Abstract
Purpose
Ependymoma is less commonly found in the supratentorial brain and has known clinical and molecular features that are unique. Our single institution series provides valuable information about disease control for supratentorial ependymoma and the complications of supratentorial irradiation in children.
Methods and Materials
A total of 50 children with newly diagnosed supratentorial ependymoma were treated with adjuvant radiation therapy (RT); 36 were using conformal methods after 1996. The median age at RT was 6.5 years (range, 1–18.9). The entire group was characterized according to sex (girls = 27), race (Caucasian = 43), extent of resection (gross-total = 46), and tumor grade (anaplastic = 28). The conformal RT group was prospectively evaluated for neurological, endocrine and cognitive effects.
Results
With a median follow-up of 9.1 years from the start of RT for survivors (range 0.2–23.2), the 10-year progression-free and overall survival were 73% + 7% and 76% + 6%, respectively. None of the evaluated factors was prognostic for disease control. Local and distant failures were evenly divided among the 16 patients who experienced progression. Eleven patients died from disease and one from CNS necrosis. Seizure disorders were present in 17 patients and 4 were considered to be clinically disabled. Clinically significant cognitive effects were limited to children with difficult to control seizures. Average values for IQ and academic achievement (reading, spelling, and math) were within the range of normal through 10 years of follow-up. Central hypothyroidism was the most commonly treated endocrinopathy.
Conclusion
RT may be administered with acceptable risks for complications in children with supratentorial ependymoma. These results suggest that outcomes for these children are improving and that complications may be limited by use of focal irradiation methods.
INTRODUCTION
Ependymoma comprises 10% to 12% of central nervous system (CNS) tumors in children (1), and fewer than one-third arise in the supratentorial compartment (2). Supratentorial ependymoma (STEP) is the only pediatric CNS tumor for which focal radiation therapy (RT) is currently administered after gross total resection (GTR), chemotherapy is not indicated, and long-term survival is expected. Post-operative RT is currently indicated for pediatric patients regardless of extent of resection, pathologic subtype, or tumor location. However, concern about side effects has resulted in a trend to omit RT after GTR without a clear understanding of the associated risks for disease progression or the incidence and magnitude of complications.
Although there is a suggestion that these children fare as well as their infratentorial counterparts (3), we performed a retrospective review of our institutional experience to confirm rates of disease control and study complications. Our series includes a cohort of children who were treated by using conformal RT and prospectively and serially evaluated for CNS effects. Our goal was to inform those who design and analyze clinical trials of children with STEP, especially those that seek to restrict the use of RT to older children.
PATIENTS AND METHODS
Patients
Between May 1972 and January 2011, a total of 50 patients with STEP received RT including 36 who were treated on a prospective institutional protocol (>1996) that included conformal RT (ClinicalTrials.gov identifier: NCT00187226). A record review was performed to obtain demographic data and information about clinical course including surgery; pathology; use of chemotherapy; CNS-directed interventions; and neurological, endocrine, and cognitive effects.
Extent of Resection
All patients underwent surgical resection before RT. GTR was defined as macroscopically complete resection with no evidence of disease on postoperative neuroimaging; subtotal resection included imaging visible residual disease.
Radiation Therapy
Among the 50 patients, 36 were treated on a protocol using a 5 or 10 mm clinical target volume margin and conformal or intensity-modulated RT. The total prescribed dose was 54 Gy (< 18 months of age at time of irradiation and gross-total resection) or 59.4 Gy. All others were treated using conventional RT with some variability in dose and volume (Table 1). Comments about RT planning and delivery are included in Appendix I.
Table 1.
Irradiation method for patients not treated by using protocol-based conformal radiation therapy.
| EBRT Dose | Method | BRT Dose | SRS Dose | CSI Dose | Whole-Brain Radiation Therapy Dose |
|---|---|---|---|---|---|
| 48.0 Gy | 32.0 Gy | ||||
| 50.8 Gy | 35.2 Gy | ||||
| 54.9 Gy | 40.4 Gy | ||||
| 48.0 Gy | 30.4 Gy | ||||
| 66.0 Gy | BRT+EBRT | 60 Gy | 49.5 Gy | ||
| 66.0 Gy | BRT+EBRT | 60 Gy | |||
| 59.4 Gy | BRT+EBRT | 50 Gy | |||
| 52.2 Gy | EBRT+SRS | 12.5 Gy | |||
| 55.8 Gy | EBRT+SRS | 10 Gy | |||
| 50.4 Gy | |||||
| 55.8 Gy | |||||
| 51.3 Gy | |||||
| 55.8 Gy | |||||
| 54.0 Gy |
Legend: EBRT=external beam radiation therapy; BRT=brachytherapy; SRS=single fraction stereotactic radiosurgery; CSI=craniospinal irradiation; WBRT=whole-brain radiation therapy.
Toxicity Assessment
We recorded seizures, use of anti-seizure medications, seizure control, and interventions for seizure disorder, hearing evaluations, visual examinations, hormonal replacement therapy, stroke and necrosis.
Cognitive Function
Cognitive testing was performed only on patients who were enrolled on a prospective trial; an age-appropriate battery was used (see Appendix II) to assess global intellectual functioning, attention, memory, academic skills, social-emotional adjustment, and adaptive functioning at baseline, at 6 months, and yearly for 5 years. Additional testing was mandated for 7 to 8 years after irradiation and 10 years after irradiation. We obtained information about individualized education programs from parental reports and medication use for attention deficits from the medical record or parental report.
Statistical Analysis and Follow-up
Progression-free survival (PFS) was defined as the time interval between initiation of RT and local or distant tumor progression. Event-free survival (EFS) was defined as the time interval between initiation of RT and date of either tumor progression (determined by MRI), death without tumor progression, or last MRI or follow-up. Overall survival (OS) was defined as the time interval between the initiation of RT and either death from any cause or the last known date of survival. Disease control was calculated by using the Kaplan–Meier method. Univariate associations were assessed by using the log-rank test and included tumor grade, tumor location, race, sex, age at the time of irradiation, total external-beam dose, extent of resection, and the use of pre-irradiation chemotherapy. Five of the survivors had not had an MRI for more than a year at last follow-up and 11 patients had not been seen in our clinics for more than one year at the time of study completion. Most of these patients have alumni status and are contacted once a year to determine their survivorship. Mean (SD) time since last contact for the surviving patients was a 1.3 (1.9) years.
The evaluation time was the number of months between the date of evaluation and RT initiation. The random coefficient mixed model was used to model the trend of cognitive function over time. Those having at least two assessments were included in the analyses. The model incorporated clinical variables one at a time to investigate the effect of each on the change in cognitive function over time. The statistical analyses did not include race, seizure surgery, or seizure control because of small sample size. Associations were investigated by using the Chi-squared and Fisher’s exact test (SAS v. 9).
RESULTS
Disease Control
Clinical and treatment characteristics are shown in Table 2. The study group was nearly evenly divided by sex and comprised predominately of Caucasian children. The median age at diagnosis and time of irradiation were 5.7 years (range, 0–18.7) and 6.5 years (1–18.9), respectively. Ten children were younger than 3 years at the time of RT. The median elapsed time to irradiation from initial surgery was 0.2 years (range, 0.2–5.68). Most patients had anaplastic WHO grade III tumors (76%) that were hemispheric (80%) and did not require cerebrospinal fluid shunting (62%) and received more than 54 Gy (88%) after gross-total resection (92%); a minority received chemotherapy prior to irradiation (22%). The most common reason for delaying RT was referral.
Table 2.
Clinical and Treatment Characteristics
| n | % | |
|---|---|---|
| Sex | ||
| M | 23 | 46 |
| F | 27 | 54 |
| Race | ||
| Caucasian | 43 | 86 |
| Non-Caucasian | 7 | 14 |
| Age at Diagnosis (years) | ||
| mean (SD) | 7.2 (5.4) | |
| median (range) | 5.7 (0–18.7) | |
| Age at CRT (years) | ||
| <3 | 10 | 20 |
| >3 | 40 | 80 |
| Age at CRT (years) | ||
| mean (SD) | 7.9 (5.3) | |
| median (range) | 6.5 (1–18.9) | |
| Elapsed years to CRT | ||
| mean (SD) | 0.7 (1.2) | |
| median (range) | 0.2 (0–5.9) | |
| Tumor Grade | ||
| Differentiated | 12 | 24 |
| Anaplastic | 38 | 76 |
| Tumor location | ||
| Hemispheric | 40 | 80 |
| Central | 10 | 20 |
| Total Prescribed Dose | ||
| <54 Gy | 6 | 12 |
| ≥54 Gy | 44 | 88 |
| Number of surgical procedures | ||
| 1 | 28 | 56 |
| 2 | 18 | 36 |
| 3 | 2 | 4 |
| 4 | 2 | 4 |
| Surgical extent | ||
| Gross-total resection | 46 | 92 |
| Sub-total resection | 4 | 8 |
| Pre-irradiation chemotherapy | ||
| Yes | 11 | 22 |
| No | 39 | 78 |
| CSF Shunt | ||
| Yes | 19 | 38% |
| No | 31 | 62% |
Abbreviation: CRT = conformed radiation therapy
During a median follow-up of 9.1 years from the start of RT for survivors (range, 0.2–23.2), 12 patients died; 11 from tumor progression and one who died of necrosis. Tumor progression was evenly divided between local (n=8) and distant failure (n=8) sites. Two patients had late recurrences, one 15 years and one 20 years after RT. Both originally had WHO grade II tumors. Two patients had histologically confirmed radiation necrosis 12 and 14 months after RT, respectively. One died of necrosis after 54Gy alone and the other was alive 20.8 years after brachytherapy (60 Gy) followed by hyper-fractionated RT (66 Gy). Two patients had stroke; one had a hemorrhagic stroke at 4 years old, one year after completing two years of chemotherapy and craniospinal RT (CSI). The other patient had a stroke 18 years after diagnosis. The latter is the same patient who survived necrosis. Incidentally, this patient had a lateral tumor, type II diabetes mellitus, and a BMI of 38.9. There were no secondary tumors in this series.
The 10-year estimate of local tumor control was 84.1% ± 5.6%. PSF, EFS, and OS rates were 70.3% ± 6.7%, 68.2% ± 6.8%, and 75.5% ± 6.4%, respectively. The median time to local treatment failure, tumor progression or an event related or unrelated to tumor progression was 2.1 years (range, 0.8–20.7), 1.7 years, (range, 0.6–20.7) and 1.8 years (range, 0.6–20.7), respectively (Figure 1). Only two patients experienced progression after 5 years. None of the clinical variables in Table 2 were predictive of outcome.
Fig. 1.
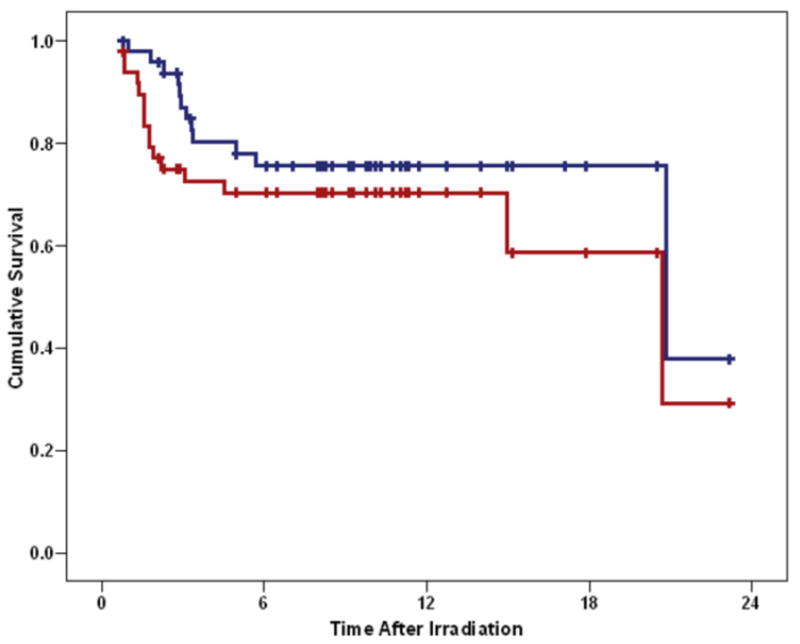
Progression-free (red) and overall survival (blue) after irradiation of supratentorial ependymoma.
Complications after Therapy
All visual impairments were stable from diagnosis, except in one patient who developed a cataract after (CSI). Hearing loss (> 25 dBHL at any frequency) was diagnosed in 7 patients, 5 were treated with chemotherapy and focal RT, 1 with CSI only, and 1 with focal RT alone that included very low doses to both cochleae. In the latter patient, we attributed hearing loss to IV antibiotics received for cranial osteomyelitis occurring after seizure surgery. Seventeen patients had seizures including 15 with hemispheric tumors. Seven of 15 had uncontrolled seizures despite medication; 3 required surgery resulting in control for 2. Eleven patients were treated with more than one anti-seizure medication, 4 patients were treated with one medication and 3 patients refused treatment. The most commonly used medications were phenytoin (n=9), tegretol (n=8), valproic acid (n=5), levetiracetam (n=4), gabapentin (n=4), carbamazepine (n=2), phenobarbital (n=2), lamotrigine (n=2), topiramate, mephobarbital and oxcarbazepine. CSF shunting was more common in patients with centrally located tumors (9 of 10) than in those with hemispheric tumors (10 of 40).
Hormone replacement therapy (HRT) was assessed for all patients; however, considering only the 38 surviving patients, 16 required some type of HRT. The most prevalent deficiency was thyroid hormone deficiency (n=9) and the same number required treatment for precocious puberty. Height (−0.33) and weight (0.39) z-scores and mean body mass index scores (15.5; range, 12.45–26.18) were within the normal range with few outliers. Toxicity assessment results are further detailed in Table 3. Neither the tumor location nor patient age at diagnosis was associated with the use of HRT.
Table 3.
Toxicity Parameters
| n | % | |
|---|---|---|
| Hormonal Replacement | ||
| Yes | 16 | 32 |
| No | 34 | 68 |
|
| ||
| No. of Replacements | ||
| 1 | 7 | 14 |
| 2 | 6 | 12 |
| 3 | 3 | 6 |
|
| ||
| Growth Hormone Replacement | ||
| Yes | 4 | 8 |
| Thyroid Hormone Replacement | ||
| Yes | 9 | 18 |
| Glucocorticoid Replacement for Adrenal Insufficiency | ||
| Yes | 2 | 4 |
| Gonadotropin-Releasing Hormone Analogue Therapy for Precocious Puberty | ||
| Yes | 9 | 18 |
| Sex Hormone Replacement | ||
| Yes | 4 | 8 |
|
| ||
| Seizures | ||
| Yes | 17 | 34 |
| No | 33 | 66 |
| Poor Control | 4 | 8 |
| Surgery | 3 | 6 |
|
| ||
| Individualized Education Program | ||
| Yes | 23 | 46 |
| No | 27 | 54 |
|
| ||
| Medication for Attention Deficit | ||
| Yes | 3 | 6 |
| No | 47 | 94 |
|
| ||
| Visual Field Impairment | ||
| cataract formation | 1 | 2 |
| visual field impairment | 13 | 26 |
| diplopia | 1 | 2 |
| normal | 35 | 70 |
|
| ||
| Hearing | ||
| Any hearing loss | 7 | 14 |
| Hearing Aids | 3 | 6 |
| normal | 43 | 86 |
|
| ||
| CSF Shunt | ||
| Yes | 19 | 38 |
| No | 31 | 62 |
Abbreviation: CSF = cerebrospinal fluid
Cognitive testing was performed prospectively in the children treated with conformal RT. Group changes over time are shown in Table 4. IQ remained in the normal range for the duration of the study. Baseline IQ values were significantly higher in older children (+1.58 points/year of age; P = .01) and those who did NOT receive pre-RT chemotherapy (+25.38 points; P = .003).
Table 4.
Trends of Neuropsychological Variables over Time
| Dependent Variable | Subjects (Evaluations) | Covariates | Estimate(SE) | 95% CI | p-value |
|---|---|---|---|---|---|
| Estimated IQ | 31(218) | Intercept | 96.1717(3.5881) | 88.8425–103.5010 | <.0001 |
| Time (months) | 0.0146(0.0267) | −0.0412–0.0703 | 0.5913 | ||
| CPT Omission | 25(179) | Intercept | 74.4205(3.6883) | 66.6475–82.1935 | <.0001 |
| Time (months) | 0.0080(0.0729) | −0.1483–0.1642 | 0.9144 | ||
| CPT Commision | 25(179) | Intercept | 34.8179(4.9217) | 24.7135–44.9223 | <.0001 |
| Time (months) | 0.1893(0.0500) | 0.0900–0.2886 | 0.0003 | ||
| CPT Reaction Time | 25(178) | Intercept | 26.4063(5.0064) | 15.9232–36.8895 | <.0001 |
| Time (months) | 0.2843(0.0622) | 0.1490–0.4196 | 0.0006 | ||
| CPT Overall Index | 21(119) | Intercept | 10.7526(0.9326) | 8.7373–12.7678 | <.0001 |
| Time (months) | 0.0085(0.0204) | −0.0345–0.0514 | 0.6823 | ||
| CVLT (Children’s) | 23(110) | Intercept | 44.6707(1.9822) | 40.4659–48.8755 | <.0001 |
| Time (months) | 0.0473(0.0319) | −0.0170–0.1117 | 0.1449 | ||
| WIAT Reading | 28(149) | Intercept | 96.1616(2.2405) | 91.5071–100.8160 | <.0001 |
| Time (months) | −0.0704(0.0349) | −0.1446–0.0039 | 0.0616 | ||
| WIAT Math | 28(149) | Intercept | 98.6489(2.6573) | 93.1086–104.1891 | <.0001 |
| Time (months) | −0.0318(0.0485) | −0.1346–0.0711 | 0.5219 | ||
| WIAT Spelling | 28(148) | Intercept | 95.1609(2.1085) | 90.8101–99.5118 | <.0001 |
| Time (months) | −0.0207(0.0359) | −0.0966–0.0552 | 0.5727 | ||
| Visual Auditory | 27(123) | Intercept | 93.5419(4.2129) | 84.6547–102.4290 | <.0001 |
| Time (months) | 0.0327(0.0528) | −0.0929–0.1583 | 0.5558 | ||
| CBCL Internal | 26(131) | Intercept | 48.6422(2.0431) | 44.3712–52.9133 | <.0001 |
| Time (months) | −0.0149(0.0306) | −0.0815–0.0517 | 0.6350 | ||
| CBCL External | 26(131) | Intercept | 49.4156(2.4733) | 44.3596–54.4715 | <.0001 |
| Time (months) | −0.0751(0.0208) | −0.1173–0.0329 | 0.0010 | ||
| CBCL Behavior Problems | 26(131) | Intercept | 49.1658(2.3665) | 44.1995–54.1321 | <.0001 |
| Time (months) | −0.0362(0.0263) | −0.0886–0.0162 | 0.1727 | ||
| CBCL Activities | 25(109) | Intercept | 45.9447(1.2624) | 43.2603–48.6291 | <.0001 |
| Time (months) | 0.0027(0.0310) | −0.0646–0.0699 | 0.9323 | ||
| CBCL Social | 25(103) | Intercept | 43.0499(2.0005) | 38.8105–47.2893 | <.0001 |
| Time (months) | 0.0375(0.0297) | −0.0257–0.1006 | 0.2261 | ||
| CBCL School | 24(102) | Intercept | 41.0651(2.0476) | 36.6188–45.5114 | <.0001 |
| Time (months) | −0.0037(0.0261) | −0.0649–0.0575 | 0.8902 | ||
| CBCL Total Social | 24(97) | Intercept | 43.1182(2.3377) | 38.1599–48.0765 | <.0001 |
| Time (months) | 0.0172(0.0324) | −0.0516–0.0859 | 0.6042 | ||
| Vineland Communication | 29(159) | Intercept | 93.3692(2.7279) | 87.7171–99.0213 | <.0001 |
| Time (months) | −0.0977(0.0459) | −0.1960–0.0006 | 0.0511 | ||
| Vineland Daily Living | 29(159) | Intercept | 93.8681(2.3789) | 88.9867–98.7495 | <.0001 |
| Time (months) | −0.0307(0.0512) | −0.1419–0.0805 | 0.5594 | ||
| Vineland Socialization | 29(159) | Intercept | 97.0114(2.9262) | 91.0026–103.0202 | <.0001 |
| Time (months) | −0.0359(0.0356) | −0.1111–0.0393 | 0.3273 | ||
| Vineland Motor | 12(49) | Intercept | 86.5567(4.5618) | 76.5282–96.5852 | <.0001 |
| Time (months) | −0.2121(0.1789) | −0.6430–0.2188 | 0.2778 | ||
| Vineland Composite | 28(157) | Intercept | 91.9031(2.9437) | 85.8428–97.9634 | <.0001 |
| Time (months) | −0.0417(0.0442) | −0.1387–0.0552 | 0.3649 |
Verbal Learning, Visual Auditory Learning and Academic Achievement
Verbal learning, as measured by California Verbal Learning Test – children’s version (CVLT-C) scores increased with time in girls; however, this difference was not significant (1.3452 points/year, P = .05). Baseline values in visual auditory learning scores from the Woodcock-Johnson Psycho-Educational Battery-Revised test (WJ-R) were higher in children who did not require seizure medications (19 points; P = .045) compared to those who were prescribed seizure medications; however, none of the clinical variables affected longitudinal scores. Baseline WIAT values of reading (15 points, P = .003), math (18 points, P = .005), and spelling (11 points, P = 0.034) were significantly higher in patients with central tumor location compared to those with hemispheric tumors. Longitudinal academic scores significantly increased when there was no history of a seizure disorder (spelling, 2.6868 points/year, P = 0.002) or use of anti-seizure medications (spelling, 2.5956 points/year, P = .004 and reading, 1.836 points/year, P = .04). Reading scores declined with time for the group as a whole (−2.1696 points/year. P = .009).
Behavioral Problem Scales, Behavioral Competence Scales and Adaptive Behavior
Externalizing behavior problems decreased for the group as a function of time (−0.9012 points/year, P = .001). Among girls, internalizing behavioral problems increased as a function of time (1.8804 points/year, P = .004). In children who did not have a seizure disorder, competence scales for social scores were significantly improved after RT (2.1072 points/year, P = .03). Social scores increased in those with no seizure history (1.6932 points/year, P = .001). Although the decline in communication scores after RT was not significant (−1.1724 points/year, P = .05); increasing age significantly increased these scores over time (0.2856 points/year of age/year, P = .03). Those who did not receive pre-RT chemotherapy had higher baseline socialization scores (14.8305 points, P = .03). Higher longitudinal scores in socialization measures were observed for those who did not take anti-seizure medications (1.8972 points/year, P = .01) or require additional educational resources (1.71 points/year, P = .03). The social findings are consistent across the behavior measures of the Vineland (interview) and CBCL (parent rating).
To assist us in interpreting the cognitive findings, we explored their association between clinical variables. Sex was not associated with the presence of seizures, the use of anti-seizure medications, the requirement of additional educational resources, the use of pre-irradiation chemotherapy or tumor location. However, the presence of seizures was highly associated with the use of anti-seizure medications (P <0.0001). Although the presence of seizures was not associated with the requirement for additional educational resources (P = .06), the use of anti-seizure medications was associated with the requirement for additional educational resources (P = .02). Younger age at diagnosis was associated with the presence of seizures (P = .0097) and the use of anti-seizure medication (P = .02) and younger patients were more likely to have been treated with pre-RT chemotherapy (P = .0001).
Discussion
This study includes one of the largest reported pediatric STEP series. It is also unique because of the large number of children under the age of 3 years at the time of RT. The results demonstrate that these children have excellent local tumor control after surgery and post-operative RT and less favorable PFS because of the propensity of ependymoma to metastasize. Children treated with surgery and post-operative RT experience a broad array of complications affecting neurological, endocrine and cognitive function; however, the frequency was low. Unique was the presence and treatment of seizure disorders which were associated with young age. Seizure disorders and their treatment, young age at the time of RT and female gender were associated with statistically significant, but not always clinically significant, lower performance on cognitive testing before and after treatment.
The 10-year PFS and OS rates are similar to those in other series (4, 5) and those of patients with infratentorial tumors (6–8). The literature is divided on the prognostic significance of supratentorial tumor location with some reports suggesting a better (9, 10) and others a worse prognosis (7, 11) compared to that of patients with infratentorial tumors. The extent of resection prior to RT is the most important prognostic factor for patients with ependymoma. The excellent outcome in our series was partially associated with the high rate of gross-total resection which has been attributed to surgical accessibility (12). The high proportion of anaplastic (12–14) and hemispheric tumors (4) were similar to those in published reports.
The molecular biology of ependymoma suggests that STEP has a different molecular signature than infratentorial ependymoma (10, 15, 16) and should be regarded as a different clinical entity with a different prognosis; however, our series shows that STEP treated with irradiation has a prognosis similar to that of infratentorial ependymoma. New histopathologic and molecular markers are emerging that may someday guide treatment decisions. Recent clinic-pathologic series showed that high mitotic count, increased cell density and the presence of 1q gain were prognostic for inferior disease control in infratentorial but not supratentorial ependymoma (17). Parenchymal invasion, assessed at the interface between ependymoma and the brain was primarily evident in supratentorial tumors and linked to poor outcome. These findings highlight that expert pathologic assessment is required to confirm diagnosis and evaluate aggressive clinical features.
Most children treated with RT for ependymoma have posterior fossa tumors. Information about long-term cognitive sequelae after focal RT of the supratentorial compartment in children is limited. In our series, most patients were younger than 5 years at the time of irradiation, with several younger than 3 three years and the overall cognitive function of these younger age subgroups appears to be preserved. Age clearly affected baseline scores suggesting that both tumor-related and surgery-related morbidity should be considered when making decisions about treatment. The evaluation of complications in our series showed that vision and hearing are rarely affected and most patients do not need hormone replacement therapy.
Age at RT, presence of seizure disorder, and the use of pre-RT chemotherapy clearly affected cognitive outcomes. Acknowledging that these are overlapping risk factors will help guide caregiver decisions, patient education and monitoring for late effects. Differences that exist at baseline should also be considered, especially those that might recover with time. For the group as a whole, reading proficiency appears to be at risk, likely because it is not an established skill in the younger patients, and girls seem to internalize symptoms suggesting two areas for intervention. The specter of side effects associated with age at the time of irradiation must be balanced against the known risk for tumor progression when observation is undertaken or when chemotherapy is administered to delay RT.
Despite good cognitive outcomes in survivors of STEP treated with contemporary RT, the tendency to treat the disease with surgery and defer irradiation will continue (18). This algorithm has been followed in recent cooperative group trials (ClinicalTrials.gov identifiers: NCT01407744 and NCT01096368) but is limited to children with WHO grade II ependymoma confirmed by central review after microscopic gross-total resection. The prevailing thought is that children with STEP fare better with tumor control than do those with infratentorial ependymoma and that irradiation of the supratentorial brain is too toxic to attempt in the very young. Our series does not provide a conclusive answer to this dilemma, and although some children were delayed in receiving RT, the numbers were insufficient to consider the effect of delay on outcome. Our series does highlight the fact that the side effects of treatment in patients treated using conformal RT methods appear to be less severe than anticipated. In the absence of a protocol, effort should be focused on achieving disease control and using methods that reduce radiation dose and the risk of complications.
The requirement for post-operative RT has been evaluated retrospectively in contemporary series. In a series of 45 adults, adjuvant RT after gross-total resection achieved a 10-year actuarial local control rate of 100% compared to a rate of only 50% for patients who underwent gross-total resection followed by observation (19). Koshy et al. data showed that OS was significantly improved for patients receiving post-operative RT (HR 0.8, 95% CL 0.6–0.9) (20). Among children younger than 3 years, the OS rate at 3 years was significantly higher for those who received post-operative RT than for those who did not (81% vs. 56%, respectively P = 0.005) (20). Although these data do not specifically address supratentorial tumors, they highlight the role and importance of RT and focus attention on the potential side effects of RT and the need to better understand the type, incidence, and severity of side effects experienced by this unique patient group.
Summary
Long-term survivorship for children with STEP treated with gross-total resection and postoperative RT is good and equal to that of children with infratentorial ependymoma. Tumor and treatment-related complications are acceptable and related to patient age at RT, the presence and treatment of seizure disorders, and the use of chemotherapy prior to irradiation.
Supplementary Material
Summary.
Compared to children with infratentorial ependymoma, those with supratentorial ependymoma have increased vulnerability to the effects of irradiation. Long-term survivorship of this group after gross-total resection and post-operative radiation therapy is good and equal to that of children with infratentorial ependymoma. Tumor and treatment-related complications of supratentorial ependymoma are acceptable and related to patient age at irradiation, the presence and treatment of seizure disorders, and the use of chemotherapy prior to irradiation.
Acknowledgments
This work was supported in part by the National Cancer Institute, Cancer Center Support Grant 5 P30 CA21765-28 and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest
There are no conflicts of interest with the authors or materials in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD: 2007. [Google Scholar]
- 2.McGuire CS, Sainani KL, Fisher PG. Both location and age predict survival in ependymoma: a SEER study. Pediatr Blood Cancer. 2009;52:65–69. doi: 10.1002/pbc.21806. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palma L, Celli P, Cantore G. Supratentorial ependymomas of the first two decades of life. Long-term follow-up of 20 cases (including two subependymomas) Neurosurgery. 1993;32:169–175. doi: 10.1227/00006123-199302000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Metellus P, Figarella-Branger D, Guyotat J, et al. Supratentorial ependymomas: prognostic factors and outcome analysis in a retrospective series of 46 adult patients. Cancer. 2008;113:175–185. doi: 10.1002/cncr.23530. [DOI] [PubMed] [Google Scholar]
- 6.Shu HK, Sall WF, Maity A, et al. Childhood intracranial ependymoma: twenty-year experience from a single institution. Cancer. 2007;110:432–441. doi: 10.1002/cncr.22782. [DOI] [PubMed] [Google Scholar]
- 7.Mansur DB, Perry A, Rajaram V, et al. Postoperative radiation therapy for grade II and III intracranial ependymoma. Int J Radiat Oncol Biol Phys. 2005;61:387–391. doi: 10.1016/j.ijrobp.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Robertson PL, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: a report of the Children’s Cancer Group. J Neurosurg. 1998;88:695–703. doi: 10.3171/jns.1998.88.4.0695. [DOI] [PubMed] [Google Scholar]
- 9.Grill J, Le Deley MC, Gambarelli D, et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19:1288–1296. doi: 10.1200/JCO.2001.19.5.1288. [DOI] [PubMed] [Google Scholar]
- 10.Andreiuolo F, Puget S, Peyre M, et al. Neuronal differentiation distinguishes supratentorial and infratentorial childhood ependymomas. Neuro Oncol. 2010;12:1126–1134. doi: 10.1093/neuonc/noq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson EL, Amdur RJ, Morris CG, et al. Intracranial ependymomas treated with radiotherapy: long-term results from a single institution. J Neurooncol. 2011;102:451–457. doi: 10.1007/s11060-010-0344-0. [DOI] [PubMed] [Google Scholar]
- 12.Ernestus RI. Prognostic relevance of localization and grading in intracranial ependymomas of childhood. 1996. [DOI] [PubMed] [Google Scholar]
- 13.Guyotat J, Metellus P, Giorgi R, et al. Infratentorial ependymomas: prognostic factors and outcome analysis in a multi-center retrospective series of 106 adult patients. Acta Neurochir (Wien ) 2009;151:947–960. doi: 10.1007/s00701-009-0417-z. [DOI] [PubMed] [Google Scholar]
- 14.Tihan T, Zhou T, Holmes E, et al. The prognostic value of histological grading of posterior fossa ependymomas in children: a Children’s Oncology Group study and a review of prognostic factors. Mod Pathol. 2008;21:165–177. doi: 10.1038/modpathol.3800999. [DOI] [PubMed] [Google Scholar]
- 15.Rogers HA, Kilday JP, Mayne C, et al. Supratentorial and spinal pediatric ependymomas display a hypermethylated phenotype which includes the loss of tumor suppressor genes involved in the control of cell growth and death. Acta Neuropathol. 2012;123:711–725. doi: 10.1007/s00401-011-0904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider D, Monoranu CM, Huang B, et al. Pediatric supratentorial ependymomas show more frequent deletions on chromosome 9 than infratentorial ependymomas: a microsatellite analysis. Cancer Genet Cytogenet. 2009;191:90–96. doi: 10.1016/j.cancergencyto.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Godfraind C, Kaczmarska JM, Kocak M, et al. Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol. 2012 doi: 10.1007/s00401-012-0981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatramani R, Dhall G, Patel M, et al. Supratentorial ependymoma in children: to observe or to treat following gross total resection? Pediatr Blood Cancer. 2012;58:380–383. doi: 10.1002/pbc.23086. [DOI] [PubMed] [Google Scholar]
- 19.Rogers L, Pueschel J, Spetzler R, et al. Is gross-total resection sufficient treatment for posterior fossa ependymomas? J Neurosurg. 2005;102:629–636. doi: 10.3171/jns.2005.102.4.0629. [DOI] [PubMed] [Google Scholar]
- 20.Koshy M, Rich S, Merchant TE, et al. Post-operative radiation improves survival in children younger than 3 years with intracranial ependymoma. J Neurooncol. 2011;105:583–590. doi: 10.1007/s11060-011-0624-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


