.6 nmol/L | ||||||
| Child urines | ΣDMP | NR | NR | NR | 62.5 nmol/L; | |
| Neurobehavioral Endpoints | ||||||
| Marks et al., 2010 | 72.4 nmol/L | |||||
| Child urines | ΣDEP | NR | NR | NR | 7.0 nmol/L; | |
| 7.2 nmol/L | ||||||
| Puertas et al., 2010 | Placental tissuet | Mirex | 0.5 ng/mL | NR | 1.4 ng/g placenta | NR |
| Sagiv et al., 2010 | Umbilical cord blood | p,p′-DDE | 0.07 ng/g | NR | 0.31 ng/g | NR |
| Sunyer et al., 2010 | Umbilical cord blood | DDT | NR | NR | NR | NR |
| Umbilical cord blood | HCB | NR | NR | NR | NR | |
| Infant blood | DDT | NR | NR | NR | NR | |
| Infant blood | HCB | NR | NR | NR | NR | |
| Bouchard et al., 2011 | Maternal urinek,r | DAP | 0.05–1.2 nmol/L | NR | 128 nmol/L | NR |
| Maternal urinek,r | ΣDMP | 0.08–1.2 nmol/L | NR | NR | NR | |
| Maternal urinek,r | ΣDEP | 0.05–0.8 nmol/L | NR | NR | NR | |
| Child urineu | DAP | NR | NR | NR | NR | |
| Child urineu | ΣDMP | NR | NR | NR | NR | |
| Child urineu | ΣDEP | NR | NR | NR | NR | |
| Engel et al., 2011 | Maternal urinev | DAP | NR | 97% | NR | NR |
| Maternal urinev | ΣDMP | NR | 90% | NR | NR | |
| Maternal urinev | ΣDEP | NR | 89% | NR | NR | |
| Maternal blood | DDE | NR | NR | NR | NR | |
| Horton et al., 2011 | Personal air sample | cis-permethrin | 0.18 ng/m3 | 18.7% | <0.18 ng/m3 | NR |
| Personal air sample | trans-permethrin | 0.36 ng/m3 | 16.5% | <0.36 ng/m3 | NR | |
| Personal air sample | PBO | 0.10 ng/m3 | 75.2% | 0.42 ng/m3 | NR | |
| Maternal blood | cis-permethrin | 1.0 pg/g | 12.9% | <1.0 pg/g | NR | |
| Maternal blood | trans-permethrin | 1.0 pg/g | 18.0% | <1.0 pg/g | NR | |
| Umbilical cord blood | cis-permethrin | 1.0 pg/g | 7.1% | <1.0 pg/g | NR | |
| Umbilical cord blood | trans-permethrin | 1.0 pg/g | 5.4% | <1.0 pg/g | NR | |
| Lovasi et al., 2011 | Umbilical cord blood | CPF | NR | NR | NR | NR |
| Rauh et al., 2011 | Umbilical cord bloodw | CPF | 0.5 pg/g (n = 93) and 1 pg/g (n = 22) | 57% | NR | 3.17 pg/g |
Abbreviations: CPF = Chlorpyrifos; DAPs = Total dialkylphosphate metabolites, including DEPs and DMPs; DDD = Dichlorodiphenyldichloroethane; DDE = Dichlorodiphenyldichloroethylene; DDT = dichlorodiphenyltrichloroethane; DEPs = Diethylphosphate metabolites, including diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP); DMPs = Dimethylphosphate metabolites, including dimethylphosphate (DMP), dimethylthiophosphate (DMPT), and dimethyldithiophosphate (DMDPT); HCB = hexachlorobenzene; β-HCH = Beta-hexachlorocyclohexane; LOD = Limit of detection MDA = Malathion dicarboxylic acid; n = Sample size; NA = Not applicable; NR = Not reported; PBA cisgender 3-phenoxybenzoic acid; PBO = Piperonyl butoxide; PNP = 4-nitrophenol; TCPy = 3,5,6-trichloro-2-pyridinol (CPF, CPF-methyl).
Chemical/metabolite had to be evaluated with respect to an outcome of interest to be included in table.
“Transplacental” exposure was measured at time of birth but it represents exposure during pregnancy.
Median value not adjusted for creatinine; median value adjusted for creatinine.
Average of two pregnancy measurements not adjusted for creatinine.
Mean level similar to that for cholinesterase.
Blood measures include those without lipids; blood measures for those with lipids available.
Total number includes samples with creatinine = 20.
Based on individual analyte value equal to or greater than LOD for any of the three metabolites in each class (1–4 nm/L, 0.2–0.5 μg/L).
Detection levels from Yan X, et al. Pesticide Concentrations in Matrices Collected in the Perinatal Period in a Population of Pregnant Women and Newborns in New Jersey, USA. Human and Ecological Risk Assessment. 2009; 15: 948–967.
Average of two pregnancy measures not adjusted for creatinine; post-delivery measurement not adjusted for creatinine.
Detection levels from Eskenazi, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Persp, 2004; 112: 1116–24.
Ribera d'Ebre cohort; Menorca cohort.
Quantifiable concentrations of DDT were detected in almost 90% of children in both cohorts. All children had quantifiable concentrations of DDE at birth. (p. 957)
6 months; 1 2 months; 24 months urinary measures not adjusted for creatinine.
Total cohort; Ribera d'Ebre cohort; Menorca cohort.
1st trimester; 2nd trimester; 3rd trimester serum levels.
Chemical concentrations in breast milk; lactational exposure metric values.
Maternal urine samples were collected twice during pregnancy and averaged.
Child's urine specimens were collected at 3.5 year visit; 5-year visit.
Median level in 27 samples that had a mirex concentration = 1 ng/mL.
Child urine specimens were collected at 6 months, 1, 2, 3.5, and 5 years.
Approximate percentage with detectable limits from entire cohort and not the cohort for analyses.
Two limits of detection were provided in participants with non-detectable CPF.
The majority of epidemiologic studies focused on evaluating OP or OC insecticides. There was substantial variability in the design (e.g., timing of exposure and health assessment, biological specimen used to measure the pesticide or metabolite) of studies that measured the same chemical or metabolite(s), limiting the ability to make direct comparisons across populations. In addition, some chemicals were measured in only one or two studies, which also limited our ability to assess consistency across multiple populations (Table 2). The nature of many of the epidemiology publications was to evaluate a health outcome, such as head circumference in newborns or behavior in children, and analyze many pesticides and other risk factors at once.
The results of these analyses and publications are organized by health outcome in Tables 3–8 with observations listed separately for each pesticide and class. Included are the adjusting variables for each analytic comparison, which may include demographics variables and exposures such as tobacco, ethanol and lead. Table 9 summarizes the results for all health outcomes and pesticides using PON1 and glutathione S-transferase. Statistically significant results are in bold blue font. The number of analyses by metabolite and pesticides with health outcomes was large. The effect estimates reported included regression coefficients from linear regression, odds ratios from logistic regression and mean differences. The tables are large and admittedly complex. However, in presenting all analytical results, a better view the scope of the data is provided. Condensed or summary tables for specific pesticides or outcomes were not presented for this reason. Some studies made additional results available in supplemental online tables (Bouchard et al., 2011; Engel et al., 2011). These data are not shown in the epidemiology tables.
TABLE 3.
Summary of results from epidemiologic studies of pesticide exposure and newborn head circumference, by chemical or metabolite
| Author, Year (Study) | Results and Sample Size for Each Analysis Statistically significant (P <.05) results in blue | Adjusting Variables |
|---|---|---|
| OP Insecticides Metabolites: DAP/DMP/DEP | ||
| Eskenazi et al., 2004 (CHAMACOS) |
Maternal log10DAP (n = 485): β = 0.32 cm (95% CI = 0.03, 0.62; P = 0.03) Maternal log10DMP (n = 485): β = 0.25 cm (95% CI = −0.02, 0.52; P = 0.07) Maternal log10DEP (n = 486): β = 0.28 cm (95% CI = −0.02, 0.59; P = 0.07) |
All analyses were adjusted for timing of urine collection, timing of entry into prenatal care, maternal age, parity, infant sex, country of birth, weight gain, body mass index, poverty level, gestational age, and (gestational age)2 |
| Wolff et al., 2007 (Mt. Sinai) |
Maternal Log10DAP (nmol/L): No creatinine adjustment (n = 318): β = −0.26 cm (SE = 0.13; P = 0.045) Creatinine adjustment (n = 318): β = −0.25 cm (SE = 0.13; P = 0.056) Maternal Log10DMP (nmol/L): No creatinine adjustment (n = 327): β = −0.16 cm (SE = 0.11; P = 0.14) Creatinine adjustment (n = 327): β = −0.15 cm (SE = 0.11; P = 0.16) Maternal Log10DEP (nmol/L): No creatinine adjustment (n = 318): β = −0.067 cm (SE = 0.12; P = 0.57) Creatinine adjustment (n = 318): β = −0.052 cm (SE = 0.12; P = 0.67) |
All analyses were adjusted for maternal age, race/ethnicity, maternal body mass index∗∗pregnancy weight gain, infant sex, and gestational age |
| OP Insecticides Metabolites: TCPy | ||
| Berkowitz et al., 2004 (Mt. Sinai) | Maternal TCPy < 11.0 μg/L (n = 216): mean = 33.8 cm (SD = 1.7) Maternal TCPy > 11.0 μg/L (n = 171): mean = 33.8 cm (SD = 1.7; P > 0.05) |
All analyses were adjusted for race/ethnicity, infant sex, and gestational age |
| Eskenazi et al., 2004 (CHAMACOS) | Maternal TCPy No detectable levels (n = 41): Referent Maternal TCPy Detectable levels < 3.3 μg/L (n = 220): β = 0.06 cm (95% CI = −0.37, 0.49; P = 0.78) Maternal TCPy Detectable levels ≥ 3.3 μg/L (n = 221): β = 0.04 cm (95% CI = −0.39, 0.47; P = 0.85) |
All analyses were adjusted for timing of urine collection, timing of entry into prenatal care, maternal age, parity, infant sex, country of birth, weight gain, body mass index, poverty level, gestational age, and (gestational age)2 |
| Organophosphate (OP) Insecticides: Chlorpyrifos (CPF) | ||
| Perera et al., 2003 (CCCEH) |
Both CPF and head circumference were log-transformed. All Participants (n = 263∗): CPF: β = −0.005 cm (P = 0.28) African American Participants (n = 116∗) CPF: β = −0.003 cm (P = 0.70) Dominican Participants (n = 146∗) CPF: β = −0.005 cm (P = 0.31) |
All analyses were adjusted for body mass index, parity, cotinine, sex of baby, gestational age, and polycyclic aromatic hydrocarbons |
| Whyatt et al., 2004 (CCCEH) |
Maternal personal air samples: log10CPF (n = 271∗): β = −0.04 cm (95% CI = −0.18, 0.10; P = 0.59) log10Suma of CPF & Diazinon (269∗): β = −0.03 cm (95% CI = −0.17, 0.11; P = 0.71) Umbilical cord plasma samples: log10CPF (n = 287∗): β = −0.01 cm (95% CI = −0.13, 0.11; P = 0.86) log10Suma of CPF & Diazinon (n = 271∗): β = −0.02 cm (95% CI = −0.15, 0.11; P = 0.73) |
All analyses were adjusted for gestational age of newborn, maternal pre-pregnancy weight, maternal net weight gain during pregnancy, newborn gender, parity, race/ethnicity, environmental tobacco smoke in the home, season of delivery, and cesarean section delivery |
| Whyatt et al., 2005 (CCCEH) | No associations were observed between head circumference and CPF or sum of CPF and diazinon in either maternal personal air or cord blood samples (per text; data not shown). | All analyses were adjusted for gestational age of newborn, maternal pre-pregnancy weight, maternal weight gain during pregnancy, newborn gender, parity, race/ethnicity, environmental tobacco smoke in the home, season of delivery, and cesarean section delivery |
| Rauh et al., 2006 (CCCEH) | log10CPF >6.17 pg/g (n = 50): mean = 34.03 cm (SD = 1.69) log10CPF ≤ 6.17 pg/g (n = 204): mean = 34.35 cm (SD = 1.84; P > 0.05) |
None |
| Barr et al., 2010 (New Jersey Study) |
Maternal serum samples (n = 148∗): CPF > 0.0007 ng/g: mean = 33.4 cm (SD = 0.6) CPF ≤ 0.0007 ng/g: mean = 35.0 cm (SD = 1.3; P = 0.229) Umbilical cord serum samples (n = 148∗): CPF > 1.32 ng/g: mean = 34.9 cm (SD = 1.4) CPF ≤ 1.32 ng/g: mean = 35.0 cm (SD = 1.2; P = 0.989) |
All analyses were adjusted for maternal age, primigravida, race, prepregnancy body-mass index, infant sex, and gestational age |
| OP Insecticides: Diazinon | ||
| Whyatt et al., 2004 (CCCEH) |
Maternal personal air samples (n = 269∗): log10Diazinon: β = −0.03 cm (95% CI = −0.14, 0.09; P = 0.67) Umbilical cord plasma samples (n = 302∗): log10Diazinon: β = −0.07 cm (95% CI = −0.30, 0.16; P = 0.53) |
All analyses were adjusted for gestational age of newborn, maternal pre-pregnancy weight, maternal net weight gain during pregnancy, newborn gender, parity, race/ethnicity, environmental tobacco smoke in the home, season of delivery, and cesarean section delivery |
| Whyatt et al., 2005 (CCCEH) | There was no association observed between head circumference and diazinon levels in either maternal personal air or cord blood samples (per text; data not shown). | All analyses were adjusted for gestational age of newborn, maternal pre-pregnancy weight, maternal weight gain during pregnancy, newborn gender, parity, race/ethnicity, environmental tobacco smoke in the home, season of delivery, and cesarean section delivery |
| OP Insecticides Metabolites: Malathion dicarboxylic acid (MDA) | ||
| Eskenazi et al., 2004 (CHAMACOS) | Maternal MDA No detectable levels (n = 233): Referent Maternal MDA Detectable levels < 0.2 μg/L (n = 74): β = −0.16 cm (95% CI = −0.52, 0.19; P = 0.37) Maternal MDA Detectable levels ≥ 0.2 μg/L (n = 75): β = 0.11 cm (95% CI = −0.24, 0.46; P = 0.53) |
All analyses were adjusted for timing of urine collection, timing of entry into prenatal care, maternal age, parity, infant sex, country of birth, weight gain, body mass index, poverty level, gestational age, and (gestational age)2 |
| Wolff et al., 2007 (Mt. Sinai) | Maternal MDA < vs. > 0.3 μg/L, no creatinine adjustment (n = 330): β = 0.15 cm (SE = 0.19; P = 0.44) Maternal MDA < vs. ≥ 0.3 μg/L, creatinine adjusted (n = 330): β = 0.23 cm (SE = 0.20; P = 0.25) |
All analyses were adjusted for maternal age, race/ethnicity, maternal body mass index∗∗pregnancy weight gain, infant sex, and gestational age |
| OP Insecticides Metabolites: 4-nitrophenol (PNP) (parathion and methyl parathion) | ||
| Eskenazi et al., 2004 (CHAMACOS) | Maternal PNP No detectable levels (n = 124): Referent Maternal PNP Detectable levels < 0.5 μg/L (n = 179): β = 0.18 cm (95% CI = −0.12, 0.48; P = 0.23) Maternal PNP Detectable levels > 0.5 μg/L (n = 179): β = 0.29 cm (95% CI = −0.01, 0.58; P = 0.06) |
All analyses were adjusted for timing of urine collection, timing of entry into prenatal care, maternal age, parity, infant sex, country of birth, weight gain, body mass index, poverty level, gestational age, and (gestational age)2 |
| Carbamates: Carbofuran | ||
| Barr et al., 2010 (New Jersey Study) |
Maternal serum samples (n = 148∗): Carbofuran > 0.007 ng/g: mean = 34.9 cm (SD = 1.5) Carbofuran ≤ 0.007 ng/g: mean = NR (P = NR) Umbilical cord serum samples (n = 148∗): Carbofuran > 8.69 ng/g: mean = 35.4 cm (SD = 1.4) Carbofuran ≤ 8.69 ng/g: mean = 34.8 cm (SD = 1.4; P = 0.099) |
All analyses were adjusted for maternal age, primigravida, race, prepregnancy body-mass index, infant sex, and gestational age |
| Carbamates: Propoxur (Isopropoxyphenol) | ||
| Whyatt et al., 2004 (CCCEH) | “No association was seen between infant head circumference and levels of propoxur or its metabolite in maternal personal air and cord blood samples (data not shown).” (p. 1130) | All analyses were adjusted for gestational age of newborn, maternal pre-pregnancy weight, maternal net weight gain during pregnancy, newborn gender, parity, race/ethnicity, environmental tobacco smoke in the home, season of delivery, and cesarean section delivery |
| Whyatt et al., 2005 (CCCEH) | There was no association observed between head circumference and propoxur (2-isopropoxyphenol) levels in either maternal personal air or cord blood samples (per text; data not shown). | All analyses were adjusted for gestational age of newborn, maternal pre-pregnancy weight, maternal weight gain during pregnancy, newborn gender, parity, race/ethnicity, environmental tobacco smoke in the home, season of delivery, and cesarean section delivery |
| Pyrethroid Insecticides: 3-Phenoxybenzoic Acid (PBA) | ||
| Berkowitz et al., 2004 (Mt. Sinai) | Maternal PBA < 15.0 μg/L (n = 142): mean = 33.7 cm (SD = 1.7) Maternal PBA > 15.0 μg/L (n = 185): mean = 33.9 cm (SD = 1.6; P > 0.05) |
All analyses were adjusted for race/ethnicity, infant sex, and gestational age |
| Organochlorine (OC) Pesticides: DDT/DDE/DDD | ||
| Rogan et al., 1986 (North Carolina Breast Milk and Formula Project Study) | There was no association observed between head circumference and transplacentalb DDE levels (per text; data not shown). | All analyses were adjusted for infant's race, infant's sex, mother's age, education, and occupation, indicators for maternal smoking, drinking and previous pregnancies, maternal weight, enrollment center, and birth weight |
| Longnecker et al., 2001 (US Collaborative Perinatal Project) | There was no association observed between head circumference and DDE levels (per text; data not shown). | All analyses were adjusted for study centre, sex, smoking habit, maternal height, maternal body mass index before pregnancy, maternal pregnancy weight gain, maternal age, socioeconomic index, parity, total cholesterol, triglycerides, index of prenatal care, and birthweight |
| Jusko et al., 2006 (Child Health and Development Study) | Mean differences comparing the 75th to 25th percentiles: p,p′-DDE 8.56 to 3.90 μg/g (n = 369): mean difference = 1 mm (95% CI = −1 to 2) p,p′-DDT 2.30 to 1.11 μg/g (n = 369): mean difference = 1 mm (95% CI = −1 to 3) o,p′-DDT 0.35 to 0.12 μg/g (n = 369): mean difference = 0 mm (95% CI = −1 to 2) ΣDDT (sum DDT) 11.15 to 5.68 μg/g (n = 369): mean difference = 1 mm (95% CI = −1 to 3) |
All analyses were adjusted for maternal body mass index, maternal height, parity, maternal race, maternal alcohol intake, father's education, and father's occupation |
| Lopez-Espinosa et al., 2007 (Environment and Childhood Study) |
p,p′-DDT (n = 150∗): ρ = 0.103 (P = 0.507) o,p′-DDT (n = 150∗); ρ = 0.306 (P = 0.044) p,p′-DDE (n = 150∗): ρ = 0.249 (P = 0.104) |
Covariates were not clearly specified for the multivariate analyses |
| Sagiv et al., 2007 (Birth-cohort at superfund site) |
p,p′-DDE Quartile 1 = 0 to 0.20 ng/g (n = 180): Referent = 0 cm p,p′-DDE Quartile 2 = 0.20 to 0.30 ng/g (n = 179): mean difference vs. quartile 1: −0.03 cm (95% CI = −0.32, 0.27) p,p′-DDE Quartile 3 = 0.30 to 0.46 (n = 180): mean difference vs. quartile 1: −0.10 cm (95% CI = −0.41, 0.20) p,p′-DDE Quartile 4 = 0.47 to 14.93 (n = 179): mean difference vs. quartile 1: −0.13 cm (95% CI = −0.47, 0.20) |
All analyses were adjusted for gestational age, infant gender, birth year, maternal age, maternal race, parity, maternal height, maternal prepregnancy body mass index, average smoking during pregnancy, and local fish consumption |
| Wolff et al., 2007 (Mt. Sinai) |
log10DDE (no lipid adjustment) (n = 178): β = −0.54 cm (SE = 0.25; P = 0.030) log10DDE (subset with lipids) (n = 160): β = −0.69 cm (SE = 0.28; P = 0.016) log10DDE (lipid adjusted) (n = 160): β = −0.69 cm (SE = 0.28; P = 0.016) |
All analyses were adjusted for maternal age, race/ethnicity, maternal body mass index∗∗pregnancy weight gain, infant sex, and gestational age |
| Tan et al., 2009 (Singapore cohort) | Data presented in a figure, PLSR coefficients (n = 41 ∗): p,p′-DDE: PLSR coefficient > 0 units not specified (95% CI includes the null value of 0) [Model 1] p,p′-DDD: PLSR coefficient > 0 units not specified (95% CI excludes the null value of 0) [Model 2] p,p′-DDT: PLSR coefficient > 0 units not specified (95% CI excludes the null value of 0) [Model 3] |
[Model 1]: β-HCH, trans-chlordane, cis-chlordane, p,p′-DDD, p,p′-DDT, PCB 28 & 31, PCB 118, PCB 132 & 153, age, height of mother, pre-pregnancy weight, post-pregnancy weight, pre-pregnancy body mass index, post-pregnancy body mass index, gestational age, parity, birth weight of infant, length of infant, ponderal index, previous breast-feeding, vegetarian, Indian, alcohol consumed by mother, and tobacco consumed by mother [Model 2]: Same covariates as in [Model 1] minus p,p′-DDD and plus p,p′-DDE [Model 3]: Same covariates as in [model 1] minus p,p′-DDT and plus p,p′-DDE |
| OC Pesticides: Hexachlorobenzene (HCB) | ||
| Sagiv et al., 2007 (Birth cohort at superfund site) | HCB Quartile 1 = 0 to 0.015 ng/g (n = 180): Referent = 0 cm HCB Quartile 2 = 0.015 to 0.023 ng/g (n = 180): mean difference vs. quartile 1: 0.66 cm (95% CI = 0.36, 0.95) HCB Quartile 3 = 0.023 to 0.032 ng/g (n = 179): mean difference vs. quartile 1: 0.22 cm (95% CI = −0.07, 0.51) HCB Quartile 4 = 0.032 to 0.658 (n = 179): mean difference vs. quartile 1: 0.30 cm (95% CI = −0.01, 0.61) |
All analyses were adjusted for gestational age, infant gender, birth year, maternal age, maternal race, parity, maternal height, maternal prepregnancy body mass index, average smoking during pregnancy, and local fish consumption |
| OC Pesticides: Beta-Hexachlorocyclohexane (β-HCH) | ||
| Tan et al., 2009 (Singapore cohort) | Data presented in a figure, PLSR coefficients (n = 41∗): β-HCH: PLSR coefficient > 0 units not specified (95% CI excludes the null value of 0) |
The analysis was adjusted for trans-chlordane, cis-chlordane, p,p′-DDE, p,p′-DDD, p,p′-DDT, PCB 28 & 31, PCB 118, PCB 132 & 153, age, height of mother, pre-pregnancy weight, post-pregnancy weight, pre-pregnancy body mass index, post-pregnancy body mass index, gestational age, parity, birth weight of infant, length of infant, ponderal index, previous breast-feeding, vegetarian, Indian, alcohol consumed by mother, and tobacco consumed by mother |
| OC Pesticides: Chlordane | ||
| Tan et al., 2009 (Singapore cohort) | Data presented in a figure: trans-chlordane (n = 41 ∗): PLSR coefficient < 0 units not specified (95% CI includes the null value of 0) [Model 1] cis-chlordane (n = 41 ∗): PLSR coefficient < 0 units not specified (95% CI includes the null value of 0) [Model 2] |
[Model 1]: β-HCH, cis-chlordane, p,p′-DDE, p,p′-DDT, p,p′-DDT, PCB 28 & 31, PCB 118, PCB 132 & 153, age, height of mother, pre-pregnancy weight, post-pregnancy weight, prepregnancy body mass index, post-pregnancy body mass index, gestational age, parity, birth weight of infant, length of infant, ponderal index, previous breast-feeding, vegetarian, Indian, alcohol consumed by mother, and tobacco consumed by mother [Model 2]: Same covariates as in [Model 1] minus cis-chlordane and plus trans-chlordane |
| OC Pesticides: Endosulphan (E) | ||
| Lopez-Espinosa et al., 2007 (Environment and Childhood Study) | E-I (n = 150∗): ρ = 0.009 (P = 0.953) E-II (n = 150∗): ρ = 0.256 (P = 0.093) E-diol (n = 150∗): ρ = 0.069 (P = 0.655) |
None |
| Other Pesticides: Dacthal | ||
| Barr et al., 2010 (New Jersey cohort) |
Maternal serum samples (n = 148∗): Dacthal > 4.01 ng/g: mean = 34.5 cm (SD = 1.9) Dacthal ≤ 4.01 ng/g: mean = 35.1 cm (SD = 1.5; P = 0.121) Umbilical cord serum samples (n = 148∗): Dacthal > 2.68 ng/g: mean = 34.7 cm (SD = 1.2) Dacthal ≤ 2.68 ng/g: mean = 35.0 cm (SD = 1.5; P = 0.963) |
All analyses were adjusted for maternal age, primigravida, race, prepregnancy body-mass index, infant sex, and gestational age |
| Other Pesticides: Dichloran | ||
| Barr et al., 2010 (New Jersey cohort) |
Maternal serum samples (n = 148∗): Dichloran > 3.22 ng/g: mean = 34.9 cm (SD = 1.5) Dichloran ≤ 3.22 ng/g: mean = 35.0 cm (SD = 1.5; P = 0.904) Umbilical cord serum samples (n = 148∗): Dichloran > 3.05 ng/g: mean = 35.0 cm (SD = 1.5) Dichloran ≤ 3.05 ng/g: mean = 35.0 cm (SD = 1.5; P = 0.417) |
All analyses were adjusted for maternal age, primigravida, race, prepregnancy body-mass index, infant sex, and gestational age |
| Other Pesticides: Metalochlor | ||
| Barr et al., 2010 (New Jersey cohort) |
Maternal serum samples (n = 148∗): Metalochlor > 0.007 ng/g: mean = 34.9 cm (SD = 1.5) Metalochlor ≤ 0.007 ng/g: mean = NR (P = NR) Umbilical cord serum samples (n = 148∗): Metalochlor > 1.99 ng/g: mean = 34.8 cm (SD = 1.2) Metalochlor ≤ 1.99 ng/g: mean = 35.0 cm (SD = 1.2; P = 0.518) |
All analyses were adjusted for maternal age, primigravida, race, pre pregnancy body-mass index, infant sex, and gestational age |
| Other Pesticides: Trifluralin | ||
| Barr et al., 2010 (New Jersey cohort) |
Maternal serum samples (n = 148∗): Trifluralin > 1.10 ng/g: mean = 35.3 cm (SD = 1.0) Trifluralin ≤ 1.10 ng/g: mean = 34.8 cm (SD = 1.3; P = 0.114) Umbilical cord serum samples (n = 148∗): Trifluralin > 2.90 ng/g: mean = 35.3 cm (SD = 1.1) Trifluralin ≤ 2.90 ng/g: mean = 34.8 cm (SD = 1.3; P = 0.101) |
All analyses were adjusted for maternal age, primigravida, race, prepregnancy body-mass index, infant sex, and gestational age |
| Other Pesticides: Diethyltoluamide | ||
| Barr et al., 2010 (New Jersey cohort) |
Maternal serum samples (n = 148∗): Diethyltoluamide > 3.23 ng/g: mean = 35.0 cm (SD = 1.4) Diethyltoluamide ≤ 3.23 ng/g: mean = 34.9 cm (SD = 1.5; P = 0.926) Umbilical cord serum samples (n = 148∗): Diethyltoluamide > 3.25 ng/g: mean = 35.2 cm (SD = 1.3) Diethyltoluamide ≤ 3.25 ng/g: mean = 34.9 cm (SD = 1.5; P = 0.247) |
All analyses were adjusted for maternal age, primigravida, race, prepregnancy body-mass index, infant sex, and gestational age |
| Other Measures: Cholinesterase | ||
| Eskenazi et al., 2004 (CHAMACOS) |
Maternal blood, pregnancy (n = 340): Cholinesterase: β = 0.06 cm (95% CI = −0.09, 0.21; P = 0.45) Butyrylcholinesterase: β = 0.12 cm (95% CI = −0.31, 0.56; P = 0.58) Maternal blood, delivery (n = 357): Cholinesterase: β = −0.07 cm (95% CI = −0.19, 0.05; P = 0.27) Butyrylcholinesterase: β = −0.07 cm (95% CI = −0.45, 0.31; P = 0.73) Umbilical cord blood (n = 292): Cholinesterase: β = −0.04 cm (95% CI = −0.23, 0.14; P = 0.65) Butyrylcholinesterase: β = −0.03 cm (95% CI = −0.50, 0.45; P = 0.91) |
All analyses were adjusted for timing of urine collection, timing of entry into prenatal care, maternal age, parity, infant sex, country of birth, weight gain, body mass index, poverty level, gestational age, and (gestational age)2 |
| Wolff et al., 2007 (Mt. Sinai study) | Butyrylcholinesterase (n = 373∗): β = 0.44 cm (SE = 1.27; P = 0.728) | The analysis was adjusted for sex, race, and gestational age |
Abbreviations: β = Beta coefficient; CI = Confidence interval; cm = Centimeters; DAP = Total dialkylphosphate metabolites, including DEP and DMP; DDD = Dichlorodiphenyldichloroethane; DDE = Dichlorodiphenyldichloroethylene; DDT = dichlorodiphenyltrichloroethane; DEP = Diethylphosphate metabolites, including diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP); DMP = Dimethylphosphate metabolites, including dimethylphosphate (DMP), dimethylthiophosphate (DMPT), and dimethyldithiophosphate (DMDPT); HCB = hexachlorobenzene; mm = millimeters; NR = Not reported; ? = Correlation coefficient; P = P-value; PCB = Polychlorinated biphenyl; PLSR = Partial least squares regression; SD = Standard deviation; SE = Standard error; TCPy = 3,5,6-trichloro-2-pyridinol (CPF, CPF-methyl).
Sample size for each analysis was not provided by the study authors and is based on available information.
Multiplication of two covariates.
Sum of CPF and diazinon is expressed in CPF equivalents, adjusted for relative potency.
“Transplacental” exposure was measured at time of birth but it represents exposure during pregnancy.
TABLE 8.
Summary of results from epidemiologic studies of pesticide exposure and behavioral assessments (ages 2 years and older), by pesticide or metabolite

TABLE 9A.
Summary of results from epidemiologic studies of potential interactions of pesticide exposure measures with metabolizing activity (PON1) and newborn head circumference, by chemical or metabolite
| Author, Year (Study) | Results and Sample Size for Each Analysis Statistically significant (P <.05) results in blue | Adjusting Variables |
|---|---|---|
| OP Insecticides Metabolites: DAP/DMP/DEP | ||
| Wolff et al., 2007 (Mt. Sinai study) | “Exposure did not modify the effect of PON1 [activity] or PON192 [genotype] to reduce head circumference … (data not shown).” (p. 248) | Covariates were not clearly specified for analyses |
| Organophosphate (OP) Insecticides Metabolites: TCPy | ||
| Berkowitz et al., 2004 (Mt. Sinai study) |
Head circumference (cm): TCPy < 11.0 μg/L Low PON1 (n = 76): β = 33.6 (SD = 1.8) Medium PON1 (n = 62): β = 33.7 (SD = 1.7) High PON1 (n = 70): β = 34.1 (SD = 1.7) TCPy > 11.0 μg/L Low PON1 (n = 47): β = 33.3 (SD = 1.5; P = 0.014) Medium PON1 (n = 57): β = 34.0 (SD = 1.5) High PON1 (n = 55): β = 34.1 (SD = 1.6) |
All analyses were adjusted for race/ethnicity, infant sex, and gestational age |
Abbreviations: β = Beta coefficient; DAP = Total dialkylphosphate metabolites, including DEP and DMP; DEP = Diethylphosphate metabolites, including diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP); DMP = Dimethylphosphate metabolites, including dimethylphosphate (DMP), dimethylthiophosphate (DMPT), and dimethyldithiophosphate (DMDPT); PON1 = Paraoxonase 1 enzyme; PON192 = Genetic polymorphism; SD = Standard deviation; TCPy = 3,5,6-trichloro-2-pyridinol (CPF, CPF-methyl).
Head Circumference
Newborn head circumference is a fetal growth endpoint of interest because smaller head circumference was suggested as a possible marker for future adverse neurobehavioral outcomes (e.g., intelligence test scores) and school performance (Perera et al., 2003; Tan et al., 2009). However, larger head circumference has also been associated with adverse outcomes, including childhood brain cancer (Samuelsen et al., 2006). Table 3 summarizes the reported associations between potential pesticide exposures and newborn head circumference. Most of the epidemiologic studies of potential in utero exposure to pesticides and newborn head circumference evaluated OP insecticides (particularly CPF) or OC pesticides (particularly DDT or DDT-related exposures).
Organophosphate (OP) Insecticides The metabolite DAP represents a possible breakdown product of up to 27 OP pesticides. Levels in maternal urine were evaluated in two birth cohort studies to estimate OP exposure (Table 3). Looking at consistency of findings across this endpoint, a coherent pattern of results is not apparent. The results for head circumference for DAP levels in the University of Berkeley Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) and Mt. Sinai Study birth cohort studies were statistically significant, but in opposite directions (Eskenazi et al., 2004; Wolff et al., 2007). Wolff et al. (2007) also evaluated potential interactions between these OP exposure biomarkers and PON1 activity or PON192 genotype, and reported no significant effect modification (Table 9A). Reasons for these divergent findings are unknown.
Whole blood cholinesterase and plasma butyrylcholinesterase are nonspecific measures of exposure to OP and N-methyl carbamate pesticides. Cholinesterase inhibition is often used to confirm exposure in occupational studies and poisoned cases. Although contact with individual pesticides may be indirect or infrequent in the urban and farm worker populations featured in many of the reviewed epidemiology studies, exposure to multiple cholinesterase inhibitors may be additive. However, there was no statistically significant association between activity of cholinesterases and newborn head circumference (Other measures, Table 3).
OP Insecticides, Metabolites with an Ethyl Group [CPF, Diazinon]
CPF, ΣDEP and TCPy As shown in Table 3, publications from the Columbia University Center for Children's Environmental Health (CCCEH) and New Jersey cohorts observed no statistically significant association of head circumference and CPF in maternal and umbilical cord blood at delivery (Whyatt et al., 2004, 2005, Perera et al., 2003; Barr et al., 2010). The CCCEH cohort study (Whyatt et al., 2004, 2005) also reported no association of CPF in maternal air samples and head circumference.
The CHAMACOS and Mt. Sinai studies estimated CPF exposure using the metabolites TCPy and ΣDEP in maternal urine prior to delivery. Head circumference was not statistically significantly associated with dichotomized TCPy levels nor with log transformed ΣDEP (Eskenazi et al., 2004; Berkowitz et al., 2004). An association of increasing maternal PON1 activity and increasing head circumference was reported in the Mt. Sinai cohort both as a main effect and for TCPy levels above the LOD, 11 μg/L (Wolff et al., 2007; Berkowitz et al., 2004) (Table 9A). However, there was little difference in the range in the magnitude of the head circumference for newborns across the PON1 activity tertiles for the TCPy < LOD group (33.6–34.1 cm) and TCPy > LOD group (33.3–34.1 cm). The interaction was not statistically significant and this raises uncertainty about the contribution CPF exposure may have with these PON1 associations. No statistically significant associations were reported in four cohort studies for head circumference and CPF, TCPy, or ΣDEP using any biological specimen regardless of the timing of exposure assessment. An in-depth review of these results specific to CPF, in addition to results for birth weight and birth length, discussed that there may be different levels of exposure in the four cohort studies (Mink et al., 2012). In summary, the data consistently fail to show a statistically significant association of CFP exposure and head circumference.
Diazinon, ΣDEP The CCCEH study reported no statistically significant associations between maternal diazinon exposure (air) or umbilical cord blood diazinon levels and newborn head circumference (Whyatt et al., 2004, 2005). As shown in Table 3 and noted earlier, statistical significance was not attained for ΣDEP as measured in the Mt. Sinai and CHAMACOS studies (Berkowitz et al., 2004; Eskenazi et al., 2004).
OP Insecticides, Metabolites With a Methyl Group [Malathion] Two studies evaluating MDA in maternal urine and newborn head circumference reported no consistent patterns or any statistically significant associations (Eskenazi et al., 2004; Wolff et al., 2007). No statistically significant results were reported for ΣDMP.
OP Insecticides, Metabolite of Parathion or Methylparathion (PNP) Eskenazi et al. (2004) observed a statistically nonsignificant association between maternal urinary PNP (biomarker for parathion and methylparathion) and increasing newborn head circumference (p = .06) in the CHAMACOS study.
Carbamates [Carbofuran and Propoxur] Barr et al. (2010) reported no statistically significant associations between carbofuran in maternal serum or umbilical cord serum and newborn head circumference (Table 3). Two reports from the CCCEH study reported no association between either propoxur or its metabolite (2-isopropoxyphenol) in maternal personal air or umbilical cord blood samples and newborn head circumference (Whyatt et al., 2004, 2005) (Table 3).
Pyrethroid Insecticides
Mean head circumference was similar among newborns with maternal 3-phenoxybenzoic acid (PBA) urine levels above and below 15.0 g/L (33.9 cm and 33.7 cm, respectively), suggesting no association with pyrethroid exposure (Table 3) (Berkowitz et al., 2004). PBA is a metabolite of cypermethrin, deltamethrin, and permethrin insecticides. Urine levels may represent exposure to any of these insecticides or to the metabolite in environment (Centers for Disease Control and Prevention [CDC], 2009).
Organochlorine (OC) Insecticides Seven publications evaluated associations between potential in utero exposure to DDT or similar chemicals, including breakdown products of DDT and DDT metabolites, such as DDE and DDD and newborn head circumference (Table 3). Most of the studies measured exposure in maternal blood collected during pregnancy (Jusko et al., 2006; Longnecker et al., 2001; Wolff et al., 2007) or umbilical cord blood (Sagiv et al., 2007; Tan et al., 2009). One study measured DDE in breast milk collected at delivery to represent exposure to the fetus during pregnancy (“transplacental” exposure) (Rogan et al., 1986), and another measured DDT and DDE in placental tissue (Lopez-Espinosa et al., 2007). In the Environment and Childhood Study, Lopez-Espinosa and colleagues observed a statistically significant positive association between o,p′-DDT concentration in placenta tissue and increasing newborn head circumference among the Grenada cohort; however, associations with the other DDT exposure measures (p,p′-DDT and p,p′-DDE) were statistically nonsignificant. In a Singapore-based study, Tan and colleagues (2009) also observed positive associations between p,p′-DDT and p,p′-DDD from umbilical cord blood and increasing head circumference (Table 3). In contrast, Wolff et al. (2007) reported statistically significant decreasing head circumference values in association with increasing (log-transformed) DDE measures, before and after lipid adjustment (Table 3). Wolff et al. (2007) noted, however, that DDE exposure in their study was low compared to other studies, including studies that observed an association between DDE and birth weight, suggesting that residual or uncontrolled confounding may have played a role, especially considering the relatively small effect size (approximately 0.5 cm change per 10-fold increase in DDE), the small sample size, and the role of other factors including maternal weight and lipids. The remaining four studies in Table 3 reported no statistically significant associations between DDT exposure measures and newborn head circumference (Jusko et al., 2006; Longnecker et al., 2001; Rogan et al., 1986; Sagiv et al., 2007).
In summary, results from the epidemiologic studies of DDT/DDE/DDD and head circumference were inconsistent, with four out of seven studies reporting no statistically significant associations (Table 3). Some findings suggested increasing exposure was associated with larger head circumferences (Lopez-Espinosa et al., 2007; Tan et al., 2009), while others suggested increasing exposure was associated with smaller head circumferences (Wolff et al., 2007). In their study of DDE, Wolff et al. (2007) noted the complexity of measuring this relationship, given that low maternal weight was also associated with small head circumference, higher DDE levels, and older maternal age in their model. Residual confounding should be considered, given that these variables, with the exception of maternal age, are likely to have been measured imprecisely, resulting in incomplete statistical control of these potential confounding factors.
There was one publication each for the following other OC pesticides: hexachlorobenzene (HCB) (Sagiv et al., 2007); beta-hexachlorocyclohexane (β-HCH) (Tan et al., 2009); chlordane (Tan et al., 2009); and endosulphan (Lopez-Espinosa et al., 2007) (Table 3). No statistically significant associations were reported for decreasing newborn head circumference and increasing exposure to HCB (Sagiv et al., 2007) or endosulphan (Lopez-Espinoza et al., 2007). Tan et al. (2009) displayed inverse associations between chlordane and newborn head circumference in a figure, but the 95% confidence intervals appeared to overlap the null value of zero. Tan et al. (2009) reported a statistically significant positive association for β-HCH, based on analysis of 41 study subjects.
Other Pesticides The New Jersey cohort study (Barr et al., 2010) also reported associations between maternal blood and umbilical cord blood levels of additional pesticides and newborn head circumference. Data did not indicate a pattern of smaller head circumference at higher exposure levels for any of the pesticides, specifically dacthal, dichloran, metalochlor, trifluralin, or diethyltoluamide (DEET), and all p values were greater than .05.
Neurobehavioral Endpoints: Neurobehavioral Testing of Newborns (BNBAS)
The Mt. Sinai cohort study (Engel et al., 2007) and the CHAMACOS study (Young et al., 2005) evaluated associations between measures of OP insecticides metabolites and the Brazelton Neonatal Behavioral Assessment Scale (BNBAS) (Table 4). The BNBAS domains were not evaluated by the CCCEH. Five publications evaluated associations between OC pesticides and the BNBAS (Engel et al., 2007; Fenster et al., 2007; Rogan et al., 1986; Sagiv et al., 2007; Stewart et al., 2000), and one study evaluated associations between DDE and performance on the Fagan Test of Infant Intelligence (FTII) at ages 6 and 12 mo (Darvill et al., 2000) (Table 4). The timing of this test was not standard across studies, thereby complicating comparisons across studies. Most of the domains were not associated with exposure levels.
TABLE 4.
Summary of results from epidemiologic studies of pesticide exposure and neurobehavioral testing of newborns, by chemical or metabolite

OP Insecticides The number of abnormal reflexes was strongly associated with OP exposure in two studies. as summarized in Table 4, a statistically significant but imprecise association was reported for the number of abnormal reflexes and maternal DAP (>3 vs. ≤3 number of reflexes, OR = 4.9, 95% CI 1.5, 16.1) in the CHAMACOS study (Young et al., 2005). The relative risk for number of abnormal reflexes in the Mt. Sinai study was smaller but more precise (RR = 1.32, 95% CI 0.99, 1.77) (Engel et al., 2007). None of the other domains attained statistical significance in either study for DAP
OP Insecticides, Metabolites With an Ethyl Group [ΣDEP] Possible exposures to diazinon or CPF per urinary ΣDEP levels were significantly associated with abnormal reflexes in both CHAMACOS and Mt. Sinai studies (Young et al., 2005; Engel et al., 2007). In the CHAMACOS study when stratified by age at BNBAS assessment, maternal ΣDEP was not statistically associated with abnormal reflexes among infants assessed within 3 d of birth (β = 0.08, 95% CI −0.16, 0.32) compared to infants assessed after 3 d (β = 0.37, 95% CI 0.09, 0.64) (Table 4). Young et al. (2005) suggested there may be “a delayed impact of in utero exposures on neurobehavioral functioning, or that the neurologic abnormalities, which the BNBAS reflex items aim to identify, may only be observed with maturity” (p. 207). Because infants were assessed at only one point in time, this explanation could not be investigated in their study. In the Mt. Sinai study, all infants were examined within 5 d of delivery, and most (86.1%) within 2 d of delivery, making direct comparisons to the CHAMACOS findings assessed after 3 d difficult (Engel et al., 2007). Although Engel et al. (2007) indicated that there appeared to be stronger associations between metabolite levels and abnormal reflexes for examinations performed after the first day of life, the interaction p value was >.1. For both studies, none of the other domains assessed on the BNBAS yielded statistically significant associations (Table 4). No interaction for PON1 was observed for ΣDEP (Table 9B).
TABLE 9B.
Summary of results from epidemiologic studies of potential interactions of pesticide exposure measures with metabolizing activity (PON1) and neurobehavioral testing of newborns (through age 12 months), by chemical or metabolite
| Author, Year (Study) | Results and Sample Size for Each Analysis Statistically significant (P <.05) results in blue | Adjusting Variables |
|---|---|---|
| Organophosphate Insecticides Metabolites: DAP/DMP/DEP | ||
| Engel et al., 2007 (Mt. Sinai study) |
Interactions among paraoxonase expression level and DAP/DMP/DEP: BNBAS Number of abnormal reflexes DAP (n = 239∗): [Model 1] PON1 tertile 1: RR = 2.38 (95% CI: 1.37, 4.15; interaction P < 0.05) PON1 tertile 2: RR = 1.75 (95% CI: 0.96, 3.17) PON1 tertile 3: RR = 0.76 (95% CI: 0.48, 1.20) DMP (n = 250∗): [Model 1] PON1 tertile 1: RR = 1.96 (95% CI: 1.27, 3.03; interaction P < 0.05) PON1 tertile 2: RR = 1.66 (95% CI: 1.03, 2.65; interaction P < 0.05) PON1 tertile 3: RR = 0.73 (95% CI: 0.56, 0.96) DEP (n = 239∗): [Model 2] PON1 tertile 1: RR = 1.78 (95% CI: 1.01, 3.14) PON1 tertile 2: RR = 1.42 (95% CI: 0.85, 2.35) PON1 tertile 3: RR = 1.56 (95% CI: 1.01, 2.39) |
[Model 1]: Examiner, anesthesia during delivery, PON1 tertiles, urinary creatinine, and overdispersion [Model 2]: [Model 1] minus overdispersion |
Abbreviations: BNBAS = Brazelton Neonatal Behavioral Assessment Scale; CI = Confidence interval; DAP = Total dialkylphosphate metabolites, including DEP and DMP; DEP = Diethylphosphate metabolites, including diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP); DMP = Dimethylphosphate metabolites, including dimethylphosphate (DMP), dimethylthiophosphate (DMPT), and dimethyldithiophosphate (DMDPT); P = P-value; PON1 = Paraoxonase 1 enzyme; RR = Relative risk.
Despite differences in the geographic location (California vs. New York) and study setting (agricultural vs. urban) of the two study populations, Young et al. (2005) and Engel et al. (2007) observed positive and statistically significant associations between ΣDEP metabolites measured in maternal urine and abnormal reflexes in neonates or infants younger than 2 mo of age. Both of these studies implemented the BNBAS, and neither reported significant findings for any of the other BNBAS measures, including motor performance. The two studies differed in the timing of the administration of the test, with 86% of the infants in the Mt. Sinai study being tested within 2 d of birth and all within 5 d (Engel et al., 2007), whereas the median age at assessment in the CHAMACOS study was 3 d, ranging from birth to 62 d after delivery (Young et al., 2005). Whether this difference affects the comparability of the studies in this regard is uncertain. Engel et al. (2007) identified two limitations that are important to consider when interpreting the findings from these studies: (1) The prognostic significance of a single measurement of infant behavior shortly after delivery (e.g., number of abnormal reflexes) is unclear; and (2) OP pesticide exposure was assessed at only one point in time using metabolites with relatively short half-lives. The implication of these findings is not clear; it will be important to evaluate the persistence of these observed associations in subsequent follow-up of these cohorts. No analyses using TCPy were reported for either study, which precludes clarification of the contribution of CPF to abnormal reflexes.
OP Insecticides, Metabolites With a Methyl Group [MDA, ΣDMP] In the New York Mt. Sinai study, Engel et al. (2007) reported a statistically significant positive association between the malathion metabolite, MDA, and increasing number of abnormal reflexes, as measured by the BNBAS within 5 d of delivery in the Mt. Sinai cohort (RR = 2.24; 95% CI 1.55, 3.24). The associations with MDA were stronger among younger infants, examined before d 2 (vs. d 2 or later). Further, in the Mt. Sinai study, the relative risk for ΣDMP within 5 d of delivery was smaller (RR = 1.13, 95% CI 0.90, 1.41). For presumed malathion exposure, similar to the results for ΣDEP, the CHAMACOS study reported statistically significant associations with maternal ΣDMP and domain for abnormal reflexes (Table 4) and when assessed in older infants ≥3 d after delivery (Young et al., 2005). In related analyses of potential interaction between ΣDMP and PON1 activity, Engel et al. (2007) did observe statistically significant positive associations between ΣDMP and number of abnormal reflexes among infants born to women in the first and second tertiles of PON1 activity (indicative of being “slower” metabolizers) but not among those born to women in the highest tertile (“fast” metabolizers) (Table 9B). The strength and precision of the association of MDA and abnormal reflexes in the Mt. Sinai Study is suggestive of a true positive. However, the consistency of the evidence using ΣDMP in the CHAMACOS study is weak. Engel et al. (2007) observed a higher association of abnormal reflexes with malathion exposure in younger infants (assessed < 2 d), whereas Young et al. (2005) observed the higher association in older infants (assessed ≥ 3 d).
OC Insecticides Of the five epidemiologic studies that evaluated exposure to DDT and newborn neurobehavioral outcomes via the BNBAS (Rogan et al., 1986; Stewart et al., 2000; Engel et al., 2007; Fenster et al., 2007; Sagiv et al., 2008), only one study reported statistically significant results (Rogan et al., 1986; Table 4). Rogan et al. (1986) indicated that there was a statistically significant association between DDE and the number of abnormal reflexes, but did not report the corresponding measure of association, confidence interval or p value. The authors did indicate that upon further investigation it appeared that the association with number of abnormal reflexes was limited to hyporeflexia (reflex not elicited or low) and not hyperreflexia (high). DDE was not associated with other scales on the BNBAS in the Rogan et al. (1986) study. Similarly, in another study (Darvill et al., 2000), the correlation coefficients between DDE and the FTII were weak (Table 4); further, the authors stated that DDE was “unrelated” to FTII performance at 6 and 12 mo. There was no strong or statistically significant association between HCB and performance on the BNBAS administered within 2 d of birth (Stewart et al., 2000).
Neurobehavioral Endpoints: Bayley Scales of Infant Development (BSID) (Ages 6–36 Months)
There were 12 studies that specifically evaluated cognitive endpoints and 13 studies that specifically evaluated psychomotor endpoints. There were no statistically significant associations reported between malathion or HCB and neurobehavioral endpoints in these studies, regardless of the timing of exposure assessment or biological specimen from which the chemical or metabolite was measured.
OP Insecticides The Bayley Scales of Infant Development (BSID) were evaluated by three cohort studies but not uniformly at the same ages. In the CHAMACOS study, the BSID were evaluated at 6, 12, and 24 mo for maternal and child DAPs. As shown in Table 5, at 24 mo, maternal urinary DAP was associated with poorer BSID Mental Development Index (MDI) scores (95% CI −6.59, −0.49), whereas child DAP were associated with better BSID:MDI scores (95% CI 0.5, 4.24) (Eskenazi et al., 2007). No other statistically significant observations were reported at any age for BSID:MDI or BSID:PDI (Psychomotor Development Index), or by PON1 genotype (Table 9C) in the CHAMACOS study (Eskenazi et al., 2010). In the Mt. Sinai study (Engel et al., 2011) the BSID scales were only reported for children at 12 mo. The pattern of associations between maternal DAP and BSID:MDI scores at age 12 mo differed significantly by race, with inverse associations observed among Black/Hispanic participants (increasing DAP associated with lower BSID:MDI scores), and positive associations observed among white participants (increasing DAP associated with higher BSID:MDI scores). In further analyses of potential PON1 interactions, Engel et al. (2011) reported statistically significant inverse associations between maternal ΣDAP and 12-mo BSID:MDI limited to Black/Hispanic participants with maternal PON1 Q192R QR/RR genotype (“fast” metabolizers) (Table 9C).
TABLE 5.
Summary of results from epidemiologic studies of pesticide exposure and performance on the Bayley Scales of Infant Development (BSID) (ages 6–36 months), by pesticide or metabolite

TABLE 9C.
Summary of results from epidemiologic studies of potential interactions of pesticide exposure measures with PON1 genotype and performance on the Bayley Scales of Infant Development (BSID) (ages 6–36 months), by pesticide or metabolite
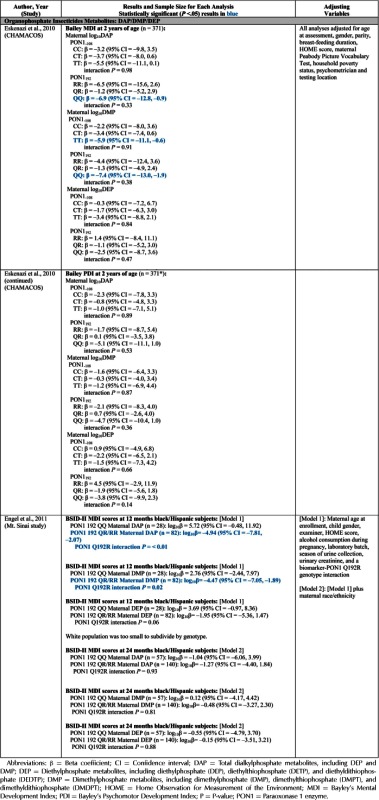
In summary, adverse associations were reported for maternal DAP and BSID:MDI assessed at 24 mo in the CHAMACOS study, and for maternal DAP and BSID:MDI assessed at 12 mo among Black/Hispanic participants in the Mt. Sinai study, particularly those with maternal PON1 Q192R QR/RR genotypes. In contrast, results for DAP were similar among those with QQ and RR genotypes in the CHAMACOS study (Eskenazi et al., 2010; Table 9C). The divergent findings for BSID by maternal and child urinary levels in the CHAMACOS study and the racial differences in the Mt. Sinai study indicate poor internal consistency for OP insecticides, in general, with these scores. The lack of consistent analyses across studies by age of testing and racial disparity further clouds the etiologic role of OP as a class and BSID scores.
CPF, DEP and TCPy None of the associations between TCPy and BSID:PDI or BSID:MDI at ages 6, 12, or 24 mo in the CHAMACOS study was statistically significant (Eskenazi et al., 2007) (Table 5). No statistically significant associations were reported for maternal ΣDEP at any age in the CHAMACOS or Mt. Sinai Study (Engel et al., 2011). Child ΣDEP at 12 mo were positively associated (that is, with better scores) with the BSID:MDI (Table 5). The authors postulated that children functioning at higher cognitive levels may be more interactive with their environments, resulting in greater opportunity for pesticide exposure. Another possible explanation is that children with higher cognitive scores consumed more fruits and vegetables and may have had increased dietary exposures to both the metabolites and pesticide residues. However, the lack of confirming associations at 24 mo indicates that this association may be a random observation among many comparisons.
Rauh and colleagues (2006) evaluated BSID at 12, 24, and 36 mo in the CCCEH study by CPF in blood. The investigators constructed multivariate regression models to evaluate BSID:MDI and BSID:PDI scores both as continuous and categorized outcomes. The BSID scores were categorized as mildly/significantly delayed (scores ≤ 85) or within normal limits/accelerated performance (scores > 85). With the exception of the BSID:PDI score at age 36 mo, the results of the linear regression analyses were not statistically significant. In contrast, the authors reported positive and significant associations between CPF levels in cord blood and both “mental delay” and “psychomotor delay” at 36 mo (OR = 2.37, 95% CI 1.08, 5.19, and OR = 4.52, 95% CI 1.61, 12.7, respectively). The associations observed at younger ages were not significant. The authors suggested that highly exposed children were more likely to need early intervention services, although they state that in New York City, children are considered developmentally delayed and referred for service if their BSID:MDI scores are less than 80 and not the cutoff point of 85 used in their study.
One drawback of using a “clinically defined” dichotomized outcome rather than the continuously distributed BSID scores is the loss of precision that results from the use of this “rare” outcome (Bellinger, 2004). This is evident in the 95% confidence intervals for the OR for “mental delay” and “psychomotor delay,” which are quite wide (1.08, 5.19) and imprecise (1.61, 12.7), respectively. Thus, although these results are statistically significant, they are relatively unstable and the role of chance remains a concern.
The results for continuous measures for the BSID:PDI and BSID:MDI at age 36 mo were similar in a subsequent analysis of the CCCEH and both estimates were statistically significant (Lovasi et al., 2011). Results showed further that adjustment for indicators of neighborhood poverty did not meaningfully change the effect estimates nor were the tests for interaction statistically significant.
The two birth cohorts (CHAMACOS and Mt. Sinai) that collected maternal urine and estimated both TCPy and ΣDEP metabolites were considered to provide relevant information for risk related to CPF exposure and neurodevelopment associations in children. Since the CCCEH study relied upon maternal and cord blood levels for exposure estimates, its results were viewed as providing more robust information than the reported associations using TCPy or ΣDEP. The results for urinary TCPy in the other cohorts were viewed as the next best evidence to confirm or refute the observations of the CCCEH because the ΣDEP levels could reflect exposure to other OP such as diazinon. If CPF exposure is causally related to neurodevelopment, the observations regarding neurodevelopment in the participating children, particularly those born in the years 1998, 1999, and 2000, would be exclusively attributed to in utero exposure because residential exposure to CPF was essentially absent in this population after 2001 due to the withdrawal of residential uses in the United States (Eaton et al., 2008; Whyatt et al., 2005).
The association of dichotomized blood CPF levels and lower BSID scores at 36 mo (Rauh et al., 2006) has not been evaluated by any other study. None of the other cohort studies included in this review modeled BSID scores as dichotomized variables or evaluated the children at 36 mo. The lack of replication in the younger ages in the CCCEH as well as the CHAMACOS and Mt. Sinai studies might indicate (1) a true effect for BSID that only manifests around 36 mo, (2) other environmental factors present in the home and neighborhood may have contributed negatively to the development of the CCCEH cohort children and been associated with insecticide use, or (3) a chance association based on a single study.
In additional statistical analyses of building disrepair and related stressors for the CCCEH cohort, CPF remained an independent factor related to the BSID:PDI scores (Lovasi et al., 2011). However, the R2 for the 6 models ranged only from 0.126 to 0.148 for BSID:PDI and from 0.263 to 0.278 for BSID:MDI, indicating that 75 to 85% of the variability remained unexplained by CPF and other measured factors. Unidentified and unmeasured confounding by factors related to poverty and home environment may be present despite the best efforts of study investigators.
Although the CCCEH data collection period spanned the restriction of residential use of CPF, less than one-third of the study children were born in 2001 and 2002 (Whyatt et al., 2005). The cohort analyses thus far have not been designed to assess the effect of the residential withdrawal. None of the publications demonstrated an improvement or rebound in neurobehavioral results among participants born after January 2001 compared to participants born earlier. For example, significant differences were noted in “pre-ban” to “mid-ban” (for BSID:MDI and BSID:PDI) but not “mid-ban” to “post-ban.” Indeed, the association reported for BSID scores and piperonyl butoxide (PBO), used as a synergist with a replacement for CPF (Horton et al., 2011), supports that an alternative explanation; for example, insecticide exposure (regardless of the chemical) may be a marker for insect infestation (and other related factors) and is not itself the causal agent driving BSID or other neurobehavioral test results.
Malathion (MDA and ΣDMP) None of the associations between MDA and BSID scores at ages 6, 12, or 24 mo in the CHAMACOS study was statistically significant (Eskenazi et al., 2007) (Table 5). Consistent with the findings for DAP, there were divergent associations at 24 mo with maternal and child ΣDMP for the BSID:MDI. No other statistically significant associations were reported for the ΣDMP. Similar to DAP results in the Mt. Sinai study (Engel et al., 2011), the pattern of associations between pregnancy metabolites and BSID:MDI scores at age 12 mo also differed significantly by race, with inverse associations observed among Black/Hispanic participants (increasing ΣDMP associated with lower BSID:MDI scores), and positive associations observed among white participants (increasing ΣDMP associated with higher BSID:MDI scores).
In further analyses of potential PON1 interactions, Engel et al. (2011) reported statistically significant inverse associations between maternal ΣDMP and 12-mo BSID:MDI limited to Black/Hispanic participants with maternal PON1 Q192R QR/RR genotype (“fast” metabolizers). Thus, the strongest evidence for potential adverse associations were for maternal DAP and ΣDMP and BSID:MDI assessed at 24 mo in the CHAMACOS study, and for maternal DAP and ΣDMP and BSID:MDI assessed at 12 mo among Black/Hispanic participants in the Mt. Sinai study, particularly those with maternal PON1 Q192R QR/RR genotypes. As with the DAP analyses, there is a lack of internal and external consistency in the two studies, suggesting that the associations observed for malathion may be due to chance, bias, or confounding
OC Insecticides In the CHAMACOS Study, associations of maternal measures of DDT and DDE with BSID:PDI and BSID:MDI at ages 6, 12, and 24 mo were variable (Table 5) (Eskenazi et al., 2006). There were statistically significant inverse associations between p,p′-DDT and the BSID:PDI at ages 6 and 12 mo, and between p,p′-DDE and BSID:PDI at age 6 mo, but none of the measures was associated with the BSID:PDI at 24 mo (Table 5). None of the measures was associated with the BSID:MDI at age 6 mo, but the two measures of maternal DDT (o,p′-DDT and p,p′-DDT) were associated inversely and significantly with the BSID:MDI at ages 12 and 24 mo. There were no statistically significant associations between p,p′-DDE and BSID:MDI at any age.
The other epidemiologic studies evaluated potential associations with DDE only. No statistically significant associations were observed in the Mt. Sinai Study (Engel et al., 2011). The North Carolina birth cohort study estimated in utero exposure based on maternal measures around the time of delivery including maternal blood, breast milk, as well as umbilical cord blood (Gladen et al., 1988; Rogan and Gladen, 1991). Exposures to the infant were based on measures of DDE in breast milk and duration of breastfeeding. In analyses of the BSID, Gladen et al. (1988) observed a positive association (higher exposure associated with higher scores) between “transplacental” exposure (estimated in utero exposure based on breast milk samples collected at the time of birth) and the BSID:MDI at 6 mo, but no association at 12 mo, or with the BSID:PDI at either 6 or 12 mo. There were no statistically significant associations between breast milk DDE (the measure of exposure to the infants that took duration of breastfeeding into account) and BSID:MDI or PDI at either 6 or 1 2 mo. Follow-up of the North Carolina birth cohort at ages 18 and 24 mo by Rogan and Gladen (1991) reported, “The effects on both scores [PDI and MDI] of transplacental DDE and of … DDE acquired through breast milk were small and not statistically significant” (data not shown, p. 409). In a Spanish birth cohort study, Ribas-Fito et al. (2003) reported statistically significant inverse associations between log-transformed p,p′-DDE measured in umbilical cord blood and BSID:MDI and PDI at age 13 mo. Torres-Sanchez et al. (2007, 2009) analyzed data from a study conducted in malaria-endemic Mexico and observed a statistically significant inverse association between log-transformed maternal DDE measured during the first trimester of pregnancy and BSID:PDI scores measured during the first 12 mo of life; however, there was no association between p,p′-DDE measures during the second and third trimester or with any of the measures and BSID:MDI (Torres-Sanchez et al., 2007). Consistent with data from the North Carolina birth cohort (Rogan and Gladen, 1991), and the CHAMACOS Study (Eskenazi et al., 2006), there were no statistically significant associations observed between DDE and the BSID:PDI or MDI after age 12 mo (Torres-Sanchez et al., 2009).
The majority of epidemiologic studies of DDT/DDE/DDD and neurobehavioral testing on newborns yielded statistically nonsignificant results. There were several statistically significant results in studies that used the BSID; however, there was not a clear pattern showing consistent decrements at particular ages. Furthermore, most of the studies that evaluated pregnancy DDT or DDE and BSID:PDI or MDI at ages 12–30 mo yielded results that were statistically nonsignificant (Table 5). Thus, if there is a true adverse association between DDT or DDE during pregnancy and performance on the BSID scales during the first 12 mo after birth, there is no strong evidence from these studies to support the persistence of such an effect after age 12 mo.
HCB The Spanish birth cohort study focused on newborns from a population that resided in the vicinity of an electrochemical factory (Ribas-Fito et al., 2003). Although levels of HCB in the atmosphere and in cord serum were high, HCB was not associated with either BSID index.
Pyrethroid Insecticides and Piperonyl Butoxide The CCCEH cohort study of inner-city New York residents reported no statistically significant associations between cis- or trans-permethrin measured in personal air samples, maternal blood, or umbilical cord blood and the BSID:MDI or PDI measured at age 36 mo (data not shown). There was also no statistically significant association between piperonyl butoxide (PBO), a synergist often used with pyrethroids in urban applications, measured in personal air samples during the third trimester of pregnancy and BSID:PDI measured at age 36 mo (Horton et al., 2011). There did not appear to be a monotonic inverse association between PBO and BSID:MDI (Table 5); however, in categorical analyses there was a statistically significant decrement of about 4 BSID:MDI points when the upper PBO quartile was compared to all other categories (Horton et al., 2011). The statistically significant decrement of PBO and BSID:MDI in the CCCEH cohort may be instructive. Due to the phase-out of CPF in 2000, CPF levels declined precipitously. Pyrethroid insecticide use increased in this cohort over time, as a replacement to the OP (Williams et al., 2008). An alternative hypothesis is that the low levels of PBO and CPF were markers of pest infestation and poor living conditions, rather than indications of a toxic exposure.
Neurobehavioral Endpoints: Intelligence Tests (Ages 3–9 Years)
OP Insecticides Two studies, the CHAMACOS Study (Bouchard et al., 2011) and the Mt. Sinai Study (Engel et al., 2011), evaluated associations between pregnancy DAP and intelligence test outcomes (Table 6). In the CHAMACOS Study, two DAP collections averaged over pregnancy were associated significantly and inversely with all WISC-IV measures reported (Table 6). The DAP collected in the first half of pregnancy were only associated with Verbal Comprehension, whereas DAP collected in the second half of pregnancy were only associated with the Full Scale IQ. Child DAP levels were not associated consistently with WISC-IV performance.
TABLE 6.
Summary of results from epidemiologic studies of pesticide exposure and performance on intelligence tests (ages 3 to 9 years), by chemical or metabolite

In the Mt. Sinai Study, participants were evaluated for intelligence testing between ages 6 and 9 yr. Children younger than age 7 yr were given the Wechsler Preschool and Primary Scale of Intelligence (WPPSI) III, and children ages 7–9 yr were administered the WISC-IV (Engel et al., 2011). Results were reported separately for the two Wechsler tests and for the combined population (ages 6–9 yr). Regardless, none of the associations of DAP with WPPSI or WISC outcomes or the outcomes for the combined populations was statistically significant (Table 6). It was difficult to compare these observations to those of the CHAMACOS study with respect to strength of association and dose response because of the log transformations. None confirmed the observations of the CHAMACOS study.
CPF and ΣDEP The CCCEH University birth cohort study evaluated potential associations between umbilical/maternal cord blood CPF and scores on the WISC IV at age 6–8 yr (Rauh et al., 2011) (Table 6). The authors reported statistically significant inverse associations between CPF and the log-transformed measures Working Memory Index and Full-Scale Intelligence Quotient (IQ), but not with Verbal Comprehension Index, Perceptual Reasoning Index, or Processing Speed Index (Table 6). Because of the log transformation of the WISC-IV outcomes, it was difficult to directly interpret the beta coefficients on these measures. Data indicated that each standard deviation increase in CPF (4.6 pg/g) was associated with a 2.8% decline in Working Memory score and a 1.4% decline in Full-Scale IQ (Rauh et al., 2011). The range of Full Scale IQ in the children as estimated from Figure 1 is 68–122, which was not dissimilar from the range measured in the mothers (60–135).
FIGURE 1.
Breakdown of animal studies by pesticide group and further breakdown of OP. A published paper may have more than one study as defined in the methods section for animal studies.
As shown in Table 6, Bouchard et al. (2011) ΣDEP levels were statistically significantly and inversely associated with Processing Speed (β = −4, 95% CI −7, −1) in the CHAMACOS children but not with Working Memory or Full Scale IQ. Supplemental analyses identified a statistically significant inverse association of maternal ΣDEP (creatinine adjusted) and Full Scale IQ (β = −3.1, 9% CI −6.1, −0.0, data not shown in Table 6). The Mt. Sinai cohort did not support these findings in their analyses of ΣDEP and PON1 genotype (Engel et al., 2011). No analyses were reported using the maternal TCPy data in either the CHAMACOS or Mt. Sinai study, making direct comparisons for CPF association unclear.
As with the CCCEH observations for BSID:PDI at 36 mo, the associations with in utero CPF exposure and childhood Working Memory and Full Scale IQ have not been sufficiently tested in other studies to confirm if these are true or false observations. Other exposures to environmental tobacco smoke and polyaromatic hydrocarbons, as well as maternal characteristics such as education, IQ, and race/ethnicity, were controlled in the regression models. On the other hand, misclassification of CPF exposure in utero could be sustained throughout all studies of the CCCEH cohort. All estimates of exposure were based upon a single sample from either umbilical cord blood or maternal blood within 2 d postpartum. Importantly, the plasma collection in the CCCEH study was not timed with an application. As a result, the timing, source, duration, and exposure level of exposure are speculative and unknown.
Malathion and ΣDMP The results for ΣDMP (and possible malathion exposure) were also inconsistent across two studies. Maternal ΣDMP were associated significantly and inversely with Working Memory, Verbal Comprehension, and Full-Scale IQ in the CHAMACOS Study but not in the Mt. Sinai study (Bouchard et al., 2011; Engel et al., 2011). Engel et al. (2011) also observed that children born to mothers with the QQ PON1 genotype (“slow” metabolizers) had statistically significant decrements in Perceptual Reasoning scores per log increase in ΣDMP, but this pattern was not observed for the QR/RR genotypes (Table 9D). None of the other interactions was statistically significant. In summary, the ΣDMP analyses were not directly comparable between the Mt. Sinai and CHAMACOS studies for WISC-IV scores and did not indicate a consistent association.
TABLE 9D.
Summary of results from epidemiologic studies of potential interactions of pesticide exposure measures with PON1 genotype and performance on intelligence tests (ages 3–9 years), by chemical or metabolite
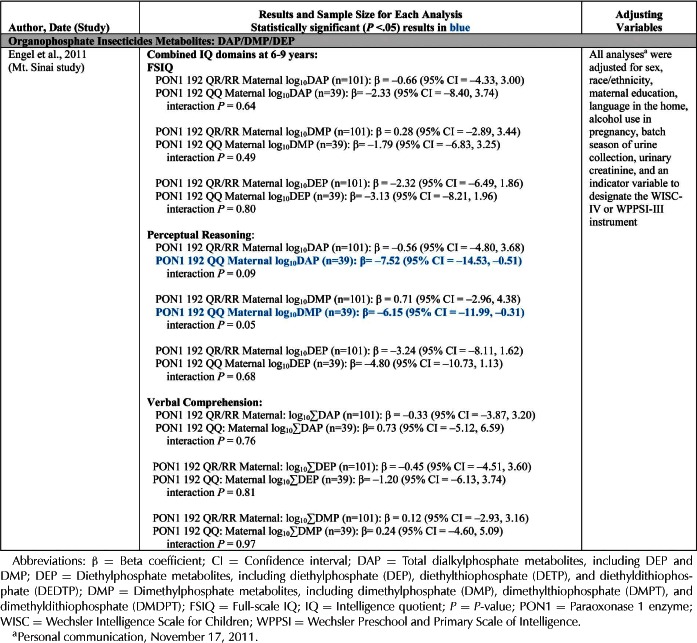
OC Insecticides
DDT and DDE The North Carolina birth cohort study evaluated potential associations of estimated in utero DDE exposure (“transplacental” DDE) and postnatal DDE exposure via breast milk with McCarthy Scales of Children's Abilities (MSCA) administered at ages 3, 4, and 5 yr and reported no statistically significant associations (Gladen and Rogan, 1991) (Table 6). In an analysis of prenatal PCB exposure and WISC-III performance at age 9 yr in a birth cohort from Oswego, New York, the investigators also measured and analyzed OC, including DDE (Stewart et al., 2008). Correlation coefficients between DDE and WISC-III outcomes were small and positive (0 < r ≤ 0.2), and the authors stated, “We measured several OC typically correlated with PCBs (DDE, HCB, mirex) in this study, and none predicted lower IQ” (Stewart et al., 2008, p. 1421). Similarly, Engel et al. (2011) reported that maternal blood DDE from the third trimester of pregnancy was not associated with either WPPSI or WISC outcomes in the Mt. Sinai study. In the Spanish birth cohort study, Ribas-Fito et al. (2006) considered several methods for characterizing DDT exposure, including DDT, DDE, DDT/DDE ratio, and DDT adjusted for DDE. Resuts showed statistically significant inverse associations between DDT and MSCA scores at age 4 yr. There was no adjusment for maternal IQ and the authors acknowledged that there may be residual confounding. Associations between DDE and MSCA scores were more modest and statistically nonsignificant, with the exception of the “Memory” area when the entire cohort was analyzed together. In subgroup analyses, associations between DDT and MSCA were stronger among girls than boys (Table 6) but consistently in the negative direction. In a similar analysis from the same Spanish study, Sunyer et al. (2010) reported statistically significant inverse associations between log-transformed DDT in umbilical cord blood and MSCA general cognition at age 4 yr, whereas the association for log-transformed DDT measured in child's blood at age 4 was positive but statistically nonsignificant (Table 6). Several inverse associations were reported in the Menorca, Spain cohort in children with a glutathione S-transferase allele (Table 9E). The relevance of these unique observations is unclear and not been reported elsewhere (Morales et al., 2008).
TABLE 9E.
Summary of results from epidemiologic studies of potential interactions of pesticide exposure measures with PON1 genotype, and behavioral assessments (ages 2 years and older), by pesticide or metabolite
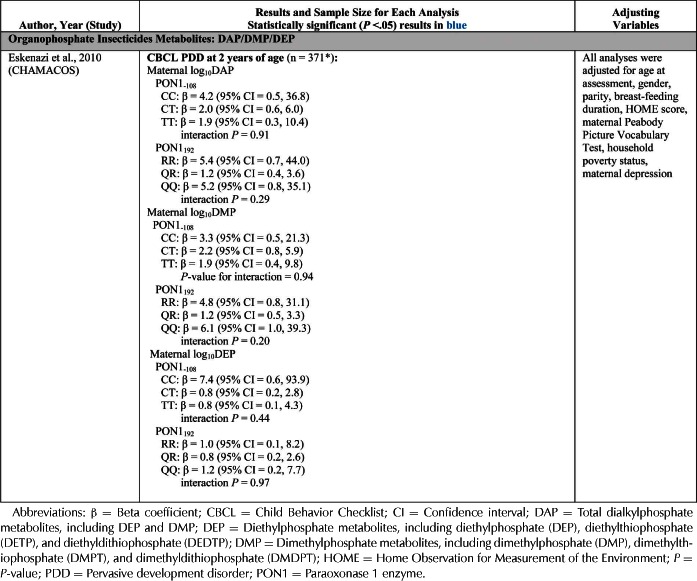
TABLE 9F.
Adjusted associations [β (SE)] between concentrations of DDT or DDE in cord serum (ng/mL) and neurodevelopment at 4 years of age, by GST polymorphisms
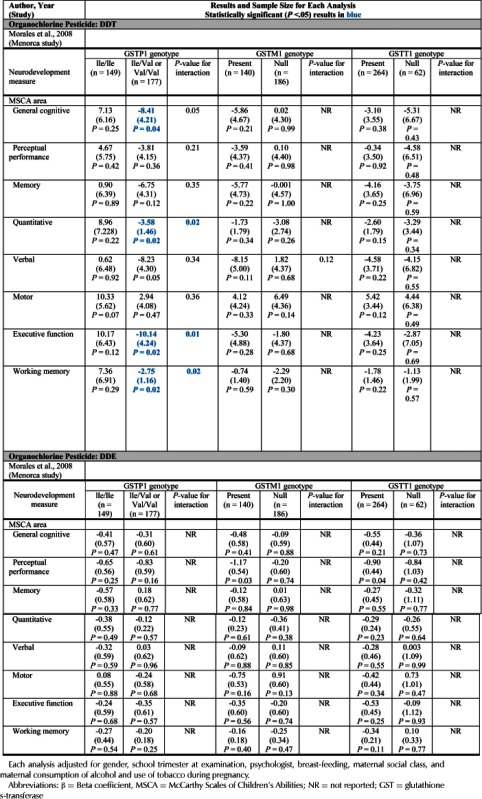
In summary, maternal DDE was not associated with either the WPPSI or WISC outcomes in the Mt. Sinai cohort study, nor was DDE associated significantly with WISC outcomes in the Oswego, New York study. The CHAMACOS study did not report on WISC outcomes and DDT/DDE levels. The Spanish birth cohort study reported statistically significant findings for DDT and the MSCA scores at age 4 yr, but associations with DDE were statistically nonsignificant.
HCB Two studies evaluated associations between HCB and performance on the MSCA; however, there was some overlap in study participants (i.e., Menorca birth cohort) (Ribas-Fito et al., 2007; Sunyer et al., 2010). Neither of these analyses observed statistically significant associations between HCB in umbilical cord blood and MCSA scores. Stewart et al. (2008) reported no statistically significant associations between HCB and WISC-III scores among participants in the Oswego cohort study.
Mirex Two studies evaluated associations of Mirex with cognitive outcomes (Table 6). Puertas et al. (2010) observed statistically significant inverse associations between Mirex in placenta and MCSA “quantitative area” scores and on working memory scores, but no significant associations with the remaining five MCSA areas or three MCSA functions. Stewart et al. (2008) noted no statistically significant associations between Mirex and WISC-III performance at age 9 yr.
Neurobehavioral Endpoints: Other Assessments
Gladen and Rogan (1991) reported no statistically significant associations between maternal DDE exposures and poorer English or math grades assessed in third grade or later among participants in the North Carolina Breast Milk and Formula Project Study (Table 7). Consistent with their results from the BSID, Ribas-Fito et al. (2003) reported statistically significant inverse associations between p,p′-DDE and the Locomotor, Social, Performance Scales of the Griffiths Mental Development Scales at age 13 mo but not with the Hearing/Language or Eye-Hand Coordination Scales. Pan et al. (2009) analyzed data from the Pregnancy, Infection, and Nutrition Babies Study and found no statistically significant associations between any of the DDT measures and outcomes assessed by the Mullen Scales of Early Learning or by the MacArthur-Bates Communicative Development Inventories (Table 7). Ribas-Fito et al. (2003) stated, “Prenatal exposure to HCB had no effect on child neurodevelopment” (p. 583).
TABLE 7.
Summary of results from epidemiologic studies of pesticide exposure and miscellaneous assessments or neurobehavioral test performance, by chemical or metabolite
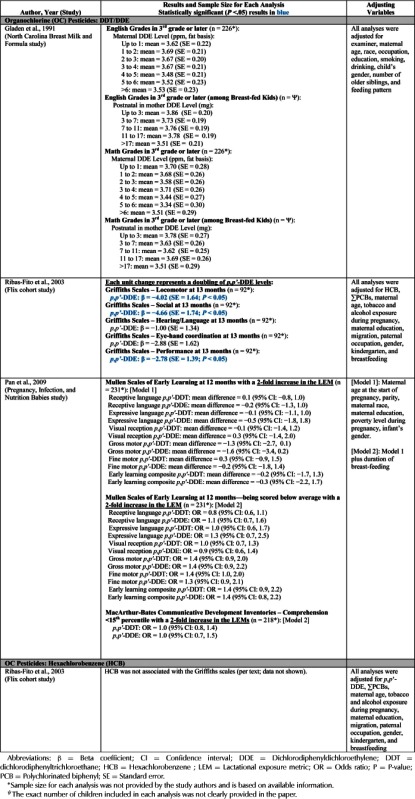
Neurobehavioral Endpoints: Behavioral Assessments (Ages 2–9.5 Years)
OP Insecticides (DAP) Only the CHAMACOS investigators Eskenazi et al. (2007) evaluated DAP and scores of the CBCL. There was a borderline significant positive association (i.e., higher scores indicate more problems) between maternal DAP and CBCL Pervasive Developmental Disorder (PDD) problems at 24 mo (OR = 2.25, 95% CI 0.99, 5.16) and between child DAP and CBCL PDD problems at 24 mo (OR = 1.71, 95% CI 1.02, 2.87). There were no statistically significant associations between maternal or child DAP and either CBCL attention problems or Attention Deficit Hyperactivity Disorder (ADHD) problems at age 24 mo (Table 9).
Marks et al. (2010) conducted many additional assessments that were unique to the CHAMACOS study. For example, ADHD was evaluated 28 times (Table 8). Analyses of maternal DAP and CBCL attention problems and ADHD problems at age 3.5 yielded elevated but imprecise and statistically nonsignificant odds ratios when data were analyzed similar to their previous publication (i.e., outcomes were categorized based on proportion above a standardized score indicating “borderline” clinical range) (Marks et al., 2010). These findings may be true but were not corroborated by other studies of associations with childhood behavior and unspecified OP exposures. Alternatively, the associations may be confounded, biased, or occurred by chance. Some of the associations were strong but lacked precision. For example, when stratified by gender, strong odds ratios for ADHD were reported for boys at age 3.5 yr (OR = 6.4, 95% CI 1.1, 39) and 5 yr (OR = 4.9, 95% CI 0.7, 33). No statistically significant associations were reported for girls. Evaluation of these outcomes in other studies with similar exposure measures will be helpful to clarify these potential associations.
CPF, TCPy, and ΣDEP Rauh et al. (2006) evaluated associations between umbilical/maternal cord blood CPF and maternal-reported outcomes on the CBCL assessed at age 36 mo among CCCEH participants (Table 8). The study investigators categorized exposure into two groups (low, <6.17 pg/g, vs. high, ≥ 6.17 pg/g) and reported statistically significant odds ratios for the following CBCL outcomes: attention problems (OR = 11.26; 95% CI 1.79, 70.99), ADHD problems (OR = 6.5; 95% CI 1.09, 38.69), and PDD problems (OR = 5.39; 95% CI 1.21, 24.11). The odds ratios reported by Rauh et al. (2006) were strong but imprecise. In contrast, there were no statistically significant associations between maternal TCPy and any CBCL outcomes (age 24 mo) in the CHAMACOS Study (Eskenazi et al., 2007). Further, there were no statistically significant associations between maternal or child ΣDEP and either attention problems or ADHD problems at age 24 mo (Table 9) (Eskenazi et al. 2007). A statistically significant positive association was reported between child ΣDEP and CBCL PDD problems at 24 mo. Marks et al. (2010) conducted additional assessments of “attentional problems” in the CHAMACOS study among children at ages 3.5 yr and 5 yr. Analyses of maternal ΣDEP and CBCL attention problems and ADHD at age 3.5 yielded OR that were elevated, but imprecise and statistically nonsignificant when data were analyzed similarly to their previous publication (i.e., outcomes were categorized based on proportion above a standardized score indicating “borderline” clinical range) (Marks et al., 2010; Eskenazi et al., 2007). The odds ratios for CBCL outcomes at age 5 yr were much closer to the null value of 1 (Table 8). Child ΣDEP were not statistically significantly associated with the CBCL outcomes at either age (Marks et al., 2010).
A coherent pattern of results was not apparent from these data. The study by Marks et al. (2010) reported the results of many analyses. Most of the associations were statistically nonsignificant, and many of the significant results lacked precision. While ADHD affects more than 2 million children in the United States and studies suggest that it may affect an equal number of non-U.S. children (Faraone et al., 2003), only two epidemiologic studies in our review evaluated attention disorders, namely, ADHD and PDD. Rauh et al. (2006) observed strong and significant associations between CPF measured in blood and attention disorders, ADHD, and PDD at 36 mo, but noted that their findings should be interpreted with caution because the CBCL criteria were obtained from the DSM-IV, which is not sensitive for this application (Ogino et al., 2005). Eskenazi et al. (2007) found no association between the metabolite most specific to CPF, TCPy, and any CBCL measure.
In summary, Rauh et al. (2006) reported inverse and imprecise associations of high CPF levels (>6.17 pg/g) and CBCL-reported ADHD problems, attention problems, and PDD problems in the CCCEH children at 36 mo. The CHAMACOS investigators did not evaluate the associations of TCPy with these CBCL outcomes after 24 mo, essentially leaving the putative effect at 36 mo untested (Eskenazi et al., 2007; Marks et al., 2010). Marks et al. (2010) reported statistically significant associations of ΣDEP at 5 yr using logistic regression but not when using linear regression. The role of CPF exposure and childhood behavior was suggestive in one study but not consistently replicated in others to date. Nevertheless, the magnitude of the odds ratios for the CBCL outcomes in the Rauh et al. (2006) study remains provocative, and it may be prudent for these investigators to evaluate further the extent to which maternal-reported CBCL problems in their study may correspond to clinical diagnoses of PDD, ADHD, or other related behavioral diagnoses.
Malathion (MDA) and ΣDMP Eskenazi et al. (2007) reported no statistically significant associations between maternal MDA and CBCL outcomes in the CHAMACOS children. There was a statistically significant positive association between maternal ΣDMP and CBCL PDD problems at 24 mo (OR = 2.19, 95% CI 1.05, 4.58). In related analyses by Marks et al. (2010), there were also statistically significant positive associations between maternal ΣDMP and both CBCL outcomes modeled as continuous variables when boys and girls were analyzed together and for boys analyzed separately at age 5 yr. Despite these apparent gender differences, none of the reported p values for interaction was less than .05. There were no other studies available to confirm or refute the observed association of in utero malathion (or other methyl OP) with CBCL problems at age 5 yr.
OC Insecticides
DDT and DDE None of the studies that evaluated associations between DDT or DDE exposure(s) and measures of attention or ADHD behaviors (Sagiv et al., 2010; Stewart et al., 2003, 2005) reported statistically significant associations with any of the measures reported (Table 8). One ecological study not included in our assessment of causality found that the risk of autism spectrum disorder was significantly higher among children who were born to mothers living near agricultural fields (OR = 6.1; 95% CI 2.4, 15.3), and that there was an exposure-response relationship between the amount of OC pesticide that was applied and the risk of autism (Roberts et al., 2007). Although notable, the results of this study were interpreted with caution due to likely misclassification of exposure, to little information about demographic or sociodemographic characteristics of the study participants being available, and to the number of children with autism spectrum disorder being small.
HCB In analyses of HCB and measures of attention at three ages in the Oswego Study, Stewart et al. (2003, 2005) observed no statistically significant associations after multivariate adjustment (Table 8). In their study of two birth cohorts in Spain, Ribas-Fito etal. (2007) noted statistically significantly elevated relative risks for measures of poor social competence and for ADHD symptoms among children assessed at 4 yr of age in the highest HCB umbilical cord serum category (≥1.5 ng/mL) as compared to those with HCB cord serum levels <0.5 ng/mL.
The epidemiology results are discussed further in the Integration section following the evaluation of animal studies.
ANIMAL STUDIES: ANIMAL LITERATURE INCLUSION CRITERIA
This review included neurobehavioral, neurophysiological, brain histopathology and brain neuropharmacology including neurotransmitter levels and receptor binding outcomes from repeated dose in vivo animal studies, in which repeated exposures to a single pesticide occurred prior to weaning. The detailed criteria used to include papers in this review were:
Experimental design: Studies in which the experimental design was not intended to make comparisons with a concu rrent control were not included (Pauluhn and Schmuck, 2003). Developmental and reproduction studies conducted according to U.S. EPA test guidelines were included only if additional neurodevelopmental endpoints were evaluated. However, reproduction studies were not included if the neurodevelopmental endpoint was measured in adults after combined gestational, lactational and adult exposures since it is not possible to determine if the associations were due to developmental exposures.
Pesticide exposure: Studies with exposures to pesticide active ingredient and not the formulation were included. Studies involving exposures to two or more pesticides combined or in series, or in which formulations were used as the test material were not included in this review.
Exposure period: The present review focuses on potential effects of early developmental exposures (gestation and prior to weaning in animals) on neurodevelopmental outcomes. Studies included in this review exposed dams and/or pups either to one or different combinations of the following developmental periods: (a) exposure to dams during gestation, (b) exposure to dams during lactation until weaning, and (c) direct exposure to pups prior to weaning. The extent of exposure to pups may not be known when dams are exposed (Holson et al., 2006). Studies involving exposures beyond weaning were only included (primarily single- or multigeneration studies) if neurodevelopmental endpoints were evaluated within 2 wk of weaning (e.g., by postnatal day [PND] 35) and exposure began prior to weaning.
Repeated exposure: This review focused on associations of repeated exposure (at least 2 days of dosing), and not the acute effects of single dose studies. An exception to this rule was made for studies measuring latent effects of neonatal exposure to a single dose of DDT (Eriksson et al., 1993; Johansson et al., 1995) and other organochlorines (OC). DDT is stored in fatty tissue and slowly released from storage thereby potentially prolonging the exposure period (ATSDR, 2002).
Route of exposure: Oral, inhalation, and dermal routes of exposure were considered to be the most relevant for human exposure. The subcutaneous (sc) and intraperitoneal (ip) routes of exposure were included in the overall analysis, but given less weight than oral studies. Studies using intravenous or direct injections into the brain were not included.
Neurodevelopmental outcomes: This review focused on studies that measured effects on neurobehavior, neurophysiology, brain histopathology, and brain morphology endpoints. Sexual behaviors were not included. Neuropharmacologic endpoints such as cholinesterase inhibition, neurotransmitter levels, and receptor binding were also included. Molecular and mechanistic endpoints such as mRNA, DNA, and protein levels, and adenylate cyclase activity were not included.
APPROACH TO EVALUATION OF ANIMAL STUDIES
The criteria used to survey the overall quality of the published studies for each pesticide included in this review were consistent with an approach used by an expert panel of developmental and reproductive toxicologists that evaluated the utility of animal developmental studies for risk assessment purposes (Chapin et al., 2008):
The Panel attended to multiple design and analysis characteristics in judging the acceptability of individual papers. It was our consensus that for a paper to be acceptable for this review process, several conditions had to be met. First, effects related to litter of origin needed to be accounted for in design and statistical procedures … Third, a minimum of 6 animals per treatment condition needed to be used to provide minimal confidence in results. Fourth, if similar tests were conducted at multiple ages, the statistical analyses needed to account for repeated measurement in order not to inflate degrees of freedom. (235–236)
These criteria are also consistent with those recommended by Adams (2010) and Maurissen (2010) for publishing developmental neurotoxicity studies and by Holson et al. (2008) for analyses of data (Li et al., 2012a). The following definitions and criteria were used to characterize published studies in summary tables 10–11 and 13–18 (shown later> and to select the most sensitive NOEL or LOEL:
TABLE 10.
Organophosphate in vivo mammalian studies
| Compound | Total Number of Papers Evaluateda | Exposure Period(s) for Which Literature Is Availableb | Total Number of Studies Evaluateda for Each Exposure Period | Species (# of studies) | Routec (# of studies) | Litter of Origin Was Unit of Analysis (# of studies) | Dose/Response within the Studyd (# of studies) | Number of Studies with ≥ 6 Litters | Brain AChE inhibition Measured | Lowest Offspring NOEL/LOEL, First Author, Year, (Neuro Endpoints) mg/kg | Offspring brain or RBC AChE inhibition NOEL or LOEL Author, Year mg/kg | Regulatory Numbers (mg/kg/day) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azinphosmethyl | 1 | Gestation only | 1 Astroff and Young, 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 2.0 Astroff and Young, 1998 NOEL (brain AChE inhibition) | 2.0 brain NOEL, Astroff and Young, 1998 | EPA RED Chronic RfD (2006): NOEL 0.149/UF 100, FQPA 1 = 0.00149 US phase-out Sept 2012 JMPR ADI (1997): 0.03; EU ADI 0.005, (2006) |
| Chlordimeform | 1 | Gestation only | 1 Olson et al., 1978 | rat (1) | oral (1) | 0 | 0 | 0 | 0 | 0.1, Olson 1978; LOEL (delay swimming) | Not measured | EPA – revoked mid 1980s JMPR ADI (1985, withdrawn 1987): 0.0001 |
| Chlorpyrifos | 60 | Gestation only | 26 See references belowe | rat (18) mouse (8) | oral (9) dermal (3) s.c. (14; thirteen DMSO) | 10 | 19 | 13 | 18 | 0.1, Abdel Rahman 2003, 2004; LOEL (increase brain AChE activity) | 0.1 brain LOEL, Abdel-Rahman 2003, 2004 | EPA RED Chronic PAD (2011): BMDL10 0.03/UF 100, FQPA 1 = 0.0003 JMPR ADI: 0.01(2004); EU: ADI 0.01 (2005) UC 100 |
| PND direct dosing to pups | 38 See references belowf | rat (34) mouse (4) | oral (12) s.c. (26, twenty-one DMSO) | 24 | 15 | 12 | 18 | 0.3, Jett 2001; LOEL (spatial learning) | 0.75 brain, RBC NOEL, Zheng 2000 | |||
| Gestation and PND | 5 See references belowg | rat (2) mouse (3) | oral (4) s.c. (1) | 5 | 5 | 4 | 4 | 0.2, Braquenier 2010; NOEL (“anxiety-like profile”) | 1.0 brain NOEL, Mattson 2000 | |||
| PND through dams | 2 Amira 2005, Tang et al., 1999 | rat (2) | oral (1) s.c. (1) | 1 | 1 | 1 | 2 | 40, Amira 2005; LOEL (abnormal cerebellar morphology, brain AChE inhibition) | 40, brain LOEL, Amira 2005 | |||
| Coumaphos | 1 | Gestation and PND | 1 Astroff et al., 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 0.29, Astroff et al., 1998; NOEL (brain AChE inhibition) | 0.29 brain and RBC NOEL, Astroff et al., 1998 | EPA RED Chronic RfD (2006): NOEL 0.025/UF 100, FQPA 1 = 0.0003 JMPR ADI (1978 withdrawn 1980): 0.0005 |
| Diazinon | 7 | Gestation only | 1 Spyker and Avery, 1977 | Mouse (1) | Oral (1) | 1 | 1 | 1 | 0 | 0.18, Spyker and Avery, 1977 LOEL (behavioral ontogeny, motor function) | Not measured | EPA RED Chronic RfD (2006): NOEL 0.02/UF 100, FQPA 1 = 0.0002 JMPR ADI (2006): 0.005, EU:ADI 0.0002 (2006) |
| PND direct dosing to pups | 6 Roegge et al., 2008, Slotkin et al., 2006a,b 2008a,b, Timofeeva et al., 2008a | rat (6) | s.c. (6; six DMSO) | 0 | 6 | 6 | 1 | 0.5, Roegge 2008, Slotkin 2006a,b, 2008a,b, Timofeeva et al., 2008a; LOEL (neurochemical and behavioral effects) | 2.0 brain LOEL, Slotkin et al., 2006b | |||
| Dichlorvos | 7 | Gestation only | 7 Dambska et al., 1979, Dambska and Maslinska, 1988, Desi and Nagymajtenyi, 1999, Maslinska et al., 1981, Maslinska and Zalewska, 1978, Mehl et al., 1994, Zalewska et al., 1977 | rat (2) rabbit (4) guinea pig (1) | oral (2) i.p. (1) not stated (5) | 3 | 4 | 1 | 4 | 1.1, Zalewska 1977; LOEL (brain AChE inhibition) | 1.1 brain LOEL, Zalewska 1977 | EPA RED Chronic RfD (2006): NOEL 0.05/UF 100, FQPA 1 = 0.0005 JMPR ADI (2010): 0.004, EU (UF 10) (tentative) ADI: 0.00008 (2006) |
| Gestation and PND | 1 Desi and Nagymajtenyi, 1999 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 4.0, Desi 1999; NOEL (electro-physiology) | 4.0 brain NOEL, Desi 1999 | |||
| Dimethoate | 4 | Gestation only | 4 DeSesso et al., 2009h, Mehl et al., 1994, Nagymajtenyi et al., 1998, Reiss and Gaylor, 2005, Srivastava and Raizada, 1996 | rat (3) guinea Pig (1) | oral (3) not stated (1) | 1 | 3 | 3 | 2 | 0.65, Reiss 2005/DeSesso 2009h; BMDL10 (brain AChE inhibition) | 0.65, brain BMDL10, Reiss 2005/DeSesso 2009h | EPA RED Chronic iRfD (2007): BMDL10 0.22/UF 100, FQPA 1 = 0.0022 JMPR ADI (2003): 0.002; EU (2007) ADI 0.001 |
| Dimethoate (continued) | Gestation and PND | 2 DeSesso et al., 2009, Nagymajtenyi et al., 1998 | rat (2) | oral (2) | 1 | 2 | 2 | 2 | 0.58, DeSesso 2009; BMDL10 (brain AChE inhibition) | 0.58 brain BMDL10, Reiss 2005/DeSesso 2009h | ||
| Fenamiphos | 2 | Gestation only | 1 Astroff and Young, 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 3.0, Astroff and Young, 1998; NOEL (brain AChE inhibition | 3.0 brain NOEL, (Astroff and Young, 1998) | EPA iRED Chronic RfD (2002): NOEL 0.01 / UF 100, FQPA 1 = 0.0001 JMPR ADI (2002): 0.0008, EU (2006) ADI 0.0008 |
| Gestation and PND | 1 Astroff et al., 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 2.78, Astroff et al., 1998; NOEL (brain AChE inhibition) | 0.62 RBC NOEL, >2.78 brain NOEL, Astroff et al., 1998 | |||
| Fenitrothion | 1 | Gestation only | 1 Struve et al., 2007 | rat (1) | oral (1) | 1 | 1 | 1 | 0 | 20, Struve 2007; LOEL (brain morphometry) | Not measured | EPA RED Chronic RfD (1999): NOEL 0.13/UF 100, FQPA 1 = 0.0013 JMPR ADI (2004): 0.005, EU (2006) ADI 0.005 |
| Fenthion | 1 | Gestation only | 1 Astroff and Young, 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 18, Astroff and Young, 1998; NOEL (brain AChE inhibition) | 18 brain NOEL, Astroff and Young, 1998 | EPA RED Chronic RfD (2001): NOEL 0.02 / UF 300, FQPA 1 = 0.00007 cancellation of all US registrations 2003 JMPR ADI (2004): 0.005, EU (2006) ADI 0.005 |
| Isofenphos | 1 | Gestation only | 1 Astroff and Young, 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 4, Astroff and Young, 1998; NOEL (brain AChE inhibition) | 4 brain NOEL, Astroff and Young, 1998 | EPA RED Chronic RfD (prior to 1999): NOEL 0.08/ UF 1000 = 0.00008 cancellation of US registrations 1999 JMPR ADI (1986): 0.001 |
| Malathion | 1 | PND through dams | 1 da Silva et al., 2006 | mouse (1) | s.c. (1) | 1 | 1 | 0 | 1 | 20, da Silva 2006; LOEL (brain AChE inhibition) | 20 brain LOEL, Da Silva, 2006 | EPA RED Chronic RfD (2009): BMDL10 NOEL 0.71/UF 100, FQPA 1 = 0.071 JMPR ADI (2004): 0.3, EU (2006) ADI 0.03 |
| Metasystox-R (oxydemeton-methyl) | 3 | Gestation only | 2 Astroff and Young, 1998, Clemens et al., 1990 | rat (2) | oral (2) | 2 | 2 | 2 | 2 | 4.5, Clemens 1990; NOEL (developmental reflexes, learning, motor activity) | 4.5 brain NOEL, Astroff and Young, 1998, Clemens 1990 | EPA RED Chronic RfD (2006): NOEL 0.013/UF 100, FQPA 1 = 0.00013 JMPR (2004) ADI 0.003, EU (2006) ADI: 0.0003 |
| Gestation and PND | 1 Astroff et al., 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 0.36, Astroff et al., 1998; NOEL (brain AChE inhibition) | 0.36 RBC, brain NOEL, Astroff et al., 1998 | |||
| Methamidophos | 2 | Gestation only | 2 de Castro et al., 2000a,b | rat (2) | oral (2) | 0 | 0 | 2 | 0 | 1, de Castro 2000b; LOEL (motor activity) | Not measured | EPA RED Chronic RfD (2006): NOEL 0.03/UF 100, FQPA 3 = 0.0001 cancellation of US registrations 2009 JMPR ADI (2004): 0.004, EU (2006) ADI 0.01 |
| Methyl parathion | 6 | Gestation only | 2 Crowder et al., 1980, Gupta et al., 1985 | rat (2) | oral (2) | 1 | 1 | 1 | 1 | 1, Gupta 1985 LOEL (motor and operant behavior) | 1 brain LOEL, Gupta 1985 | EPA RED Chronic RfD (2006): NOEL 0.021 / UF 100, FQPA 10 = 0.00002 cancellation of US registrations December 2012 JMPR ADI (2003): 0.003 |
| PND direct dosing to pups | 4 Eells and Brown, 2009, Guo-Ross et al., 2007, Liu et al., 1999, Tang et al., 2003 | rat (4) | oral (3) s.c. (1) | 1 | 3 | 1 | 3 | 0.2, Guo-Ross 2007; LOEL (muscarinic receptor binding) | 0.2 brain NOEL, Guo-Ross 2007 | |||
| Parathion | 13 | Gestation only | 4 al-Hachim and Fink, 1968a,b,c, Veronesi and Pope, 1990 | mouse (3) rat (1) | oral (4) | 0 | 0 | 2 | 1 | 0.882, Veronesi 1990; LOEL (brain AChE inhibition, histopathology, muscarinic receptor binding) | 0.882 brain LOEL, Veronesi 1990 | EPA RED Chronic RfD 0.006, POD not available cancellation of US registrations 2006 JMPR ADI (2000): 0.004, EU (2001) ADI 0.0006 |
| PND direct dosing to pups | 9 Levin et al. 2010, Slotkin et al. 006a, b, 2008, 2009a,b, Stamper et al 1988, Timofeeva et al. 2008b, Dvergsten and Meeker 1994 | rat (9) | s.c. (9; seven DMSO) | 1 | 9 | 8 | 3 | 0.02, Slotkin 2006b; NOEL (brain AChE inhibition) | 0.02 brain NOEL, Slotkin 2006b | |||
| Quinalphos | 2 | Gestation only | 2 Srivastava and Raizada, 1999, Srivastava et al., 1992 | rat (2) | oral (2) | 1 | 2 | 2 | 2 | 0.5, Srivastava 1992; LOEL (brain AChE inhibition) | 0.5 brain LOEL, Srivastava 1992, (not repeated by Srivastava 1999: NOEL 2.0) | EPA IRIS Chronic RfD (1992): NOEL 0.05/UF 100, FQPA 1 = 0.0005 JMPR ADI: none. EU Pesticides Database: no ADI, withdrawn 2002. |
| Tribufos | 2 | Gestation only | 1 Astroff and Young, 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 28, Astroff and Young, 1998; NOEL (brain AChE inhibition) | 28 brain NOEL, Astroff and Young, 1998 | EPA RED Chronic RfD (2006): NOEL 0.1 / UF 100, FQPA 10 = 0.0001 JMPR ADI: not reviewed. EU: no entry. |
| Gestation and PND | 1 Astroff et al., 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 2.08, Astroff et al., 1998; NOEL (brain AChE inhibition) | 2.08 brain NOEL, Astroff et al., 1998 | |||
| Trichlorfon | 7 | Gestation only | 8 Berge et al., 1987a,b, Hjelde et al., 1998, Mehl et al., 1994, 2007, Pope et al., 1986, | pig (5) guinea pig (3) | oral (4) s.c. (2) i.p. (1) not stated (1) | 0 | 2 | 1 | 2 | 50, Hjelde 1998; NOEL (regional brain weight) | 125 brain LOEL, Mehl 2007 | EPA RED Chronic RfD (2006): NOEL 0.2/UF 100, FQPA 10 = 0.0002 JMPR ADI (2003, 2006): 0.002; EU (2001): ADI 0.045 |
| PND direct dosing to pups | 1 Berge et al., 1987b | pig (1) | i.p. (1) | 0 | 0 | 0 | 0 | 35–50i, Berge et al., 1987b LOEL (cerebellar pathology) | Measured but results not reported | |||
| Gestation and PND | 1 Astroff et al., 1998 | rat (1) | oral (1) | 1 | 1 | 1 | 1 | 7, Astroff et al., 1998 repro tox; NOEL (brain AChE inhibition) | 7 brain NOEL, Astroff et al., 1998 |
One paper may contain more than one study if it includes more than one exposure period, exposure route, or species.
Four exposure periods were defined: gestation only, PND direct dosing to pups, gestation and PND, and PND through dams.
Number of studies using DMSO as a vehicle is recorded.
Two or more doses tested per exposure period.
Chlorpyrifos gestation-only references: Abdel-Rahman et al., 2003, 2004, Abou-Donia et al., 2006, Billauer-Haimovitch et al., 2009, Chen et al., 2011b, Haviland et al., 2010, Lassiter et al., 1998, 1999, Ricceri et al., 2006, Turgeman et al., 2011, Venerosi et al., 2006, 2009, 2010, Aldridge et al., 2005a,b,c, Chanda and Pope, 1996, Farag et al., 2003, Icenogle et al., 2004, Levin et al., 2002, Qiao et al., 2002, 2003, 2004, Richardson and Chambers, 2003, 2004, Slotkin and Seidler, 2007.
Chlorpyrifos PND direct dosing references: Betancourt and Carr, 2004, 2007, Carr et al., 2001, 2011, Carr and Nail, 2008, Eells and Brown, 2009, Guo-Ross et al., 2007, Johnson et al., 2009, Richardson and Chambers, 2005, Zheng et al., 2000, Aldridge et al., 2003, 2004, 2005a, b, c, Chakraborti et al., 1993, Dam et al., 1999, 2000, Jett et al., 2001, Levin et al., 2001, Liu et al., 1999, Raines et al., 2001, Rhodes et al., 2004, Roy et al., 2004, 2005, Ricceri et al., 2006, Slotkin et al., 2001, 2002, 2004, 2005, 2006a, b, Slotkin and Seidler, 2007, Song et al., 1997, Venerosi et al., 2006, 2009, 2010, Turgeman et al., 2011.
Chlorpyrifos Gestation and PND dosing references: Braquenier et al., 2010, Mattsson et al., 2000, Maurissen et al., 2000, Ricceri et al., 2006, Venerosi et al., 2006.
DeSesso/Reiss are considered one study because they are based on the same dataset.
There was a range of individual doses used in a single sow.
TABLE 11.
Comparison of neurobehavioral effects following PND 1–4 sc injections of parathion, diazinon or chlorpyrifos
| Parathion (Timofeeva et al., 2008b) | Parathion (Levin et al., 2010, Slotkin et al., 2006b) | Diazinon (Timofeeva et al., 2008a, Roegge et al., 2008) | Chlorpyrifos (Aldridge et al., 2005a) | Chlorpyrifos (Levin et al., 2001) | ||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg, s.c, DMSO) | 0.1 | 0.2 | 0.1 | 0.2 | 0.5 | 2.0 | 0.1 | 0.1 |
| Mortality | 0 | ↑ | 0 | ↑ | 0 | 0 | 0 | 0 |
| Chocolate milk preference | 0 | 0 | n.a. | n.a. | M↓FO | 0 | ↓ | n.a. |
| Elevated plus maze | 0 | Open arms: ↑ Center crosses: ↑ | n.a. | n.a. | 0 | Open arms: M↓FO | Open arms: M↑ F0 Center crosses: M↑ F0 | n.a. |
| Motor activity | 0 | 0 | n.a. | n.a. | 0 | 0 | n.a. | 0 |
| Radial Arm Maze (RAM) Working memory (# errors 18 trials) | ↓ | 0 | M↑FO | M↑FO | ↑ | 0 | MOF↓ | M↑FO |
| RAM Reference memory (# errors 18 trials) | 0 | 0 | M↑FO | M↑FO | 0 | 0 | M↑F↓ | M↑F↓ |
| Spontaneous alternation (T-maze, no reinforcement) | 0 | 0 | n.a. | n.a. | 0 | 0 | n.a. | n.a. |
| Latency to initiate choice on spontaneous alternation test | 0 | 0 | n.a. | n.a. | ↓ | ↓ | n.a. | n.a. |
| Startle reflex | 0 | ↓ | n.a. | n.a. | 0 | 0 | n.a. | n.a. |
| Pre –pulse inhibition of startle | 0 | 0 | n.a. | n.a. | M↓FO | M↓FO | n.a. | n.a. |
| RAM Ketanserin challenge | 0 | 0 | n.a. | n.a. | 0 | 0 | ↑ errors | n.a. |
| RAM Scopolamine challenge | 0 | 0 | n.a. | n.a. | ↑ (enhance) | 0 | n.a. | 0 |
| RAM Dizoclipine challenge | ↓ | 0 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| RAM Mecamylamine challenge | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0 |
TABLE 13.
2,4-D in vivo mammalian studies
| Compound | Total Number of Papers Evaluateda | Exposure Period(s)b for Which Literature Is Available | Total Number of Studies Evaluateda for Each Exposure Period | Species (# of studies) | Routec (# of studies) | Litter of Origin was unit of analysis (# of studies) | Dose/Response within the Studyd | Number of Studies with ≥6 Litters | Lowest Offspring NOEL/LOEL, First Author, Year, (Neuro Endpoint) | Regulatory Numbers (mg/kg/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2,4-D | 13 | Gestation only | 1 de Duffard et al., 1990 | rat (1) | oral (1) | 0 | 0 | 0 | 70, de Duffard 1990; NOAEL (serotonin neurochemistry) | EPA RED chronic PAD (2005): NOAEL 5.0/1,000 UF, FQPA 1 = 0.005 JMPR ADI (2001): 0.01; EU (2001) ADI 0.05 |
| PND direct dosing to pups | 1 Rosso et al., 2000 | rat (1) | s.c. (1) | 0 | 1 | 0 | 70, Rosso 2000; LOEL (myelin staining and motor activity) | |||
| Gestation and PND | 6 Bortolozzi et al., 1999, 2002, 2003, 2004, de Duffard et al., 1990, Garcia et al., 2001 | rat (6) | oral (6) | 0 | 0 | 0 | 70, Bortolozzi 1999, 2002, 2003, 2004, Garcia 2001; LOEL (developmental reflexes, neurobehavior, dopamine and serotonin neurochemistry) | |||
| PND through dams | 6 Duffard et al., 1996, Evangelista de Duffard 1995, Ferri et al., 2000, Garcia et al. 2004, 2006, Konjuh et al., 2008 | rat (6) | i.p. (6; five DMSO vehicle) | 1 | 2 | 0 | 70, Evangelista de Duffard 1995, Garcia 2004, 2006, Konjuh 2008; LOEL (serotonin, catecholamine and myelin immunostaining, myelin levels) |
One paper may have more than one study if there is more than one exposure period, exposure route, or species.
Four exposure periods were defined: gestation only, PND direct dosing to pups, gestation and PND, and PND through dams.
Number of studies using DMSO as a vehicle is recorded.
Two or more doses tested per exposure period.
TABLE 18.
Miscellaneous pesticides with fewer than 5 in vivo mammalian studies
| Compound (Class) | Total Number of Papers Evaluateda | Exposure Period(s) for Which Literature Is Availableb | Total Number of Studiesa Evaluated for Each Exposure Period | Species (# of studies) | Route (# of studies) | Litter of Origin was Unit of Analysis (# of studies) | Dose/Response within the Studyc (# of studies) | Number of Studies with ≥6 Litters | Overall Offspring NOEL/LOEL with Author and Year Using Neuro Endpoints mg/kg | Regulatory Numbers mg/kg/day |
|---|---|---|---|---|---|---|---|---|---|---|
| Amitraz (formamidine) | 1 | PND through dams | 1 Palermo-Neto et al., 1997 | rat (1) | oral (1) | 0 | 0 | 1 | 10, Palermo-Neto 1997; LOEL (motor activity) | EPA Acuted RfD (2010): NOEL 0.125 / UF 100, FQPA1 = 0.00125 JMPR ADI (1998): 0.01 |
| Atrazine (chlorotriazine) | 2 | Gestation only | 2 Belloni et al., 2011, Fraites et al., 2011 | rat (1) mouse (1) | oral (2) | 2 | 2 | 2 | 0.001, Belloni 2011; LOEL (social behaviors, learning and memory) | EPA chronic RfD 0.018 (2006): NOEL 1.8 / UF 100, FQPA 10 for chronic PAD (dietary) |
| Azadirachtin (tetranortriterpenoid) | 1 | Gestation and PND | 1 Srivastava and Raizada, 2007 | rat (1) | oral (1) | 0 | 1 | 1 | 50, Srivastava 2007; NOEL (neuropathology) | No EPA RfD (registration review ongoing), not reviewed by JMPR |
| Benomyl (benzimidazole / carbamate) | 2 | Gestation only | 2 Ellis et al., 1987, Zeman et al., 1986 | rat (2) | oral (2) | 1 | 1 | 2 | 31.2, Zeman 1986, Ellis 1987; LOEL (neuropathology) | EPA IRIS RfD (1989): NOEL 5.0/UF 100, MF 1 = 0.05 (cancellation of US registrations 2002) JMPR ADI (1995): 0.1 |
| Carbendazim (benz imidazole) | 1 | Gestation only | 1 Vergieva, 1985 | rat (1) | oral (1) | 0 | 1 | 0 | 15.6, Vergieva 1985; LOEL (developmental reflex) | EPA RED chronic PAD (2005): NOEL 2.5 / UF 100, FQPA 10 = 0.0025 JMPR ADI (1995, 2005): 0.03 |
| Chlordimeform (formamidine) | 1 | Gestation and PND | 1 Olson et al., 1978 | rat (1) | oral (1) | 0 | 0 | 1 | 0.1, Olson 1979; LOEL (motor behavior) | No EPA data available (cancellation of US registrations 1987) JMPR ADI (1985): 0.0001, withdrawn 1987 |
| Dinocap (dinitrophenol) | 2 | Gestation only | 4 Gray et al., 1986, 1988 | rat (1) mouse (2) hamster (1) | oral (4) | 1 | 4 | 4 | 6, Gray 1988; NOEL (developmental reflex) | EPA RED Chronic PAD (2003): NOEL 0.375 / UF 100, FQPA 10 = 0.004 JMPR ADI (1998, 2000): 0.008 |
| Emamectin (avermectin) | 1 | Gestation and PND | 1 Wise et al., 1997 | rat (1) | oral (1) | 1 | 1 | 1 | 0.6e Wise 1997, NOEL (neurohistology, auditory startle, motor activity, learning and memory) | EPA RED Chronic PAD (2008): NOEL 0.075 / UF 100, FQPA 10 = 0.000075 JMPR: not reviewed |
| Imazalil (conazole) | 1 | Gestation and PND | 1 Tanaka, 1995 | mouse (1) | oral (1) | 0 | 1 | 1 | 21f, Tanaka 1995; LOEL (developmental reflex) | EPA RED (2003): chronic oral NOEL 2.5 JMPR ADI (2000, 2005): 0.03 |
| Imidacloprid (nicotinoid) | 1 | Gestation only | 1 Abou-Donia et al., 2008 | rat (1) | i.p. (1) | 1 | 0 | 0 | 337, Abou-Donia 2008; LOEL (motor activity, neurochemistry) | EPA Chronic PAD (2006): NOEL 5.7 / UF 100, FQPA 1 = 0.057; JMPR ADI (2001): 0.06 |
| Mancozeb (dithiocarbamate) | 2 | Gestation only | 1 Miranda-Contreras et al., 2005 | mouse (1) | i.p. (1) | 1 | 0 | 1 | 30, Miranda-Contreras 2005; LOEL (motor activity, neurochemistry) | EPA RED Chronic PAD (2005): NOEL 4.83 / UF 100, FQPA 1 = 0.05 JMPR ADI (1993): 0.03; |
| Gestation and PND | 1 Axelstad et al., 2011 | rat (1) | oral (1) | 1 | 1 | 1 | 100, Axelstad 2011; NOEL (motor activity, auditory startle, learning and memory) | |||
| Maneb (dithiocarbamate) | 4 | Gestation only | 1 Barlow et al., 2004 | mouse (1) | s.c. (1) | 0 | 0 | 1 | 1, Barlow 2004; LOEL (neurohistology) | EPA RED Chronic PAD (2005): NOEL 5 / UF 100, FQPA 1 = 0.05 (cancellation of US registrations 2010) JMPR ADI (1993): 0.03 |
| Gestation and PND through dams | 1 Chernoff et al., 1979 | rat (1) | oral (1) | 1 | 1 | 1 | 480, Chernoff 1979; NOEL (open field behavior, developmental reflexes) | |||
| PND direct dosing to pups | 2 Thiruchelvam et al., 2002, Sobotka et al., 1972 | rat (1) mouse (1) | oral (1) i.p. (1) | 1 | 1 | 2 | 1, Thiruchelvam 2002; LOEL (dopamine histochemistry) 0.5 ppm Sobotka 1971, LOEL (AChE inhibition) | |||
| Paclobutrazol (triazole) | 1 | PND through dams | 1 de Castro et al., 2004 | rat (1) | oral (1) | 1 | 0 | 1 | 1, de Castro 2004; LOEL (motor activity, auditory startle) | EPA 2007: chronic dietary RfD is not required because paclobutrazol is not used for food JMPR ADI (1988): 0.1 |
| Paraquat (quaternary ammonium) | 4 | Gestation only | 2 Barlow et al., 2004, Miranda-Contreras et al., 2005 | mouse (2) | i.p.(1) s.c. (1) | 1 | 0 | 2 | 0.3, Barlow 2004; NOEL (motor activity, dopamine histochemistry) | EPA RED Chronic RfD (1997): NOEL 0.45 / UF 100, FQPA 1 = 0.0045 JMPR ADI (2003): 0.005 |
| PND direct dosing to pups | 2 Fredriksson et al., 1993, Thiruchelvam et al., 2002 | mouse (2) | oral (1) i.p. (1) | 1 | 1 | 1 | 0.07, Fredriksson 1993; LOEL (motor activity, dopamine neurochemistry) | |||
| Piperonyl butoxide (synergist) | 2 | Gestation and PND | 2 Tanaka, 1992, Tanaka, 2003 | mouse (2) | oral (2) | 0 | 2 | 2 | 17g, Tanaka 2003; LOEL (developmental reflex) | EPA RED Chronic PAD (2006): NOEL 15.5/ UF 100, FQPA 1 = 0.16 JMPR ADI (1995, 2001): 0.02 |
| Prochloraz (amide/conazole) | 1 | Gestation and PND | 1 Vinggaard et al., 2005 | rat (1) | oral (1) | 1 | 0 | 1 | 30, Vinggard 2005; LOEL (motor activity) | EPA IRIS RfD (1989): NOEL 0.9 / UF 100, MF 1 = 0.009; (cancellation of US registrations) JMPR ADI (2001): 0.01 |
| Sulfentrazone (anilide/triazolone) | 1 | Gestation only | 1 de Castro et al., 2007 | rat (1) | oral (1) | 1 | 1 | 1 | 25, de Castro 2007; LOEL (motor activity, developmental reflex) | EPA RED Chronic PAD (2003): NOEL 14 / UF 100, FQPA 1 = 0.14 JMPR: not reviewed |
| Thiabendazole (benzimidazole/thiazole) | 1 | Gestation and PND | 1 Tanaka, 2001 | mouse (1) | oral (1) | 1 | 1 | 1 | 50h, Tanaka 2001; LOEL (developmental reflex) | EPA RED Chronic PAD (2002): NOEL 10 / UF 100, FQPA 1 = 0.1, JMPR ADI (1997, 2006): 0.1 |
| Thiobencarb (thiocarbamate) | 1 | PND direct dosing to pups | 1 Pentyala and Chetty, 1993 | rat (1) | oral (1) | 0 | 1 | 0 | 20, Pentyala 1993; LOEL (AChE inhibition, enzyme activity) | EPA RED Chronic RfD (1997): NOEL 1 / UF 100, MF 1 = 0.01 JMPR: not reviewed |
| Triadimefon (conazole) | 1 | PND direct dosing to pups | 1 Reeves et al., 2004 | mouse (1) | i.p. (1) | 1 | 0 | 1 | 25, Reeves 2004; LOEL (dopamine neurochemistry) | EPA RED Chronic PAD (2006): NOEL 3.4 / UF 100, FQPA 10 = 0.0034 JMPR ADI (2004) 0.03 |
| Tributyltin oxide (organotin) | 1 | Gestation only | 1 Crofton et al., 1989 | rat (1) | oral (1) | 0 | 1 | 1 | 2.5, Crofton 1989; LOEL (motor activity) | EPA Chronic RfD (2008): BMD10 0.03 / UF 100, MF 10 = 0.00003 JMPR: not reviewed |
| PND direct dosing to pups | 1 Crofton et al., 1989 | rat (1) | oral (1) | 0 | 1 | 1 | 40, Crofton 1989; LOEL (auditory startle) | |||
| Vinclozolin (dichlorophenyl dicarboximide/oxazole) | 4 | PND direct dosing to pups | 1 Hotchkiss et al., 2003 | rat (1) | s.c. (1) | 1 | 0 | 1 | 200, Hotchkiss 2003; LOEL (social behavior) | EPA RED Chronic PAD (2000): NOEL 1.2 / UF 100, FQPA 10 = 0.0012 JMPR ADI (1995): 0.01; |
| Gestation and PND | 3 Bisenius et al., 2006, Colbert et al., 2005, Flynn et al., 2001 | rat (2) rabbit (1) | oral (3) | 2 | 2 | 2 | 3, Colbert 2005; NOEL (social behavior) | |||
| Zineb (dithiocarbamate/zinc) | 1 | PND direct dosing to pups | 1 Jia and Misra, 2007 | mouse (1) | i.p. (1) | 0 | 0 | 0 | 5, Jia 2007; NOEL (dopamine neurochemistry) | EPA IRIS Chronic RfD (1988): LOEL 25 / UF 500, MF 1 = 0.05 (cancellation of US registrations 1989) JMPR ADI (1993): 0.03 |
One paper may contain more than one study if it includes more than one exposure period, exposure route, or species.
Four exposure periods were defined: gestation only, PND direct dosing to pups, gestation and PND, and PND through dams.
Two or more doses tested per exposure period.
The single-dose human oral endpoint was considered to be protective for all exposure durations. (EPA 2010; EPA-HQ-OPP-2009-1015-0004).
The chronic POD is based on EPA's NOEL for Wise et al. 1997 which is lower than the author's conclusions.
The dose is based on the low dose chemical intake of 21 mg/kg calculated by the author during gestation and listed in Table 1, page 283.
The dose is based on the low dose chemical intake of 17 mg/kg calculated by the author during gestation and listed in Table 1, page 210.
This dose is based on the low dose chemical intake of 50 mg/kg calculated by the author during gestation and listed in Table 1, page 378 There were no injection studies that used DMSO vehicle.
Number of “papers.” A “paper” was defined as a peer-reviewed publication in the English language accessible through MEDLINE. The inclusion criteria for papers were described earlier.
Exposure period. The studies were categorized into four main exposure periods: (1) gestational exposure only; (2) postnatal exposure directly to pups and prior to weaning; (3) gestational and postnatal exposure prior to weaning (including direct pup exposure and/or lactational exposure); or (4) postnatal exposure to dam during lactation.
Number of “studies” in each category. A paper could include more than one “study” if different species, exposure periods (as defined earlier) and/or routes of exposure were tested in different groups of animals in the same paper.
Litter of origin was the experimental unit of analysis. Maternal influences during gestation and lactation may exert significant effects on developmental outcomes. Therefore, the litter of origin needs to be accounted for in evaluating neurodevelopmental outcomes for both gestational and postnatal exposures (DeSesso et al., 2009; Holson et al., 2008; Holson and Pearce, 1992). Studies that selected one offspring per original litter exposed or included litter of origin as a factor in the statistical analyses met this criterion. Studies that repeatedly randomized pups and dams so that the litter of origin could no longer be discerned did not meet this criterion for gestational exposures. For postnatal exposures, it is unclear what the impact on study outcome is for experimental designs involving repeated randomization of pups in litters and rotation of dams among reconstituted litters. Thus, this practice was not considered a strength or weakness for postnatal exposure studies. One rationale for this practice is “to distribute any maternal caretaking differences randomly across litters and treatment groups” (Aldridge et al., 2003). This rationale may not fully consider that maternal caretaking has much more impact during early lactation when pups are fully dependent on the dam (DeSesso et al., 2009). It is also possible for maternal care in treated groups to be disproportionally impacted by the combination of (a) repeated disruption of dams and reconstituted litters and (b) sc injection of pups postnatally with test material that might cause acute toxicity in pups (e.g., 75% AChE inhibition 2 h after PND 1 dosing with 1 mg/kg CPF in Dam et al., 2000) as well as potentially causing the pups to taste or smell different. Studies that selected one male and one female from each litter and combined data from both sexes in the analysis but did not include litter as a variable in the statistical analysis did not meet this criterion.
Number of litters greater than or equal to 6. If at least one treated dose group included animals from six or more litters for at least one endpoint, then the study met this criterion for tabulation, regardless of whether or not the litter of origin was the experimental unit of analysis. For example, studies with two or more pups per litter from six litters per dose group or those that cross-fostered pups following gestational exposure met this criterion as long as pups were selected from six or more litters. Meeting this minimal criterion was not synonymous with meeting criterion for a robust study. The Organization for Economic Cooperation and Development (OECD) test guideline for developmental neurotoxicity requires a minimum of 10 to 20 litters/dose group for neuropathology or neurobehavioral endpoints (Carr et al., 2011). Thus, 10 litters/dose group with at least 1 pup/sex/litter was required in order to be considered a robust study.
Two or more dose levels tested. For the purposes of characterizing studies for this survey, studies with only one dose level tested were considered to have very limited utility.
Vehicle and route of exposure: Studies that used vehicles that potentially cause developmental effects were given lower weight. Postnatal sc injections of 0.3 ml/kg dimethyl sulfoxide (DMSO) produced widespread apoptosis in the developing mouse brain (Hanslick et al., 2009; Uphouse et al., 1982). DMSO alters pharmacokinetics of the test chemical by significantly enhancing permeability of cell membranes (Gurtovenko and Anwar, 2007). Thus, studies using sc injections of pesticides with DMSO as the vehicle are of low utility for deriving the point-of-departure for risk assessment, but could aid in characterizing potential hazards. For example, if both an oral and sc/DMSO study measured similar endpoints, the NOEL or LOEL from the oral study may be given more weight than from the sc/DMSO study.
NOEL or LOEL for most sensitive DNT endpoint. This survey tabulated the lowest NOEL or LOEL from all the studies in the same exposure period category (e.g., gestation only). The lowest LOEL was reported only if there was no NOEL. The general categories of neurodevelopmental effects, namely, behavior, pathology, neuropharmacology, were tabulated for the lowest LOEL. Only neurodevelopmental endpoints were considered in this survey and were tabulated without any critical analyses of all the evidence. Thus, even though effects on body weight or other endpoints may have been reported in the paper, the NOELs or LOELs for these broader endpoints were not included in this table.
NOELs and LOELs for RBC or brain AChE inhibition (Table 10). For the OF; the lowest NOEL or LOEL for brain or RBC AChE inhibition in the offspring was included because both of these endpoints were used for risk assessment for OP.
U.S. EPA chronic reference dose (RfD) and other regulatory values. The U.S. EPA Office of Pesticides Program (OPP) website for Pesticide Reregistration (http://www.epa.gov/opp00001/reregistration/status.htm) was searched for chronic RfDs and the point-of-departure (POD) was used to derive the chronic RfD. The POD is the lowest no-observed-adverse-effect level (NOAEL) or lowest-observed-adverse-effect level (LOAEL) from repeated dose mouse, rat or dog toxicity and reproductive studies. Comparison with the POD was a convenient way to compare the NOELs or LOELs from developmental neurotoxicity endpoints published in the peer-reviewed literature with the NOELs or LOELs for other toxicity endpoints. In some cases, more recent U.S. EPA OPP decisions on PODs were found in other U.S. EPA summary documents as cited in Tables 10 and later in Tables 13–18. European regulatory numbers were also included, but were not the primary point of comparison.
Critical analyses–-2nd tier evaluation As discussed in point 7 of this section, all studies regardless of whether they met any of the aforementioned criteria were considered in the selection of the lowest NOELs or LOELs. Critical analysis of studies was reserved for those pesticides in which the NOELs or LOELs were numerically lower than the PODs used for chronic RfDs. In this second-tier evaluation, attention was paid to the criteria already described for experimental designs of higher utility, for a pattern of changes from the concurrent control for all parameters tested, and for the experimental and statistical methods used to evaluate these parameters. Although a concurrent control group was most relevant, the historical control data from the same laboratory can provide additional perspective when there were limited or no dose-response data (e.g., only one dose level tested). Additional analyses of some behavioral endpoints were also provided for a few selected pesticides associated with neurobehavioral outcomes relevant to the epidemiologic review.
Animal Studies Literature Search
A comprehensive search of the published English language literature in MEDLINE was conducted using the following core search criteria: (“offspring” OR “neonatal” OR “maternal” OR “in utero” OR “development” OR “developmental” OR “pregnancy” OR “pregnant” OR “gestational” OR “newborn” OR “prenatal” OR “perinatal” OR “teratology” OR “fetus” OR “fetal” OR “maternal” OR “age-dependent” OR “age dependent” OR “age sensitivity”) AND (“brain” OR “neuron” OR “nervous” OR “neurotoxic∗” OR “neurolog∗” OR “neurobehavior∗” OR “neurodevelopment” OR “developmental neurotoxic∗” OR “motor” OR “cognition” OR “cognitive” OR “behavior” OR “receptor” OR “neurotransmitter” OR “cholinesterase” OR “cerebellum” OR “hippocampus” OR “striatum” OR “cortex”). These search criteria were combined with (“pesticide” OR “insecticide” OR “herbicide” OR “fumigant” OR “rodenticide” OR “fungicide” OR “carbamate” OR “organophosphate” OR “pyrethroid”). In addition, the core search terms were also used in combination with the following specific pesticides: acibenzolar-S-methyl, aldicarb, allethrin, benomyl, bioallethrin, bis(tri-n-butyltin)oxide), carbaryl, chlordecone, chlorpyrifos, cypermethrin, DEET, deltamethrin, diazinon, dieldrin, heptachlor, lindane, maneb, methylparathion, parathion, ethyl parathion, permethrin, tebuconazole, trichlorfon, abamectin, acephate, acetamiprid, amicarbazone (MKH3586), atrazine, azinphos methyl, BAS 510 (boscalid), BAS 670H, bifenthrin, busulfan, carbofuran, chlordane, chlordimeform, chlorfenapyr, clodinafoppropargyl, clothianidin, coumaphos, cyfluthrin, cyhalothrin, cymoxanil, DDT, dichlorvos, DDVP; dicrotophos, dimethoate, dinoseb, disulfoton, emamectin, endosulphan, endrin, EPTC, S-ethyl dipropylthiocarbamate, etofenprox, fenamiphos, fenitrothion, fenvalerate, flufenacet, thiafluamide, glufosinate ammonium, glyphosate, glyphosate trimesium, imidacloprid, ivermectin, malathion, mancozeb, methamidaphos, MNDA, n-methyl neodecanamide, molinate, naled, phorate, BAS 2251, profenofos, prothioconazole, spirodiclofen, terbufos, thiamethoxam, and tribufos. These pesticides were selected because they were listed as having at least one report (published or unpublished) on neurodevelopmental outcome on a 2009 poster at the Society of Toxicology Meeting entitled “Building a Database of Neurotoxicants: Evidence from Human and Animal Studies” authored by U.S. EPA scientists (Mundy et al., 2009). Reference lists in recent reviews for pyrethroids and CPF on this topic were reviewed to identify any relevant papers that may have been missed by our electronic search.
RESULTS AND DISCUSSION OF ANIMAL STUDIES
In total, 225 peer-reviewed papers available on MEDLINE on or before April 30, 2011, studying the effects of repeated developmental exposure (2 or more days) to 59 different pesticides on neurobehavioral, neuropharmacologic, or neuropathologic outcomes, met our inclusion criteria (Tables 10–18). These papers included results from 248 different studies (as defined in the Methods section, a paper could include more than one study) that exposed rats, mice, guinea pigs, hamsters, or rabbits to a single pesticide during gestation only, during gestation and postnatally (direct or through lactation), directly to pups postnatally only, or to the dams during lactation only (Figure 1). The individual pesticides with the greatest number of studies were CPF (71), chlordecone (15), 2,4-D (14), parathion (13), DDT (11), trichlorfon (10), and dichlorvos (8). Three of these pesticides (chlordecone, parathion, and DDT) have been withdrawn from U.S. registration.
The large majority of studies were conducted on OP (Figure 1). This included 139 studies on 19 OP, more than half of which included CPF as the test chemical (Table 10, Figure 1). Fourteen studies were conducted on 2,4-D (Table 13). The next largest groups included OC pesticides (31 studies on 9 pesticides including DDT; Table 14 and 15), and pyrethroids (13 studies for three type I pyrethroids and four type II pyrethroids; Tables 16 and 17). There were 40 studies on 23 different pesticides from different classes of chemistry that did not fit into any of the aforementioned classes of pesticides. These were grouped together in a table of miscellaneous pesticides, each of which had fewer than 5 published papers (Table 18). There were no published animal studies on n-methyl carbamates that met the inclusion criteria.
TABLE 14.
Organochlorine in vivo mammalian studies
| Compound | Total Number of Papers Evaluateda | Exposure Periods for Which Literature Is Availableb | Total Number of Studiesa Evaluated for Each Exposure Period | Species (# of studies) | Routec (# of studies) | Litter of Origin was Unit of Analysis (# of studies) | Dose/Response within the Studyd (# of studies) | Number of Studies with ≥6 Litters | Overall Offspring NOEL/LOEL with Author and Year Using Neuro Endpoints mg/kg | Regulatory Numbers mg/kg/day |
|---|---|---|---|---|---|---|---|---|---|---|
| Chlordane | 1 | Gestation only | 1 Al-Hachim and Al-Baker, 1973 | mouse (1) | oral (1) | 0 | 1 | 1 | 1, Al-Hachim 1973; LOEL (learning and memory) | Withdrawn in US 1983, worldwide 2004 EPA IRIS RfD (1997): NOEL 0.15/UF 300, MF none = 0.0005 JMPR ADI (1994): 0.0005 |
| Chlordecone | 14 | Gestation only | 1 Laessig et al., 2007 | rat (1) | i.p. (1) | 1 | 0 | 1 | 5, Laessig 2007; (gonadectomized) LOEL (motor activity) | Withdrawn in US 1975, worldwide 2009 EPA IRIS RfD (2009): BMDL10 0.08 / UF 300, no MF = 0.0003 JMPR no ADI or RfD |
| PND direct dosing to pups | 7 Gray, 1982, Hong and Ali, 1982, Mactutus and Tilson, 1985, Mactutus et al., 1982, 1984, Tilson et al., 1982, Uphouse et al., 1982 | rat (6) hamster (1) | s.c. (7, all DMSO) | 3 | 3 | 5 | 0.25, Gray 1982 LOEL (motor activity) | |||
| Chlordecone (continued) | Gestation and PND | 5 Cooper et al., 1985, Rosecrans et al., 1982, 1984, Seth et al., 1981, Squibb and Tilson, 1982 | rat (5) | oral (4) i.p. (1) | 0 | 2 | 3 | 0.05; Squibb 1982; LOEL (motor activity) | ||
| PND through dams | 1 Jinna et al., 1989 | rat (1) | oral (1) | 0 | 0 | 1 | 2.5; LOEL Jinna 1989 (brain enzyme activity) | |||
| Endosulfan | 3 | PND direct dosing to pups | 2 Jia and Misra, 2007, Lakshmana and Raju, 1994 | rat (1) mouse (1) | oral (1) i.p. (1) | 0 | 0 | 2 | 0.155 Jia 2007 NOEL (dopamine neurochemistry, AChE) | Under review to withdraw as a persistent organic pollutant EPA RED Chronic PAD (2002): NOEL 0.6 / UF 100, FQPA 10 = 0.0006 JMPR ADI (1998): 0.006 |
| Gestation and PND | 1 Cabaleiro et al., 2008 | rat (1) | oral (1) | 0 | 1 | 1 | 0.6; Cabaleiro 2008 LOEL (neurochemistry) | |||
| Endrin | 1 | Gestation only | 2 Gray et al., 1981 | rat (1) hamster (1) | oral (2) | 0 | 2 | 2 | 0.075 Gray 1981 NOEL (motor activity) | Withdrawn in US 1986, worldwide 2004 EPA IRIS RfD (1991): NOEL 0.025/UF 100, MF 1 = 0.0003 JMPR ADI (1994): 0.0002 |
| Heptachlor | 4 | Gestation only | 1 Purkerson-Parker et al., 2001 | rat (1) | oral (1) | 1 | 1 | 0 | 4.2 Purkerson-Parker 2001 LOEL (dopamine neurochemistry) | Withdrawn in US 1988, worldwide 2004 EPA IRIS RfD (1991): NOEL 0.15/UF 300, MF 1 = 0.0005 JMPR ADI (1994): 0.0001 |
| Gestation and PND | 4 Caudle et al., 2005, Moser et al., 2001, Purkerson-Parker et al., 2001, Richardson et al., 2008 | rat (2) mouse (2) | oral (4) | 4 | 2 | 1 | 0.3 NOEL Moser 2001 NOEL (developmental reflexes, motor activity, learning and memory) | |||
| Lindane | 1 | Gestation only | 1 Johri et al., 2007 | rat (1) | oral (1) | 0 | 1 | 0 | 0.06 Johri 2007 NOEL (motor activity, enzyme activity) | Withdrawn in US 2007, worldwide 2009 EPA RED Chronic RfD (2002): NOEL 0.47 / UF 300 = 0.0005; withdrawn 2007 JMPR ADI (2002): 0.005 |
| Methoxychlor | 5 | Gestation only | 3 Palanza et al., 2001, 2002, vom Saal et al., 1995 | mouse (3) | oral (3) | 2 | 3 | 3 | 0.02 Palanza 2002 LOEL (developmental reflexes; inconsistent with Palanza 2001) | Withdrawn in US 2004, not available in EU, in use elsewhere (2010) EPA IRIS RfD (1991): NOEL 5.0/UF 1000, MF 1 = 0.005; withdrawn 2003 JMPR ADI (1977): 0.1 |
| Gestation and PND | 2 Flynn et al., 2005, Gioiosa et al., 2007 | rat (1) mouse (1) | oral (2) | 1 | 1 | 2 | 0.02 Gioiosa 2007 LOEL (gender behavioral differences) |
One paper may contain more than one study if it includes more than one exposure period, exposure route, or species.
Four exposure periods were defined: gestation only, PND direct dosing to pups, gestation and PND, and PND through dams.
Number of studies using DMSO as a vehicle is recorded.
Two or more doses tested per exposure period.
TABLE 15.
DDT in vivo mammalian studies
| Compound | Total Number of Papers Evaluateda | Exposure Period(s) for Which Literature Is Availableb | Total Number of Studies Evaluateda for Each Exposure Period | Species (# of studies) | Routec (# of studies) | Litter of Origin was unit of analysis (# of studies) | Dose/Response within the Studyd (# of studies) | Number of Studies with ≥6 Litters | Overall Offspring NOEL/LOEL with Author and Year Using Neuro Endpoints mg/kg | Regulatory Number (mg/kg/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| DDT | 11 | Gestation onlye | 5 al-Hachim and Fink, 1968a,b,c, Palanza et al., 2001, vom Saal et al., 1995 | mouse (5) | oral (5) | 1 | 2 | 5 | 0.02f, vom Saal 1995; LOEL (urine marking behavior) | Withdrawn US 1972, worldwide 2004 except for mosquito control (malaria) EPA IRIS chronic oral RfD (1996): NOEL 0.05 / UF 100, FQPA 1 = 0.0005 JMPR ADI (2000): 0.01 |
| PND direct dosing to pups | 6 Eriksson et al., 1984, 1990a,b, 1992, 1993, Johansson et al., 1995 | mouse (6) | oral (6) | 1 | 0 | 0 | 0.5, Eriksson 1984, 1990a,b, 1992, 1993, Johansson 1995; LOEL (motor activity, muscarinic receptor density) |
One paper may have more than one study if there is more than one exposure period, exposure route, or species.
Four exposure periods were defined: gestation only, PND direct dosing to pups, gestation and PND, and PND through dams.
Oral includes gavage, dietary, and through drinking water.
Two or more doses tested per exposure period.
Although animals were dosed only during gestation, there was likely residual postnatal exposure due to long half-life of DDT.
Calculated based on reported dose level (1 μg/day) and average (55 g) of reported range of body weight (“45 to 65 g from days 11–17”).
TABLE 16.
Type I Pyrethroid in vivo mammalian studies
| Compound | Total Number of Papers Evaluateda | Exposure Period(s) for Which Literature Is Availableb | Total Number of Studies Evaluateda for Each Exposure Period | Species (# of studies) | Routec (# of studies) | Litter of Origin was Unit of Analysis (# of studies) | Dose/Response within the Studyd (# of studies) | Number of Studies with ≥6 Litters | Overall Offspring NOEL/LOEL with Author and year Using Neuro Endpoints mg/kg | Regulatory Numbers (mg/kg/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| Allethrin | 1 | PND direct dosing to pups | 1 Tsuji et al., 2002 | mouse (1) | inhalation (1) | 0 | 1 | 1 | 120, Tsuji 2002; NOEL (muscarinic receptor binding, motor activity, learning/memory) | EPA incbidental intermediate RfD (2009): BMDL10 8.0, UF 1000 = 0.008 no JMPR ADI or EU Pesticides database entry |
| Bioallethrin | 4 | PND direct dosing to pups | 4 Ahlbom et al., 1994, Eriksson and Fredriksson, 1991, Eriksson and Nordberg, 1990, Talts et al., 1998 | mouse (4) | oral (4) | 0 | 2 | 0 | 0.21, Ahlbom 1994; LOEL (motor activity, muscarinic receptor density) | EPA incidental intermediate RfD (2009): BMDL10 8.0, UF 1000 = 0.008 no JMPR ADI or EU Pesticides database entry |
| Permethrin | 1 | PND direct dosing to pups | 1 Nasuti et al., 2007 | rat (1) | oral (1) | 1 | 0 | 1 | 34, Nasuti 2007; LOEL (motor activity) | EPA RED Chronic RfD (2009): NOEL 25 / UF 100, FQPA 1 = 0.25 JMPR ADI (2002): 0.05 EU: no values derived |
One paper may contain more than one study if it includes more than one exposure period, exposure route, or species.
Four exposure periods were defined: gestation only, PND direct dosing to pups, gestation and PND, and PND through dams.
Preweaning inhalation studies are counted as direct exposure since both dams and pups are exposed in the chamber; oral is gavage, dietary, or through drinking water.
Two or more doses tested per exposure period.
TABLE 17.
Type II Pyrethroid in vivo mammalian studies
| Compound | Total Number of Papers Evaluateda | Exposure Period(s) for Which Literature is Availableb | Total Number of Studies Evaluateda for Each Exposure Period | Species (# of studies) | Routec (# of studies) | Litter of Origin was unit of analysis (# of studies) | Dose/Response within the Studyd (# of studies) | Number of Studies with ≥6 Litters | Overall Offspring NOEL/LOEL with Author and Year Using Neuro Endpoints (mg/kg when available) | Regulatory Numbers (mg/kg/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyhalothrin | 1 | PND through dams | 1 Moniz et al., 1990 | rat (1) | oral (1) | 1 | 0 | 1 | 0.02% in water (estimate 15 mg/kge), Moniz 1990; LOEL (learning) | EPA (2010)f: NOEL 0.1 / UF 100 = 0.001 JMPR ADI (2007): 0.02 |
| Cypermethrin | 1 | PND direct dosing to pups | 1 Nasuti et al., 2007 | rat (1) | oral (1) | 1 | 0 | 1 | 1.49, Nasuti 2007; LOEL (motor activity, dopamine neurochemistry) | EPA RED Chronic RfD (2008): NOEL 6 / UF 100, FQPA 1 = 0.06 JMPR ADI (2009): 0.02 |
| Deltamethrin | 3 | PND direct dosing to pups | 3 Eriksson and Fredriksson, 1991, Eriksson and Nordberg, 1990, Patro et al., 2009 | rat (1) mouse (2) | oral (2) i.p. (1 DMSO vehicle) | 0 | 1 | 0 | 0.7, Eriksson 1990, 1991, Patro 2009; LOEL (muscarinic and nicotinic receptor binding, motor activity, neuron morphology) | EPA (2010)f: NOEL 1 / UF 100 = 0.01 JMPR ADI (2002): 0.01, EUADI (2002): 0.01 |
| Fenvalerate | 2 | Gestation and PND | 2 Moniz et al., 1999, 2005 | rat (2) | i.p. (2) | 2 | 0 | 2 | 10, Moniz 1999; LOEL (immobility time) NOEL Moniz 2005 (immobility time and motor activity) | EPA withdrawn 2008 EPA (2010)f: NOEL = 1.75/UF 1000 = 0.002 JMPR ADI (1986): 0.02 |
One paper may contain more than one study if it includes more than one exposure period, exposure route, or species.
Four exposure periods were defined: gestation only, PND direct dosing to pups, gestation and PND, and PND through dams.
Oral is gavage, dietary, or through drinking water; number of studies with DMSO as the vehicle was recorded.
Two or more doses tested per exposure period.
Charles River Technical Bulletin (Spring 1999) indicates rats drink 8–11 ml water/100 gm body weight. Assuming 270 g as the average body weight during lactation, 10 ml water/0.1 and 0.02% = 0.2g/liter, then 0.2 g/liter ∗0.02 liter/0.27 kg bw = 0.015 g/kg, or 15 mg/kg cyhalothrin intake.
EPA, 2010. Pyrethroids: Evaluation of data from developmental neurotoxicity studies and consideration of comparative sensitivity. EPA Memorandum from Edward Scollon to Cathryn OConnell dated January 20, 2010. P. 16 for cyhalothrin, P. 19 for deltamethrin, P. 21 for fenvalerate (using esfenvalerate values). http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2008-0331-0028
The lowest NOAELs and LOAELs listed in Tables 10 and 13–18 were tabulated regardless of “quality” of study or whether authors of the paper or authors of this review considered the findings to be of questionable biological significance. An analysis was included if the pesticide is registered in the United States and the lowest published NOAEL or LOAEL was numerically less than the POD for the U.S. EPA's chronic RfD. Analysis of the data was also included if human studies suggested a potential association for specific pesticide exposure and neurobehavioral outcomes. A neurobehavioral study could not be considered robust unless the study included 10 litters/dose level (10–20 required by U.S. EPA guidelines), 2 dose levels not including control (3 required by U.S. EPA guidelines), litter of origin as the experimental unit or included as a variable in the statistical analysis, and clear description of statistical and behavioral methods and results (Li et al., 2012a).
Several of the lowest NOAELs or LOAELs in summary tables 10 and 13–18, were based on studies using dimethyl sulfoxide (DMSO) sc vehicle injections. While this is appropriate as a first-pass evaluation in this survey of the animal literature, it is important to emphasize that comparison with the POD should be interpreted cautiously. Although there are many routes of administration used in the literature that are legitimate and justified for investigative mechanistic studies, the route of exposure should match the primary exposure route for humans if the studies are to be used for risk assessment purposes. The sc injection was justified by some authors because it “avoids the potential confounds of different rates of gastrointestinal absorption between compounds or ages and first-pass effects on bioavailability” (Slotkin et al., 2006a). However, these first-pass effects on bioavailability are relevant for establishing the PODs for human health risk assessment. In the case of CPF, sc injections prolonged inhibition of AChE activity in the brain relative to oral exposure due to slow release of CPF from the injection site depot (Smith et al., 2009), and increased toxicity and extent of AChE inhibition by CPF (Carr and Nail, 2008). In addition, Rosencrans et al. (1984) and Uphouse et al. (1982) reported effects of DMSO used as the vehicle on neuropharmacologic outcomes following developmental exposures. Uphouse et al. (1982) concluded, “We hope that these examples will draw attention to the possible complications arising from the use of DMSO and discourage its continued use in toxicological studies.” In summary, the resultant effects of DMSO as a vehicle for sc injection may produce developmental neurotoxicity in animal studies that are not representative of human exposures to the test chemical.
OP Insecticides (Tables 10, 11, 12)
TABLE 12.
Comparison of serotonergic effects from two comparable studies following PND 11–14 sc injection (Aldridge et al. 2004, 2005b)
| Brain Region | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebral cortex | Hippocampus | Striatum | Midbrain | Brainstem | |||||||||||||
| Author | Dose (mg/kg) | Age at Testing | 5HT1A | 5HT2 | 5HTT | 5HT1A | 5HT2 | 5HTT | 5HT1A | 5HT2 | 5HTT | 5HT1A | 5HT2 | 5HTT | 5HT1A | 5HT2 | 5HTT |
| (Aldridge et al., 2005b) | 5 | PND 45 | n.s.b | n.s. | n.s. | M↑9%; Fn.s. | n.s. | n.s. | M↓18%; Fn.s. | n.s. | n.s. | M↑25%; Fn.s. | M↑11%; Fn.s. | M↑12%; F↑10% | M↑14% F↑15% | M↑17%; F↑15% | M↑28%; F↑21% |
| 5 | PND 60 | M↑25%; F↑13% | M↑15%; F↑16% | n.s. | – | – | – | – | – | – | – | – | – | – | – | – | |
| Aldridge et al., 2004 | 5 | PND 60 | M↑22%; Fn.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | Mn.s.; F↓15% | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
Note: Statistical significance was based on an ANOVA analysis in which data from at least three different factors were combined together (e.g., sex, brain region, pharmacologic endpoints). The statistical analyses were guided by the significance of the interaction terms. In some instances, there was no statistically significant interaction between dose and these factors, so all the findings were considered statistically significant based on the overall main effect without any lower order analysis conducted for each brain region or serotonergic endpoint.
“n.s.” was used for changes that were not statistically significant, or less than 10% if there were no lower order statistics due to lack of statistically significant interactions between chlorpyrifos and other factors (e.g., brain region, pharmacologic measure, sex)
If lower order statistical significance was reported for the specific measure, then these results were reported even if the changes were less than 10% of control.
General Background and Overview OP are among the most widely used pesticides in the world, with major uses in agriculture. OP exert toxicity by binding to the active site of acetylcholinesterase (AChE), the enzyme that cleaves the bond between the acetyl and the choline subunits in the neurotransmitter acetylcholine. The lack of enzymatic cleavage of the acetylcholine leads to increased acetylcholine levels and hyperstimulation of cholinergic receptors. The inhibition of AChE activity is considered the classic mechanism of action of OP for acute clinical signs of neurotoxicity. In some cases the molecules may be reactivated. In other cases, inhibition can only be reversed by synthesis of new AChE molecules. Noncholinesterase effects have also been postulated to be involved in cognitive and other behavioral effects in adult animals (Pancetti et al., 2007; Pope, 1999; Eaton et al., 2008; Garcia et al., 2003). For the developing nervous system, hypotheses that may or may not involve AChE activity include the following (Slotkin, 2004; Makris et al., 2009; Eaton et al., 2008; Prueitt et al., 2011; Carr et al., 2011; Costa et al., 2008; Richardson and Chambers, 2004, 2005; Eells and Brown, 2009; Guo-Ross et al., 2007; Slotkin et al., 2009):
Inhibition of AChE and disruption of cholinergic signaling through cholinergic receptors by an increase in acetylcholine and/or direct interaction of OP with cholinergic receptors.
Alteration of noncholinergic systems (serotonergic, dopaminergic, endocannabinoid systems) either as a downstream effect of, or independent of, AChE inhibition and subsequent perturbations of the cholinergic system.
Induction of oxidative stress and production of reactive oxygen species resulting in cellular damage.
Alteration in the expression or structure of the AChE protein leading to disruption of the morphogenic function of AChE to regulate axon or dendritic growth.
Binding of OP or their oxon metabolites to various proteins essential to neuronal development and function and axonal transport, such as tubulin and microtubule-associated proteins.
These and other hypotheses have been studied with in vitro, in alternative animal models (i.e., zebrafish), or in vivo systems and predominately with CPF as the test chemical. In general, when tested in zebrafish, rats, or mice, the dose levels of OP producing alterations of noncholinergic endpoints also produce AChE inhibition (Yang et al., 2011; Eaton et al., 2008; Prueitt et al., 2011; Li et al., 2012b). Given the importance of AChE itself as a potential morphogen for proper brain development, even daily brief bursts of acetylcholine-based cholinergic hyperexcitation may result in the longer term effects observed in the literature based on activation of various signaling pathways and/or alterations of cholinergic or noncholinergic neurotransmitter systems (Eells and Brown, 2009). Thus, it cannot be ruled out that AChE inhibition may be a precursor event to downstream cholinergic and noncholinergic modes of action. It is also possible that independent modes of action are in force that together produce disparate effects of OP on the developing nervous system as described in the next section.
The present review focused on comparing exposure levels of pesticides producing developmental outcomes on brain function and structure (i.e., neuropharmacologic, neuropathologic, neurobehavioral outcomes) with the U.S. EPA's POD for chronic risk assessment. OP are regulated based on AChE inhibition in red blood cell (RBC), brain in offspring, or brain in adults. U.S. EPA requires time of peak effect studies following single acute and repeated exposures in fetuses, pups and adults. RBC AChE inhibition is generally a more sensitive endpoint than brain AChE inhibition as assessed by the number of chronic RfD's based on RBC AChE inhibition (Table 10). When brain or RBC AChE inhibition data were not available, plasma butyrylcholinesterase inhibition has been used for risk assessment, and is considered a marker of OP exposure.
Pups and fetuses demonstrate greater brain AChE inhibition at higher acute doses of CPF (>10 mg/kg/d), and diminished difference in sensitivity at lower acute doses (Atterberry et al., 1997; Moser et al., 1998; Zheng et al., 2000; Pope and Chakraborti, 1992; Pope et al., 1991; Liu et al., 1999). This appears to be due to a more rapid rate of protein synthesis of new AChE molecules in pups and fetuses compared to adults (Lassiter et al., 1999; Ashry et al., 2002). Capturing the timing of the peak AChE inhibition during developmental exposures is exceptionally challenging in the fetus and young pup due to the rapid synthesis of AChE. These factors may explain the observation that maternal brain AChE inhibition is greater than that of fetuses or pups following repeated gestational exposures (Lassiter et al., 1998, 1999; Mattsson et al., 2000; Richardson and Chambers, 2003, 2004).
Survey of Literature for OP There were 122 published papers on 19 OP that included neurodevelopmental outcomes meeting our inclusions criteria. Half (60) of these papers included CPF as a test chemical (Table 10). There were 5 or more studies for diazinon, dichlorvos, dimethoate, methylparathion, parathion, and trichlorfon (Table 10). A wide range of behavioral, developmental reflex, electrophysiological, neuropharmacologic (including AChE inhibition), and morphologic effects in offspring were evaluated following different combinations of gestational, direct dosing to pups and/or exposure to dams during lactation.
The lowest LOELs/NOELs for neurodevelopmental outcomes were consistently higher than the POD for the chronic RfD for OP that are still registered in the United States. Many of the U.S. EPA's PODs for chronic risk assessment are based on NOAELs or U.S. EPA's benchmark dose (BMD) calculations for RBC or brain AChE inhibition in pups or adults, whichever is lowest. The BMD calculations are based on the lower limit of a one-sided 95% confidence interval on the BMD response of 10% decrease. This suggests that AChE inhibition, as measured in U.S. EPA required guideline studies for OP, is a sensitive endpoint for risk assessment for OP compared to neurodevelopmental outcomes published in the literature.
For 5 of the OP (azinphos-methyl, fenamiphos, fenthion, isofenphos, oxydemeton-methyl), the LOELs/NOELs were higher than POD for chronic RfD because brain AChE in the published papers was not measured within 24 h of dosing (Astroff and Young, 1998). In other words, timing of measurement makes a difference to sensitivity of this measurement. The studies for these 5 OP measured brain AChE levels in offspring 5 d after the last day of repeated exposure to pregnant dams in developmental studies when AChE levels recovered to control levels (Astroff and Young, 1998). However, RBC and brain AChE inhibition in dams was detected 1 and 5 d after the last day of exposure (Astroff and Young, 1998). Although these studies met basic quality criteria for studies (e.g., three dose levels, litter was the unit of analysis, adequate sample size), the timing of AChE levels was not optimized to detect the effects that occur after animals are dosed every day.
Similarly, Astroff et al. (1998) may not have optimized measurement of AChE inhibition in the five different dietary multigeneration reproductive toxicology studies on tribufos, oxydemeton-methyl, trichlorfon, fenamiphos, and coumaphos. Although substantial (up to 70–80%) brain AChE inhibition was measured in dams after 8 wk of exposure, there was much less brain AChE inhibition in pups on LD 4 and 21. The lower AChE inhibition could be due to higher turnover and replacement of AChE at younger stages of development, lower exposure to pups through lactation, or difficulty in detecting peak levels following dietary exposures. Thus, the published papers for several OP had relatively high LOELs because the test conditions were not optimal for detecting the maximum effect.
This review focused on in vivo studies with neuropathologic, neuropharmacologic, and behavioral endpoints. The following sections highlight those studies conducted at the lower range of dose levels tested.
CPF The U.S. EPA's POD for chronic risk assessment is 0.03 mg/kg/d based on the BMDL10 for RBC AChE inhibition. The lowest LOEL reported is 0.3 mg/kg/d based on cognitive effects reported by Jett et al. (2001). Based on an in-depth analysis of the published literature the LOEL/NOAEL for developmental neurobehavioral outcomes is 1 mg/kg/d (Li et al., 2012b). AChE inhibition (RBC or brain from adult or offspring) is a sensitive endpoint that is protective of neurobehavioral, neuropharmacologic, and morphologic alterations (Li et al., 2012b). Indeed, the U.S. EPA's POD for the chronic RfD is 0.03 mg/kg/d based on RBC AChE inhibition in adults.
Selected examples of developmental reflex, learning and memory and serotonergic endpoints are described to (a) illustrate the approach in evaluating the weight-of-evidence, (b) discuss hypotheses tested in rats and mice (in vivo) at lower dose levels on possible modes of action, and (c) summarize selected effects that may be relevant to epidemiologic studies on CPF and OR Only key studies that help define the LOEL/NOAEL of 1 mg/kg/d are discussed. A comprehensive in depth analysis of neurobehavioral and neuropharmacologic endpoints can be found in Li et al. (2012b), and brief summaries of all significant findings for all mammalian endpoints were tabulated by Eaton et al. (2008).
Learning and Memory Mixed effects (decreased errors, increased errors, no effect) on learning and memory tests were reported in the literature. CPF produced treatment-related effects on learning and memory at doses that produced significant AChE inhibition. A neurobehavioral finding that has been replicated in more than one lab is an improvement in female performance and a decrement in male performance on the radial arm maze (RAM) following direct dosing to pups.
Gestational Exposures Maurissen et al. (2000) and Icenogle et al. (2004) reported no marked effects of CPF at 1 mg/kg/d on spatial-delayed alternation or outcome measures in the RAM. Both of these studies also tested a 5 mg/kg/d dose, with no effects observed by Maurissen et al. (2000) following oral exposure to CPF from gestation day (GD) 6 to PND 10. However, Icenogle et al. (2004) found increased working memory and reference memory errors at 5 but not 1 mg/kg/d (GD 17–20). The different outcomes at 5 mg/kg/d may be related to different routes (sc vs. oral), age of testing, and periods of administration, and the use of different behavioral tasks to assess learning and memory. Both authors dosed dams during gestation, but Maurissen et al. (2000) extended dosing to PND 10.
In contrast, Levin et al. (2002) reported increased working memory errors in females at 1 but not 5 mg/kg/d CPF administered to dams on GD 17–20. In this study the 1-mg/kg/d males performed better (fewer errors) than the 1-mg/kg/d females when tested on RAM 2–3 mo after exposure, but there was little difference between control males and females. In other RAM studies from the same group of collaborators (Levin et al., 2001; Aldridge et al., 2005a), Aldridge et al. (2005a) observed that control males “consistently” have fewer errors than control females in their lab. Although the concurrent controls are of primary importance, the control data from these other studies provides perspective that the pattern and number of errors in males and females in the 1 mg/kg/d group are similar to normal control behavior reported by this lab. Thus, in our opinion, the rise in error in the 1-mg/kg/d GD 1 7–20 females in Levin et al. (2002) is not an adverse effect (Li et al. 2005).
The spatial-delayed alternation paradigm (Maurissen et al. 2000) was a more robust test for working memory because there were different delay periods between alternating choice of arms in a maze. In contrast, the RAM paradigm (Icenogle et al., 2004; Levin et al., 2002) did not include a delay period, and rats could potentially maximize rewards simply by repeatedly turning the same direction to each arm of the maze (chaining). Although the sample size for both studies was 10 pups/gender/dose, only Maurissen et al. (2000) designed the study so that the litter of origin was the experimental unit, included 3 dose levels, and measured AChE inhibition in both dams and offspring within 24 h of the last gestational dose. Levin et al. (2002) randomized pups to different dams in the same dose group on a repeated basis after birth so that the litter of origin was unknown and may not be the experimental unit of analysis. These study design features, together with the sc route of exposure and use of DMSO as a vehicle reduced the weight given to this study for risk assessment purposes. Taken together, the lowest LOEL/NOAEL for learning and memory was 1 mg/kg/d for gestational exposures.
Postnatal Exposures With regard to effects on learning and memory tests following postnatal exposures, Johnson et al. (2009) measured increased working errors on the RAM in males but not females following PND 1–21 oral exposures directly to pups. Rats were allowed 5 min to retrieve reinforcers from 8 of 12 arms. The low dose of 1 mg/kg/d produced 17% brain AChE inhibition. The mid- and high-dose regimens involved incremental doses up to 4 and 6 mg/kg/d, respectively, and caused greater than 46% brain AChE inhibition (Johnson et al. 2009). A statistically significant increase in working memory errors (3 errors versus 2.7 errors for first 8 arm visits in controls) in males at the 1 mg/kg/d dose level was found during wk 4 but not wk 1, 2, or 3 of testing. The mid- and high-dose groups produced the same magnitude of error (3–3.1 errors for the first 8 arm visits). The rationale for limiting the analysis to the first eight arm visits was not provided, and may have contributed to the small number of errors measured. The mean number of errors for all 4 wk of trials resulted in significant dose-dependent increases in errors in males at the mid- and high-dose levels but not at the 1-mg/kg/d dose level. Based on the slight difference in mean working memory errors from control, lack of dose-dependent differences in number of errors, and the lack of effects on cumulative working or reference memory, 1 mg/kg/d was considered a LOEL/NOAEL. Johnson et al. (2009) found that females performed better on the RAM (fewer reference errors) at 3 and 5 mg/kg/d but not 1 mg/kg/d. A similar outcome was observed following postnatal sc injections of 1 mg/kg/d (the only dose level tested for PND 1–4), namely, decreases in reference and working errors in females using the radial arm maze in other studies (Aldridge et al., 2005c; Levin et al., 2001). Brain AChE inhibition was not measured in these two sc studies, but in other studies by the same group of collaborators, CPF produced brain AChE inhibition of 25% at 24 h after PND 1–4 sc injection and 75% at 2 h after single sc injection on PND 1 (Song et al., 1997; Dam et al., 2000). In light of the longer exposure period and more relevant oral route of exposure of the Johnson et al. (2009), these results supported a LOEL/NOAEL at 1 mg/kg/d.
As previously described in detail (Li et al. 2012b), other studies that reported effects on cognitive behaviors at 1 mg/kg/d or lower were not considered robust due to an insufficient number of litters used (e.g., 2–4 litters/dose in Jett et al. [2001]), control animals that did not appear to adequately learn the task (Haviland et al., 2010; Jett et al., 2001), methods and statistical results that were not adequately reported (Billauer-Haimovitch et al., 2009; Haviland et al., 2010), or the pattern of alternation from control not being consistent with an adverse effect at 1 mg/kg/d (Billauer-Haimovitch et al., 2009; Li et al., 2012b).
Neurodevelopmental Reflex CPF exposures did not produce a pattern of adverse effects on neurodevelopmental reflex at doses below 6 mg/kg/d. The robust rat oral study by Johnson et al. (2009) described earlier (PND 1–21 oral doses; 20 litters/dose) resulted in no effects on surface righting, an inclined plane test referred to as “negative geotaxis,” cliff avoidance, free fall righting, and acoustic startle, following 3 oral doses that produced brain cholinesterase inhibition ranging from 14% (1 mg/kg/d) to 53% (1.5 increasing incrementally to 6 mg/kg/d during lactation). A much weaker rat sc study by Dam et al. (2000) found decrements in righting reflex and inclined plane test in females but not males. The Dam et al. study (2000) included only one dose level, and did not include litter as a variable in the statistical analysis (4 pups/litter tested). Venerosi et al. (2009) found no statistically significant effects on neurodevelopmental reflexes in mouse offspring following 6 mg/kg/d oral exposure to 16 dams on GD 14–17.
Cholinergic Effects of CPF or Methylparathion Several labs measured the effects of OP on the cholinergic system following repeated postnatal exposures to methylparathion or CPF (Eells and Brown, 2009; Guo-Ross et al., 2007; Liu et al., 1999; Tang et al., 2003). The most important finding relevant to risk assessment is that oral and sc exposure to CPF or methylparathion produced substantial AChE inhibition at doses below or equal to dose levels that produced smaller but longer-lasting decreases in muscarinic acetylcholine receptor (mAChR) binding and high-affinity choline uptake transporter (HAChT) levels. The persistent overstimulation of mAChRs, which occurs during repeated OP exposure, frequently results in a decrease in receptor number, which is a physiological response to the exposure (Guo-Ross et al., 2007). Similarly, reduction in HAChT levels following gestational exposures (GD 6–20) to doses of 3 mg/kg CPF and above were found at PND 6 and persisted through PND 30 in a higher dosage group (7 mg/kg) (Richardson and Chambers, 2004). Qiao et al. (2003) found a similar persistent effect on HAChT following late gestational exposure to CPF. Longer-term effects on HAChT and other components of presynaptic cholinergic neurons have also been reported in studies involving postnatal exposures to sc or oral doses ranging from 1 to 6 mg/kg/d that produced marked brain cholinesterase inhibition (Dam et al., 1999; Richardson and Chambers, 2005; Slotkin et al., 2001; Song et al., 1997). The functional significance of the decreases in mAChR binding and HAChT levels, especially those less than 20% at lower doses and without clear dose-response relationship, requires further research. These studies underscore the need for a better understanding of the brief periods of cholinergic hyperexcitation after CPF exposure and the potential for this to impact neurochemistry and behavior later in life.
Comparison of Effects of Parathion, Diazinon, and CPF on Neurodevelopmental Outcomes Slotkin and collaborators (see Table 11 for references) tested the effects of parathion, diazinon and CPF on a wide range of molecular, pharmacologic, behavioral, and morphometric endpoints. As discussed in the previous section, CPF was extensively studied following two different gestational and two different postnatal periods (Table 10). In contrast, parathion and diazinon were studied following PND 1–4 exposures, allowing for comparison across these three OP (Table 11). One of the conclusions made by Slotkin and collaborators (2008a, 2008b) is that OP produced different patterns of alterations in serotonergic, cholinergic and behavioral endpoints at doses that “straddle the threshold for barely detectable” AChE inhibition. These differences were postulated to be due to differential effects on molecular or pharmacologic endpoints that were not a result of AChE inhibition. The authors emphasized effects on the serotonergic system as an alternative mode of action independent of AChE inhibition that was correlated to potential behavioral effects. However, the majority of these studies did not measure AChE inhibition concurrently in the experiments. In the few papers that did report AChE inhibition, it was not measured at all dose levels or at the time of peak effect after last injection. When pup brain AChE inhibition was measured 24 h after the last dose, the full magnitude of an effect may have been missed as evidenced by 30 and 15% brain AChE inhibition, respectively, at 2 and 24 h after dosing with 2 mg/kg diazinon on PND1–4 (Slotkin et al., 2006b). Unfortunately, AChE was measured only at 24 and not 2 h for the lower 1 mg/kg diazinon dose level used in this study (Slotkin et al., 2006a).
5HT System For CPF, alterations in serotonergic endpoints (5HT levels, turnover, 5HT1A and 5HT2 receptor and 5HT transporter [5HTT] binding levels) occurred in different directions and included effects at just the low dose (1 mg/kg), just the high dose (5 mg/kg), or of equal magnitude for both doses depending on the study, the specific parameter, age of exposure, age of sacrifice, or brain regions. Although the authors claimed effects on 5HT-related behavioral assessments, there was no direct experimental evidence that the changes in the behaviors were 5HT-mediated. In some cases, pharmacological probes with effects on more than just the 5HT system were used.
An important principle in scientific research to establish the validity of findings is the replication of said findings by more than a single lab. There were no instances in which more than one lab replicated the serotonergic effects. However, there was one instance in which a study was repeated to some extent in two different experiments conducted by the same lab using similar experimental conditions and dose regimens (sc DMSO PND 11–14; Aldridge et al., 2004; 2005c). Aldridge and colleagues (2004, 2005c) measured the effects of PND 11–14 exposures to CPF on brain 5HT parameters in pups sacrificed at PND 45 or PND60. CPF (5 mg/kg) produced an increase in 5HT1A binding in males at PND 60 in cerebral cortex in these 2 studies (despite a difference in control values), but the findings in other serotonergic endpoints were not in the same direction or they lacked statistical significance. Comparing PND 45 with PND 60 results for more brain regions, there were both similarities and differences in findings for each endpoint for these two ages of sacrifice, and no consistent dose-response patterns or gender related findings (Table 12). It is possible that a consistent pattern may not be present due differences in developmental stage of the 5HT system in adolescent versus adult ages. Nevertheless, a consistent pattern of effects on serotonergic endpoints has not yet emerged based on the studies published thus far.
Nonmonotonic dose responses that occurred for some but not all neuropharmacologic or behavioral endpoints were hypothesized to be due to AChE inhibition or systemic toxicity at higher doses, offsetting noncholinergic effects that produced lower dose effects either directly through cholinergic activation or other neurotransmitter systems. Based on these data, Slotkin et al. (2008a) concluded that “these results point to the inadequacy of cholinesterase measurements alone as a biomarker for defining the safe exposure limits for developmental neurotoxicity of organophosphates.” This conclusion appears unfounded when comparing the PODs based on AChE inhibition with the developmental exposures levels for diazinon, parathion, methylparathion or CPF necessary to elicit noncholinergic effects.
It is notable that the chronic POD for CPF and diazinon were lower than doses reported to exert effects on serotonergic and other noncholinergic endpoints, before taking into account the sc route of exposure and use of DMSO as the vehicle (Table 10). A U.S. EPA chronic RfD for parathion could not be found (parathion is no longer registered in the United States). However, 15% brain AChE inhibition was measured in pups 24 h following sc 0.1 mg/kg parathion in DMSO, with 0.2 mg/kg parathion causing deaths (Slotkin et al., 2006a). Taken together, these data suggest that AChE inhibition as regulated by U.S. EPA using plasma, RBC, or brain AChE inhibition in pups or adults is likely to be protective of behavioral and neuropharmacologic effects of OP in pups, provided that AChE inhibition is measured at the time of peak effect after exposure.
Behavioral Effects The behavioral effects of CPF at lower dose levels tested were discussed in the previous section and more comprehensively by Li et al. (2012b). Slotkin and collaborators (Table 11 for references) tested the effects of PND 1–4 exposure to parathion, diazinon, and CPF on the same behavioral endpoints at similar ages of testing. These studies are of special interest because the dose levels tested were lower than the doses used in most of the other papers published by other investigators. Timofeeva et al. (2008b) noted that parathion affected a “smaller number of behaviors” and showed “smaller magnitude of effects in most tests” compared to CPF and diazinon. Parathion failed to produce effects on several behaviors even at lethal doses of 0.2 mg/kg/d compared to CPF and diazinon (Table 11). Parathion improved performance on RAM (decreased errors) at approximately 4 mo (Timofeeva et al., 2008b), but impaired performance (increased errors) in a different study at approximately 14 mo (Levin et al., 2010). These differences might be due to the age of testing, but highlight the need for replication of studies.
Diazinon produced nonmonotonic impairment on RAM working memory (increased errors) and enhanced nonmonotonic effects of pharmacologic challenge of scopolamine to increase errors on RAM working memory (Table 11). Diazinon also produced decreased latency on spontaneous alternation during the early trials, decreased prepulse inhibition, diminished latency to begin eating in males in a novel environment, and reduced preference for chocolate milk in males. In some cases, the findings are of uncertain biological significance for the following reasons:
Decreased latency (3–4 s) for spontaneous alternation at 0.5 mg/kg diazinon occurred only in the first two trials of a test designed primarily to evaluate spontaneous alternation, which was not affected (Timofeeva et al., 2008a). The authors conclude that this reflects “hyperactivity in the situation that is novel and challenging.” However, there were no effects on motor activity of the figure-8 test of locomotion (a novel environment), including on the pattern of intersession activity within the test session.
Decreased chocolate preference in males at 0.5 but not 2 mg/kg diazinon was confounded by very low chocolate preference in controls. The authors stated that these results may have been confounded by introduction of fluid bottles for the first time as part of this test (Roegge et al., 2008). Decreased latency to begin eating by 1 min in males, measured in both 0.5- and 2.0-mg/kg diazinon groups in the novelty-suppression feeding test, was not accompanied by any effects on the number of eating bouts, total time spent eating, or total amount of food eaten within the 10-min test period. There was a large gender difference in control males and females in the concurrent control for diazinon, with control males (110 s) having shorter latency than control females (160 s). This gender difference disappeared at both dose levels of diazinon, and both males and females had a latency similar to control females (approximately 160 s). However, comparison of control values for this endpoint with that from the contemporary study on parathion indicates that this endpoint is highly variable in males (Roegge et al., 2008). In the Roegge et al. study (2008), there were no gender differences in controls. Given the completely different pattern of male control data in two comparable studies, it is premature to conclude that these are adverse effects on the sexual differentiation of the brain.
Timofeeva et al. (2008a) concluded that the disparate effects of CPF, diazinon, and parathion on behavioral and neuropharmacologic endpoints support the “concept that the developmental neurotoxicity of OP do not reflect a single, common mechanism and that the various agents can differ in their neurobehavioral outcomes.” It was postulated that the nonmonotonic effects of diazinon were due to noncholinergic effects at lower doses, presumably serotonergic effects that are offset by a small degree of cholinesterase inhibition at higher doses (Slotkin et al. 2008b, Timofeeva et al. 2008a). The effects of parathion in the opposite direction were attributed to cholinergic effects at lower doses that are offset by systemic toxicity at higher parathion doses (Slotkin et al. 2006a, b). Further studies are needed to test this hypothesis.
In summary, several neuropharmacological and neurobehavioral alterations have been measured in adolescence and adulthood following sc injections of low doses of diazinon, parathion, and CPF on PND1–4. The general conclusions linking a noncholinergic mode of action (e.g., serotonergic effects) directly with behavioral alterations were hypothetical without direct evidence. These lower doses of parathion and diazinon also produced AChE inhibition after the last dose was administered. This increases confidence that risk assessments based on AChE inhibition are likely to be protective of these effects.
2,4-Dichlorophenoxyacetic Acid (2,4-D) (Table 13)
2,4-D is a common chlorophenoxy herbicide used for agriculture, forestry, and lawn care, including residential uses. Exposure can occur via inhalation, ingestion, and dermal contact. Dermal exposure during occupational exposure is considered to be a route of entry into the body. The POD for the chronic RfD is 5 mg/kg/d (U.S. Environmental Protection Agency, 2005).
2,4-D is primarily excreted in the urine as the parent compound. Saturation of renal clearance appears to occur at 50 or 60 mg/kg (Kennepohl and Munro, 2001). Therefore, studies using repeated doses of 50 mg/kg/d or higher are conducted at levels that saturate renal clearance, leading to different toxicokinetics than would be seen at lower exposure levels. Thus, toxicity noted at doses at or near those saturating renal clearance are not regarded as relevant to human health risk (U.S. Environmental Protection Agency, 2005).
Fourteen studies on 2,4-D conducted primarily by the Evangelista de Duffard lab met our inclusion criteria (Table 13 for references). The de Duffard studies (Table 13) covered all four developmental periods. All of them were conducted at high doses of 70 or 100 mg/kg/d to characterize effects rather than to address human-relevant dose levels. Seven of these studies were considered to be of low utility for risk assessment purposes because of the route of exposure (6 ip, of which 5 used DMSO, 1 sc water/ethanol). Six studies exposed dams in the diet throughout gestation and lactation. Although many of these papers were published within the last decade, only one used litter as the unit of analysis. The majority of studies did not report litter data (number of litters/dams and pup assignment to dose groups or measurements tested), and included only one dose level, thereby reducing their utility for risk assessment (Table 13).
Effects of 2,4-D treatment were seen at 70 mg/kg/d, the lowest dose tested in all three exposure periods. Developmental reflexes were delayed in rat pups from dams given 2,4-D in their diet beginning on GD16 and continuing to weaning, and abnormal motor activity was reported (Bortolozzi et al., 1999). Later studies by the same lab reported changes in membrane characteristics and 5HT and dopamine neurotransmitter parameters in PND exposed pups. In most of these studies no marked effects were detected in dams, but few measurements were made. However, one study by the Evangelista de Duffard lab measured altered parameters associated with maternal behaviors, increased catecholamine levels, decreased indolamine levels in the arcuate nucleus, and diminished prolactin levels in dams following dietary exposures to 15, 25, or 50 mg/kg/d (Sturtz et al., 2008). These studies suggest that high doses of 2,4-D produce effects in offspring at doses that also induce perturbations in neuropharmacologic and maternal parameters in dams and saturate renal clearance. These dose levels exceed the POD for the chronic RfD.
OC Insecticides (Table 14)
The OC pesticides are a large group of compounds that have largely been taken off the market, although research continues into their mechanisms of action. Typically, lipophilic OC pesticides persist in the environment and within the body, leading to bioaccumulation in tissues of long-lived species such as humans. In 2001 the Stockholm Convention on Persistent Organic Pollutants was signed by almost 100 countries to eliminate the use of persistent chemicals like OC, but in 2006 the World Health Organization recognized the continuing need for compounds with specific purposes, like DDT that is the most effective alternative for mosquito control in malarial regions. OC pesticide exposure remains relevant to children because children under 5 yr and pregnant women are the populations most susceptible to severe malaria, and who most often use DDT-treated mosquito netting (http://www.cdc.gov/malaria/control_prevention/vector_control.htm#itn).
Forty-two studies on eight OC insecticides met our inclusion criteria (see Tables 14 and 15 for references). These included studies in which a single (acute) dose was administered but effects were measured at long intervals after dose administration. Chemicals tested were chlordane, chlordecone, DDT, endosulfan, endrin, heptachlor, lindane, and methoxychlor. The chemicals with the greatest number of studies meeting our inclusion criteria were DDT (11 studies; references listed in Table 15) and chlordecone (14 studies; references listed in Table 14). Studies testing aldrin did not meet our inclusion criteria because they were all formulations.
As a group, the published LOELs were generally at or above the same order of magnitude as the dose levels last determined to be the POD for chronic risk assessment prior to withdrawal from the market. A wide range of endpoints were studied including neuropharmacologic (e.g. dopamininergic, muscarinic, GABAergic) and neuroendocrine analyses, learning and memory tests, motor activity, and play and other social behaviors. Due to concerns about effects of some chemicals on the endocrine system, several studies evaluated the ability of the OC pesticides to disrupt sexually dimorphic behaviors including salt/saccharine intake, play behavior, sexual behaviors (not included in this review), and motor activity tests such as the running wheel. Motor activity was among the more commonly studied endpoints for this class of pesticide.
Methoxychlor was the only compound with at least two robust studies from more than one lab at oral dose levels close to the POD for the chronic RfD (Flynn et al., 2005; Gioiosa et al., 2007; Table 14). Flynn et al. (2005) dosed rats from GD 7 through gestation, PND life, and through adulthood at doses of 0.8, 8, and 80 mg/kg/d, and tested open field and motor activity 3 times up to 67 d of age. Gioiosa et al. (2007) dosed mice from GD11 through gestation to PND 8 at a single dose of 0.02 mg/kg/d, then tested motor activity at PND 70. Neither group found effects different from control at any dose, but Gioiosa et al. (2007) reported a reduction in gender differences with treatment in two of eight behaviors. That same lab tested 3 dose levels (0.02, 0.2, and 2 mg/kg/d) and saw effects at 0.02 mg/kg/d that were not apparent at the two higher doses (Palanza et al., 2002). In addition, an effect level of 0.03 mg/kg/d was reported by vom Saal (1995) for adult male mouse urine marking behavior, but this preliminary study has not been repeated.
In total, 14 papers on the effects of chlordecone on a variety of behavioral, neuroendocrine, and neuropharmacologic endpoints were published in the 1980s, with some demonstrating effects on the sexual differentiation of the brain (Gray, 1982; Cooper et al., 1985). Seven studies, many from U.S. EPA or other U.S. government labs, used sc injections of chlordecone in DMSO. The authors considered their results to be confounded by the use of DMSO because sc injection of DMSO to PND4 pups itself affected some behavioral and neuroendocrine parameters as compared to a water-based control (Uphouse et al., 1982; Rosecrans et al., 1984; Sierra and Uphouse, 1986). Nonetheless, the lowest dose level at which an effect was reported was comparable to the POD for the chronic RfD (Table 14).
Of the few studies that tested learning and memory tasks after any OC exposure, and considered the litter as the experimental unit or a factor in the analysis, no adverse effects on learning tasks were seen for chlordane (Cassidy et al., 1994; high dose 100× below regulatory POD), while heptachlor showed effects on GABAergic neurotransmission, water maze latency, and probe performance at 3 mg/kg/d (Moser et al., 2001) compared to the regulatory NOAEL of 0.15 mg/kg/d.
DDT (Dichlorodiphenyltrichloroethane) (Table 15)
DDT is the most well-known OC insecticide. A section is devoted to DDT because several epidemiological studies have attributed a variety of neurobehavioral outcomes to prenatal DDT/DDE exposures, and pregnant women and children in malarial areas of Africa and Southeast Asia continue to use DDT-impregnated mosquito control nets. Further, although human exposure to DDT has markedly decreased over the past 30 yr, the persistence of DDT and its metabolite DDE in the environment has led to continued concern over potential adverse health effects, particularly as the possibility of low levels of DDT acting as an endocrine disruptor have been studied. DDT exerts its pesticidal activity by delaying the closure of fast-action sodium channels, resulting in hyperexcitation of the nervous system in a similar manner as noted with pyrethroid pesticides (Narahashi, 2000). Developing animals were found to be less sensitive to the acute toxic effects of DDT (Henderson and Woolley, 1969). The NOEL used for the 1996 U.S. EPA risk assessment is 0.05 mg/kg/d based on liver lesions in a 6-mo rat dietary study, resulting in a chronic oral RfD of 0.0005 mg/kg/d.
All 11 studies were oral studies conducted in mice (references listed in Table 15). Three of these studies from al-Hachim's lab exposed mice to 2.5 mg/kg/d by gavage (corn oil) during either the “1st, 2nd or 3rd trimester” of mouse pregnancy and found no effects on “behavior-conditioned avoidance” (active avoidance), electroshock seizure threshold, and 3-min open field activity (al-Hachim and Fink, 1968a, b, c). These studies were limited in terms of description of experimental methods, clear definition of “trimester” period, and inclusion of litter as the experimental unit of analysis (8–10 pups from 5–9 litters). It is possible that all papers are from the same cohort of animals, and these studies are considered to be inadequate for risk assessment purposes.
Two studies from vom Saal's laboratory were conducted on pregnant mice exposed by gavage from GD11–17 (Palanza et al., 2001; vom Saal et al., 1995). Palanza et al. (2001) included 6–10 litters/dose group with all 8 pups/litter tested and 5 dose levels over 4 orders of magnitude (0.02, 0.2, 2, 20, and 100 mg/kg/d in corn oil). The litter was included as a main effect in the statistical analysis. The authors indicated an interest in examining DDT as an endocrine disrupter, and therefore provided an additional diethylstilbestrol treatment group as a positive control. There were no effects on neurodevelopmental endpoints of cliff avoidance and righting reflex at any dose tested, vom Saal et al. (1995) found that all dose levels of DDT (0.018, 1.81, and 90.9 mg/kg) produced an increase in male urine marking, suggesting this to be an estrogen mediated behavior. This study used 6–10 litters/group with 2 male pups/litter tested, but the litter did not appear to be included as a factor in the statistical analysis. The LOEL was below, but within an order of magnitude of, the 1996 POD for the chronic RfD; however, the reliability and relevance of male urine marking for risk assessment are uncertain.
Eriksson and Talts (2000) consistently demonstrated increased locomotor activity and rearing in mice exposed to a single oral dose (0.5 mg/kg) of DDT on postnatal day 10, which was suggested to be the result of delayed or absent habituation (Eriksson, 1996). These studies are of low utility for risk assessment because litter was not controlled for as a variable in the analysis, few litters were represented (3–4 pups/litter from 3 litters), and only one dose level was tested. However, this rise in activity was reported in five separate studies, thereby increasing confidence in the results (Eriksson et al., 1990a, b, 1992, 1993; Johansson et al., 1995), although all five studies were performed by the same lab. There was no effect of DDT on a Morris water maze learning and memory test (Johansson et al., 1995).
Eriksson et al. (1992) determined that PND 10, which is the beginning of the “brain growth spurt” in rodents, is particularly sensitive to insult, as exposure of PND 3 and PND 19 animals to the same dose of DDT does not result in measurable effects on locomotor activity or rearing. Although the mechanism for this effect is not clear, studies from the Eriksson group (1997) demonstrated small alterations in muscarinic receptor density and affinity state. However, there are currently few experimental data showing that these small alterations in mAChR levels lead to changes in locomotor behavior or habituation. In contrast, PND 10 rats were less sensitive to the acute toxic effects of DDT and accumulate lower levels of DDT in the brain than rats at PND 60 (Henderson and Woolley, 1969).
In summary, DDT increased motor activity in adult mice after oral administration of 0.5 mg/kg directly to pups in studies with small numbers of litters tested. In a study with larger number of litters tested, there were no effects on righting and cliff avoidance reflexes in mice following 6 gestational doses of 0.02–100 mg/kg to dams (Palanza, 2001). Similarly, no marked effects were reported on conditioned active avoidance, 3-min open field test and electroshock seizure threshold in limited studies after gestational exposure using an adequate number of litters but poorly described methods (al-Hachim and Fink, 1968a, b, c). The LOEL for male urine marking behavior is 0.018 mg/kg, but is not a clear adverse effect. Overall, the U.S. EPA POD of 0.05 appeared to be protective of adverse developmental neurotoxic effects of DDT reported in the literature.
Pyrethroids (Table 16 and 17)
General Background and Overview Pyrethroid insecticides are one of the most widely used pesticide classes, with 16 pyrethroid compounds registered as pesticides in the United States and pyrethroid use comprising a quarter of the world pesticide market as early as 1995 (Casida and Quistad, 1998). Pyrethroid use has increased since the cancellation or reduction in use of some of the OP insecticides, particularly for residential applications. Although pyrethroids are used extensively on crops, particularly vegetables and stored foods, they also have major uses in household applications, public health, and commercial usage. In urban areas, pyrethroids have been used extensively for mosquito control following outbreaks of the West Nile Virus and most recently, pyrethroids have become the pesticide of choice to combat bed bug infestations (Agency for Toxic Substances and Disease Registry, 2003). Urban usage for structural and landscaping sites (particularly termiticides), as well as home and garden applications may account for up to 70% of the use of synthetic pyrethroids, even in highly agricultural regions like California (Spurlock and Lee, 2008). Indeed, Williams et al. (2008) demonstrated that biomarkers of pyrethroid pesticide exposure significantly increased, while biomarkers of CPF exposure decreased as it was being phased out for household use.
Pyrethroids are categorized as either type I or type II compounds, based on differences in signs of acute toxicity following administration to rodents, and also based on structural differences, most notably the inclusion of a cyano group on the phenoxybenzyl moiety (Ray and Fry, 2006; Soderlund et al., 2002). The acute toxicity of pyrethroids in adult animals was studied extensively (Bradberry et al., 2005; Wolansky and Harrill, 2008; Weiner et al., 2009). Pyrethroids exert their toxic effects on the nervous system by holding open voltage gated sodium channels (VGSC) (Vijverberg and van den Bercken, 1990; Narahashi, 2000; Soderlund et al., 2002), which results in persistent depolarization and repeated firing of neurons (Narahashi, 2000; Soderlund et al., 2002). In addition to sodium channels, GABAA receptors and calcium channels have also been reported to be molecular targets of some pyrethroids (Hildebrand et al., 2004; Shafer and Meyer, 2004; Soderlund et al., 2002; Lawrence and Casida, 1983; Crofton and Reiter, 1987), and recent data supported the idea that these alternate targets may contribute to differences in toxicity observed with type I and type II compounds (Breckenridge et al., 2009). Although these alternate targets of pyrethroid pesticides may contribute to the acute toxicological response at higher doses, their potential role in the effects of lower level repeated exposures remains to be established.
Pyrethroids, particularly the type II pyrethroids, are more acutely toxic to developing animals than adults. For example, deltamethrin has an acute oral LD50 of 12 mg/kg in weanling rats compared to 80 mg/kg in adult rats (Sheets et al., 1994). Similarly, Cantalamessa (1993) found that postnatal day 8 rats were much more sensitive to the acute toxic effects of cypermethrin and permethrin. However, Sheets (2000) reported no age-dependent toxicity for two type I pyrethroids, cismethin and permethrin. When age-related sensitivity to pyrethroids existed, it appeared to be influenced by dose and pyrethroid type (Sheets, 2000), but more studies are needed to demonstrate this (Shafer et al., 2005).
At higher doses, differences in toxicity are most likely the result of the pyrethroid-specific metabolism by carboxylesterase and cytochrome P450 enzymes, which are expressed at much lower levels in the developing mammal (Atterberry et al., 1997; Ross et al., 2006). Indeed, the use of esterase and P450 inhibitors was observed to alter the toxicity of pyrethroids in adult animals (Cantalamessa, 1993). However, there are also data to suggest that other factors might contribute to or work in concert with immature metabolic capacity of developing animals to increase their susceptibility to pyrethroids. Meacham and coworkers (2008) demonstrated that the NaV 1.3 isoform of the mammalian sodium channel, which is highly expressed during development in rodents, is more sensitive to some pyrethroids. Data suggest that there may be higher sensitivity of young animals to pyrethroids based on a differential expression of target molecules. Therefore, understanding the neurotoxic consequences of developmental exposure to pyrethroids and relative sensitivity of mammals at lower dose levels is of importance to risk assessment.
Survey of Pyrethroid Studies Shafer et al. (2005) evaluated the strengths and limitations of 22 pyrethroid studies for effects of either the formulation (10 studies) or active ingredient (12 studies) on neurodevelopmental endpoints. Our review includes 13 studies of active ingredients only, 12 of which were included in the comprehensive review by Shafer et al. (2005). These included studies on allethrin, bioallethrin, permethrin, cyhalothrin, cypermethrin, deltamethrin, and fenvalerate (see Tables 16 and 17 for references). Studies on pyrethroid formulations were excluded because they contain solvents, emulsifying agents, or petroleum distillates that make it impossible to determine if the effects are due to the pyrethroid (Shafer and Crofton, 2011). The use of formulated products may provide a more real-life exposure situation for some routes of exposure (i.e., immediate inhalation or direct dermal contact through occupational and residential applications), but they are less likely to be relevant for dietary and other oral exposures to children in the general population. The present review builds on the Shafer et al. (2005) review by relating the effects reported in the literature to regulatory values used for chronic risk assessment.
In general, the published studies were of low utility for risk assessment because the litter was not the experimental unit or litter as a variable was not controlled for in the analysis (allethrin, bioallethrin, deltamethrin), there was no dose-response assessment (permethrin, cypermethrin, fenvalerate), and/or the sample size was inadequate (<6, bioallethrin, deltamethrin). Of the 7 pyrethroids tested, the lowest LOEL or NOEL for allethrin, permethrin, deltamethrin, or fenvalerate was numerically higher than the POD for the chronic RfD. However, the LOELs for bioallethrin, cypermethrin, and deltamethrin were lower than the POD. A reliable comparison could not be made for cyhalothrin because Moniz et al. (1990) only reported concentration in water but not water consumption. As discussed below, more robust DNT studies conducted according to U.S. EPA guidelines (20 litters/dose level, 3 dose levels plus control, 10–20 pups/gender/level for behavioral tests) were conducted on 6 pyrethroids (bifenthrin, cyfluthrin, cyhalothrin, cypermethrin, fenpropathrin, and deltamethrin). The NOELs for all six of these DNT studies were above the POD for chronic RfD (U.S. Environmental Protection Agency, 2010b).
The following sections on type I and type II pyrethroids discuss the findings from the published literature with a focus on studies with LOELs below the POD for the chronic RfD.
Type I Pyrethroids Bioallethrin (one of the allethrin isomers) produced effects at 0.21 mg/kg which is an order of magnitude below the POD of 8 mg/kg/d for allethrins (Table 16). Bioallethrin produced dose-dependent decreases in muscarinic receptor binding and increases in motor activity in mice following PND10–16 direct oral dosing (0.2, 0.4, 0.7 mg/kg/d), but not at the higher dose level of 42 mg/kg/d (Ahlbom et al., 1994). The motor activity test was conducted on an unstated number of pups from 3–4 different litters and the muscarinic receptor assays were conducted on 8–13 pups from 3–4 litters. The experimental design has low utility for risk assessment purposes because litter was not properly controlled for as a variable thereby increasing the probability of a type I statistical error (Shafer and Crofton, 2011). However, enhanced motor activity was repeated by the authors at 0.7 mg/kg/d (only dose tested) in other experiments with similarly limited designs (Eriksson and Fredriksson, 1991; Talts et al., 1998). In contrast, elevated muscarinic receptor binding was not reproducible: There were increases (Ahlbom et al., 1994), decreases (Eriksson and Fredriksson, 1991) or no change (Talts et al., 1998) in cerebral cortex (4 to 5 mo) following PND 10–16 gavage dosing to bioallethrin at 0.7 mg/kg/d.
Increased motor activity was also observed in unpublished replicate studies for bioallethrin performed by Muhammad and Ray reported in a review by Shafer et al. (2005). These results on bioallethrin were not reproduced by Tsuji et al. (2002) following inhalation exposures to mouse dams and their pups on PND10–16. The estimated doses were 0.7, 2.2, 5.7, and 120.2 mg/kg/d based on inhalation exposures of 0.43, 1.35, 3.49, and 74.2 mg/m3, respectively. This study selected three mice from five different litters per dose level for behavioral and neuropharmacological testing, but did not consider litter as an experimental variable in the analysis. Prior to measurements of mAChR, the reliability of this method was confirmed by measuring greater than 80% decrease in mAChR density following diisopropyl fluorophosphates (DFP) to mice on PND 10, 12, 14, and 16 as a positive control. The neonatal exposures to d-allethrin by inhalation did not markedly induce effects either on brain mAChR density and motor activity at 17 d and 4 mo, or on performance in the learning/memory test at the age of 11 mo.
Shafer et al. (2005) reviewed an unpublished “Ivens et al.” study in mice submitted to the U.S. EPA using the inhalation route of exposure to d-allethrin (0.15, 4, or 100 mg/m3, 6 hr/d). Based on Tsuji et al. (2002) dose conversions to mg/kg/d, these exposure levels were comparable to exposure levels in Ahlbom et al. (1994). Shafer et al. (2005) reported that the Ivens et al. study closely replicated methodology of Eriksson's lab except with better experimental design including larger group sizes of 10, and litter as the statistical unit of analysis. A limitation of the study was that some biochemical measurements were variable and not dose related (Shafer et al., 2005). According to Shafer et al. (2005), effects on motor activity at PND 17 were not dose related and there were no effects on motor activity at 4 mo. Increases in muscarinic receptors were measured at PND 17 (dose level not reported), but not at 4 mo.
Taken together, the literature on bioallethrin/allethrins indicated conflicting results on motor activity and muscarinic receptors at lower doses that could be due to different routes of exposure. Less weight should be given to the single report of slight effects following oral exposure to 0.21 mg/kg/d. There was more consistent evidence of long-lasting increases in activity following 0.7 mg/kg/d oral exposures. Whether considering 0.21 or 0.7 mg/kg/d to be a possible effect level, these effects were based on studies that were limited in experimental design and statistical analysis. Thus, although these dose levels are numerically lower than the POD of 8 mg/kg/d for the chronic RfD, the studies are of low utility for risk assessment purposes.
Oral doses of 34 mg/kg/d permethrin to pups (PND 6–15) produced increases in 5-min motor activity levels and decreases in dopamine (DA) levels and other perturbations of the DA system on PND 35 but not PND 21 (Nasuti et al., 2007). Thus, the LOEL for these effects are comparable to the POD of 25 mg/kg/d for the chronic RfD.
Type II Pyrethroids The developmental neurotoxicity studies fortype II pyrethroids contain little overlap of endpoints that would allow comparison across studies. Although most studies tested an activity parameter, incomplete methods sections and contradictory results do not give a clear indication of effects. However, a developmental window at PND 10 that is relevant to adult motor activity was proposed by the Eriksson lab, and can be examined for consistency across cyhalothrin, cypermethrin, and deltamethrin. Eriksson and Fredriksson (1991) reported that male mice dosed in utero with 0.7 mg/kg/d (oral) deltamethrin showed elevated motor activity during the final 20 min of an hr activity session at 4 mo of age, whereas Patro et al. (2009) found increased activity in rats at PND 21 but decreased activity at PND 90 using 0.7 mg/kg/d ip dose. Moniz (1999) found no change in motor activity at PND 97 and PND104 using a 0.02% cyclohathrin in water that produced a reduction in passive avoidance latency (Table 17, estimated dose 15 mg/kg/d). Nasuti et al. (2007) measured a rise in motor activity at PND 35 using a cypermethrin dose level of 10 mg/kg/d that lowered levels of DA in striatum on the same day. Therefore, effects on motor activity are not consistent across the type 11 pyrethroids, even at doses that could affect other neurodevelopmental parameters.
Cypermethrin was the only type II pyrethroid with a LOEL numerically below the POD for the chronic RfD (Table 17). This LOEL of 1.49 is based on increased motor activity and decreased striatal DA levels at PND 35 but not PND 21 (Nasuti et al., 2007). This study had no dose-response data, but defined litter as the experimental unit and had a sample size of 10 pups (gender of animals was not clearly identified).
Pyrethroid Discussion The most consistent finding was increased locomotor behavior at adult time points following a single neonatal exposure with bioallethrin and deltamethrin (Eriksson and Nordberg, 1990; Eriksson and Fredriksson, 1991; Ahlbom et al., 1994; Talis et al., 1998). The elevated locomotor activity observed following neonatal treatment differs from that noted in adult rodents, which typically display reduced locomotor activity following an acute dose of a number of pyrethroids (Wolansky and Harrill, 2008). Whether this difference was the result of a developmental sensitivity to this particular outcome or whether the reduced locomotor activity in adults was merely a pharmacological effect of the treatment remains to be established. The other finding of the single dose neonatal studies was alteration in mAChR density, which the authors related to the increased locomotor activity found in these studies. However, alterations of mAChR (often 5–25%) were not consistently replicated (Ahlbom et al., 1994; Eriksson and Fredriksson, 1991; Eriksson and Nordberg, 1990; Talis et al., 1998). In addition, other labs have not consistently replicated this effect (Shafer et al., 2005). The other study that examined motor activity and neurochemistry was Nasuti et al. (2007), who found concurrent increased motor activity and decreased striatal DA. These studies suggest that motor activity may be a sensitive endpoint for DNT effects of pyrethroids.
The relationship between muscarinic receptor alterations and locomotor activity is not clear. For example, M1 and M4 mAChR knockout mice displayed basal increases in locomotor behavior, but little effect was observed with mice heterozygous for the receptors (Gomeza et al., 1999; Gerber et al., 2001). However, there were few data to suggest that elevated mAChR levels would enhance locomotor activity. Shafer et al. (2005) similarly concluded that the relationship, if any, between biochemical and behavioral changes has not been established, particularly when the magnitude of these effects is small. Further, comparison of differences between studies reporting locomotor changes was often hampered by lab variation in the conduct of studies (Crofton et al., 1991), and even when steps are taken to minimize lab variation, significant differences in rodent behavior still occur (Crabbe et al., 1998). Thus, further standardized study design is required, including attention to controlling experimental testing conditions such as balancing the time of testing across dose group, ensuring adequate sample sizes of pups from a minimum of 10 litters for behavioral studies, and including litter as a factor in the statistical analysis. As discussed in the introductory section for pyrethroids, a primary mode of action for acute pyrethroid neurotoxicity involves VGSC. Currently, the potential role of VGSC in developmental neurotoxicity of pyrethroids has not been directly examined (Shafer et al., 2005).
The developmental neurotoxicity studies on pyrethroids were conducted in either rats or mice. The most consistent evidence of pyrethroid effects was found in studies in which mice were used as the test species. Therefore, there may be significant species differences in the effects observed. Unfortunately, little is known about the metabolism of pyrethroids in mice compared to other rodents and humans. In vitro studies demonstrated significant differences in the metabolism of pyrethroids between rat and human liver microsomes (Godin et al., 2006; Scollon et al., 2009), which raises questions about the proper species to use for human health risk assessment. This issue underscores the need for additional measurements of pyrethroid levels in biological samples following dosing so that the dose administered can be compared to blood and/or tissue levels of the compound. In this manner, data can be generated that may help to extrapolate the relevance of the doses administered to concentrations found in the human population and aid in comparison among animal studies.
There are several other common issues to these studies that need to be addressed in the future to aid in comparison. First, formulations need to be avoided, and the source and purity of the pyrethroid should always be stated with the purest form of the compound utilized. Further, the isomer composition of the compounds often was not described. Since the toxicity of pyrethroids is stereospecific (Soderlund et al., 2002), differences in the isomer composition of compounds may exert profound effects on toxicity. Volume of dosing solution also needs to be considered since it affected adult toxicity (Wolansky et al., 2007).
In conclusion, there are a number of published studies on pyrethroids in which the LOELs were close to or below the PODs used for chronic (or intermediate) risk assessments. The most common changes, observed primarily by one lab, were increased activity and alterations in mAChR density, although there were important limitations in experimental design that preclude use of these studies for risk assessment purposes.
N-Methyl Carbamates
The majority of carbamate insecticides are N-methyl carbamates, which include aldicarb, carbaryl, carbofuran, formetanate hydrochloride, methiocarb, methomyl, oxamyl, primicarb, propoxur, and thiodicarb. Not all of these pesticides are being reregistered for all or some uses in the United States (e.g., methomyl, carbofuran, primicarb). Humans may be exposed to N-methyl carbamates through food and drinking water and in and around residences, schools, and commercial buildings. Therefore, oral, dermal, and inhalation routes of exposure are of relevance to pregnant women, infants, and children (U.S. Environmental Protection Agency, 2007).
There were no papers on N-methyl carbamates that met our inclusion criteria for repeated exposures prior to weaning. However, a recent paper by Moser et al. (2010) measured acute effects of N-methyl carbamates in PND 17 pups on AChE inhibition and motor activity. They compared these results with acute effects on adults published in a separate study (McDaniel et al., 2007). The N-methyl carbamates tested were carbaryl, carbofuran, formetenate, methiocarb, methomyl, oxamyl, and propoxur. As acknowledged by the authors, these comparisons need to be interpreted cautiously, because age-related differences in dose-response curves are best described using systematic comparisons in the same lab (Moser et al., 2010). These data, together with publically available dose-response and time-course data on offspring and adults submitted to the U.S. EPA, are being used as the basis for risk assessments for N-methyl carbamates (U.S. Environmental Protection Agency, 2007). These submitted data are discussed below under the section on unpublished data. Although acute toxicity studies did not meet our general inclusion criteria, well-conducted acute studies are relevant for N-methyl carbamates because the primary mode of action is rapidly reversible AChE inhibition that “peaks within minutes to hours after a single oral dose and recovered by 24 hs” (Moser et al. 2010). Therefore, one of the most sensitive endpoints for offspring is AChE inhibition following acute exposures. U.S. EPA has concluded for several N-methyl carbamates such as carbofuran that the acute RfD is considered protective of chronic exposures, given that ChE activity is reversible (within 24 h). The longer term exposures could be considered a series of acute exposures (http://www.epa.gov/oppsrrd1/reregistration/REDs/carbofuran_red.pdf).
The insecticidal N-methyl carbamates, similar to OP, exert their effects by inhibiting AChE. Following exposure to OP, the phosphorylated enzyme is reactivated slowly, on the order of days to weeks (Moser et al., 2010). In contrast, N-methyl carbamates showed maximal AChE inhibition at 15–45 min, with most inhibition recovered by 24 h. Since decarbamylation is favored by dilution and increased temperature, assay conditions that might be appropriate for OP may induce further decarbamylation, reducing the sensitivity of accurately measuring the full extent of AChE inhibition produced by N-methyl carbamates in vivo (Nostrandt et al., 1993). As discussed in great detail in the previous section on OP, brain AChE inhibition was associated with compensatory effects on the cholinergic system or potential downstream effects on noncholinergic alterations. Therefore, based on mode of action and the OP literature, the effects of acute exposures to AChE inhibition in offspring were likely to be one of the most sensitive endpoints for developmental neurotoxicity in offspring (Moser et al., 2010). In conclusion, although there are no published data on DNT studies for N-methyl carbamates that met our inclusion criteria, there are many unpublished studies on the acute effects of AChE inhibition to offspring and adults that have been used as the basis for risk assessments by U.S. EPA. These data have been published by U.S. EPA as part of the N-methyl carbamate cumulative risk assessment (U.S. Environmental Protection Agency, 2007), which is in many ways more transparent than the published literature in that all the individual data on AChE inhibition from all studies were released by the U.S. EPA.
Miscellaneous Pesticides With Fewer Than Five Papers (Table 18)
There were 23 chemicals with fewer than 5 peer-reviewed papers that did not fall into the well-studied pesticide classes of chemistry such as OP, OC insecticides, or pyrethroids (Table 18). Altogether there were 40 studies from 37 papers that met our inclusion criteria (see Table 18 for references). The litter was the experimental unit for half of these studies (22) and most of them (34) used at least 6 litters to derive their results. More than half of these studies (24) included two or more dose levels. For many of these pesticides the LOEL or NOEL reported by the authors for each pesticide was numerically higher than the POD for chronic risk assessment, when available. The exceptions were atrazine, maneb, paraquat, piperonyl butoxide, and zineb. As discussed previously, these general comparisons provide some perspective on the dose levels at which effects were reported relative to effect levels from regulatory toxicity databases. As illustrated in examples discussed for OP, OC, and pyrethroids, effect levels as reported by the authors may not necessarily be adverse effects appropriate for risk assessments depending on the quality of study, pattern of effect across multiple parameters measured, consistency of effect, comparison with other studies, and/or relevance of route of exposure. Only the studies for pesticides with NOELs or LOELs numerically below the POD for the established chronic RfD were evaluated critically based on a priori criteria discussed in the methods section and pattern of effects within and across studies.
Atrazine Atrazine has been examined for disruption of reproductive and endocrine functions. Two studies from the peer-reviewed literature evaluated the effects of atrazine on similar social interaction behaviors following oral exposures. Fraites et al. (2011) exposed rats in utero (GD 14–21) at doses of 1, 5, 20, or 100 mg/kg/d) and examined social behaviors in male offspring at age PND 30–33. The litter was the experimental unit of analysis. Twelve litters per dose level were tested and all behavioral observations were made by a single observer unaware of treatment level. There were no effects of atrazine on any of the social behavior parameters, including rough-and-tumble play. In controls, the duration of play for females was shorter than for males. However, there were no significant gender differences in frequency of play or social interaction. The lack of effects in the Fraites et al. (2011) rat study is in contrast to a mouse study by Belloni et al. (2011), who evaluated locomotor and exploratory activity (PND 16), social interactions (PND 31) and cognitive performance (PND 60) in male and female offspring following maternal oral exposure to 0.001 (low dose) or 0.1 mg/kg/d (high dose) from GD 14 to PND 21. Specifically, Belloni et al. (2011) measured higher frequency (but not duration) of exploratory behavior at the low but not at the higher dose level. There were no significant differences at either dose level in frequency or duration of all five other related open field parameters. Both doses of atrazine increased frequency and duration of sniffing a novel object by similar magnitude despite the 100-fold difference in dose levels. The low-dose but not high-dose level of atrazine produced an increase in social (mid dose) and nonsocial (high dose) exploration in the second of three 5-min blocks based on statistical analysis of factors (groupings of behavioral parameters) defined by a principal component analysis. The high but not the low dose of atrazine improved performance during the learning session of the passive avoidance test. The authors considered the principal component analysis to be “an exploratory rather than an analytical technique.” This, together with the lack of consistent dose-response pattern described earlier, suggests that it is premature to consider these effects to be biologically significant adverse effects. This conclusion is further supported by the lack of findings on similar behaviors in the Fraites et al. (2011) paper, although these difference could be due, in part, to differences in the species tested (mice vs. rats), the developmental period of exposure (GD + PND vs. GD only), different dose levels, and the method of statistical analysis. Nevertheless, they occurred at 2–3 orders of magnitude below the POD for the chronic RfD, based on a study that included 2 dose levels, litter as the experimental unit, and litter sizes of 10.
Maneb Maneb also had an effect level from the literature (1 mg/kg/d) that was below the POD for risk assessment (5 mg/kg/d). Thiruchelvam et al. (2002) dosed mouse pups daily with ip injections of 1 mg/kg/d maneb on PND 5–19 (15 total injections). The animals were also given challenge injections twice weekly from 6.5 to 7.5 mo of age (7 total injections). No effects were seen on motor activity at 6 wk or 6 mo before the adult challenge injections, or at 8 mo of age after the challenge injections, but a statistically significant decrease in tyrosine hydroxylase staining neurons in the substantia nigra was seen at 8 mo of age. Based on inspection of graphs in the original paper, these changes were approximately a 10% reduction after PND treatment alone, and 25% reduction when combined with adult challenge (Figure 5 in Thiruchelvam et al. 2002). However, no changes in levels of DA or its metabolites were measured in the striatum at 8 mo of age. Barlow et al. (2004) from the same lab dosed mice prenatally with 1 mg/kg/d sc maneb from GD 10–17 and administered an adult challenge using sc injections of 10 mg/kg/d paraquat, 30 mg/kg/d maneb, or saline daily on PND 47–55. Results showed reduced activity one week later (on PND 62) in males challenged with paraquat but not maneb. Dopamine and its metabolite DOPAC were reduced in the striatum of males treated postnatally with maneb and challenged with paraquat as adults, as were TH+ neuronal counts in two dopaminergic brain nuclei, the substantia nigra and ventral tegmental area. Although the LOELs for these studies were numerically lower than the POD for chronic risk assessment, they were based on ip or sc injections, which are not an appropriate route of exposure for risk assessment purposes.
Paraquat (and Maneb) Paraquat is one of the pesticides where effects were noted at an oral dose level that was below the oral POD of 0.45 mg/kg/d used for chronic risk assessment (Table 18). Fredriksson et al. (1993) dosed mice orally on PND 10 and 11 at 0.36 and 0.07 mg/kg/d, and reported a decrease in activity (1-h automated test) at both dose levels on PND 18. This study is of low utility for risk assessment because 12 mice from only 3 litters were assigned to each treatment group. Dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic (HVA) levels were lowered in the striatum at 0.36 mg/kg/d, but only a slight reduction of HVA was seen at 0.07 mg/kg/d. In a study in which the litter was the experimental unit and an adequate number of litters were used (n = 10), there were no motor activity effects measured in mice dosed daily on PND5–19 with 0.3 mg/kg/d ip injections either before or after adult challenge with paraquat (Thiruchelvam et al., 2002). Dopamine and metabolites (n = 10) and tyrosine hydroxylase positive (TH+) staining neurons in the substantia nigra (n = 4) were decreased at 0.3 mg/kg/d (ip). Another study from the same lab by Barlow et al. (2004) injected 0.3 mg/kg/d sc to dams during GD10–17, then tested the offspring for motor activity at 6 wk of age (this study was described in the maneb section earlier). A pesticide challenge dose, similar to a pharmacologic challenge, was injected (sc) daily on PND 47–55 (30 mg/kg/d maneb, 5 mg/kg/d paraquat). Dopamine, HVA, and DOPAC were measured on PND 62 after motor activity recording the same day. Gestational paraquat did not affect adult motor activity without adult challenge, or change DA and metabolite levels in the striatum. Despite the similar mg/kg/d dose level, comparison of the conflicting results on dopaminergic pharmacology from the Fredriksson et al. (1993) and Barlow et al. (2004) studies was confounded by different routes of exposure and different developmental periods of exposure. Intraperitoneal injections of paraquat were much more acutely toxic than oral doses, with mean lethal doses for ip and oral doses to be approximately 16 and 112 mg/kg, respectively (Lock and Wilks, 2001). Both studies have low utility for risk assessment purposes.
Later publications in adult mice by the first author of this dual pesticide model (e.g., Thiruchelvam et al., 2005) have been retracted due to fabrication of data (http://www.gpo.gov/fdsys/pkg/FR-2012-06-28/html/2012–15887.htm; Fed. Reg. 77 (125): 38632–38633, June 28, 2012). To date, this developmental model has not been replicated by other labs, although Kachroo et al. (2010) found that adult dosing of paraquat and maneb decreased TH+ staining cells.
Piperonyl Butoxide (PBO) It is relevant to include piperonyl butoxide (PBO) in this analysis because, while not a pesticide, it is used as a synergistic agent with pyrethroids, pyrethrins, rotenone, and carbamates. Horton et al. (2011) studied PBO as “an indicator of pyrethroid insecticide use.” Piperonyl butoxide inhibits the metabolic P450 enzymes that break down the pesticides, thereby prolonging the systemic concentration of the pesticide and making it more effective at a lower dose. The POD for the U.S. EPA's chronic RfD is 15.5 mg/kg/d (Table 18). However, in a two-generation toxicity study, Tanaka (2003) found an effect on olfactory orientation in PND 14 female mice that had been exposed to the lowest (0.01%) dietary exposure level of PBO (chemical consumption during gestation and lactation 17–59 mg/kg/d). Olfactory orientation was defined as the time taken for mice to enter the compartment with home cage flakes. This effect is not considered an adverse finding because (a) there were no marked effects at two higher dose levels, and (b) there were no significant effects on other indices of development, including inclined plane test (negative geotaxis test), cliff avoidance, swimming behavior (direction, head angle, number of limbs moving), and exploratory behavior. In contrast, surface righting was delayed and olfactory orientation depressed in males at the two higher dietary exposure levels in the first but not second generation. The chemical consumption for the mid and high dose levels during gestation and lactation were, respectively, 50–163 and 145–524 mg/kg/d (Tanaka, 2003). Thus, the LOAEL exceeds the POD for the chronic RfD.
Zineb The lowest NOEL of 5 mg/kg/d from Jia and Misra (2007) is numerically lower than the U.S. EPA POD of 25 mg/kg/d for the chronic oral RfD, not taking into account the different route of exposure (Table 18). Jia and Misra (2007) dosed mice on PND 5–19 with 5 mg/kg/d zineb ip. At 8 mo of age, the animals were challenged with 7 injections of 50 mg/kg zineb over 2 wk. Seven days after the last dose, the mice were sacrificed and the brains were dissected to allow measurement of dopamine, DOPAC, and HVA in the striatum, and AChE in the cerebral cortex. Treatment on PND 5–19 alone produced no difference from controls. The adult challenge reduced DA and DOPAC levels below the respective control, but HVA level and AChE activity were not affected. There was no concurrent group dosed only as adults. Therefore, these effects may be a result of adult exposure to zineb, and cannot be attributed to the PND 5–19 ip doses alone. This study was not acceptable for risk assessment in that the number of litters and selection process for pups from the litters were not reported (Table 18).
In summary, there were 23 pesticides in this miscellaneous category of pesticides with fewer than 5 studies that included developmental neurotoxicity-like endpoints. Adverse effects noted in these studies were generally at or above the levels used as PODs. Four pesticides (atrazine, maneb, paraquat, and zineb) and one pesticide synergist (piperonyl butoxide) had at least one study in which the LOEL was numerically lower than the POD for chronic risk assessment, not taking into consideration routes of exposures or utility of the study for risk assessment purposes. The sensitive studies for three of the pesticides (maneb, paraquat, and zineb) were ip studies, which compromises comparisons with the oral POD. In the case of atrazine, Belloni et al. (2011), but not Fraites et al. (2011), found perturbations in social behaviors. These results have not been replicated and it is premature to consider these effects to be adverse.
Unpublished Animal DNT Studies
This survey has the disadvantage of not including unpublished regulatory studies that have been submitted to U.S. EPA and are being used in the risk assessments. These include DNT studies conducted according to U.S. EPA OPPTS guidelines (U.S. EPA, 1998; http://www.epa.gov/oppts/pubs/frs/publications/Test_Guidelines/series870.htm) and comparative AChE inhibition studies following single and/or repeated doses of OP or N-methyl carbamates directly to pups and adults.
U.S. EPA DNT Guideline Studies on Pesticides Only two guideline DNT studies on pesticides have been published (Maurissen et al., 2000; Wise et al., 1997). U.S. EPA guidelines for DNT studies require exposures to occur on GD6–PND11, although U.S. EPA OPP currently requests that exposure continue until PND21. The DNT guideline requires sample sizes of 20 litters/dose group with at least 3 treated dose groups plus concurrent control. At least 1 male or 1 female from each litter must be selected for behavioral tests such that there are 10 pups/gender/dose group. The subjective behavioral tests must be conducted by trained observers who are unaware of treatment level and demonstrated the ability to detect adverse effects in positive control studies. The time of testing must be balanced across treatment level. The tests include motor activity (PND13, 17, 21, 60), auditory startle habituation (weaning and adult), learning and memory tests (weaning and adult), perfusion fixation histopathology, and morphometric measurements (weaning or PND 11 and adult).
Although the majority of submitted DNT studies have not been published in the peer-reviewed literature, a review by Makris et al. (2009) indicates that as of August 2006, approximately 75 DNT studies conducted according to U.S. EPA guidelines have been submitted to the U.S. EPA OPP in support of pesticide registrations. Some of these pesticides are listed in Table 19. For 58 of the 75 pesticides, the DNT study was considered in the review of the toxicology database (Makris et al., 2009). The DNT study was used to set the POD for risk assessments for 8 of 75 chemicals, of which 4 were based on critical effects solely related to offspring behavioral and neuropathology parameters that are not evaluated in other guideline studies (i.e., motor activity, Functional Observational Battery [FOB], auditory startle, morphometric measurements) (Makris et al., 2009). Raffaele et al. (2010) reviewed the same U.S. EPA OPP DNT database as Makris et al. (2009) and concluded that 15 of 69 acceptable DNT studies were used to determine the POD for one or more risk assessment scenarios, 13 of which had body weight changes as one of the effects at the LOEL. Relevant to this present review paper, only two DNT studies (Flufanacet and Tebuconazole) were used for chronic risk assessment, both of which had body weight changes at the LOEL. Although this indicates that the DNT study has been useful for risk assessment decisions, it also suggests that in most cases the DNT endpoints have not been more sensitive than other endpoints from other regulatory toxicity studies.
TABLE 19.
Examples of pesticides tested using the U.S. EPA DNT guideline or OECD TG 426 published by Makris et al. (2009)
| Abamectin | Dicrotophos | n-Methylneodecanamide |
| Acephate | Dimethoate | Molinate |
| Acetamiprid | Disulfoton | Naled |
| Acibenzolar-s-methyl | Emamectin | Phorate (2) |
| AE-0172747 | s-Ethyldipropylthiocarbamate | Prochloraz |
| Aldicarb | Ethoprophos | Profenofos |
| Amicarbazone | Etofenprox | Pymetrozine |
| Azinphos methyl | Fenamidone | Pyrasulfotole |
| BAS 510F | Fenamiphos | Spirodiclofen |
| BAS 670H | Fentin hydroxide | Prothioconazole |
| Bifenthrin | Fipronil | Tebuconazole |
| Carbaryl | Flubendiamide | Terbufos |
| Carbofuran | Flufenacet | Tetrachlorvinphos |
| Chlorfenapyr | Glufosinate ammonium | Thiamethoxam |
| Chlorpyrifos | Glyphosate trimesium | Thiocloprid |
| Clodinafop propargyl | Imidacloprid | Thiram |
| Clothianidin | Indoxacarb | Triallate |
| Coumaphos | Isopropanol | Tribufos |
| λ-Cyhalothrin | Isoxaflutole | Trichlorfon |
| β-Cyfluthrin | Lindane | Ziram |
| Cymoxanil | Malathion | |
| θ-Cypermethrin | Methamidaphos | |
| p,p-DDT | p-Methane-3,8-diol | |
| DEET (N,N-diethyl-meta-toluamide) | Methimazole (6) | |
| Deltamethrin | Methyl parathion |
The results of the Makris et al. (2009) and Raffaele et al. (2010) retrospective reviews are similar to those reported by Middaugh et al. (2003) on 175 compounds. Middaugh et al. (2003) conducted a survey of studies including neurobehavioral assessments on second-generation (F1) offspring following developmental exposures. The survey included 175 compounds that were primarily pharmaceuticals (81%) but also pesticides (7%), industrial chemicals (1 %), and other undefined (10%) substances. This review found that F1 behavioral parameters (i.e., neurodevelopmental reflex, activity level, and learning and memory), along with other parameters, defined the chronic POD in 17 of 113 studies (15%) and solely defined this POD in 3 of 113 (2.6%) of the studies examined (Middaugh et al., 2003).
Unpublished DNT Studies for Pyrethroids In January 2010, U.S. EPA (2010b) summarized data from six U.S. EPA guideline DNT studies for one type I (bifenthrin), four type II (cyfluthrin, lambda-cyhalothrin, zeta-cypermethrin, deltamethrin), and one mixed syndrome pyrethroid (fenpropathrin). Data suggested that neurobehavioral and morphometric endpoints from a DNT study design are not more sensitive than other endpoints from repeated dose developmental, reproductive or chronic studies (U.S. Environmental Protection Agency, 2010b). Indeed, in evaluating the DNT studies for pyrethroids, the U.S. EPA observed that “weight changes are a more sensitive indicator of toxicity in the pups than are neurological effects,” and there were no changes on learning or memory at any dose in any of the six DNT studies on pyrethroids (U.S. Environmental Protection Agency, 2010b).
The DNT studies provide sufficient evidence to support redirecting resources toward study design focused on evaluating relative sensitivity to acute neurotoxic effects. Indeed, comparative age sensitivity studies on the acute effects of pyrethroids on auditory startle are underway (U.S. Environmental Protection Agency, 2011). Auditory startle is a sensitive endpoint that distinguishes between type I and type II pyrethroids (Crofton and Reiter, 1984). Another endpoint that is considered sensitive to acute effects of pyrethroids is motor activity, even though type I and type II pyrethroids both decrease activity (Wolansky and Harrill, 2008).
Comparative AChE Inhibition Data for N-Methyl Carbamates and OP in Pups and Adults Studies on AChE inhibition following acute and repeated direct dosing to pups have been conducted on a number of OP and N-methyl carbamates, and the results of these unpublished studies were reported in the cumulative risk assessments for these classes of pesticides (U.S. EPA, 2002; U.S. EPA, 2007). As of 2007, data indicated that the BMD10S for brain AChE inhibition in PND11 rat pups dosed with N-methyl carbamates were generally two- to fourfold lower than BMD10S for adult brain AChE inhibition, and the lowest BMD estimates for brain, RBC or plasma butyrylcholinesterase inhibition were used to derive PODs for individual N-methyl carbamate risk assessments. Thus, although few guideline DNT studies on OP and N-methyl carbamates have been published, there are AChE inhibition data for pups and adults for N-methyl carbamates and OP that were used as sensitive endpoints for risk assessment purposes.
INTEGRATION OF ANIMAL AND EPIDEMIOLOGY STUDIES AND CONCLUSIONS
The main objective of this review was to answer the two following questions: (1) What is the evidence for causality between exposure to specific pesticides (or classes of pesticides) during critical periods of brain development and neurobehavioral outcomes in the epidemiologic literature? (2) What are the lowest dose levels for adverse functional neurodevelopmental effects in animals in the published literature, and how do they compare with effect levels from repeat dose toxicity studies used to derive the chronic RfD? This integration section addresses these two questions.
What Is the Evidence of Causality?
Our objective in evaluating the epidemiology studies was to assess whether there is a causal relationship between exposure to specific pesticides during pregnancy or early childhood and adverse neurodevelopmental outcomes measured during early childhood. Neurobehavioral outcomes, including head circumference, were evaluated from 16 epidemiological cohort studies published in nearly 50 papers. More than 400 associations from these studies were reported in the literature, the majority of which were related to biomarkers of exposures to OP and OC insecticides. A previous review listed the adverse findings for pesticides and neurotoxic effects in children (Bjorling-Poulsen et al., 2008). Many of these were related to OP and OC pesticide exposure and were also presented in this review in bold blue type in Tables 3–9. Thus on a macroscopic level, the epidemiology literature contains multiple observations that support the hypothesis of adverse neurobehavioral outcomes in children with certain pesticide exposures. However, the type of evaluation that focuses on adverse associations only cannot make a valid conclusion regarding causality. Further, a summary table of effect sizes would omit the findings “not shown” in many publications and over-represent publications for which dozens of comparisons were reported (such as Marks et al., 2010, in Table 8). It is important to take into consideration the totality of findings and evaluate consistency within and across studies of both statistically significant and nonsignificant associations, specificity of exposure metrics (e.g., child vs. maternal; specific vs. nonspecific indices of exposure), and exposure response patterns.
As emphasized in the animal section, replication of findings by more than one lab or population is an important scientific principle. Despite the many analytical comparisons reported in Tables 3–9, the number of comparisons that evaluated the same pesticide (or its metabolite) and the same health endpoint was small. A number of statistically significant observations have not been “tested” in the available published literature thereby limiting the ability to determine consistency across independent studies. For example, the CCCEH was the only study to conduct the BSID tests in children at 36 mo, the only age at which the investigators observed statistically significant associations with CPF levels (Rauh et al., 2006). Similarly, CPF levels were strongly associated with CBCL-assessed ADHD problems in the CCCEH study (OR = 6.50, 95% CI 1.09–38.69) (Rauh et al., 2006), but not for TCPy levels in the CHAMACOS study (OR = 0.59, 95% CI 0.21–1.68) (Eskenazi et al., 2007). ADHD was not evaluated by the Mt. Sinai study. Some associations were strong but imprecise (i.e., confidence intervals were wide). Further, it was also difficult to compare the experimental evidence of childhood development using developmental indices with lab tests in animals.
Another hallmark for interpreting data in both animal and human studies is exposure response. Few of the epidemiology data were reported in this context. Many of the cohorts reported linear regression coefficients for urine or blood levels and head circumference (Table 3), but log-transformed data hindered direct interpretation of coefficients. Others used only two exposure levels (Barr et al., 2010; Wolff et al., 2007; Berkowitz et al., 2004; Rauh et al., 2006; Jusko et al., 2006). The CHAMACOS study did stratify exposure by three urinary levels (< detection, < median, ≥ median) for TCPy and MDA (Eskenazi et al., 2004). Uniquely, Sagiv et al. (2007) evaluated exposure and head circumference at four levels for DDE and HCB.
A key strength in the reviewed epidemiology studies was that the pesticides or their metabolites were measured in one or more biological specimen(s), which eliminated the possibility of recall bias associated with questionnaire- or interview-based methods of assessing current or previous exposure to pesticides. Nevertheless, collection of biological specimens did not eliminate the possibility of exposure measurement error. The longer half-life of the OC insecticides makes the timing of data collection less critical than for the OP, for which the half-life in the body is a matter of hours or days. Insecticide use, both in urban and agricultural settings, is episodic. The exposure during pregnancy is unlikely to be a steady state. Further, a blood or urine sample collected at delivery, typically in a hospital setting many hours after leaving the home environment, may poorly estimate the typical exposure levels during a critical period of pregnancy.
The methods of exposure assessment can be improved and standardized to facilitate comparison of results across studies. Arcury et al. (2006) noted that for studies of farm worker populations in particular, sensitive and reliable lab techniques are not widely available, and investigators using the same analytic labs use different analytical methods, making comparisons across studies difficult. Timing of the collection with an application would reduce the need for complex models to recreate putative exposure sources. Collection of and analysis of biospecimens at multiple times during pregnancy would improve information about the stability of exposure to a given pesticide and about potential relevant windows of exposure. Similarly, comparisons across studies would be facilitated if investigators identified the most relevant neurodevelopmental assessment tools for identifying adverse effects of pesticide exposure and used them in multiple study settings.
This review of the epidemiology literature analyzed the evidence for causality between pesticide exposure and developmental neurobehavioral outcomes. By using guideposts of consistency, specificity, and dose-response, as well as considering the relevant animal evidence, our approach required that conclusions on causality be corroborated by more than one analytic study and for specific classes of pesticides. A systematic critical presentation of all findings from epidemiologic and animal literature builds a strong scientific foundation for regulatory decision making. The approach taken in this review provided a comprehensive view of the epidemiologic literature and aided in identifying potential research needs to evaluate the hypothesis of whether there is a causal relation between specific pesticides and neurobehavioral outcomes.
The methodological design, the prospective cohort, of the many studies reviewed here is one of the most robust approaches to epidemiology research. However, there were also important limitations in evaluating exposures, outcomes, and their associations. The collections of blood or urine were timed for convenience, birth or prenatal visits, rather than with use or application of a pesticide. Similarly, since many pesticides can be analytically identified in a single sample, the number of analytical comparisons became large, as demonstrated by the large tables in this review, making it cumbersome to summarize results. A number of the cohorts also investigated other early life exposures, such as lead, alcohol, and environmental tobacco smoke, often in independent publications. The cohorts with websites, such as the CHAMACOS (http://cerch.org/research-programs/chamacos/index.html) and CCCEH (http://ccceh.org/), provide the motivated reader with more information about the cohort, research on other exposures, and a list of publications.
Using consistency of specific exposures, strength of association, and exposure response as a framework for evaluating the epidemiology literature, data from epidemiologic studies as a whole do not strongly implicate any particular pesticide as being causally related to adverse neurodevelopmental outcomes in infants and children. Our rationale for this conclusion for each pesticide was discussed in detail in the epidemiologic section of this review article. Most of the publications evaluated OP (in general), CPF and/or DDT. Therefore, summaries for each are discussed in relation to the animal data in order to evaluate the biological plausibility of the epidemiologic associations.
OP There are substantial challenges to a definitive conclusion for a clear causal relationship for OP as a class of pesticide. Urinary DAP levels were considered to reflect exposure to OP as a class. The epidemiology literature was limited to the CHAMACOS and Mt. Sinai birth cohort studies. Thus, consistency of results in both studies was evaluated. The number of abnormal reflexes in newborns was the only health outcome statistically associated with maternal DAP levels in both studies (Young et al., 2005; Engel et al., 2007). The specific pesticides contributing to urinary DAP levels may be different for the California farm worker participants (CHAMACOS) and the urban New York City participants (Mt. Sinai Study). A spot sample on any given day may reflect one or more of the OP that metabolize to DAP, and may be from contact with the parent pesticide or its environmental residue. Without repeated samples, it is not possible to attribute the contribution of one specific pesticide versus another to the observed level. For this reason, caution is recommended when evaluating DAP levels for interpretation of an individual pesticide. At best, the DAP metabolites provide a general range of overall access to OP pesticides. The biological plausibility for a causal effect of OP exposure on neurobehavioral outcomes collectively is not well-supported by the recent animal literature. The animal data at lower dose ranges tested suggest that effects on neurobehavioral outcomes of different OP, such as diazinon, parathion, and CPF are not consistent as described earlier in the Animals section (Table 11). In fact, the authors of the only studies that allow comparison of three OP on similar serotonergic, molecular, cognitive, motor, and emotional outcomes conclude that the neurobehavioral and neuropharmacologic data suggested that OP exerted disparate effects of noncholinergic modes of action on the developing nervous system. Other studies demonstrating a more consistent dose-response effect on cholinergic systems provide more compelling evidence of a potential common mode of action based on downstream effects of AChE inhibition. However, these effects occur at dose levels that also produce AChE inhibition. In the case of CPF, human exposures in the CHAMACOS and Columbia cohorts are estimated to be four orders of magnitude lower than the 1-mg/kg/d LOEL/NOAEL for neurodevelopmental effects of CPF (Mink et al., 2012; McKone et al., 2007; Lowe et al., 2009; Eaton et al., 2008). If humans and rodents are equally sensitive, then the biological plausibility of effects in humans is weak based on the animal studies. Some behavioral observations, such as those of ADHD, require additional research in children with exposure metrics more targeted to sources of exposure, and differentiation of in utero versus early childhood exposures and contributing factors.
CPF There were seven neurodevelopmental outcomes statistically significantly associated with in utero CPF exposure, all in the CCCEH study. The outcomes include BSID:PDI (36 mo), BSID:MDI (36 mo), ADHD, attention problems, PDD, and WISC-IV scores for Working Memory and Full Scale IQ. These associations were not supported by other independent studies when using other exposure measures, albeit less specific to CPF. The New Jersey study was the only other study to evaluate CPF in blood, but findings have not shown any health outcome other than those observed at birth (Barr et al., 2010). Although the CCCEH results were based on a robust exposure measure, CPF in blood, one cannot rule out alternative hypotheses because of lack of replication in independent studies. Since statistically significant health outcomes continue to be observed as the CCCEH children age, one hypothesis is that maternal and/or cord blood levels are correlated with other complex factors such as, but not limited to, poverty, pest infestation, maternal stress, and child care. Other exposures such as to lead, tobacco, and air pollution are concurrent foci of this cohort and reflect the complexity of human development. Since there was little to no CPF exposure in the cohort children born after 2000 (due to withdrawal of urban use registration in the United States), further analyses stratifying this unique cohort by birth year would be interesting.
Other recently published reviews on CPF (Li et al., 2012b; Mink et al., 2012; Prueitt et al., 2011; Eaton et al., 2008) discuss the epidemiology data in further detail and conclude the current data do not support a causal relationship between CPF and adverse neurodevelopmental outcomes and head circumference in infants or young children. Li et al. (2012b) pointed out the difficulty in determining exposure in utero, particularly given the short half-life of CPF in the body. Further exposure reconstruction based upon the timing of the data collection is recommended. Since the maternal blood samples were collected within 1 d postpartum and cord blood at delivery, the degree of correlation between the samples may be dependent upon when the participant left the source of exposure, the insecticide-treated home.
Similarly, other reviews of animal studies conclude the evidence does not support an effect of CPF on neurodevelopmental reflex and motor activity, and there were mixed results on learning and memory tests (Li et al., 2012b; Prueitt et al., 2011). Specifically, 1-mg/kg/d injections of CPF in DMSO vehicle directly to young pups (PND1–4) improve performance of females and produce deficits in males on RAM as adults. However, in a superior spatial-delayed alternation learning and memory test compared to RAM, there were no marked effects in weanling or adult offspring following oral exposures of 1–5 mg/kg/d CPF to dams (GD6–PND11) (Maurissen et al., 2000). As discussed in detail in Li et al. (2012b), the LOEL/NOAEL for neurobehavioral outcomes is 1 mg/kg/d, based on an analysis that considers the utility of the study for risk assessment purposes. Regardless of agreement with our analysis, the fact remains that neurobehavioral, neuropharmacologic and neuropathologic findings were reported only at doses that produce statistically significant RBC or brain AChE inhibition in dams or pups. These doses (≥1 mg/kg/d) are estimated to be three orders of magnitude higher than the human exposures estimated in these epidemiology studies (Mink et al., 2012; Eaton et al., 2008). Thus, the biological plausibility based on animal data alone is weak because of the wide discrepancy in exposure levels producing effects in animals compared to human exposures in the different cohort studies.
DDT/DDE Several epidemiological studies attributed a variety of neurobehavioral effects to prenatal DDT/DDE exposure, whereas others have not. However, it should be noted that the concentrations measured in the populations studied are several times higher than that observed in the general population of the United States. For example, recent research from the CHAMACOS study identified decrements in neurodevelopment in the children of farmworkers from California (Eskenazi et al., 2006; Fenster et al., 2007). The measured levels of serum DDE and DDT in the California studies was 1.1 μg/g lipids, whereas a representative sample from the United States reported levels of 0.1 μg/g lipids (Centers for Disease Control and Prevention [CDC], 2005). The animal studies meeting our inclusion requirements did not provide data on internal concentrations following animal exposures to make comparisons. The most consistent behavioral effect measured in animals that was associated with developmental exposures to DDT was an increase in activity at 0.3 mg/kg oral doses. These changes in activity are not directly relevant to the cognitive outcomes in the human studies, but may be general evidence of an effect on the development of the brain. These effects were measured in studies with limited experimental design, but were repeated in more than one study conducted by the same lab. Taken together, evidence to date from epidemiologic studies did not indicate strong or consistent findings that would signify that maternal exposure at current population levels to DDT (or DDE) is associated with neurobehavioral decrements in infants or young children.
Other Pesticides There were relatively few epidemiology studies of other pesticides, including, but not limited to, N-methyl carbamates and pyrethroid insecticides. There were no consistent adverse associations of any of these pesticides or pesticide classes with head circumference or with any of the neurobehavioral outcomes reported. Further interpretation is limited by the paucity of data.
How Does the Lowest Effect Level Compare With the POD for the Chronic RfD?
Two hundred and forty-eight animal studies (225 papers) on 58 pesticides with developmental neurotoxicity endpoints representing a wide range of classes of chemistry met our inclusion criteria, with the majority of studies conducted on OP, and on CPF in particular (Figure 1). There were no published reports on developmental neurotoxicity of N-methyl carbamates that met our inclusion criteria. However, acute AChE inhibition following postnatal exposures to N-methyl carbamates was one of the most relevant and sensitive endpoints, based on our current understanding of the primary mode of action, even when taking into consideration the rapidly reversible effects of N-methyl carbamates on AChE inhibition. For OP, AChE inhibition as regulated by the U.S. EPA (based on the most sensitive RBC and brain AChE inhibition endpoints) was also the most sensitive endpoint, even when compared with potential neurodevelopmental outcomes in animal studies published in the literature.
In most of the animal studies, effects were noted at dose levels that were within the same order of magnitude or higher compared to the POD for chronic risk assessments. There were 14 pesticides with at least 1 study with a LOEL or NOEL that was numerically below the POD for the chronic RfD. However, when examining the available evidence, this was due, in part, to effects from studies with low utility for risk assessment purposes due to route of exposure (maneb, paraquat), weak experimental design (chlordecone, endosulfan, lindane, bioallethrin, deltamethrin), or the lowest dose level effects being not adverse when considering the weight of evidence (atrazine, zineb, DDT, cypermethrin, PBO). Two of these pesticides have been canceled (methoxychlor and lindane) by the U.S. EPA. As discussed previously, EPA DNT guideline studies for cypermethrin and deltamethrin were not more sensitive than other repeat dose studies and further acute neurobehavioral testing is underway.
A wide variety of endpoints including biochemical, behavioral, or physiological outcomes were evaluated in the animal studies, many of which are not included in guideline DNT studies. However, there was a significant gap in understanding the relationship between the variety of behavioral, pharmacologic and physiological endpoints, including the better studied pesticides CPF, DDT, and 2,4-D. Interpretation of whether neuropharmacologic alterations were adverse effects was especially challenging. The lack of studies directly testing hypotheses associating neuropharmacologic effects with behavioral effects, together with other limitations discussed previously with experimental methods, made it difficult to determine whether alterations in some of the neuropharmacologic parameters were clear evidence of adverse effects, especially when not repeated in other studies by the same or different labs.
Taking into consideration the results of our survey and that of reviews by two expert panels convened by the International Life Science Institute (Makris et al., 2009; Middaugh et al., 2003), developmental neurotoxicity endpoints have been useful in characterizing effects that were considered in risk assessment decisions, but not been shown to be routinely more sensitive compared to other endpoints measured in repeated dose toxicity studies for those pesticides where toxicology guideline requirements for registration have been satisfied. Raffaele et al. (2010) found that 40% of DNT studies (28 of 69) were either already used or anticipated to be used as a critical study in an OPP risk assessment. However, only 2 of the 15 DNT studies that were confirmed as the critical study had neurobehavioral or pathology endpoints solely affected (authors did not provide data for the remaining 13 studies). In the majority of cases, body weight or preputial separation was either equally as sensitive as or more sensitive than the behavioral and morphometric measures.
The vast majority of developmental neurotoxicity effects, as reported by the original authors of the primary papers, were measured at dose levels well above or within an order of magnitude of the chronic POD. As discussed previously, this is a crude comparison based on selection of the lowest effect level reported without any critical evaluation of the data, consideration of route of exposure, or comparison with other studies. While this should not detract from the usefulness of DNT endpoints to characterize important potential hazards to the developing nervous system over the range of dose levels tested (Middaugh et al., 2003; Raffaelle et al., 2010), it does provide support for a tiered approach to DNT testing so that resources can be focused on those chemicals with a greater likelihood of exerting effects on DNT endpoints at the lower dose levels.
The National Academy of Science report on the future of toxicity testing in the 21st century concluded that the design and scope of toxicity testing should serve risk management needs with human exposure guiding the depth of toxicity testing (Krewski et al., 2010). The “goal of toxicity testing should be to focus resources on evaluation of the more sensitive adverse effects of exposures rather than full characterization of all adverse effects irrespective of relevance for risk assessment needs” (Krewski et al., 2010). Because DNT-related endpoints from the published literature seldom resulted in more sensitive adverse effects than other repeated dose guideline studies (based on comparison with the POD for chronic risk assessment), this reduces concern that this test needs to be conducted routinely for all pesticides, in the view of the authors. Instead, more focused testing of sensitive endpoints such as acute effects of AChE inhibition for OP and N-methyl carbamates in pups, or acute effects of auditory startle for pyrethroids in pups, may be a much more effective and scientifically defensible way to focus resources toward sensitive endpoints that will be protective of potential developmental neurotoxicity. Similarly, it may be more effective to invest resources in developing reliable measures of thyroid or other hormonal adverse effects that might lead to downstream developmental neurotoxicity, rather than test these chemicals using DNT protocols that require large numbers of animals and lab resources but that may or may not be sensitive to the hormonal effects (Llorens et al., 2012). While this analysis suggests that a tiered approach to DNT testing is appropriate, improvements in evaluating the nervous system in first-tier screening tests using new technologies need to evolve as they become validated (Llorens et al., 2012).
CONCLUSION
In conclusion, epidemiologic evidence for causality between exposure to specific pesticides during critical periods of brain development and neurobehavioral outcomes is not compelling. Using guidelines for consistency, strength of association, dose response, and biological plausibility the data suggest few if any adverse associations. A few associations were unique for a health outcome, and specific pesticide and alternative hypotheses could not be ruled out. Notably, the exposure levels in the human participants were well below the POD based on repeat dose adult and reproductive guideline toxicity studies used for establishing human RfDs. Attention to exposure assessment and testing at consistent ages would help to clarify the observations currently reported in the literature.
In animals, functional, neuropathologic, or neuropharmacologic endpoints published in the neurodevelopmental literature meeting our inclusion criteria were not routinely more sensitive than the U.S. EPA's POD for chronic risk assessment. This comparison is consistent with other comparisons of unpublished guideline DNT studies with the POD for chronic risk assessment. While this does not negate the importance of DNT-like endpoints to characterize the effects of pesticides, these results suggest that targeted testing using a tiered approach would be a more effective use of animal and regulatory resources. For some pesticides such as pyrethroids, N-methyl carbamates, and OP where there is concern about direct exposure to children, a more effective approach to identifying a sensitive POD for risk assessment may be to focus on acute effects of exposure to pups using AChE inhibition or sensitive behaviors as endpoints rather than routinely requiring guideline DNT testing.
REFERENCES
- Abdel-Rahman A. Dechkovskaia A. Mehta-Simmons H. Guan X. Khan W. Abou-Donia M. Increased expression of glial fibrillary acidic protein in cerebellum and hippocampus: Differential effects on neonatal brain regional acetylcholinesterase following maternal exposure to combined chlorpyrifos and nicotine. J. Toxicol. Environ. Health A. 2003;66:2047–2066. doi: 10.1080/713853982. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman A. Dechkovskaia A. M. Mehta-Simmons H. Sutton J. M. Guan X. Khan W. A. Abou-Donia M. B. Maternal exposure to nicotine and chlorpyrifos, alone and in combination, leads to persistently elevated expression of glial fibrillary acidic protein in the cerebellum of the offspring in late puberty. Arch. Toxicol. 2004;78:467–476. doi: 10.1007/s00204-004-0560-5. [DOI] [PubMed] [Google Scholar]
- Abou-Donia M. B. Goldstein L. B. Bullman S. Tu T. Khan W. A. Dechkovskaia A. M. Abdel-Rahman A. A. Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J. Toxicol. Environ. Health A. 2008;71:119–130. doi: 10.1080/15287390701613140. [DOI] [PubMed] [Google Scholar]
- Abou-Donia M. B. Khan W. A. Dechkovskaia A. M. Goldstein L. B. Bullman S. L. Abdel-Rahman A. In utero exposure to nicotine and chlorpyrifos alone, and in combination produces persistent sensorimotor deficits and Purkinje neuron loss in the cerebellum of adult offspring rats. Arch. Toxicol. 2006;80:620–631. doi: 10.1007/s00204-006-0077-1. [DOI] [PubMed] [Google Scholar]
- Achenbach T. Rescorla L. Manual for the ASEBA preschool forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- Adams J. Editor's note by editor-in-chief. Neurotoxicol. Teratol. 2010;32:iii. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological profile for DDT, DDE, and DDD. 2002. http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=81&tid=20. [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological profile for pyrethrins and pyrethroids. 2003. http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=787&tid=153. [PubMed]
- Ahlbom J. Fredriksson A. Eriksson P. Neonatal exposure to a type-I pyrethroid (bioallethrin) induces dose-response changes in brain muscarinic receptors and behaviour in neonatal and adult mice. Brain Res. 1994;645:318–324. doi: 10.1016/0006-8993(94)91666-7. [DOI] [PubMed] [Google Scholar]
- Al-Hachim G. M. Al-Baker A. Effects of chlordane on conditioned avoidance response, brain seizure threshold and open-field performance of prenatally-treated mice. Br. J. Pharmacol. 1973;49:311–315. doi: 10.1111/j.1476-5381.1973.tb08377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hachim G. M. Fink G. B. Effect of DDT or parathion on open-field behavior of offspring from DDT- or parathion-treated mothers. Psychol. Rep. 1968a;22:1193–1196. doi: 10.2466/pr0.1968.22.3c.1193. [DOI] [PubMed] [Google Scholar]
- Al-Hachim G. M. Fink G. B. Effect of DDT or parathion on the minimal electroshock seizure threshold of offspring from DDT or parathion-treated mothers. Psychopharmacologia. 1968b;13:408–412. doi: 10.1007/BF00404955. [DOI] [PubMed] [Google Scholar]
- Al-Hachim G. M. Fink G. B. Effect of DDT or paration on condition avoidance response of offspring from DDT or paration treated mothers. Psychopharmacologia. 1968c;12:424–427. doi: 10.1007/BF00401347. [DOI] [PubMed] [Google Scholar]
- Aldridge J. E. Levin E. D. Seidler F. J. Slotkin T. A. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect. 2005a;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge J. E. Meyer A. Seidler F. J. Slotkin T. A. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ. Health Perspect. 2005b;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge J. E. Meyer A. Seidler F. J. Slotkin T. A. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol. Appl. Pharmacol. 2005c;203:132–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Aldridge J. E. Seidler F. J. Meyer A. Thillai I. Slotkin T. A. Serotonergic systems targeted by developmental exposure to chlorpyrifos: Effects during different critical periods. Environ. Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge J. E. Seidler F. J. Slotkin T. A. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: Critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Amira M. S. Hamadi F. Kamel J. Khansa C. Feriel E. Fadhel G. Najiba A. Impact of chlorpyrifos on cerebrum and cerebellum maturity in suckling rats. Toxicol Env Chem. 2005;87:551–558. [Google Scholar]
- Arcury T. A. Quandt S. A. Barr D. B. Hoppin J. A. Mccauley L. Grzywacz J. G. Robson M. G. Farmworker exposure to pesticides: Methodologic issues for the collection of comparable data. Environ. Health Perspect. 2006;114:923–928. doi: 10.1289/ehp.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashry K. M. Abu-Qare A. W. Saleem F. R. Hussein Y. A. Hamza S. M. Kishk A. M. Abou-Donia M. B. Inhibition and recovery of maternal and fetal cholinesterase enzymes following a single oral dose of chlorpyrifos in rats. Arch. Toxicol. 2002;76:30–39. doi: 10.1007/s00204-001-0314-6. [DOI] [PubMed] [Google Scholar]
- Astroff A. B. Freshwater K. J. Eigenberg D. A. Comparative organophosphate-induced effects observed in adult and neonatal Sprague-Dawley rats during the conduct of multigeneration toxicity studies. Reprod. Toxicol. 1998;12:619–645. doi: 10.1016/s0890-6238(98)00044-6. [DOI] [PubMed] [Google Scholar]
- Astroff A. B. Young A. D. The relationship between maternal and fetal effects following maternal organophosphate exposure during gestation in the rat. Toxicol. Ind. Health. 1998;14:869–889. doi: 10.1177/074823379801400608. [DOI] [PubMed] [Google Scholar]
- Atterberry T. T. Burnett W. T. Chambers J. E. Age-related differences in parathion and chlorpyrifos toxicity in male rats: Target and nontarget esterase sensitivity and cytochrome P450-mediated metabolism. Toxicol. Appl. Pharmacol. 1997;147:411–418. doi: 10.1006/taap.1997.8303. [DOI] [PubMed] [Google Scholar]
- Axelstad M. Boberg J. Nellemann C. Kiersgaard M. Jacobsen P. R. Christiansen S. Hougaard K. S. Hass U. Exposure to the widely used fungicide mancozeb causes thyroid hormone disruption in rat dams but no behavioral effects in the offspring. Toxicol. Sci. 2011;120:439–446. doi: 10.1093/toxsci/kfr006. [DOI] [PubMed] [Google Scholar]
- Barlow B. K. Richfield E. K. Cory-Slechta D. A. Thiruchelvam M. A fetal risk factor for Parkinson's disease. Dev. Neurosci. 2004;26:11–23. doi: 10.1159/000080707. [DOI] [PubMed] [Google Scholar]
- Barr D. B. Allen R. Olsson A. O. Bravo R. Caltabiano L. M. Montesano A. Nguyen J. Udunka S. Walden D. Walker R. D. Weerasekera G. Whitehead R. D., Jr. Schober S. E. Needham L. L. Concentrations of selective metabolites of rganophosphorus pesticides in the United States population. Environ. Res. 2005;99:314–326. doi: 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Barr D. B. Ananth C. V. Yan X. Lashley S. Smulian J. C. Ledoux T. A. Hore P. Robson M. G. Pesticide concentrations in maternal and umbilical cord sera and their relation to birth outcomes in a population of pregnant women and newborns in New Jersey. Sci. Total Environ. 2010;408:790–795. doi: 10.1016/j.scitotenv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Barr D. B. Angerer J. Potential uses of biomonitoring data: A case study using the organophosphorus pesticides chlorpyrifos and malathion. Environ. Health Perspect. 2006;114:1763–1769. doi: 10.1289/ehp.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr D. B. Bravo R. Weerasekera G. Caltabiano L. M. Whitehead R. D., Jr. Olsson A. O. Caudill S. P. Schober S. E. Pirkle J. L. Sampson E. J. Jackson R. J. Needham L. L. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ. Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D. C. Assessing environmental neurotoxicant exposures and child neurobehavior: confounded by confounding? Epidemiology. 2004;15:383–384. doi: 10.1097/01.ede.0000129525.15064.a4. [DOI] [PubMed] [Google Scholar]
- Belloni V. Dessi-Fulgheri F. Zaccaroni M. Di Consiglio E. De Angelis C. Testai E. Santochirico M. Alleva E. Santucci D. Early exposure to low doses of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology. 2011;279:19–26. doi: 10.1016/j.tox.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Benasich A. A. Bejar I. I. The Fagan test of inteligence: A critical review. J. Appl. Dev. Psychol. 1992;13:153–171. [Google Scholar]
- Berge G. N. Fonnum F. Brodal P. Neurotoxic effects of prenatal trichlorfon administration in pigs. Acta Vet. Scand. 1987a;28:321–332. doi: 10.1186/BF03548600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge G. N. Fonnum F. Soli N. E. Sognen E. Neurotoxicological examination of the piglet brain after prenatal and postnatal exposure to trichlorfon. Acta Vet. Scand. 1987b;28:313–320. doi: 10.1186/BF03548599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. S. Obel J. Deych E. Lapinski R. Godbold J. Liu Z. Landrigan P. J. Wolff M. S. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ. Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. S. Wetmur J. G. Birman-Deych E. Obel J. Lapinski R. H. Godbold J. H. Holzman I. R. Wolff M. S. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ. Health Perspect. 2004;112:388–391. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt A. M. Carr R. L. The effect of chlorpyrifos and chlorpyrifos-oxon on brain cholinesterase, muscarinic receptor binding, and neurotrophin levels in rats following early postnatal exposure. Toxicol. Sci. 2004;77:63–71. doi: 10.1093/toxsci/kfh003. [DOI] [PubMed] [Google Scholar]
- Betancourt A. M. Filipov N. M. Carr R. L. Alteration of neurotrophins in the hippocampus and cerebral cortex of young rats exposed to chlorpyrifos and methyl parathion. Toxicol. Sci. 2007;100:445–455. doi: 10.1093/toxsci/kfm248. [DOI] [PubMed] [Google Scholar]
- Billauer-Haimovitch H. Slotkin T. A. Dotan S. Langford R. Pinkas A. Yanai J. Reversal of chlorpyrifos neurobehavioral teratogenicity in mice by nicotine administration and neural stem cell transplantation. Behav. Brain Res. 2009;205:499–504. doi: 10.1016/j.bbr.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisenius E. S. Veeramachaneni D. N. Sammonds G. E. Tobet S. Sex differences and the development of the rabbit brain: effects of vinclozolin. Biol. Reprod. 2006;75:469–476. doi: 10.1095/biolreprod.106.052795. [DOI] [PubMed] [Google Scholar]
- Bjorling-Poulsen M. Andersen H. R. Grandjean P. Potential developmental neurotoxicity of pesticides used in Europe. Environ. Health. 2008;7:50–71. doi: 10.1186/1476-069X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. M. Matula K. Essentials of Bayley Scales of Infant Development—II Assessment. New York, NY: John Wiley and Sons; 2000. [Google Scholar]
- Bortolozzi A. Duffard R. Antonelli M. Evangelista De Duffard A. M. Increased sensitivity in dopamine D (2)-like brain receptors from 2, 4-dichlorophenoxyacetic acid (2, 4-D)-exposed and amphetamine-challenged rats. Ann. NY Acad. Sci. 2002;965:314–323. doi: 10.1111/j.1749-6632.2002.tb04173.x. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A. Duffard R. De Duffard A. M. Asymmetrical development of the monoamine systems in 2, 4-dichlorophenoxyacetic acid treated rats. Neurotoxicology. 2003;24:149–157. doi: 10.1016/s0161-813x(02)00156-0. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A. A. Duffard R. O. Evangelista De Duffard A. M. Behavioral alterations induced in rats by a pre- and postnatal exposure to 2, 4-dichlorophenoxyacetic acid. Neurotoxicol. Teratol. 1999;21:451–465. doi: 10.1016/s0892-0362(98)00059-2. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A. A. Evangelista De Duffard A. M. Duffard R. O. Antonelli M. C. Effects of 2, 4-dichlorophenoxyacetic acid exposure on dopamine D2-like receptors in rat brain. Neurotoxicol. Teratol. 2004;26:599–605. doi: 10.1016/j.ntt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bouchard M. F. Chevrier J. Harley K. G. Kogut K. Vedar M. Calderon N. Trujillo C. Johnson C. Bradman A. Barr D. B. Eskenazi B. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environ. Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry S. M. Cage S. A. Proudfoot A. T. Vale J. A. Poisoning due to pyrethroids. Toxicol. Rev. 2005;24:93–106. doi: 10.2165/00139709-200524020-00003. [DOI] [PubMed] [Google Scholar]
- Braquenier J. B. Quertemont E. Tirelli E. Plumier J. C. Anxiety in adult female mice following perinatal exposure to chlorpyrifos. Neurotoxicol. Teratol. 2010;32:234–239. doi: 10.1016/j.ntt.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Brazelton T. B. Nugent J. K. Neonatal Behavioral Assessment Scale. 3rd ed. London, UK: Mac Keith Press; 1995. [Google Scholar]
- Breckenridge C. B. Holden L. Sturgess N. Weiner M. Sheets L. Sargent D. Soderlund D. M. Choi J. S. Symington S. Clark J. M. Burr S. Ray D. Evidence for a separate mechanism of toxicity for the type I and the type II pyrethroid insecticides. Neurotoxicology. 2009;30(suppl. 1):S17–S31. doi: 10.1016/j.neuro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Bruckner J. V. Differences in sensitivity of children and adults to chemical toxicity: The NAS panel report. Regul. Toxicol. Pharmacol. 2000;31:280–285. doi: 10.1006/rtph.2000.1393. [DOI] [PubMed] [Google Scholar]
- Cabaleiro T. Caride A. Romero A. Lafuente A. Effects of in utero and lactational exposure to endosulfan in prefrontal cortex of male rats. Toxicol. Lett. 2008;176:58–67. doi: 10.1016/j.toxlet.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Cantalamessa F. Acute toxicity of two pyrethroids, permethrin, and cypermethrin in neonatal and adult rats. Arch. Toxicol. 1993;67:510–513. doi: 10.1007/BF01969923. [DOI] [PubMed] [Google Scholar]
- Carr R. L. Borazjani A. Ross M. K. Effect of developmental chlorpyrifos exposure, on endocannabinoid metabolizing enzymes, in the brain of juvenile rats. Toxicol. Sci. 2011;122:112–120. doi: 10.1093/toxsci/kfr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R. L. Chambers H. W. Guarisco J. A. Richardson J. R. Tang J. Chambers J. E. Effects of repeated oral postnatal exposure to chlorpyrifos on open-field behavior in juvenile rats. Toxicol. Sci. 2001;59:260–267. doi: 10.1093/toxsci/59.2.260. [DOI] [PubMed] [Google Scholar]
- Carr R. L. Nail C. A. Effect of different administration paradigms on cholinesterase inhibition following repeated chlorpyrifos exposure in late preweanling rats. Toxicol. Sci. 2008;106:186–192. doi: 10.1093/toxsci/kfn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida J. E. Quistad G. B. Golden age of insecticide research: Past, present, or future? Annu. Rev. Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- Cassidy R. A. Vorhees C. V. Minnema D. J. Hastings L. The effects of chlordane exposure during pre- and postnatal periods at environmentally relevant levels on sex steroid-mediated behaviors and functions in the rat. Toxicol. Appl. Pharmacol. 1994;126:326–337. doi: 10.1006/taap.1994.1123. [DOI] [PubMed] [Google Scholar]
- Caudle W. M. Richardson J. R. Wang M. Miller G. W. Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology. 2005;26:721–728. doi: 10.1016/j.neuro.2004.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Third national report on human exposure to environmental chemicals. Atlanta, GA: Center for Disease Control, Department of Health and Human Services; 2005. [Google Scholar]
- Centers for Disease Control and Prevention. Fourth national report on human exposure to environmental chemicals. Atlanta, GA: Center for Disease Control, Department of Health and Human Services; 2009. [Google Scholar]
- Chakraborti T. K. Farrar J. D. Pope C. N. Comparative neurochemical and neurobehavioral effects of repeated chlorpyrifos exposures in young and adult rats. Pharmacol. Biochem. Behav. 1993;46:219–224. doi: 10.1016/0091-3057(93)90344-s. [DOI] [PubMed] [Google Scholar]
- Chanda S. M. Pope C. N. Neurochemical and neurobehavioral effects of repeated gestational exposure to chlorpyrifos in maternal and developing rats. Pharmacol. Biochem. Behav. 1996;53:771–776. doi: 10.1016/0091-3057(95)02105-1. [DOI] [PubMed] [Google Scholar]
- Chapin R. E. Adams J. Boekelheide K. Gray L. E., Jr. Hayward S. W. Lees P. S. Mcintyre B. S. Portier K. M. Schnorr T. M. Selevan S. G. Vandenbergh J. G. Woskie S. R. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. B Dev Reprod. Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Chen T. H. Wang Y. H. Wu Y. H. Developmental exposures to ethanol or dimethylsulfoxide at low concentrations alter locomotor activity in larval zebrafish: implications for behavioral toxicity bioassays. Aquat Toxicol. 2011a;102:162–166. doi: 10.1016/j.aquatox.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Chen X. P. Wang X. Dong J. Y. Different reaction patterns of dopamine content to prenatal exposure to chlorpyrifos in different periods. J. Appl. Toxicol. 2011b;31:355–359. doi: 10.1002/jat.1598. [DOI] [PubMed] [Google Scholar]
- Chernoff N. Kavlock R.J. Rogers E.H. Carver B.D. Murray S. Perinatal toxicity of maneb, ethylene thiourea, and ethylenebisisothiocyanate sulfide in rodents. J. Toxicol. Environ. Health. 1979;5:821–834. doi: 10.1080/15287397909529792. [DOI] [PubMed] [Google Scholar]
- Clemens G. R. Hartnagel R. E. Bare J. J. Thyssen J. H. Teratological, neurochemical, and postnatal neurobehavioral assessment of METASYSTOX-R, an organophosphate pesticide in the rat. Fundam. Appl. Toxicol. 1990;14:131–143. doi: 10.1016/0272-0590(90)90239-g. [DOI] [PubMed] [Google Scholar]
- Colbert N. K. Pelletier N. C. Cote J. M. Concannon J. B. Jurdak N. A. Minott S. B. Markowski V. P. Perinatal exposure to low levels of the environmental antiandrogen vinclozolin alters sex-differentiated social play and sexual behaviors in the rat. Environ. Health Perspect. 2005;113:700–707. doi: 10.1289/ehp.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T. B. Walter B. J. Shih D. M. Tward A. D. Lusis A. J. Timchalk C. Richter R. J. Costa L. G. Furlong C. E. Toxicity of chlorpyrifos and chlorpyrifos oxon in a transgenic mouse model of the human paraoxonase (PON1) Q192R polymorphism. Pharmacogenet. Genomics. 2005;15:589–598. doi: 10.1097/01.fpc.0000167327.08034.d2. [DOI] [PubMed] [Google Scholar]
- Cooper J. R. Vodicnik M. J. Gordon J. H. Effects of perinatal Kepone exposure on sexual differentiation of the rat brain. Neurotoxicology. 1985;6:183–90. [PubMed] [Google Scholar]
- Costa L. G. Giordano G. Guizzetti M. Vitalone A. Neurotoxicity of pesticides: A brief review. Front. Biosc. 2008;13:1240–1249. doi: 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- Costa L.G. Giordano G. Cole T.B. Marsillach J. Furlong C. E. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology. 2012. http://dx.doi.org/10.1016/i.tox.2012.07.011. [DOI] [PMC free article] [PubMed]
- Crabbe J. C. Gallaher E. J. Cross S. J. Belknap J. K. Genetic determinants of sensitivity to diazepam in inbred mice. Behav. Neurosci. 1998;112:668–677. doi: 10.1037//0735-7044.112.3.668. [DOI] [PubMed] [Google Scholar]
- Crofton K. M. Dean K. F. Boncek V. M. Rosen M. B. Sheets L. P. Chernoff N. Reiter L. W. Prenatal or postnatal exposure to bis (tri-N-butyltin)oxide in the rat: Postnatal evaluation of teratology and behavior. Toxicol. Appl. Pharmacol. 1989;97:113–123. doi: 10.1016/0041-008x(89)90060-4. [DOI] [PubMed] [Google Scholar]
- Crofton K. M. Howard J. L. Moser V. C. Gill M. W. Reiter L. W. Tilson H. A. Macphail R. C. Interlaboratory comparison of motor activity experiments: Implications for neurotoxicological assessments. Neurotoxicol. Teratol. 1991;13:599–609. doi: 10.1016/0892-0362(91)90043-v. [DOI] [PubMed] [Google Scholar]
- Crofton K. M. Reiter L. W. Effects of two pyrethroid insecticides on motor activity and the acoustic startle response in the rat. Toxicol. Appl. Pharmacol. 1984;75:318–328. doi: 10.1016/0041-008x(84)90214-x. [DOI] [PubMed] [Google Scholar]
- Crofton K. M. Reiter L. W. Pyrethroid insecticides and the gamma-aminobutyric acidA receptor complex: Motor activity and the acoustic startle response in the rat. J. Pharmacol. Exp. Ther. 1987;243:946–954. [PubMed] [Google Scholar]
- Crowder L. A. Lanzaro G. C. Whitson R. S. Behavioral effects of methyl parathion and toxaphene exposure in rats. J. Environ. Sci. Health B. 1980;15:365–378. doi: 10.1080/03601238009372189. [DOI] [PubMed] [Google Scholar]
- Curl C. L. Fenske R. A. Kissel J. C. Shirai J. H. Moate T. F. Griffith W. Coronado G. Thompson B. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ. Health Perspect. 2002;110:A787–A792. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin B. D. Hein M. J. Sanderson W. T. Striley C. Heederik D. Kromhout H. Reynolds S. J. Alavanja M. C. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa. Ann. Occup. Hyg. 2007;51:53–65. doi: 10.1093/annhyg/mel062. [DOI] [PubMed] [Google Scholar]
- Da Silva A. P. Meotti F. C. Santos A. R. Farina M. Lactational exposure to malathion inhibits brain acetylcholinesterase in mice. Neurotoxicology. 2006;27:1101–1105. doi: 10.1016/j.neuro.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Dam K. Garcia S. J. Seidler F. J. Slotkin T. A. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Brain Res. Dev. Brain Res. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Dam K. Seidler F. J. Slotkin T. A. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res. Dev. Brain Res. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Dambska M. Iwanowski L. Kozlowski P. The effect of transplacental intoxication with dichlorvos on the development of cerebral cortex in newborn rabbits. Neuropatol. Pol. 1979;17:571–576. [PubMed] [Google Scholar]
- Dambska M. Maslinska D. Morphological changes after acetylcholinesterase (AChE) inhibition by dichlorvos (DDVP) in young rabbit brain. J. Hirnforsch. 1988;29:569–571. [PubMed] [Google Scholar]
- Darvill T. Lonky E. Reihman J. Stewart P. Pagano J. Prenatal exposure to PCBs and infant performance on the fagan test of infant intelligence. Neurotoxicology. 2000;21:1029–1038. [PubMed] [Google Scholar]
- De Castro V. L. Chiorato S. H. Pinto N. F. Biological monitoring of embrio-fetal exposure to methamidophos or chlorothalonil on rat development. Vet. Hum. Toxicol. 2000a;42:361–365. [PubMed] [Google Scholar]
- De Castro V. L. Chiorato S. H. Pinto N. F. Relevance of developmental testing of exposure to methamidophos during gestation to its toxicology evaluation. Toxicol. Lett. 2000b;118:93–102. doi: 10.1016/s0378-4274(00)00271-x. [DOI] [PubMed] [Google Scholar]
- De Castro V. L. Destefani C. R. Diniz C. Poli P. Evaluation of neurodevelopmental effects on rats exposed prenatally to sulfentrazone. Neurotoxicology. 2007;28:1249–1259. doi: 10.1016/j.neuro.2007.06.001. [DOI] [PubMed] [Google Scholar]
- De Castro V. L. Goes K. Chiorato S. Developmental toxicity potential of paclobutrazol in the rat. Int. J. Environ. Health Res. 2004;14:371–380. doi: 10.1080/09603120400004055. [DOI] [PubMed] [Google Scholar]
- De Duffard A. M. De Alderete M. N. Duffard R. Changes in brain serotonin and 5-hydroxyindolacetic acid levels induced by 2, 4-dichlorophenoxyacetic butyl ester. Toxicology. 1990;64:265–270. doi: 10.1016/0300-483x(90)90119-2. [DOI] [PubMed] [Google Scholar]
- Desesso J. M. Developmental and reproductive toxicology: A practical approach. 3rd ed. New York, NY: Informa; 2012. Comparative gestational milestones in vertebrate development. chap. 6. [Google Scholar]
- Desesso J. M. Scialli A. R. Holson J. F. Apparent lability of neural tube closure in laboratory animals and humans. Am. J. Med. Genet. 1999;87:143–162. doi: 10.1002/(sici)1096-8628(19991119)87:2<143::aid-ajmg6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Desesso J. M. Watson R. E. Keen C. L. Hazelden K. P. Haws L. C. Li A. A. Analysis and integration of developmental neurotoxicity and ancillary data into risk assessment: A case study of dimethoate. J. Toxicol. Environ. Health A. 2009;72:94–109. doi: 10.1080/15287390802477452. [DOI] [PubMed] [Google Scholar]
- Desi I. Nagymajtenyi L. Electrophysiological biomarkers of an organophosphorous pesticide, dichlorvos. Toxicol. Lett. 1999;107:55–64. doi: 10.1016/s0378-4274(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Duffard R. Garcia G. Rosso S. Bortolozzi A. Madariaga M. Di Paolo O. Evangelista De Duffard A. M. Central nervous system myelin deficit in rats exposed to 2, 4-dichlorophenoxyacetic acid throughout lactation. Neurotoxicol. Teratol. 1996;18:691–696. doi: 10.1016/s0892-0362(96)00087-6. [DOI] [PubMed] [Google Scholar]
- Dvergsten C. Meeker R. B. Muscarinic cholinergic receptor regulation and acetylcholinesterase inhibition in response to insecticide exposure during development. Int J Devl Neurosci. 1994;12:63–75. doi: 10.1016/0736-5748(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Eaton D. L. Daroff R. B. Autrup H. Bridges J. Buffler P. Costa L. G. Coyle J. Mckhann G. Mobley W. C. Nadel L. Neubert D. Schulte-Hermann R. Spencer P. S. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008;38(suppl. 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Eells J. B. Brown T. Repeated developmental exposure to chlorpyrifos and methyl parathion causes persistent alterations in nicotinic acetylcholine subunit mRNA expression with chlorpyrifos altering dopamine metabolite levels. Neurotoxicol. Teratol. 2009;31:98–103. doi: 10.1016/j.ntt.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Ellis W. G. Semple J. L. Hoogenboom E. R. Kavlock R. J. Zeman F. J. Benomyl-induced craniocerebral anomalies in fetuses of adequately nourished and protein-deprived rats. Teratogen. Carcinogen. Mutagen. 1987;7:357–375. doi: 10.1002/tcm.1770070404. [DOI] [PubMed] [Google Scholar]
- Engel S. M. Berkowitz G. S. Barr D. B. Teitelbaum S. L. Siskind J. Meisel S. J. Wetmur J. G. Wolff M. S. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am. J. Epidemiol. 2007;165:1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel S. M. Wetmur J. Chen J. Zhu C. Barr D. B. Canfield R. L. Wolff M. S. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ. Health Perspect. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P. Developmental neurotoxicology in the neonate—Effects of pesticides and polychlorinated organic substances. Arch. Toxicol. Suppl. 1996;18:81–88. doi: 10.1007/978-3-642-61105-6_9. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Developmental neurotoxicity of environmental agents in the neonate. Neurotoxicology. 1997;18:719–726. [PubMed] [Google Scholar]
- Eriksson P. Ahlbom J. Fredriksson A. Exposure to DDT during a defined period in neonatal life induces permanent changes in brain muscarinic receptors and behaviour in adult mice. Brain Res. 1992;582:277–281. doi: 10.1016/0006-8993(92)90144-x. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Archer T. Fredriksson A. Altered behaviour in adult mice exposed to a single low dose of DDT and its fatty acid conjugate as neonates. Brain Res. 1990a;514:141–142. doi: 10.1016/0006-8993(90)90446-i. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Falkeborn Y. Nordberg A. Slanina P. Effects of DDT on muscarine- and nicotine-like binding sites in CNS of immature and adult mice. Toxicol. Lett. 1984;22:329–334. doi: 10.1016/0378-4274(84)90109-7. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Fredriksson A. Neurotoxic effects of two different pyrethroids, bioallethrin and deltamethrin, on immature and adult mice: Changes in behavioral and muscarinic receptor variables. Toxicol. Appl. Pharmacol. 1991;108:78–85. doi: 10.1016/0041-008x(91)90270-o. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Johansson U. Ahlbom J. Fredriksson A. Neonatal exposure to DDT induces increased susceptibility to pyrethroid (bioallethrin) exposure at adult age—Changes in cholinergic muscarinic receptor and behavioural variables. Toxicology. 1993;77:21–30. doi: 10.1016/0300-483x(93)90134-e. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Nilsson-Hakansson L. Nordberg A. Aspberg A. Fredriksson A. Neonatal exposure to DDT and its fatty acid conjugate: Effects on cholinergic and behavioural variables in the adult mouse. Neurotoxicology. 1990b;11:345–354. [PubMed] [Google Scholar]
- Eriksson P. Nordberg A. Effects of two pyrethroids, bioallethrin and deltamethrin, on subpopulations of muscarinic and nicotinic receptors in the neonatal mouse brain. Toxicol. Appl. Pharmacol. 1990;102:456–463. doi: 10.1016/0041-008x(90)90041-r. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Talts U. Neonatal exposure to neurotoxic pesticides increases adult susceptibility: A review of current findings. Neurotoxicology. 2000;21:37–47. [PubMed] [Google Scholar]
- Eskenazi B. Harley K. Bradman A. Weltzien E. Jewell N. P. Barr D. B. Furlong C. E. Holland N. T. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ. Health Perspect. 2004;112:1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B. Huen K. Marks A. Harley K. G. Bradman A. Barr D. B. Holland N. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environ. Health Perspect. 2010;118:1775–1781. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B. Marks A. R. Bradman A. Fenster L. Johnson C. Barr D. B. Jewell N. P. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics. 2006;118:233–241. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- Eskenazi B. Marks A. R. Bradman A. Harley K. Barr D. B. Johnson C. Morga N. Jewell N. P. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista De Duffard A. M. Brusco A. Duffard R. Garcia G. Pecci Saavedra J. Changes in serotonin-immunoreactivity in the dorsal and median raphe nuclei of rats exposed to 2, 4-dichlorophenoxyacetic acid through lactation. Mol. Chem. Neuropathol. 1995;26:187–193. doi: 10.1007/BF02815012. [DOI] [PubMed] [Google Scholar]
- Fagan J. F. The Fagan Test of Infant Intelligence manual. Cleveland, OH: Case Western Reserve University; 2005. [Google Scholar]
- Fagan J. P. Detterman D. K. The Fagan test of infant intelligence: A technical summary. J. Appl. Dev. Psychol. 1992;13:173–193. [Google Scholar]
- Farag A. T. El Okazy A. M. El-Aswed A. F. Developmental toxicity study of chlorpyrifos in rats. Reprod. Toxicol. 2003;17:203–208. doi: 10.1016/s0890-6238(02)00121-1. [DOI] [PubMed] [Google Scholar]
- Faraone S. V. Sergeant J. Gillberg C. Biederman J. The worldwide prevalence of ADHD: Is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- Fenske R. A. Lu C. Barr D. Needham L. Children's exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environ. Health Perspect. 2002;110:549–553. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster L. Eskenazi B. Anderson M. Bradman A. Hubbard A. Barr D. B. In utero exposure to DDT and performance on the Brazelton neonatal behavioral assessment scale. Neurotoxicology. 2007;28:471–477. doi: 10.1016/j.neuro.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Ferri A. Bortolozzi A. Duffard R. De Duffard A. M. E. Monoamine levels in neonatal rats lactationally exposed to 2, 4-dichlorophenoxyacetic acid. Biogenic Amines. 2000;16:73–100. [Google Scholar]
- Flynn K. M. Delclos K. B. Newbold R. R. Ferguson S. A. Behavioral responses of rats exposed to long-term dietary vinclozolin. J. Agric. Food Chem. 2001;49:1658–1665. doi: 10.1021/jf0008893. [DOI] [PubMed] [Google Scholar]
- Flynn K. M. Delclos K. B. Newbold R. R. Ferguson S. A. Long term dietary methoxychlor exposure in rats increases sodium solution consumption but has few effects on other sexually dimorphic behaviors. Food Chem. Toxicol. 2005;43:1345–1354. doi: 10.1016/j.fct.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Fraites M. J. Narotsky M. G. Best D. S. Stoker T. E. Davis L. K. Goldman J. M. Hotchkiss M. G. Klinefelter G. R. Kamel A. Qian Y. Podhorniak L. Cooper R. L. Gestational atrazine exposure: Effects on male reproductive development and metabolite distribution in the dam, fetus, and neonate. Reprod. Toxicol. 2011;32:52–63. doi: 10.1016/j.reprotox.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Fredriksson A. Fredriksson M. Eriksson P. Neonatal exposure to paraquat or MPTP induces permanent changes in striatum dopamine and behavior in adult mice. Toxicol. Appl. Pharmacol. 1993;122:258–264. doi: 10.1006/taap.1993.1194. [DOI] [PubMed] [Google Scholar]
- Furlong C. E. Cole T. B. Jarvik G. P. Pettan-Brewer C. Geiss G. K. Richter R. J. Shih D. M. Tward A. D. Lusis A. J. Costa L. G. Role of paraoxonase (PON1) status in pesticide sensitivity: Genetic and temporal determinants. Neurotoxicology. 2005;26:651–659. doi: 10.1016/j.neuro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Furlong C. E. Richter R. J. Seidel S. L. Motulsky A. G. Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon. Am. J. Hum. Genet. 1988;43:230–238. [PMC free article] [PubMed] [Google Scholar]
- Garcia G. Tagliaferro P. Bortolozzi A. Madariaga M. J. Brusco A. Evangelista De Duffard A. M. Duffard R. Saavedra J. P. Morphological study of 5-HT neurons and astroglial cells on brain of adult rats perinatal or chronically exposed to 2, 4-dichlorophenoxyacetic acid. Neurotoxicology. 2001;22:733–741. doi: 10.1016/s0161-813x(01)00059-6. [DOI] [PubMed] [Google Scholar]
- Garcia G. Tagliaferro P. Ferri A. Evangelista De Duffard A. M. Duffard R. Brusco A. Study of tyrosine hydroxylase immunoreactive neurons in neonate rats lactationally exposed to 2, 4-dichlorophenoxyacetic acid. Neurotoxicology. 2004;25:951–957. doi: 10.1016/j.neuro.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Garcia G. B. Konjuh C. Duffard R. O. Evangelista De Duffard A. M. Dopamine-beta-hydroxylase immunohistochemical study in the locus coeruleus of neonate rats exposed to 2, 4-dichlorophenoxyacetic acid through mother's milk. Drug Chem. Toxicol. 2006;29:435–442. doi: 10.1080/01480540600838058. [DOI] [PubMed] [Google Scholar]
- Garcia S.J. Abu-Qare A.W. Meeker-O'Connell W.A. Borton A.J. Abou-Donia M.B. Methyl parathion: A review of health effects. J. Toxicol. Environ. Health B. 2003;6:185–210. doi: 10.1080/10937400306471. [DOI] [PubMed] [Google Scholar]
- Gerber D. J. Sotnikova T. D. Gainetdinov R. R. Huang S. Y. Caron M. G. Tonegawa S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 2001;98:15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioiosa L. Fissore E. Ghirardelli G. Parmigiani S. Palanza P. Developmental exposure to low-dose estrogenic endocrine disrupters alters sex differences in exploration and emotional responses in mice. Horm. Behav. 2007;52:307–316. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Gladen B. C. Rogan W. J. Effects of perinatal polychlorinated biphenyls and dichlorodiphenyl dichloroethene on later development. J. Pediatr. 1991;119:58–63. doi: 10.1016/s0022-3476(05)81039-x. [DOI] [PubMed] [Google Scholar]
- Gladen B. C. Rogan W. J. Hardy P. Thullen J. Tingelstad J. Tully M. Development after exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene transplacentally and through human milk. J. Pediatr. 1988;113:991–995. doi: 10.1016/s0022-3476(88)80569-9. [DOI] [PubMed] [Google Scholar]
- Godin S. J. Scollon E. J. Hughes M. F. Potter P. M. Devito M. J. Ross M. K. Species differences in the in vitro metabolism of deltamethrin and esfenvalerate: Differential oxidative and hydrolytic metabolism by humans and rats. Drug Metab. Dispos. 2006;34:1764–1771. doi: 10.1124/dmd.106.010058. [DOI] [PubMed] [Google Scholar]
- Gomeza J. Zhang L. Kostenis E. Felder C. Bymaster F. Brodkin J. Shannon H. Xia B. Deng C. Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M (4) muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P. Landrigan P. J. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr. Neonatal chlordecone exposure alters behavioral sex differentiation in female hamsters. Neurotoxicology. 1982;3:67–79. [PubMed] [Google Scholar]
- Gray L. E., Jr. Kavlock R. J. Chernoff N. Gray J. A. Mclamb J. Perinatal toxicity of endrin in rodents. III. Alterations of behavioral ontogeny. Toxicology. 1981;21:187–202. doi: 10.1016/0300-483x(81)90155-4. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr. Rogers J. M. Kavlock R. J. Ostby J. S. Ferrell J. M. Gray K. L. Prenatal exposure to the fungicide dinocap causes behavioral torticollis, ballooning and cleft palate in mice, but not rats or hamsters. Teratogen. Carcinogen. Mutagen. 1986;6:33–43. doi: 10.1002/tcm.1770060105. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr. Rogers J. M. Ostby J. S. Kavlock R. J. Ferrell J. M. Prenatal dinocap exposure alters swimming behavior in mice due to complete otolith agenesis in the inner ear. Toxicol. Appl. Pharmacol. 1988;92:266–273. doi: 10.1016/0041-008x(88)90386-9. [DOI] [PubMed] [Google Scholar]
- Griffiths R. The Griffiths Mental Development Scales. Oxon, England: The Test Agency Limited; 1996. [Google Scholar]
- Guo-Ross S. X. Chambers J. E. Meek E. C. Carr R. L. Altered muscarinic acetylcholine receptor subtype binding in neonatal rat brain following exposure to chlorpyrifos or methyl parathion. Toxicol. Sci. 2007;100:118–127. doi: 10.1093/toxsci/kfm195. [DOI] [PubMed] [Google Scholar]
- Gupta R. C. Rech R. H. Lovell K. L. Welsch F. Thornburg J. E. Brain cholinergic, behavioral, and morphological development in rats exposed in utero to methylparathion. Toxicol. Appl. Pharmacol. 1985;77:405–413. doi: 10.1016/0041-008x(85)90180-2. [DOI] [PubMed] [Google Scholar]
- Gurtovenko A. A. Anwar J. Modulating the structure and properties of cell membranes: The molecular mechanism of action of dimethyl sulfoxide. J. Phys. Chem. B. 2007;111:10453–10460. doi: 10.1021/jp073113e. [DOI] [PubMed] [Google Scholar]
- Hanslick J. L. Lau K. Noguchi K. K. Olney J. W. Zorumski C. F. Mennerick S. Farber N. B. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol. Dis. 2009;34:1–10. doi: 10.1016/j.nbd.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass U. The need for developmental neurotoxicity studies in risk assessment for developmental toxicity. Reprod. Toxicol. 2006;22:148–156. doi: 10.1016/j.reprotox.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Haviland J. A. Butz D. E. Porter W. P. Long-term sex selective hormonal and behavior alterations in mice exposed to low doses of chlorpyrifos In utero. Reprod. Toxicol. 2010;29:74–79. doi: 10.1016/j.reprotox.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Henderson G. L. Woolley D. E. Studies on the relative insensitivity of the immature rat to the neurotoxic effects of 1, 1, 1-trichloro-2, 2-bis (p-chlorophenyl)ethane (DDT) J. Pharmacol. Exp. Ther. 1969;170:173–180. [PubMed] [Google Scholar]
- Hildebrand M. E. Mcrory J. E. Snutch T. P. Stea A. Mammalian voltage-gated calcium channels are potently blocked by the pyrethroid insecticide allethrin. J. Pharmacol. Exp. Ther. 2004;308:805–813. doi: 10.1124/jpet.103.058792. [DOI] [PubMed] [Google Scholar]
- Hill A. B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelde T. Mehl A. Schanke T. M. Fonnum F. Teratogenic effects of trichlorfon (Metrifonate) on the guinea-pig brain. Determination of the effective dose and the sensitive period. Neurochem. Int. 1998;32:469–477. doi: 10.1016/s0197-0186(97)00125-3. [DOI] [PubMed] [Google Scholar]
- Holson R. R. Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol. Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Holson J. F. Nemec M. D. Stump D. G. Kaufman L. E. Lindstrom P. Varsho B. J. Significance, reliability, and interpretation of developmental and reproductive study findings. In: Hood D., editor. Developmental and reproductive toxicology: A practical approach. 2nd ed. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- Holson R. R. Freshwater L. Maurissen J. P. Moser V. C. Phang W. Statistical issues and techniques appropriate for developmental neurotoxicity testing: A report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints. Neurotoxicol. Teratol. 2008;30:326–348. doi: 10.1016/j.ntt.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Hong J. S. Ali S. F. Chlordecone (Kepone) exposure in the neonate selectively alters brain and pituitary endorphin levels in prepuberal and adult rats. Neurotoxicology. 1982;3:111–118. [PubMed] [Google Scholar]
- Horton M. K. Rundle A. Camann D. E. Boyd Barr D. Rauh V. A. Whyatt R. M. Impact of prenatal exposure to piperonyl butoxide and permethrin on 36-month neurodevelopment. Pediatrics. 2011;127:e699–e706. doi: 10.1542/peds.2010-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss A. K. Ostby J. S. Vandenbergh J. G. Gray L. E., Jr. An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiol. Behav. 2003;79:151–156. doi: 10.1016/s0031-9384(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Icenogle L. M. Christopher N. C. Blackwelder W. P. Caldwell D. P. Qiao D. Seidler F. J. Slotkin T. A. Levin E. D. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol. Teratol. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Ivanovic D. M. Leiva B. P. Perez H. T. Olivares M. G. Diaz N. S. Urrutia M. S. Almagia A. F. Toro T. D. Miller P. T. Bosch E. O. Larrain C. G. Head size and intelligence, learning, nutritional status and brain development. Head, IQ, learning, nutrition and brain. Neuropsychologia. 2004;42:1118–1131. doi: 10.1016/j.neuropsychologia.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L. Jacobson S. W. Padgett R. J. Brummitt G. A. Billings L. Effects of prenatal PCB exposure on cognitive processing efficiency and sustained attention. Dev. Psychol. 1992;28:297–306. [Google Scholar]
- Jett D. A. Navoa R. V. Beckles R. A. Mclemore G. L. Cognitive function and cholinergic neurochemistry in weanling rats exposed to chlorpyrifos. Toxicol. Appl. Pharmacol. 2001;174:89–98. doi: 10.1006/taap.2001.9198. [DOI] [PubMed] [Google Scholar]
- Jia Z. Misra H. P. Developmental exposure to pesticides zineb and/or endosulfan renders the nigrostriatal dopamine system more susceptible to these environmental chemicals later in life. Neurotoxicology. 2007;28:727–735. doi: 10.1016/j.neuro.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Jinna R. R. Uzodinma J. E. Desaiah D. Age-related changes in rat brain ATPases during treatment with chlordecone. J. Toxicol. Environ. Health. 1989;27:199–208. doi: 10.1080/15287398909531291. [DOI] [PubMed] [Google Scholar]
- Johansson U. Fredriksson A. Eriksson P. Bioallethrin causes permanent changes in behavioural and muscarinic acetylcholine receptor variables in adult mice exposed neonatally to DDT. Eur. J. Pharmacol. 1995;293:159–166. doi: 10.1016/0926-6917(95)00012-7. [DOI] [PubMed] [Google Scholar]
- Johnson F. O. Chambers J. E. Nail C. A. Givaruangsawat S. Carr R. L. Developmental chlorpyrifos and methyl parathion exposure alters radial-arm maze performance in juvenile and adult rats. Toxicol. Sci. 2009;109:132–142. doi: 10.1093/toxsci/kfp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A. Yadav S. Dhawan A. Parmar D. Overexpression of cerebral and hepatic cytochrome P450s alters behavioral activity of rat offspring following prenatal exposure to lindane. Toxicol. Appl. Pharmacol. 2007;225:278–292. doi: 10.1016/j.taap.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Julvez J. Grandjean P. Neurodevelopmental toxicity risks due to occupational exposure to industrial chemicals during pregnancy. Ind Health. 2009;47:459–468. doi: 10.2486/indhealth.47.459. [DOI] [PubMed] [Google Scholar]
- Jusko T. A. Koepsell T. D. Baker R. J. Greenfield T. A. Willman E. J. Charles M. J. Teplin S. W. Checkoway H. Hertz-Picciotto I. Maternal DDT exposures in relation to fetal and 5-year growth. Epidemiology. 2006;17:692–700. doi: 10.1097/01.ede.0000232226.06807.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A. Irizarry M. C. Schwarzschild M. A. Caffeine protects against combined paraquat and maneb-induced dopaminergic neuron degeneration. Exp. Neurol. 2010;223:657–661. doi: 10.1016/j.expneurol.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennepohl E. Munro I. C. Phenoxy herbicides (2, 4-D) Tox. Stud. 2001;2:1623–1638. [Google Scholar]
- Konjuh C. Garcia G. Lopez L. De Duffard A. M. Brusco A. Duffard R. Neonatal hypomyelination by the herbicide 2, 4-dichlorophenoxyacetic acid. Chemical and ultrastructural studies in rats. Toxicol. Sci. 2008;104:332–340. doi: 10.1093/toxsci/kfn085. [DOI] [PubMed] [Google Scholar]
- Krewski D. Acosta D., Jr. Andersen M. Anderson H. Bailar J. C., 3rd Boekelheide K. Brent R. Charnley G. Cheung V. G. Green S., Jr. Kelsey K. T. Kerkvliet N. I. Li A. A. Mccray L. Meyer O. Patterson R. D. Pennie W. Scala R. A. Solomon G. M. Stephens M. Yager J. Zeise L. Toxicity testing in the 21st century: A vision and a strategy. J. Toxicol. Environ. Health B. 2010;13:51–138. doi: 10.1080/10937404.2010.483176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laessig S. A. Auger A. P. McCarthy M. M. Silbergeld E. K. Effects of prenatal chlordecone on sexually differentiated behavior in adult rats. Neurotoxicol. Teratol. 2007;29:255–263. doi: 10.1016/j.ntt.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Lakshmana M. K. Raju T. R. Endosulfan induces small but significant changes in the levels of noradrenaline, dopamine and serotonin in the developing rat brain and deficits in the operant learning performance. Toxicology. 1994;91:139–150. doi: 10.1016/0300-483x(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Lassiter T. L. Barone S., Jr. Moser V. C. Padilla S. Gestational exposure to chlorpyrifos: Dose response profiles for cholinesterase and carboxylesterase activity. Toxicol. Sci. 1999;52:92–100. doi: 10.1093/toxsci/52.1.92. [DOI] [PubMed] [Google Scholar]
- Lassiter T. L. Padilla S. Mortensen S. R. Chanda S. M. Moser V. C. Barone S., Jr. Gestational exposure to chlorpyrifos: Apparent protection of the fetus? Toxicol. Appl. Pharmacol. 1998;152:56–65. doi: 10.1006/taap.1998.8514. [DOI] [PubMed] [Google Scholar]
- Lawrence L. J. Casida J. E. Stereospecific action of pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore complex. Science. 1983;221:1399–1401. doi: 10.1126/science.6310756. [DOI] [PubMed] [Google Scholar]
- Levin E. D. Addy N. Baruah A. Elias A. Christopher N. C. Seidler F. J. Slotkin T. A. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol. Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin E. D. Addy N. Nakajima A. Christopher N. C. Seidler F. J. Slotkin T. A. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Brain Res. Dev. Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin E. D. Timofeeva O. A. Yang L. Petro A. Ryde I. T. Wrench N. Seidler F. J. Slotkin T. A. Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behav. Brain Res. 2010;208:319–327. doi: 10.1016/j.bbr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. A. Levine T. E. Burns C. J. Kent Anger W. Integration of epidemiology and animal neurotoxicity data for risk assessment. Neurotoxicology. 2012a;33:823–832. doi: 10.1016/j.neuro.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Li A. A. Lowe K. A. Mcintosh L. J. Mink P. J. Evaluation of epidemiology and animal data for risk assessment: chlorpyrifos developmental neurobehavioral outcomes. J. Toxicol. Environ. Health B. 2012b;15:109–184. doi: 10.1080/10937404.2012.645142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Olivier K. Pope C. N. Comparative neurochemical effects of repeated methyl parathion or chlorpyrifos exposures in neonatal and adult rats. Toxicol. Appl. Pharmacol. 1999;158:186–196. doi: 10.1006/taap.1999.8693. [DOI] [PubMed] [Google Scholar]
- Llorens J. Li A. A. Ceccatelli S. Sunol C. Strategies and tools for preventing neurotoxicity: To test, to predict and how to do it. Neurotoxicology. 2012;33:796–804. doi: 10.1016/j.neuro.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Lock E. A. Wilks M. F. Paraquat. In: Krieger R. I., editor. Handbook of pesticide toxicology. 2nd ed. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Longnecker M. P. Klebanoff M. A. Zhou H. Brock J. W. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet. 2001;358:110–114. doi: 10.1016/S0140-6736(01)05329-6. [DOI] [PubMed] [Google Scholar]
- Longnecker M. P. Rogan W. J. Lucier G. The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu Rev Public Health. 1997;18:211–244. doi: 10.1146/annurev.publhealth.18.1.211. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M. J. Granada A. Carreno J. Salvatierra M. Olea-Serrano F. Olea N. Organochlorine pesticides in placentas from Southern Spain and some related factors. Placenta. 2007;28:631–638. doi: 10.1016/j.placenta.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Lovasi G. S. Quinn J. W. Rauh V. A. Perera F. P. Andrews H. F. Garfinkel R. Hoepner L. Whyatt R. Rundle A. Chlorpyrifos exposure and urban residential environment characteristics as determinants of early childhood neurodevelopment. Am. J. Public Health. 2011;101:63–70. doi: 10.2105/AJPH.2009.168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe E. R. Poet T. S. Rick D. L. Marty M. S. Mattsson J. L. Timchalk C. Battels M. J. The effect of plasma lipids on the pharmacokinetics of chlorpyrifos and the impact on interpretation of blood biomonitoring data. Toxicol. Sci. 2009;108:258–272. doi: 10.1093/toxsci/kfp034. [DOI] [PubMed] [Google Scholar]
- Mactutus C. F. Tilson H. A. Evaluation of long-term consequences in behavioral and/or neural function following neonatal chlordecone exposure. Teratology. 1985;31:177–186. doi: 10.1002/tera.1420310202. [DOI] [PubMed] [Google Scholar]
- Mactutus C. F. Unger K. L. Tilson H. A. Neonatal chlordecone exposure impairs early learning and memory in the rat on a multiple measure passive avoidance task. Neurotoxicology. 1982;3:27–44. [PubMed] [Google Scholar]
- Mactutus C. F. Unger K. L. Tilson H. A. Evaluation of neonatal chlordecone neurotoxicity during early development: initial characterization. Neurobehav. Toxicol. Teratol. 1984;6:67–73. [PubMed] [Google Scholar]
- Makris S. L. Raffaele K. Allen S. Bowers W. J. Hass U. Alleva E. Calamandrei G. Sheets L. Amcoff P. Delrue N. Crofton K. M. A retrospective performance assessment of the developmental neurotoxicity study in support of OECD test guideline 426. Environ. Health Perspect. 2009;117:17–25. doi: 10.1289/ehp.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel J. S. Alexander B. H. Baker B. A. Acquavella J. F. Chapman P. Honeycutt R. Biomonitoring for farm families in the farm family exposure study. Scand. J. Work Environ. Health. 2005;31(suppl. 1):98–104. discussion 6–65. [PubMed] [Google Scholar]
- Marks A. R. Harley K. Bradman A. Kogut K. Barr D. B. Johnson C. Calderon N. Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ. Health Perspect. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslinska D. Lewandowska I. Prokopczyk J. Effect of prolonged acetylcholinesterase inhibition on postnatal brain development in rabbit. I. Level of serotonin in different brain regions. Acta Neuropathol. Suppl. 1981;7:52–55. doi: 10.1007/978-3-642-81553-9_16. [DOI] [PubMed] [Google Scholar]
- Maslinska D. Zalewska Z. Effect of dichlorvos, administered to the pregnant rabbits, on the colinesterases activity in the progeny. Folia Histochem. Cytochem. (Krakow) 1978;16:335–341. [PubMed] [Google Scholar]
- Mattsson J. L. Maurissen J. P. Nolan R. J. Brzak K. A. Lack of differential sensitivity to cholinesterase inhibition in fetuses and neonates compared to dams treated perinatally with chlorpyrifos. Toxicol. Sci. 2000;53:438–446. doi: 10.1093/toxsci/53.2.438. [DOI] [PubMed] [Google Scholar]
- Maurissen J. Practical considerations on the design, execution and analysis of developmental neurotoxicity studies to be published in Neurotoxicology and Teratology. Neurotoxicol. Teratol. 2010;32:121–123. doi: 10.1016/j.ntt.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Maurissen J. P. Hoberman A. M. Garman R. H. Hanley T. R., Jr. Lack of selective developmental neurotoxicity in rat pups from dams treated by gavage with chlorpyrifos. Toxicol. Sci. 2000;57:250–263. doi: 10.1093/toxsci/57.2.250. [DOI] [PubMed] [Google Scholar]
- McCarthy D. Manual for the McCarthy Scales of Chilren's Abilities. New York, NY: Psychological Corporation; 1972. [Google Scholar]
- McCauley L. A. Lasarev M. R. Higgins G. Rothlein J. Muniz J. Ebbert C. Phillips J. Work characteristics and pesticide exposures among migrant agricultural families: A community-based research approach. Environ. Health Perspect. 2001;109:533–538. doi: 10.1289/ehp.01109533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel K. L. Padilla S. Marshall R. S. Phillips P. M. Podhorniak L. Qian Y. Moser V. C. Comparison of acute neurobehavioral and cholinesterase inhibitory effects of N-methylcarbamates in rat. Toxicol. Sci. 2007;98:552–560. doi: 10.1093/toxsci/kfm114. [DOI] [PubMed] [Google Scholar]
- McKone T. E. Castorina R. Harnly M. E. Kuwabara Y. Eskenazi B. Bradman A. Merging models and biomonitoring data to characterize sources and pathways of human exposure to organophosphorus pesticides in the Salinas Valley of California. Environ. Sci. Technol. 2007;41:3233–3240. doi: 10.1021/es0618447. [DOI] [PubMed] [Google Scholar]
- Meacham C. A. Brodfuehrer P. D. Watkins J. A. Shafer T. J. Developmentally-regulated sodium channel subunits are differentially sensitive to alpha-cyano containing pyrethroids. Toxicol. Appl. Pharmacol. 2008;231:273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Mehl A. Schanke T. M. Johnsen B. A. Fonnum F. The effect of trichlorfon and other organophosphates on prenatal brain development in the guinea pig. Neurochem. Res. 1994;19:569–574. doi: 10.1007/BF00971332. [DOI] [PubMed] [Google Scholar]
- Mehl A. Schanke T. M. Torvik A. Fonnum F. The effect of trichlorfon and methylazoxymethanol on the development of guinea pig cerebellum. Toxicol. Appl. Pharmacol. 2007;219:128–135. doi: 10.1016/j.taap.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Mendola P. Selevan S. G. Gutter S. Rice D. Environmental factors associated with a spectrum of neurodevelopmental deficits. MRDD Res. Rev. 2002;8:188–197. doi: 10.1002/mrdd.10033. [DOI] [PubMed] [Google Scholar]
- Middaugh L. D. Dow-Edwards D. Li A. A. Sandler J. D. Seed J. Sheets L. P. Shuey D. L. Slikker W., Jr. Weisenburger W. P. Wise L. D. Selwyn M. R. Neurobehavioral assessment: A survey of use and value in safety assessment studies. Toxicol. Sci. 2003;76:250–261. doi: 10.1093/toxsci/kfg211. [DOI] [PubMed] [Google Scholar]
- Mink P. J. Kimmel C. A. Li A. A. Potential effects of chlorpyrifos on fetal growth outcomes: implications for risk assessment. J. Toxicol. Environ. Health B. 2012;15:281–316. doi: 10.1080/10937404.2012.672150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Contreras L. Davila-Ovalles R. Benitez-Diaz P. Pena-Contreras Z. Palacios-Pru E. Effects of prenatal paraquat and mancozeb exposure on amino acid synaptic transmission in developing mouse cerebellar cortex. Brain Res. Dev. Brain Res. 2005;160:19–27. doi: 10.1016/j.devbrainres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Moniz A. C. Bernardi M. M. Souza-Spinosa H. S. Palermo-Neto J. Effects of exposure to a pyrethroid insecticide during lactation on the behavior of infant and adult rats. Braz. J. Med. Biol. Res. 1990;23:45–48. [PubMed] [Google Scholar]
- Moniz A. C. Cruz-Casallas P. E. Oliveira C. A. Lucisano A. Florio J. C. Nicolau A. A. Spinosa H. S. Bernardi M. M. Perinatal fenvalerate exposure: behavioral and endocrinology changes in male rats. Neurotoxicol. Teratol. 1999;21:611–618. doi: 10.1016/s0892-0362(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Moniz A. C. Cruz-Casallas P. E. Salzgeber S. A. Varoli F. M. Spinosa H. S. Bernardi M. M. Behavioral and endocrine changes induced by perinatal fenvalerate exposure in female rats. Neurotoxicol. Teratol. 2005;27:609–614. doi: 10.1016/j.ntt.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Morales E. Sunyer J. Castro-Giner F. Estivill X. Julvez J. Ribas-Fito N. Torrent M. Grimalt J. O. De Cid R. Influence of glutathione S-transferase polymorphisms on cognitive functioning effects induced by P, p′-DDT among preschoolers. Environ. Health Perspect. 2008;116:1581–1585. doi: 10.1289/ehp.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser V. C. Dose-response and time-course of neurobehavioral changes following oral chlorpyrifos in rats of different ages. Neurotoxicol. Teratol. 2000;22:713–723. doi: 10.1016/s0892-0362(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Moser V. C. Chanda S. M. Mortensen S. R. Padilla S. Age- and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol. Sci. 1998;46:211–222. doi: 10.1006/toxs.1998.2526. [DOI] [PubMed] [Google Scholar]
- Moser V. C. Mcdaniel K. L. Phillips P. M. Lowit A. B. Time-course, dose-response, and age comparative sensitivity of N-methyl carbamates in rats. Toxicol. Sci. 2010;114:113–123. doi: 10.1093/toxsci/kfp286. [DOI] [PubMed] [Google Scholar]
- Moser V. C. Shafer T. J. Ward T. R. Meacham C. A. Harris M. W. Chapin R. E. Neurotoxicological outcomes of perinatal heptachlor exposure in the rat. Toxicol. Sci. 2001;60:315–326. doi: 10.1093/toxsci/60.2.315. [DOI] [PubMed] [Google Scholar]
- Mundy W. P. S. Shafer T. Gilbert M. Breier J. Cowden J. Crofton K. Herr D. Jensen K. Raffaele K. Radio N. Schumacher K. Building a database of neurotoxicants: Evidence from human and animal studies. 2009. Poster presentad at Society of Toxicology Meeting, U.S. EPA.
- Nagymajtenyi L. Schulz H. Papp A. Desi I. Developmental neurotoxicological effects of lead and dimethoate in animal experiments. Neurotoxicology. 1998;19:617–622. [PubMed] [Google Scholar]
- Narahashi T. Neuroreceptors and ion channels as the basis for drug action: Past, present, and future. J. Pharmacol. Exp. Ther. 2000;294:1–26. [PubMed] [Google Scholar]
- Nasuti C. Gabbianelli R. Falcioni M. L. Di Stefano A. Sozio P. Cantalamessa F. Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology. 2007;229:194–205. doi: 10.1016/j.tox.2006.10.015. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences. Research on children and adolescents with mental, behavioral, and developmental disorders. Washington, DC: National Academy Press; 1988. [Google Scholar]
- National Academy of Sciences. Scientific frontiers in developmental toxicity and risk assessment. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Nostrandt A. C. Duncan J. A. Padilla S. A modified spectrophotometric method appropriate for measuring cholinesterase activity in tissue from carbaryl-treated animals. Fundam. Appl. Toxicol. 1993;21:196–203. doi: 10.1006/faat.1993.1089. [DOI] [PubMed] [Google Scholar]
- O'rourke M. K. Lizardi P. S. Rogan S. P. Freeman N. C. Aguirre A. Saint C. G. Pesticide exposure and creatinine variation among young children. J. Expos. Anal. Environ. Epidemiol. 2000;10:672–681. doi: 10.1038/sj.jea.7500119. [DOI] [PubMed] [Google Scholar]
- Ogino T. Hattori J. Abiru K. Nakano K. Oka E. Ohtsuka Y. Symptoms related to ADHD observed in patients with pervasive developmental disorder. Brain Dev. 2005;27:345–348. doi: 10.1016/j.braindev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Olson K. L. Boush G. M. Matsumura F. Behavioral effects of perinatal exposure of chlordimeform in rats. Bull. Environ. Contam. Toxicol. 1978;20:760–768. doi: 10.1007/BF01683597. [DOI] [PubMed] [Google Scholar]
- Palanza P. Morellini F. Parmigiani S. Vom Saal F. S. Ethological methods to study the effects of maternal exposure to estrogenic endocrine disrupters: A study with methoxychlor. Neurotoxicol. Teratol. 2002;24:55–69. doi: 10.1016/s0892-0362(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Palanza P. Parmigiani S. Vom Saal F. S. Effects of prenatal exposure to low doses of diethylstilbestrol, O, p′-DDT, and methoxychlor on postnatal growth and neurobehavioral development in male and female mice. Horm. Behav. 2001;40:252–265. doi: 10.1006/hbeh.2001.1697. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J. Sakate M. Florio J. C. Developmental and behavioral effects of postnatal amitraz exposure in rats. Braz. J. Med. Biol. Res. 1997;30:989–997. doi: 10.1590/s0100-879x1997000800013. [DOI] [PubMed] [Google Scholar]
- Pan I. J. Daniels J. L. Goldman B. D. Herring A. H. Siega-Riz A. M. Rogan W. J. Lactational exposure to polychlorinated biphenyls, dichlorodiphenyltrichloroethane, and dichlorodiphenyldichloroethylene and infant neurodevelopment: An analysis of the pregnancy, infection, and nutrition babies study. Environ. Health Perspect. 2009;117:488–494. doi: 10.1289/ehp.0800063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancetti F. Olmos C. Dagnino-Subiabre A. Rozas C. Morales B. Noncholinesterase effects induced by organophosphate pesticides and their relationship to cognitive processes: Implications for the action of acylpeptide hydrolase. J. Toxicol. Environ. Health B. 2007;10:623–630. doi: 10.1080/10937400701436445. [DOI] [PubMed] [Google Scholar]
- Patro N. Shrivastava M. Tripathi S. Patro I. K. S100beta upregulation: A possible mechanism of deltamethrin toxicity and motor coordination deficits. Neurotoxicol. Teratol. 2009;31:169–176. doi: 10.1016/j.ntt.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Schmuck G. Critical analysis of potential body temperature confounders on neurochemical endpoints caused by direct dosing and maternal separation in neonatal mice: A study of bioallethrin and deltamethrin interactions with temperature on brain muscarinic receptors. J. Appl. Toxicol. 2003;23:9–18. doi: 10.1002/jat.873. [DOI] [PubMed] [Google Scholar]
- Pentyala S. N. Chetty C. S. Comparative study on the changes in AChE and ATPase activities in neonate and adult rat brains under thiobencarb stress. J. Appl. Toxicol. 1993;13:39–42. doi: 10.1002/jat.2550130109. [DOI] [PubMed] [Google Scholar]
- Perera F. P. Rauh V. Tsai W. Y. Kinney P. Camann D. Barr D. Bernert T. Garfinkel R. Tu Y. H. Diaz D. Dietrich J. Whyatt R. M. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ. Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole C. Low P-values or narrow confidence intervals: Which are more durable? Epidemiology. 2001;12:291–294. doi: 10.1097/00001648-200105000-00005. [DOI] [PubMed] [Google Scholar]
- Pope A. M. Heavner J. E. Guarnieri J. A. Knobloch C. P. Trichlorfon-induced congenital cerebellar hypoplasia in neonatal pigs. J. Am. Vet. Med. Assoc. 1986;189:781–783. [PubMed] [Google Scholar]
- Pope C. N. Organophosphorus pesticides: Do they all have the same mechanism of toxicity? J. Toxicol. Environ. Health B. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Pope C. N. Chakraborti T. K. Dose-related inhibition of brain and plasma cholinesterase in neonatal and adult rats following sublethal organophosphate exposures. Toxicology. 1992;73:35–43. doi: 10.1016/0300-483x(92)90168-e. [DOI] [PubMed] [Google Scholar]
- Pope C. N. Chakraborti T. K. Chapman M. L. Farrar J. D. Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68:51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- Prueitt R. L. Goodman J. E. Bailey L. A. Rhomberg L. R. Hypothesis-based weight-of-evidence evaluation of the neurodevelopmental effects of chlorpyrifos. Crit. Rev. Toxicol. 2011;41:822–903. doi: 10.3109/10408444.2011.616877. [DOI] [PubMed] [Google Scholar]
- Puertas R. Lopez-Espinosa M.-J. Cruz F. Ramos R. Freire C. Perez-Garcia M. Abril A. Julvez J. Salvatierra M. Campoy C. Olea N. Prenatal exposure to mirex impairs neurodevelopment at age of 4 years. Neurotoxicology. 2010;31:154–160. doi: 10.1016/j.neuro.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Purkerson-Parker S. Mcdaniel K. L. Moser V. C. Dopamine transporter binding in the rat striatum is increased by gestational, perinatal, and adolescent exposure to heptachlor. Toxicol. Sci. 2001;64:216–223. doi: 10.1093/toxsci/64.2.216. [DOI] [PubMed] [Google Scholar]
- Qiao D. Seidler F. J. Abreu-Villaca Y. Tate C. A. Cousins M. M. Slotkin T. A. Chlorpyrifos exposure during neurulation: cholinergic synaptic dysfunction and cellular alterations in brain regions at adolescence and adulthood. Brain Res. Dev. Brain Res. 2004;148:43–52. doi: 10.1016/j.devbrainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Qiao D. Seidler F. J. Padilla S. Slotkin T. A. Developmental neurotoxicity of chlorpyrifos: What is the vulnerable period? Environ. Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D. Seidler F. J. Tate C. A. Cousins M. M. Slotkin T. A. Fetal chlorpyrifos exposure: Adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ. Health Perspect. 2003;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele K. C. Rowland J. May B. Makris S. L. Schumacher K. Scarano L. J. The use of developmental neurotoxicity data in pesticide risk assessments. Neurotoxicol. Teratol. 2010;32:563–572. doi: 10.1016/j.ntt.2010.04.053. [DOI] [PubMed] [Google Scholar]
- Raines K. W. Seidler F. J. Slotkin T. A. Alterations in serotonin transporter expression in brain regions of rats exposed neonatally to chlorpyrifos. Brain Res. Dev. Brain Res. 2001;130:65–72. doi: 10.1016/s0165-3806(01)00211-5. [DOI] [PubMed] [Google Scholar]
- Rauh V. Arunajadai S. Horton M. Perera F. Hoepner L. Barr D. B. Whyatt R. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ. Health Perspect. 2011;119:1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V. A. Garfinkel R. Perera F. P. Andrews H. F. Hoepner L. Barr D. B. Whitehead R. Tang D. Whyatt R. W. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. E. Fry J. R. A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacol. Ther. 2006;111:174–193. doi: 10.1016/j.pharmthera.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Reeves R. Thiruchelvam M. Richfield E. K. Cory-Slechta D. A. The effect of developmental exposure to the fungicide triadimefon on behavioral sensitization to triadimefon during adulthood. Toxicol. Appl. Pharmacol. 2004;200:54–63. doi: 10.1016/j.taap.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Reiss R. Gaylor D. Use of benchmark dose and meta-analysis to determine the most sensitive endpoint for risk assessment for dimethoate. Regul. Toxicol. Pharmacol. 2005;43:55–65. doi: 10.1016/j.yrtph.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Rescorla L. A. Assessment of young children using the Achenbach System of Empirically Based Assessment (ASEBA) Merit. Retard. Dev. Disabil. Res. Rev. 2005;11:226–237. doi: 10.1002/mrdd.20071. [DOI] [PubMed] [Google Scholar]
- Rhodes M. C. Seidler F. J. Qiao D. Tate C. A. Cousins M. M. Slotkin T. A. Does pharmacotherapy for preterm labor sensitize the developing brain to environmental neurotoxicants? Cellular and synaptic effects of sequential exposure to terbutaline and chlorpyrifos in neonatal rats. Toxicol. Appl. Pharmacol. 2004;195:203–217. doi: 10.1016/j.taap.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Ribas-Fito N. Cardo E. Sala M. Eulalia De Muga M. Mazon C. Verdu A. Kogevinas M. Grimalt J. O. Sunyer J. Breastfeeding, exposure to organochlorine compounds, and neurodevelopment in infants. Pediatrics. 2003;111:e580–e585. doi: 10.1542/peds.111.5.e580. [DOI] [PubMed] [Google Scholar]
- Ribas-Fito N. Torrent M. Carrizo D. Julvez J. Grimalt J. O. Sunyer J. Exposure to hexachlorobenzene during pregnancy and children's social behavior at 4 years of age. Environ. Health Perspect. 2007;115:447–450. doi: 10.1289/ehp.9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Fito N. Torrent M. Carrizo D. Munoz-Ortiz L. Julvez J. Grimalt J. O. Sunyer J. In utero exposure to background concentrations of DDT and cognitive functioning among preschoolers. Am. J. Epidemiol. 2006;164:955–962. doi: 10.1093/aje/kwj299. [DOI] [PubMed] [Google Scholar]
- Ricceri L. Venerosi A. Capone F. Cometa M. F. Lorenzini P. Fortuna S. Calamandrei G. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol. Sci. 2006;93:105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Rice D. Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108(suppl. 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D. C. Assessing the effects of environmental toxicant exposure in developmental epidemiological studies: issues for risk assessment. Neurotoxicology. 2005;26:483–489. doi: 10.1016/j.neuro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Richardson J. Chambers J. Effects of gestational exposure to chlorpyrifos on postnatal central and peripheral cholinergic neurochemistry. J. Toxicol. Environ. Health A. 2003;66:275–289. doi: 10.1080/15287390306369. [DOI] [PubMed] [Google Scholar]
- Richardson J. R. Caudle W. M. Wang M. Z. Dean E. D. Pennell K. D. Miller G. W. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) in a gender-specific manner. Neurotoxicology. 2008;29:855–863. doi: 10.1016/j.neuro.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. R. Chambers J. E. Neurochemical effects of repeated gestational exposure to chlorpyrifos in developing rats. Toxicol. Sci. 2004;77:83–90. doi: 10.1093/toxsci/kfh014. [DOI] [PubMed] [Google Scholar]
- Richardson J. R. Chambers J. E. Effects of repeated oral postnatal exposure to chlorpyrifos on cholinergic neurochemistry in developing rats. Toxicol. Sci. 2005;84:352–359. doi: 10.1093/toxsci/kfi081. [DOI] [PubMed] [Google Scholar]
- Roberts E. M. English P. B. Grether J. K. Windham G. C. Somberg L. Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ. Health Perspect. 2007;115:1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegge C. S. Timofeeva O. A. Seidler F. J. Slotkin T. A. Levin E. D. Developmental diazinon neurotoxicity in rats: Later effects on emotional response. Brain. Res. Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan W. J. Gladen B. C. PCBs, DDE, and child development at 18 and 24 months. Ann. Epidemiol. 1991;1:407–413. doi: 10.1016/1047-2797(91)90010-a. [DOI] [PubMed] [Google Scholar]
- Rogan W. J. Gladen B. C. Mckinney J. D. Carreras N. Hardy P. Thullen J. Tinglestad J. Tully M. Neonatal effects of transplacental exposure to PCBs and DDE.; Pediatr. 1986;109:335–341. doi: 10.1016/s0022-3476(86)80397-3. [DOI] [PubMed] [Google Scholar]
- Rosecrans J. A. Hong J. S. Squibb R. E. Johnson J. H. Wilson W. E. Tilson H. A. Effects of perinatal exposure to chlordecone (Kepone) on neuroendocrine and neurochemical responsiveness of rats to environmental challenges. Neurotoxicology. 1982;3:131–142. [PubMed] [Google Scholar]
- Rosecrans J. A. Johnson J. H. Tilson H. A. Hong J. S. Hypothalamic-pituitary adrenal (HPAA) axis function in adult Fischer-344 rats exposed during development to neurotoxic chemicals perinatally. Neurobehav. Toxicol. Teratol. 1984;6:281–288. [PubMed] [Google Scholar]
- Ross M. K. Borazjani A. Edwards C. C. Potter P. M. Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases. Biochem. Pharmacol. 2006;71:657–669. doi: 10.1016/j.bcp.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Rosso S. B. Garcia G. B. Madariaga M. J. Evangelista De Duffard A. M. Duffard R. O. 2, 4-Dichlorophenoxyacetic acid in developing rats alters behaviour, myelination and regions brain gangliosides pattern. Neurotoxicology. 2000;21:155–163. [PubMed] [Google Scholar]
- Roy T. S. Seidler F. J. Slotkin T. A. Morphologic effects of subtoxic neonatal chlorpyrifos exposure in developing rat brain: Regionally selective alterations in neurons and glia. Brain Res. Dev. Brain Res. 2004;148:197–206. doi: 10.1016/j.devbrainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Roy T. S. Sharma V. Seidler F. J. Slotkin T. A. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Brain Res. Dev. Brain Res. 2005;155:71–80. doi: 10.1016/j.devbrainres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Sagiv S. K. Nugent J. K. Brazelton T. B. Choi A. L. Tolbert P. E. Altshul L. M. Korrick S. A. Prenatal organochlorine exposure and measures of behavior in infancy using the Neonatal Behavioral Assessment Scale (NBAS) Environ. Health Perspect. 2008;116:666–673. doi: 10.1289/ehp.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv S. K. Thurston S. W. Bellinger D. C. Tolbert P. E. Altshul L. M. Korrick S. A. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am. J. Epidemiol. 2010;171:593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv S. K. Tolbert P. E. Altshul L. M. Korrick S. A. Organochlorine exposures during pregnancy and infant size at birth. Epidemiology. 2007;18:120–129. doi: 10.1097/01.ede.0000249769.15001.7c. [DOI] [PubMed] [Google Scholar]
- Samuelsen S. O. Bakketeig L. S. Tretli S. Johannesen T. B. Magnus P. Head circumference at birth and risk of brain cancer in childhood: A population-based study. Lancet Oncol. 2006;7:39–42. doi: 10.1016/S1470-2045(05)70470-8. [DOI] [PubMed] [Google Scholar]
- Sattler J. M. Assessment of children: Cognitive applications. La Mesa, CA: Jerome M. Sattler, Publisher; 2001. [Google Scholar]
- Sattler J. M. Wechser Preschool and Primary Scale of Intelligence—Third edition (WPPSI-III): Description. In: Sattler J. M., editor. Assessment of children: Cognitive foundations. 5th ed. San Diego, CA: Jerome M. Sattler, Publisher; 2008a. pp. 403–440. [Google Scholar]
- Sattler J. M. Wechsler Intelligence Scale for Children—Fourth edition (WISC-IV): Description. In: Sattler J. M., editor. Assessment of children: Cognitive foundations. 5th ed. San Diego, CA: Jerome M. Sattler, Publisher; 2008b. pp. 265–315. [Google Scholar]
- Sattler J. M. Weyandt L. Willis J. O. Attention-deficit/hyperactivity disorder. In: Sattler J. M., editor; Hoge R. D., editor. Assessment of children: Behavioral, social, and clinical foundations. 5th ed. San Diego, CA: Jerome M. Sattler, Publisher; 2006. pp. 374–389. [Google Scholar]
- Scollon E. J. Starr J. M. Godin S. J. Devito M. J. Hughes M. F. In vitro metabolism of pyrethroid pesticides by rat and human hepatic microsomes and cytochrome P450 isoforms. Drug Metab. Dispos. 2009;37:221–228. doi: 10.1124/dmd.108.022343. [DOI] [PubMed] [Google Scholar]
- Seth P. K. Agrawal A. K. Bondy S. C. Biochemical changes in the brain consequent to dietary exposure of developing and mature rats to chlordecone (Kepone) Toxicol. Appl. Pharmacol. 1981;59:262–267. doi: 10.1016/0041-008x(81)90197-6. [DOI] [PubMed] [Google Scholar]
- Shafer T. J. Crofton K. M. Comments on: ‘Perinatal toxicity of cyfluthrin in mice: Developmental and behavioral effects’ by Soni and colleagues. Hum. Exp. Toxicol. 2011;30:1112–1113. doi: 10.1177/0960327111411500. [DOI] [PubMed] [Google Scholar]
- Shafer T. J. Meyer D. A. Effects of pyrethroids on voltage-sensitive calcium channels: A critical evaluation of strengths, weaknesses, data needs, and relationship to assessment of cumulative neurotoxicity. Toxicol. Appl. Pharmacol. 2004;196:303–318. doi: 10.1016/j.taap.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Shafer T. J. Meyer D. A. Crofton K. M. Developmental neurotoxicity of pyrethroid insecticides: Critical review and future research needs. Environ. Health Perspect. 2005;113:123–136. doi: 10.1289/ehp.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets L. P. A consideration of age-dependent differences in susceptibility to organophosphorus and pyrethroid insecticides. Neurotoxicology. 2000;21:57–63. [PubMed] [Google Scholar]
- Sheets L. P. Doherty J. D. Law M. W. Reiter L. W. Crofton K. M. Age-dependent differences in the susceptibility of rats to deltamethrin. Toxicol. Appl. Pharmacol. 1994;126:186–190. doi: 10.1006/taap.1994.1106. [DOI] [PubMed] [Google Scholar]
- Sierra V. Uphouse L. Long-term consequences of neonatal exposure to chlordecone. Neurotoxicology. 1986;7:609–621. [PubMed] [Google Scholar]
- Slotkin T. A. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. Bodwell B. E. Levin E. D. Seidler F. J. Neonatal exposure to low doses of diazinon: Long-term effects on neural cell development and acetylcholine systems. Environ. Health Perspect. 2008a;116:340–348. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A. Cousins M. M. Tate C. A. Seidler F. J. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. Lassiter T. L. Ryde I. T. Wrench N. Levin E. D. Seidler F. J. Consumption of a high-fat diet in adulthood ameliorates the effects of neonatal parathion exposure on acetylcholine systems in rat brain regions. Environ Health Persp. 2009a;117:916–922. doi: 10.1289/ehp.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A. Levin E. D. Seidler F. J. Comparative developmental neurotoxicity of organophosphate insecticides: Effects on brain development are separable from systemic toxicity. Environ. Health Perspect. 2006a;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A. Levin E. D. Seidler F. J. Developmental neurotoxicity of parathion: Progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol. Teratol. 2009b;31:11–17. doi: 10.1016/j.ntt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A. Oliver C. A. Seidler F. J. Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Brain Res. Dev. Brain Res. 2005;157:172–180. doi: 10.1016/j.devbrainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. Ryde I. T. Levin E. D. Seidler F. J. Developmental neurotoxicity of low dose diazinon exposure of neonatal rats: Effects on serotonin systems in adolescence and adulthood. Brain Res. Bull. 2008b;75:640–647. doi: 10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A. Seidler F. J. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: Critical periods for regional and sex-selective effects. Reprod. Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. Seidler F. J. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: Chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol. Appl. Pharmacol. 2008;233:211–219. doi: 10.1016/j.taap.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A. Southard M. C. Adam S. J. Cousins M. M. Seidler F. J. Alpha7 nicotinic acetylcholine receptors targeted by cholinergic developmental neurotoxicants: Nicotine and chlorpyrifos. Brain Res. Bull. 2004;64:227–235. doi: 10.1016/j.brainresbull.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. Tate C. A. Cousins M. M. Seidler F. J. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Brain Res. Dev. Brain Res. 2002;133:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. Tate C. A. Ryde I. T. Levin E. D. Seidler F. J. Organophosphate insecticides target the serotonergic system in developing rat brain regions: Disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ. Health Perspect. 2006b;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. N. Campbell J. A. Busby-Hjerpe A. L. Lee S. Poet T. S. Barr D. B. Timchalk C. Comparative chlorpyrifos pharmacokinetics via multiple routes of exposure and vehicles of administration in the adult rat. Toxicology. 2009;261:47–58. doi: 10.1016/j.tox.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Sobotka T. J. Brodie R. E. Cook M. P. Behavioral and neuroendocrine effects in rats of postnatal exposure to low dietary levels of maneb. Dev. Psychobiol. 1972;5:137–148. doi: 10.1002/dev.420050207. [DOI] [PubMed] [Google Scholar]
- Soderlund D. M. Clark J. M. Sheets L. P. Mullin L. S. Piccirillo V. J. Sargent D. Stevens J. T. Weiner M. L. Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Song X. Seidler F. J. Saleh J. L. Zhang J. Padilla S. Slotkin T. A. Cellular mechanisms for developmental toxicity of chlorpyrifos: Targeting the adenylyl cyclase signaling cascade. Toxicol. Appl. Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Spyker J. M. Avery D.L. Neurobehavioral effects of prenatal exposure to the organophosphate diazinon in mice. J. Toxicol. Environ. Health. 1977;3:989–1002. doi: 10.1080/15287397709529633. [DOI] [PubMed] [Google Scholar]
- Spurlock P. Lee M. Synthetic pyrethroid use patterns, properties, and environmental effects. Washington, DC: American Chemical Society; 2008. [Google Scholar]
- Squibb R. E. Tilson H. A. Effects of gestational and perinatal exposure to chlordecone (Kepone) on the neurobehavioral development of Fischer-344 rats. Neurotoxicology. 1982;3:17–26. [PubMed] [Google Scholar]
- Srivastava M. K. Raizada R. B. Development effect of technical dimethoate in rats: Maternal and fetal toxicity evaluation. Indian J Exp Biol. 1996;34:329–333. [PubMed] [Google Scholar]
- Srivastava M. K. Raizada R. B. Assessment of the no-observed-effect level (NOEL) of quinalphos in pregnant rats. Food Chem. Toxicol. 1999;37:649–653. doi: 10.1016/s0278-6915(99)00036-8. [DOI] [PubMed] [Google Scholar]
- Srivastava M. K. Raizada R. B. Lack of toxic effect of technical azadirachtin during postnatal development of rats. Food Chem. Toxicol. 2007;45:465–471. doi: 10.1016/j.fct.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Srivastava M. K. Raizada R. B. Dikshith T. S. Fetotoxic response of technical quinalphos in rats. Vet. Hum. Toxicol. 1992;34:131–133. [PubMed] [Google Scholar]
- Stamper C. R. Balduini W. Murphy S. D. Costa L. G. Behavioral and biochemical effects of postnatal parathion exposure in the rat. Neurotoxicol Teratol. 1988;10:261–266. doi: 10.1016/0892-0362(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Stewart P. Fitzgerald S. Reihman J. Gump B. Lonky E. Darvill T. Pagano J. Hauser P. Prenatal PCB exposure, the corpus callosum, and response inhibition. Environ. Health Perspect. 2003;111:1670–1677. doi: 10.1289/ehp.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. Reihman J. Gump B. Lonky E. Darvill T. Pagano J. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol. Teratol. 2005;27:771–780. doi: 10.1016/j.ntt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Stewart P. Reihman J. Lonky E. Darvill T. Pagano J. Prenatal PCB exposure and neonatal behavioral assessment scale (NBAS) performance. Neurotoxicol. Teratol. 2000;22:21–29. doi: 10.1016/s0892-0362(99)00056-2. [DOI] [PubMed] [Google Scholar]
- Stewart P. W. Lonky E. Reihman J. Pagano J. Gump B. B. Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ. Health Perspect. 2008;116:1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. Sherman E. M. S. Spreen O. A compendium of neuropsychological tests: Administration, norms and commentary. 3rd ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Struve M. F. Turner K. J. Dorman D. C. Preliminary investigation of changes in the sexually dimorphic nucleus of the rat medial preoptic area following prenatal exposure to fenitrothion. J. Appl. Toxicol. 2007;27:631–636. doi: 10.1002/jat.1267. [DOI] [PubMed] [Google Scholar]
- Sturtz N. Deis R. P. Jahn G. A. Duffard R. Evangelista De Duffard A. M. Effect of 2, 4-dichlorophenoxyacetic acid on rat maternal behavior. Toxicology. 2008;247:73–79. doi: 10.1016/j.tox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Sudakin D. L. Stone D. L. Dialkyl phosphates as biomarkers of organophosphates: The current divide between epidemiology and clinical toxicology. Clin. Toxicol. (Phila.) 2011;49:771–781. doi: 10.3109/15563650.2011.624101. [DOI] [PubMed] [Google Scholar]
- Sunyer J. Basagana X. Gonzalez J. R. Julvez J. Guerra S. Bustamante M. De Cid R. Anto J. M. Torrent M. Early life environment, neurodevelopment and the interrelation with atopy. Environ. Res. 2010;110:733–738. doi: 10.1016/j.envres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Talts U. Fredriksson A. Eriksson P. Changes in behavior and muscarinic receptor density after neonatal and adult exposure to bioallethrin. Neurobiol. Aging. 1998;19:545–552. doi: 10.1016/s0197-4580(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Tan J. Loganath A. Chong Y. S. Obbard J. P. Exposure to persistent organic pollutants in utero and related maternal characteristics on birth outcomes: A multivariate data analysis approach. Chemosphere. 2009;74:428–433. doi: 10.1016/j.chemosphere.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Tanaka H. Yan S. Miura N. Shoyama Y. Preparation of anti-2, 4-dichlorophenol and 2, 4-dichlorophenoxyacetic acid monoclonal antibodies. Cytotechnology. 2003;42:101–107. doi: 10.1023/B:CYTO.0000009919.62901.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Effects of piperonyl butoxide on F1 generation mice. Toxicol. Lett. 1992;60:83–90. doi: 10.1016/0378-4274(92)90050-t. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Reproductive and neurobehavioral effects of imazalil administered to mice. Reprod. Toxicol. 1995;9:281–288. doi: 10.1016/0890-6238(95)00010-8. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Reproductive and neurobehavioural effects of thiabendazole administered to mice in the diet. Food Addit. Contam. 2001;18:375–383. doi: 10.1080/02652030118327. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Reproductive and neurobehavioural effects of piperonyl butoxide administered to mice in the diet. Food Addit. Contam. 2003;20:207–214. doi: 10.1080/0265203021000050617. [DOI] [PubMed] [Google Scholar]
- Tang J. Carr R. L. Chambers J. E. Changes in rat brain cholinesterase activity and muscarinic receptor density during and after repeated oral exposure to chlorpyrifos in early postnatal development. Toxicol. Sci. 1999;51:265–272. doi: 10.1093/toxsci/51.2.265. [DOI] [PubMed] [Google Scholar]
- Tang J. Carr R. L. Chambers J. E. The effects of repeated oral exposures to methyl parathion on rat brain cholinesterase and muscarinic receptors during postnatal development. Toxicol. Sci. 2003;76:400–406. doi: 10.1093/toxsci/kfg245. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M. Prokopenko O. Cory-Slechta D. A. Buckley B. Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. J. Biol. Chem. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M. Richfield E. K. Goodman B. M. Baggs R. B. Cory-Slechta D. A. Developmental exposure to the pesticides paraquat and maneb and the Parkinson's disease phenotype. Neurotoxicology. 2002;23:621–633. doi: 10.1016/s0161-813x(02)00092-x. [DOI] [PubMed] [Google Scholar]
- Thomas K. W. Dosemeci M. Hoppin J. A. Sheldon L. S. Croghan C. W. Gordon S. M. Jones M. L. Reynolds S. J. Raymer J. H. Akland G. G. Lynch C. F. Knott C. E. Sandler D. P. Blair A. E. Alavanja M. C. Urinary biomarker, dermal, and air measurement results for 2, 4-D and chlorpyrifos farm applicators in the Agricultural Health Study. J. Expos. Sci. Environ. Epidemiol. 2010;20:119–134. doi: 10.1038/jes.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilson H. A. Squibb R. E. Burne T. A. Neurobehavioral effects following a single dose of chlordecone (Kepone) administered neonatally to rats. Neurotoxicology. 1982;3:45–57. [PubMed] [Google Scholar]
- Timchalk C. Nolan R. J. Mendrala A. L. Dittenber D. A. Brzak K. A. Mattsson J. L. A Physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol. Sci. 2002;66:34–53. doi: 10.1093/toxsci/66.1.34. [DOI] [PubMed] [Google Scholar]
- Timofeeva O. A. Roegge C. S. Seidler F. J. Slotkin T. A. Levin E. D. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol. Teratol. 2008a;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva O. A. Sanders D. Seemann K. Yang L. Hermanson D. Regenbogen S. Agoos S. Kallepalli A. Rastogi A. Braddy D. Wells C. Perraut C. Seidler F. J. Slotkin T. A. Levin E. D. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res. Bull. 2008b;77:404–411. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sanchez L. Rothenberg S. J. Schnaas L. Cebrian M. E. Osorio E. Del Carmen Hernandez M. Garcia-Hernandez R. M. Del Rio-Garcia C. Wolff M. S. Lopez-Carrillo L. In utero P, p′-DDE exposure and infant neurodevelopment: A perinatal cohort in Mexico. Environ. Health Perspect. 2007;115:435–439. doi: 10.1289/ehp.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sanchez L. Schnaas L. Cebrian M. E. Hernandez Mdel C. Valencia E. O. Garcia Hernandez R. M. Lopez-Carrillo L. Prenatal dichlorodiphenyldichloroethylene (DDE) exposure and neurodevelopment: A follow-up from 12 to 30 months of age. Neurotoxicology. 2009;30:1162–1165. doi: 10.1016/j.neuro.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji R. Kobayashi K. Ikeda M. Yoshioka T. Yamada T. Seki T. Okuno Y. Nakatsuka I. Tsuruo Y. Kishioka S. Lack of changes in brain muscarinic receptor and motor activity of mice after neonatal inhalation exposure to D-allethrin. J. Appl. Toxicol. 2002;22:423–429. doi: 10.1002/jat.881. [DOI] [PubMed] [Google Scholar]
- Turgeman G. Pinkas A. Slotkin T. A. Tfilin M. Langford R. Yanai J. Reversal of chlorpyrifos neurobehavioral teratogenicity in mice by allographic transplantation of adult subventricular zone-derived neural stem cells. J. Neurosci. Res. 2011;89:1185–1193. doi: 10.1002/jnr.22631. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Reregistration eligibility decision for 2, 4-D. Washington, DC: U.S. EPA, Office of Pesticide Programs; 2005. [Google Scholar]
- U.S. Environmental Protection Agency. Revised N-methyl carbamate cumulative risk assessment. Washington, DC: U.S. EPA, Office of Pesticide Programs; 2007. [Google Scholar]
- U.S. Environmental Protection Agency. Draft framework for incorporating human epidemiologic, and incident data in health risk assessment. Washington, DC: U.S. EPA, Office of Pesticide Programs; 2010a. [Google Scholar]
- U.S. Environmental Protection Agency. Pyrethroids: Evaluation of data from developmental neurotoxicity studies and consideration of comparative sensitivity. Washington, DC: U.S. EPA, Office of Prevention; 2010b. [Google Scholar]
- U.S. Environmental Protection Agency. Pyrethroid cumulative risk assessment. Washington, DC: U.S. EPA, Office of Prevention; 2011. [Google Scholar]
- Uphouse L. Mason G. Bondy S. Comments concerning the use of dimethylsulfoxide as a solvent for studies of chlordecone neurotoxicity. Neurotoxicology. 1982;3:149–154. [PubMed] [Google Scholar]
- Venerosi A. Calamandrei G. Ricceri L. A social recognition test for female mice reveals behavioral effects of developmental chlorpyrifos exposure. Neurotoxicol. Teratol. 2006;28:466–471. doi: 10.1016/j.ntt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Venerosi A. Ricceri L. Rungi A. Sanghez V. Calamandrei G. Gestational exposure to the organophosphate chlorpyrifos alters social-emotional behaviour and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology (Berlin) 2010;208:99–107. doi: 10.1007/s00213-009-1713-2. [DOI] [PubMed] [Google Scholar]
- Venerosi A. Ricceri L. Scattoni M. L. Calamandrei G. Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in CD-1 mouse pups. Environ. Health. 2009;8:12. doi: 10.1186/1476-069X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergieva T. Behavioral teratology–Results achieved and perspectives of development. J. Hyg. Epidemiol. Microbiol. Immunol. 1985;29:121–127. [PubMed] [Google Scholar]
- Veronesi B. Pope C. The neurotoxicity of parathion-induced acetylcholinesterase inhibition in neonatal rats. Neurotoxicology. 1990;11:609–626. [PubMed] [Google Scholar]
- Vijverberg H. P. Van Den Bercken J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit. Rev. Toxicol. 1990;21:105–126. doi: 10.3109/10408449009089875. [DOI] [PubMed] [Google Scholar]
- Vinggaard A. M. Christiansen S. Laier P. Poulsen M. E. Breinholt V. Jarfelt K. Jacobsen H. Dalgaard M. Nellemann C. Hass U. Perinatal exposure to the fungicide prochloraz feminizes the male rat offspring. Toxicol. Sci. 2005;85:886–897. doi: 10.1093/toxsci/kfi150. [DOI] [PubMed] [Google Scholar]
- Vom Saal F. S. Nagel S. C. Palanza P. Boechler M. Parmigiani S. Welshons W. V. Estrogenic pesticides: binding relative to estradiol in MCF-7 cells and effects of exposure during fetal life on subsequent territorial behaviour in male mice. Toxicol. Lett. 1995;77:343–350. doi: 10.1016/0378-4274(95)03316-5. [DOI] [PubMed] [Google Scholar]
- Watson R. E. Desesso J. M. Hurtt M. E. Cappon G. D. Postnatal growth and morphological development of the brain: A species comparison. Birth Defects Res. B Dev. Reprod. Toxicol. 2006;77:471–484. doi: 10.1002/bdrb.20090. [DOI] [PubMed] [Google Scholar]
- Weiner M. L. Nemec M. Sheets L. Sargent D. Breckenridge C. Comparative functional observational battery study of twelve commercial pyrethroid insecticides in male rats following acute oral exposure. Neurotoxicology. 2009;30(suppl. 1):S1–S16. doi: 10.1016/j.neuro.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Weselek M. Arbuckle T.E. Foster W. Pesticide exposures and developmental outcomes: the epidemiological evidence. J. Toxicol. Environ. Health B. 2007;10:41–80. doi: 10.1080/10937400601034571. [DOI] [PubMed] [Google Scholar]
- Whyatt R. M. Camann D. Perera F. P. Rauh V. A. Tang D. Kinney P. L. Garfinkel R. Andrews H. Hoepner L. Barr D. B. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol. Appl. Pharmacol. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Whyatt R. M. Rauh V. Barr D. B. Camann D. E. Andrews H. F. Garfinkel R. Hoepner L. A. Diaz D. Dietrich J. Reyes A. Tang D. Kinney P. L. Perera F. P. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ. Health Perspect. 2004;112:1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle D. T. Arbuckle T. E. Walker M. Wade M. G. Liu S. Krewski D. Environmental hazards: Evidence for effects on child health. J. Toxicol. Environ. Health B. 2007;10:3–39. doi: 10.1080/10937400601034563. [DOI] [PubMed] [Google Scholar]
- Wigle D. T. Arbuckle T. E. Turner M.C. Bérubé A. Yang Q. Liu S. Krewski D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J. Toxicol. Environ. Health B. 2008;11:373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- Williams M. K. Rundle A. Holmes D. Reyes M. Hoepner L. A. Barr D. B. Camann D. E. Perera F. P. Whyatt R. M. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ. Health Perspect. 2008;116:1681–1688. doi: 10.1289/ehp.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise L. D. Allen H. L. Hoe C. M. Verbeke D. R. Gerson R. J. Developmental neurotoxicity evaluation of the avermectin pesticide, emamectin benzoate, in Sprague-Dawley rats. Neurotoxicol. Teratol. 1997;19:315–326. doi: 10.1016/s0892-0362(97)00002-0. [DOI] [PubMed] [Google Scholar]
- Wolansky M. J. Harrill J. A. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: A critical review. Neurotoxicol. Teratol. 2008;30:55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Wolansky M. J. Mcdaniel K. L. Moser V. C. Crofton K. M. Influence of dosing volume on the neurotoxicity of bifenthrin. Neurotoxicol. Teratol. 2007;29:377–384. doi: 10.1016/j.ntt.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Wolff M. S. Engel S. Berkowitz G. Teitelbaum S. Siskind J. Barr D. B. Wetmur J. Prenatal pesticide and PCB exposures and birth outcomes. Pediatr. Res. 2007;61:243–250. doi: 10.1203/pdr.0b013e31802d77f0. [DOI] [PubMed] [Google Scholar]
- Yang D. Lauridsen H. Buels K. Chi L. H. La Du J. Bruun D. A. Olson J. R. Tanguay R. L. Lein P. J. Chlorpyrifosoxon disrupts zebrafish axonal growth and motor behavior. Toxicol. Sci. 2011;121:146–159. doi: 10.1093/toxsci/kfr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. G. Eskenazi B. Gladstone E. A. Bradman A. Pedersen L. Johnson C. Barr D. B. Furlong C. E. Holland N. T. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26:199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zalewska Z. Rakowska I. Matraszek G. Sitkiewicz D. Effect of dichlorvos on some enzyme activities of the rat brain during postnatal development. I. Cholinesterases. Neuropatol. Pol. 1977;15:255–262. [PubMed] [Google Scholar]
- Zeman F. J. Hoogenboom E. R. Kavlock R. J. Semple J. L. Effects on the fetus of maternal benomyl exposure in the protein-deprived rat. J. Toxicol. Environ. Health. 1986;17:405–417. doi: 10.1080/15287398609530835. [DOI] [PubMed] [Google Scholar]
- Zheng Q. Olivier K. Won Y. K. Pope C. N. Comparative cholinergic neurotoxicity of oral chlorpyrifos exposures in preweanling and adult rats. Toxicol. Sci. 2000;55:124–132. doi: 10.1093/toxsci/55.1.124. [DOI] [PubMed] [Google Scholar]


