Abstract
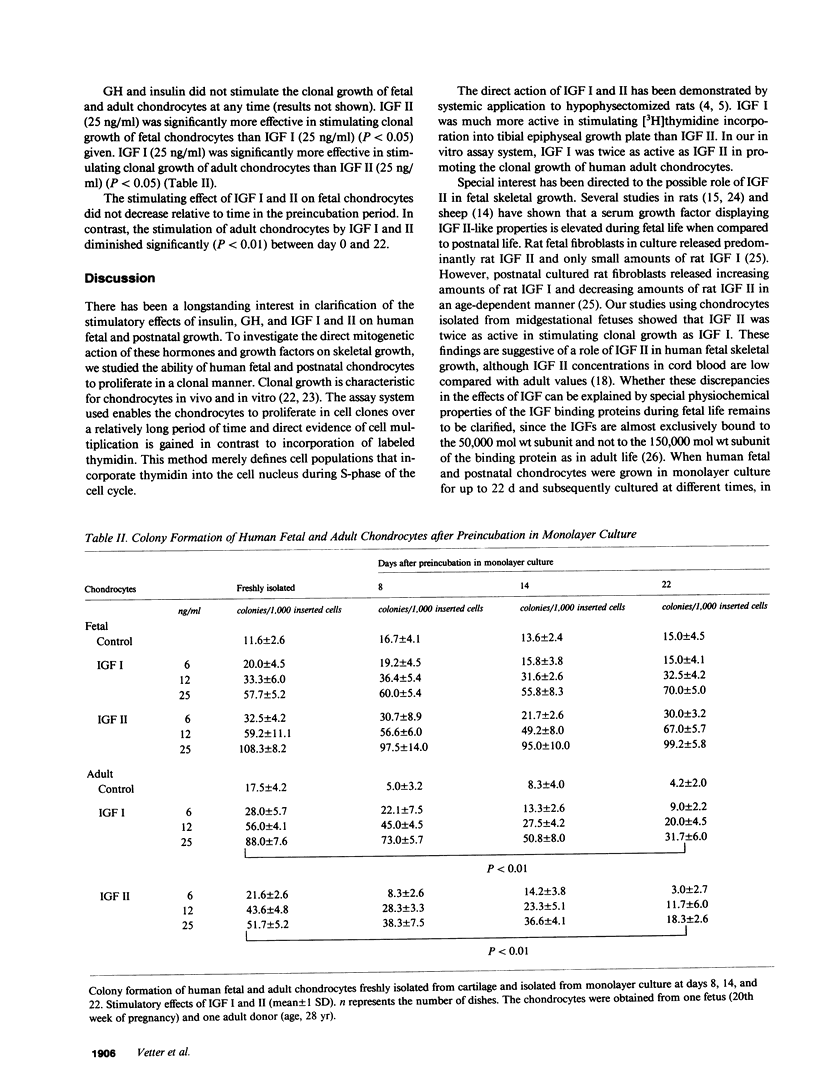
Clonal proliferation of freshly isolated human fetal chondrocytes and adult chondrocytes in response to human insulinlike growth factors I and II (IGF I, IGF II), human biosynthetic insulin, and human growth hormone (GH) was assessed. IGF I (25 ng/ml) stimulated clonal growth of fetal chondrocytes (54 +/- 12 colonies/1,000 inserted cells, mean +/- 1 SD), but IGF II (25 ng/ml) was significantly more effective (106 +/- 12 colonies/1,000 inserted cells, P less than 0.05, unstimulated control: 14 +/- 4 colonies/1,000 inserted cells). In contrast, IGF I (25 ng/ml) was more effective in adult chondrocytes (42 +/- 6 colonies/1,000 inserted cells) than IGF II (25 ng/ml) (21 +/- 6 colonies/1,000 inserted cells; P less than 0.05, unstimulated control: 6 +/- 3 colonies/1,000 inserted cells). GH and human biosynthetic insulin did not affect clonal growth of fetal or adult chondrocytes. The clonal growth pattern of IGF-stimulated fetal and adult chondrocytes was not significantly changed when chondrocytes were first grown in monolayer culture, harvested, and then inserted in the clonal culture system. However, the adult chondrocytes showed a time-dependent decrease of stimulation of clonal growth by IGF I and II. This was not true for fetal chondrocytes. The results are compatible with the concept that IGF II is a more potent stimulant of clonal growth of chondrocytes during fetal life, whereas IGF I is more effective in stimulating clonal growth of chondrocytes during postnatal life.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. O., Nissley S. P., Handwerger S., Rechler M. M. Developmental patterns of insulin-like growth factor-I and -II synthesis and regulation in rat fibroblasts. Nature. 1983 Mar 10;302(5904):150–153. doi: 10.1038/302150a0. [DOI] [PubMed] [Google Scholar]
- Ashton I. K., Zapf J., Einschenk I., MacKenzie I. Z. Insulin-like growth factors (IGF) 1 and 2 in human foetal plasma and relationship to gestational age and foetal size during midpregnancy. Acta Endocrinol (Copenh) 1985 Dec;110(4):558–563. doi: 10.1530/acta.0.1100558. [DOI] [PubMed] [Google Scholar]
- Bembenek M. E., Willis D. H., Jr, Liberti J. P. The effect of insulin on collagen production in isolated chondrosarcoma chondrocytes. Biochem Biophys Res Commun. 1982 May 31;106(2):338–345. doi: 10.1016/0006-291x(82)91115-9. [DOI] [PubMed] [Google Scholar]
- Bennett A., Wilson D. M., Liu F., Nagashima R., Rosenfeld R. G., Hintz R. L. Levels of insulin-like growth factors I and II in human cord blood. J Clin Endocrinol Metab. 1983 Sep;57(3):609–612. doi: 10.1210/jcem-57-3-609. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Coon H. G. Clonal stability and phenotypic expression of chick cartilage cells in vitro. Proc Natl Acad Sci U S A. 1966 Jan;55(1):66–73. doi: 10.1073/pnas.55.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ercole A. J., Willson D. F., Underwood L. E. Changes in the circulating form of serum somatomedin-C during fetal life. J Clin Endocrinol Metab. 1980 Sep;51(3):674–676. doi: 10.1210/jcem-51-3-674. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Raben M. S., Salmon W. D., Jr, van den Brande J. L., van Wyk J. J. Somatomedin: proposed designation for sulphation factor. Nature. 1972 Jan 14;235(5333):107–107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Parker K. A., Borowsky S., Trivedi B., Kapadia M. Measurement of somatomedin-related peptides in fetal, neonatal, and maternal rat serum by insulin-like growth factor (IGF) I radioimmunoassay, IGF-II radioreceptor assay (RRA), and multiplication-stimulating activity RRA after acid-ethanol extraction. Endocrinology. 1982 Feb;110(2):575–581. doi: 10.1210/endo-110-2-575. [DOI] [PubMed] [Google Scholar]
- Gauss V., Müller P. K. Change in the expression of collagen genes in dividing and nondividing chondrocytes. Biochim Biophys Acta. 1981 Jan 29;652(1):39–47. doi: 10.1016/0005-2787(81)90206-9. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Butler J. H. Parturition-related changes in insulin-like growth factors-I and -II in the perinatal lamb. J Endocrinol. 1983 Nov;99(2):223–232. doi: 10.1677/joe.0.0990223. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Johnson-Barrett J. J., Butler J. H., Edgar B. W., Gunn T. R. Studies of insulin-like growth factor -I and -II by specific radioligand assays in umbilical cord blood. Clin Endocrinol (Oxf) 1983 Sep;19(3):405–413. doi: 10.1111/j.1365-2265.1983.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Hill D. J. Stimulation of cartilage zones of the calf costochondral growth plate in vitro by growth hormone dependent rat plasma somatomedin activity. J Endocrinol. 1979 Nov;83(2):219–227. doi: 10.1677/joe.0.0830219. [DOI] [PubMed] [Google Scholar]
- Isaksson O. G., Jansson J. O., Gause I. A. Growth hormone stimulates longitudinal bone growth directly. Science. 1982 Jun 11;216(4551):1237–1239. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Smith M. D., Canalis E., Raisz L. G. Characterization of the effect of insulin on collagen synthesis in fetal rat bone. Endocrinology. 1985 Jan;116(1):296–302. doi: 10.1210/endo-116-1-296. [DOI] [PubMed] [Google Scholar]
- Lebovitz H. E., Eisenbarth G. S. Hormonal regulation of cartilage growth and metabolism. Vitam Horm. 1975;33:575–648. doi: 10.1016/s0083-6729(08)60973-5. [DOI] [PubMed] [Google Scholar]
- Madsen K., Friberg U., Roos P., Edén S., Isaksson O. Growth hormone stimulates the proliferation of cultured chondrocytes from rabbit ear and rat rib growth cartilage. Nature. 1983 Aug 11;304(5926):545–547. doi: 10.1038/304545a0. [DOI] [PubMed] [Google Scholar]
- Madsen K., Makower A. M., Friberg U., Edén S., Isaksson O. Effect of human growth hormone on proteoglycan synthesis in cultured rat chondrocytes. Acta Endocrinol (Copenh) 1985 Mar;108(3):338–342. doi: 10.1530/acta.0.1080338. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Thrasher A. Z., Weinberg E. H., Harris W. H. Dissociation between the effect of bovine growth hormone in articular cartilage and in bone of the adult dog. J Bone Joint Surg Am. 1978 Dec;60(8):1071–1075. [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. Primary structure of human insulin-like growth factor II. FEBS Lett. 1978 May 15;89(2):283–286. doi: 10.1016/0014-5793(78)80237-3. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978 Apr 25;253(8):2769–2776. [PubMed] [Google Scholar]
- SALTER J., BEST C. H. Insulin as a growth hormone. Br Med J. 1953 Aug 15;2(4832):353–356. doi: 10.1136/bmj.2.4832.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Hauri C., Steiner T., Froesch E. R. Comparison of in vivo effects of insulin-like growth factors I and II and of growth hormone in hypophysectomized rats. Acta Endocrinol (Copenh) 1985 Feb;108(2):167–174. doi: 10.1530/acta.0.1080167. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Humbel R. E., Froesch E. R. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982 Mar 18;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- Spencer G. S., Hill D. J., Garssen G. J., Macdonald A. A., Colenbrander B. Somatomedin activity and growth hormone levels in body fluids of the fetal pig: effect of chronic hyperinsulinaemia. J Endocrinol. 1983 Jan;96(1):107–114. doi: 10.1677/joe.0.0960107. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Nissley S. P., Kimura J. H., Rechler M. M., Caplan A. I., Hascall V. C. Effects of insulin and multiplication-stimulating activity on proteoglycan biosynthesis in chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 Feb 25;256(4):2045–2052. [PubMed] [Google Scholar]
- Straus D. S. Growth-stimulatory actions of insulin in vitro and in vivo. Endocr Rev. 1984 Spring;5(2):356–369. doi: 10.1210/edrv-5-2-356. [DOI] [PubMed] [Google Scholar]
- Susa J. B., Widness J. A., Hintz R., Liu F., Sehgal P., Schwartz R. Somatomedins and insulin in diabetic pregnancies: effects on fetal macrosomia in the human and rhesus monkey. J Clin Endocrinol Metab. 1984 Jun;58(6):1099–1105. doi: 10.1210/jcem-58-6-1099. [DOI] [PubMed] [Google Scholar]
- Vetter U., Heit W., Helbing G., Heinze E., Pirsig W. Growth of the human septal cartilage: cell density and colony formation of septal chondrocytes. Laryngoscope. 1984 Sep;94(9):1226–1229. doi: 10.1288/00005537-198409000-00016. [DOI] [PubMed] [Google Scholar]
- Watanabe K. Changes in the capacity for clonal growth and differentiation in vitro of the vertebral cartilage cells with embryonic development. II. Vitalizing effect of conditioned medium on the cells of younger embryos. Dev Growth Differ. 1971 Aug;13(2):107–118. doi: 10.1111/j.1440-169x.1971.00107.x. [DOI] [PubMed] [Google Scholar]
- Zapf J., Rinderknecht E., Humbel R. E., Froesch E. R. Nonsuppressible insulin-like activity (NSILA) from human serum: recent accomplishments and their physiologic implications. Metabolism. 1978 Dec;27(12):1803–1828. doi: 10.1016/0026-0495(78)90267-6. [DOI] [PubMed] [Google Scholar]
- von der Mark H., von der Mark K., Gay S. Study of differential collagen synthesis during development of the chick embryo by immunofluorescence. I. Preparation of collagen type I and type II specific antibodies and their application to early stages of the chick embryo. Dev Biol. 1976 Feb;48(2):237–249. doi: 10.1016/0012-1606(76)90088-9. [DOI] [PubMed] [Google Scholar]




