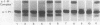Abstract
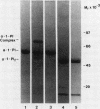
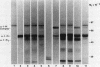
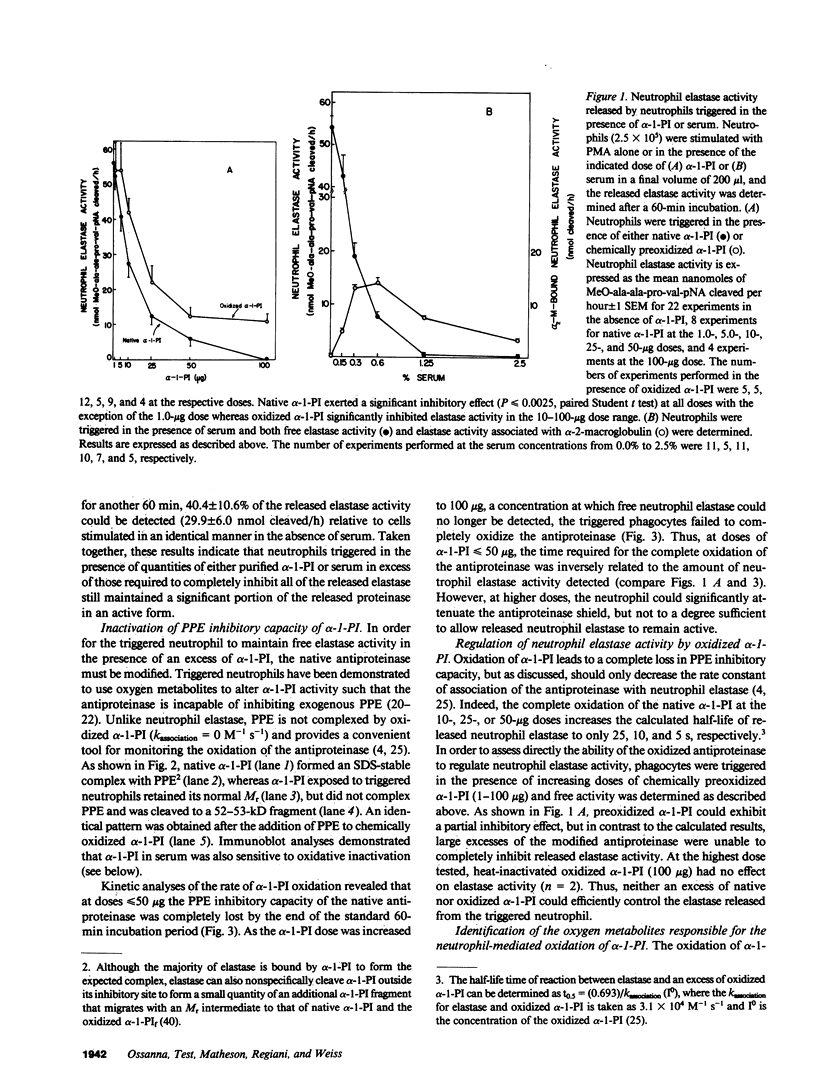
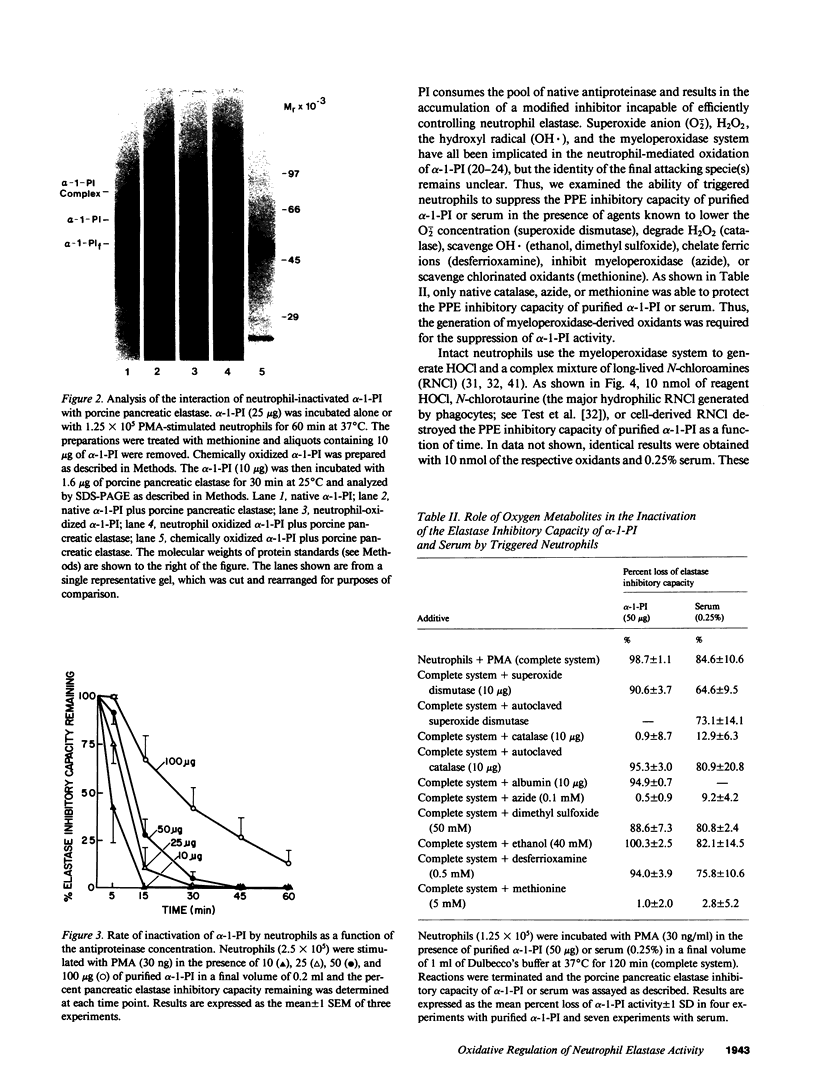
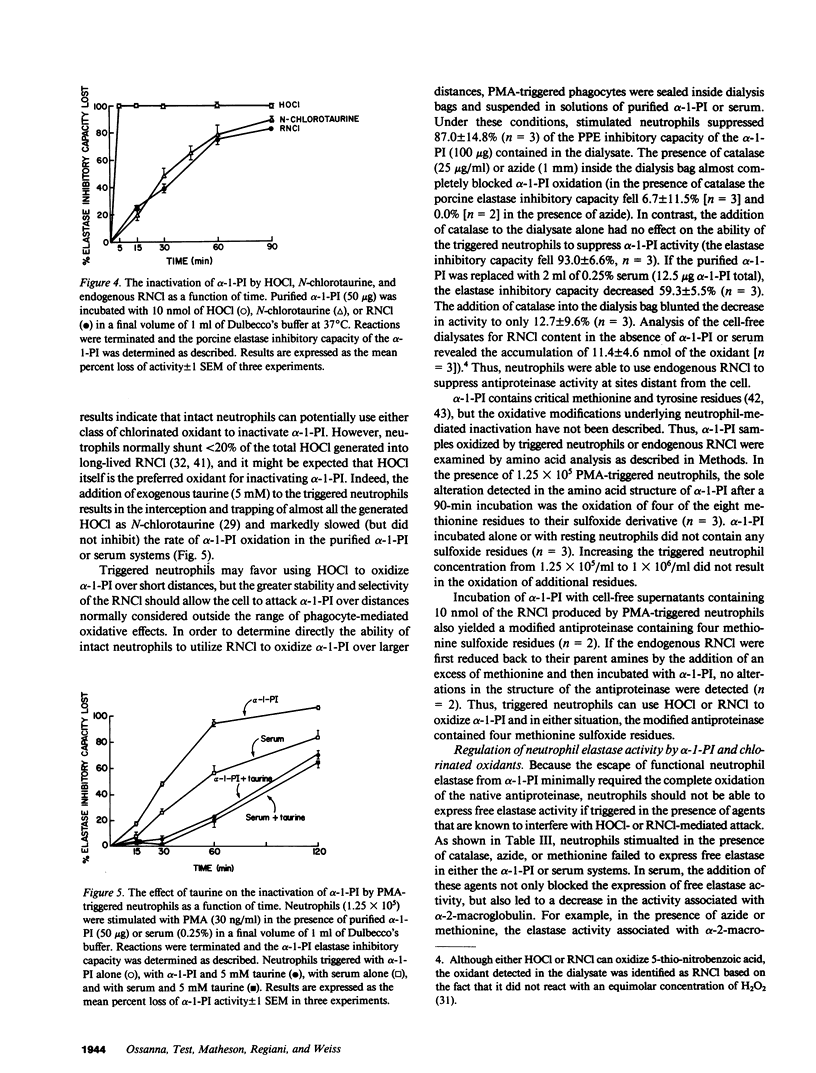
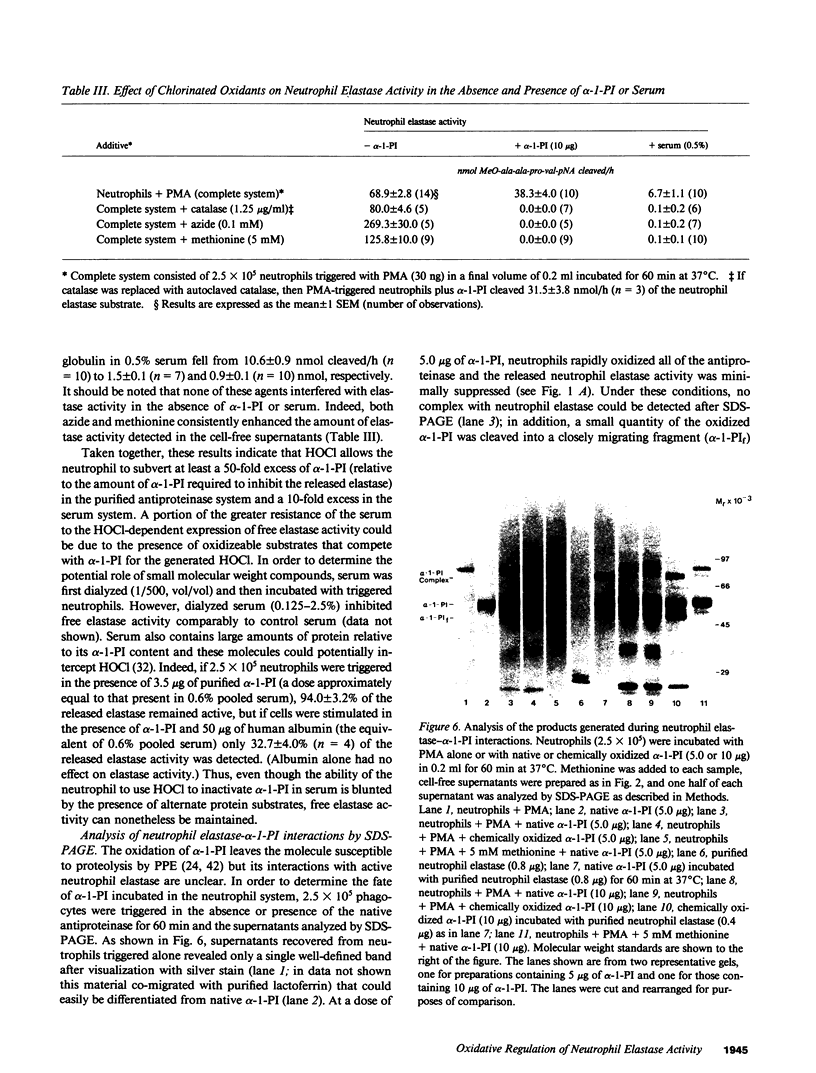
Triggered human neutrophils were able to maintain released elastase in an active form in the presence of purified alpha-1-proteinase inhibitor (alpha-1-PI), serum or bronchoalveolar lavage fluid (BAL). The accumulation of free elastase activity was associated with a decrease in the ability of the alpha-1-PI to inhibit porcine pancreatic elastase, an increase in proteinase activity associated with alpha-2-macroglobulin, and the oxidation of alpha-1-PI to a molecule containing four methionine sulfoxide residues. Neutrophils used both hypochlorous acid and long-lived N-chloroamines to oxidize the alpha-1-PI, but hypochlorous acid was preferentially used for suppressing the activity of the antiproteinase over short distances whereas the N-chloroamines were effective even when the phagocytes and alpha-1-PI were physically separated. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified alpha-1-PI, serum, or BAL that had been incubated with triggered neutrophils revealed that the released neutrophil elastase was not complexed with the antiproteinase and that a portion of the alpha-1-PI had undergone proteolysis. These data suggest that the presence of free neutrophil elastase as well as inactive, oxidized, and proteolyzed alpha-1-PI in fluids recovered from inflammatory sites in vivo could be directly mediated by triggered neutrophils alone.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams W. R., Fein A. M., Kucich U., Kueppers F., Yamada H., Kuzmowycz T., Morgan L., Lippmann M., Goldberg S. K., Weinbaum G. Proteinase inhibitory function in inflammatory lung disease. I. Acute bacterial pneumonia. Am Rev Respir Dis. 1984 May;129(5):735–741. doi: 10.1164/arrd.1984.129.5.735. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Schnyder J., Bretz U., Dewald B., Ruch W. Cellular mechanisms of proteinase release from inflammatory cells and the degradation of extracellular proteins. Ciba Found Symp. 1979;(75):105–121. doi: 10.1002/9780470720585.ch7. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Clark E. J., Werb Z. Limited proteolysis by macrophage elastase inactivates human alpha 1-proteinase inhibitor. J Exp Med. 1980 Dec 1;152(6):1563–1570. doi: 10.1084/jem.152.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda M. J., Clark E. J., Werb Z. Regulation of alpha 1 proteinase inhibitor function by rabbit alveolar macrophages. Evidence for proteolytic rather than oxidative inactivation. J Clin Invest. 1985 Jun;75(6):1758–1762. doi: 10.1172/JCI111887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Beatty K., Matheson N., Travis J. Kinetic and chemical evidence for the inability of oxidized alpha 1-proteinase inhibitor to protect lung elastin from elastolytic degradation. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):731–736. doi: 10.1515/bchm2.1984.365.2.731. [DOI] [PubMed] [Google Scholar]
- Bieth J. G., Meyer J. F. Temperature and pH dependence of the association rate constant of elastase with alpha 2-macroglobulin. J Biol Chem. 1984 Jul 25;259(14):8904–8906. [PubMed] [Google Scholar]
- Brower M. S., Harpel P. C. Proteolytic cleavage and inactivation of alpha 2-plasmin inhibitor and C1 inactivator by human polymorphonuclear leukocyte elastase. J Biol Chem. 1982 Aug 25;257(16):9849–9854. [PubMed] [Google Scholar]
- Brower M. S., Levin R. I., Garry K. Human neutrophil elastase modulates platelet function by limited proteolysis of membrane glycoproteins. J Clin Invest. 1985 Feb;75(2):657–666. doi: 10.1172/JCI111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett D., Stockley R. A. The electrophoretic mobility of alpha 1-antitrypsin in sputum and its relationship to protease inhibitory capacity, leucocyte elastase concentrations and acute respiratory infection. Hoppe Seylers Z Physiol Chem. 1980 May;361(5):781–789. doi: 10.1515/bchm2.1980.361.1.781. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Miller F., Hoidal J. R., Janoff A. Potential mechanism of emphysema: alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2041–2045. doi: 10.1073/pnas.79.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W., Owen M. C. Plakalbumin, alpha 1-antitrypsin, antithrombin and the mechanism of inflammatory thrombosis. Nature. 1985 Oct 24;317(6039):730–732. doi: 10.1038/317730a0. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L. Comparison of live human neutrophil and alveolar macrophage elastolytic activity in vitro. Relative resistance of macrophage elastolytic activity to serum and alveolar proteinase inhibitors. J Clin Invest. 1984 Nov;74(5):1693–1700. doi: 10.1172/JCI111586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Stone P. J., El Hag A., Calore J. D., Franzblau C. Myeloperoxidase-catalyzed inactivation of alpha 1-protease inhibitor by human neutrophils. J Biol Chem. 1981 Apr 10;256(7):3348–3353. [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feste A., Gan J. C. Selective loss of elastase inhibitory activity of alpha 1-proteinase inhibitor upon chemical modification of its tyrosyl residues. J Biol Chem. 1981 Jun 25;256(12):6374–6380. [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Squier S. U., Wood R. E., Gee J. B., Reynolds H. Y. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Invest. 1984 Jul;74(1):236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P. M., Vissers M. C., Travis J., Winterbourn C. C., Carrell R. W. A genetically engineered mutant of alpha 1-antitrypsin protects connective tissue from neutrophil damage and may be useful in lung disease. Lancet. 1984 Dec 22;2(8417-8418):1426–1428. doi: 10.1016/s0140-6736(84)91623-4. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Jefferson M. M., Melton D. F., Thomas E. L. Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J Biol Chem. 1984 Aug 25;259(16):10404–10413. [PubMed] [Google Scholar]
- Havemann K., Gramse M. Physiology and pathophysiology of neutral proteinases of human granulocytes. Adv Exp Med Biol. 1984;167:1–20. doi: 10.1007/978-1-4615-9355-3_1. [DOI] [PubMed] [Google Scholar]
- Heifets L., Imai K., Goren M. B. Expression of peroxidase-dependent iodination by macrophages ingesting neutrophil debris. J Reticuloendothel Soc. 1980 Oct;28(4):391–404. [PubMed] [Google Scholar]
- Hornebeck W., Schnebli H. P. Effect of different elastase inhibitors on leukocyte elastase pre-adsorbed to elastin. Hoppe Seylers Z Physiol Chem. 1982 Apr;363(4):455–458. [PubMed] [Google Scholar]
- James H. L., Cohen A. B. Mechanism of inhibition of porcine elastase by human alpha-1-antitrypsin. J Clin Invest. 1978 Dec;62(6):1344–1353. doi: 10.1172/JCI109255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Travis J. Inactivation of human alpha 1-proteinase inhibitor by thiol proteinases. Biochem J. 1977 Jun 1;163(3):639–641. doi: 10.1042/bj1630639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977 Mar;89(3):675–686. [PubMed] [Google Scholar]
- Kobayashi M., Tanaka T., Usui T. Inactivation of lysosomal enzymes by the respiratory burst of polymorphonuclear leukocytes. Possible involvement of myeloperoxidase-H2O2-halide system. J Lab Clin Med. 1982 Dec;100(6):896–907. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeRoy E. C., Ager A., Gordon J. L. Effects of neutrophil elastase and other proteases on porcine aortic endothelial prostaglandin I2 production, adenine nucleotide release, and responses to vasoactive agents. J Clin Invest. 1984 Sep;74(3):1003–1010. doi: 10.1172/JCI111467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. T., Fein A. M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981 Jan 22;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- Martin W. J., Taylor J. C. Abnormal interaction of alpha 1-antitrypsin and leukocyte elastolytic activity in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1979 Aug;120(2):411–419. doi: 10.1164/arrd.1979.120.2.411. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Schuyler M., Travis J. Interaction of human alpha-1-proteinase inhibitor with neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):331–336. doi: 10.1021/bi00505a016. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- McGuire W. W., Spragg R. G., Cohen A. B., Cochrane C. G. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest. 1982 Mar;69(3):543–553. doi: 10.1172/JCI110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt T. A., Cochrane C. G., Holcomb K., Bohl B., Hallman M., Strayer D., Edwards D. K., 3rd, Gluck L. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983 Aug;72(2):656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Powers J. C., Ashe B. M., Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979 May 25;254(10):4027–4032. [PubMed] [Google Scholar]
- Ogden B. E., Murphy S. A., Saunders G. C., Pathak D., Johnson J. D. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis. 1984 Nov;130(5):817–821. doi: 10.1164/arrd.1984.130.5.817. [DOI] [PubMed] [Google Scholar]
- Plow E. F. Leukocyte elastase release during blood coagulation. A potential mechanism for activation of the alternative fibrinolytic pathway. J Clin Invest. 1982 Mar;69(3):564–572. doi: 10.1172/JCI110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. C., Gupton B. F., Harley A. D., Nishino N., Whitley R. J. Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Nov 23;485(1):156–166. doi: 10.1016/0005-2744(77)90203-0. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Travis J. The degradation of human lung elastin by neutrophil proteinases. Biochim Biophys Acta. 1980 Jan 24;621(1):147–157. doi: 10.1016/0005-2795(80)90070-7. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Barrett A. J. Identification of proteinases in rheumatoid synovium. Detection of leukocyte elastase cathepsin G and another serine proteinase. Biochim Biophys Acta. 1980 Sep 9;615(1):167–177. doi: 10.1016/0005-2744(80)90020-0. [DOI] [PubMed] [Google Scholar]
- Sanders E., Coles G. A., Davies M. Lysosomal enzymes in human urine: evidence for polymorphonuclear leucocyte proteinase involvement in the pathogenesis of human glomerulonephritis. Clin Sci Mol Med. 1978 Jun;54(6):667–672. doi: 10.1042/cs0540667. [DOI] [PubMed] [Google Scholar]
- Sharman M. C., Mudd J. B. Ozone inactivation of anti-elastase activity of chicken ovoinhibitor and human alpha-1-proteinase inhibitor. Biochem Biophys Res Commun. 1981 Sep 30;102(2):640–645. doi: 10.1016/s0006-291x(81)80180-5. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Burstein Y., Patchornik A. Selective oxidation of methionine residues in proteins. Biochemistry. 1975 Oct 7;14(20):4497–4503. doi: 10.1021/bi00691a025. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., McNeil V. M., Jesaitis A. J., Painter R. G., Cochrane C. G. A continuous, spectroscopic analysis of the kinetics of elastase secretion by neutrophils. The dependence of secretion upon receptor occupancy. J Biol Chem. 1982 May 25;257(10):5471–5475. [PubMed] [Google Scholar]
- Speer C. P., Pabst M. J., Hedegaard H. B., Rest R. F., Johnston R. B., Jr Enhanced release of oxygen metabolites by monocyte-derived macrophages exposed to proteolytic enzymes: activity of neutrophil elastase and cathepsin G. J Immunol. 1984 Oct;133(4):2151–2156. [PubMed] [Google Scholar]
- Stockley R. A., Afford S. C. Qualitative studies of lung lavage alpha 1-proteinase inhibitor. Hoppe Seylers Z Physiol Chem. 1984 Apr;365(4):503–510. doi: 10.1515/bchm2.1984.365.1.503. [DOI] [PubMed] [Google Scholar]
- Stockley R. A., Afford S. C. The effect of leucocyte elastase on the immunoelectrophoretic behaviour of alpha 1-antitrypsin. Clin Sci (Lond) 1984 Feb;66(2):217–224. doi: 10.1042/cs0660217. [DOI] [PubMed] [Google Scholar]
- Stockley R. A., Hill S. L., Morrison H. M., Starkie C. M. Elastolytic activity of sputum and its relation to purulence and to lung function in patients with bronchiectasis. Thorax. 1984 Jun;39(6):408–413. doi: 10.1136/thx.39.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. J., Calore J. D., Snider G. L., Franzblau C. Role of alpha-macroglobulin-elastase complexes in the pathogenesis of elastase-induced emphysema in hamsters. J Clin Invest. 1982 Apr;69(4):920–931. doi: 10.1172/JCI110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. J., Franzblau C., Kagan H. M. Proteolysis of insoluble elastin. Methods Enzymol. 1982;82(Pt A):588–605. doi: 10.1016/0076-6879(82)82089-2. [DOI] [PubMed] [Google Scholar]
- Stone P. J. The elastase-antielastase hypothesis of the pathogenesis of emphysema. Clin Chest Med. 1983 Sep;4(3):405–412. [PubMed] [Google Scholar]
- Takaki A., Enfield D. L., Thompson A. R. Cleavage and inactivation of Factor IX by granulocyte elastase. J Clin Invest. 1983 Nov;72(5):1706–1715. doi: 10.1172/JCI111130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Test S. T., Lampert M. B., Ossanna P. J., Thoene J. G., Weiss S. J. Generation of nitrogen-chlorine oxidants by human phagocytes. J Clin Invest. 1984 Oct;74(4):1341–1349. doi: 10.1172/JCI111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Giles P. J., Porcelli L., Reilly C. F., Baugh R., Powers J. Human leucocyte elastase and cathepsin G: structural and functional characteristics. Ciba Found Symp. 1979;(75):51–68. doi: 10.1002/9780470720585.ch4. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Twumasi D. Y., Liener I. E. Proteases from purulent sputum. Purification and properties of the elastase and chymotrypsin-like enzymes. J Biol Chem. 1977 Mar 25;252(6):1917–1926. [PubMed] [Google Scholar]
- Virca G. D., Travis J. Kinetics of association of human proteinases with human alpha 2-macroglobulin. J Biol Chem. 1984 Jul 25;259(14):8870–8874. [PubMed] [Google Scholar]
- Voetman A. A., Weening R. S., Hamers M. N., Meerhof L. J., Bot A. A., Roos D. Phagocytosing human neutrophils inactivate their own granular enzymes. J Clin Invest. 1981 May;67(5):1541–1549. doi: 10.1172/JCI110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtfogel Y. T., Kucich U., James H. L., Scott C. F., Schapira M., Zimmerman M., Cohen A. B., Colman R. W. Human plasma kallikrein releases neutrophil elastase during blood coagulation. J Clin Invest. 1983 Nov;72(5):1672–1677. doi: 10.1172/JCI111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Lampert M. B., Test S. T. Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science. 1983 Nov 11;222(4624):625–628. doi: 10.1126/science.6635660. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Peppin G., Ortiz X., Ragsdale C., Test S. T. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985 Feb 15;227(4688):747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984 May;73(5):1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. S., Travis J. Isolation and properties of oxidized alpha-1-proteinase inhibitor from human rheumatoid synovial fluid. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1449–1454. doi: 10.1016/0006-291x(80)90113-8. [DOI] [PubMed] [Google Scholar]
- Wretlind B., Pavlovskis O. R. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S998–1004. doi: 10.1093/clinids/5.supplement_5.s998. [DOI] [PubMed] [Google Scholar]
- Zaslow M. C., Clark R. A., Stone P. J., Calore J. D., Snider G. L., Franzblau C. Human neutrophil elastase does not bind to alpha 1-protease inhibitor that has been exposed to activated human neutrophils. Am Rev Respir Dis. 1983 Sep;128(3):434–439. doi: 10.1164/arrd.1983.128.3.434. [DOI] [PubMed] [Google Scholar]