Abstract
The endocytic and exocytic/secretory pathways are two major intracellular membrane trafficking routes that regulate numerous cellular functions in a variety of cell types. Osteoblasts and osteoclasts, two major bone cells responsible for bone remodeling and homeostasis, are no exceptions. During the past few years emerging evidence has pinpointed a critical role for endocytic and secretory pathways in osteoblast and osteoclast differentiation and function. The endosomal membrane provides a platform to integrate bone tropic signals of hormones and growth factors in osteoblasts. In osteoclasts, endocytosis, followed by transcytosis, of degraded bone matrix promotes bone resorption. Secretory pathways, especially lysosome secretion, not only participate in bone matrix deposition by osteoblasts and degradation of mineralized bone matrix by osteoclasts; they may also be involved in the coupling of bone resorption and bone formation during bone remodeling. More importantly, mutations in genes encoding regulatory factors within the endocytic and secretory pathways have been identified as causes for bone diseases. Identification of the molecular mechanisms of these genes in bone cells may provide new therapeutic targets for skeletal disorders.
Keywords: intracellular membrane trafficking, endocytosis, exocytosis, osteoblast, osteoclast, bone remodeling, bone formation, bone resorption
Introduction
Maintenance of skeletal integrity and mass in adult life relies on bone remodeling, a process in which old or damaged bone matrix is removed by osteoclasts and replaced by new bone that is synthesized and secreted from osteoblasts [1, 2] (Figure 1). An imbalance in remodeling can lead to loss of bone mass, as in osteoporosis, or an aberrant accumulation of structurally compromised bone, as in osteopetrosis and osteosclerosis.
Figure 1. A schematic illustration of bone-remodeling process.
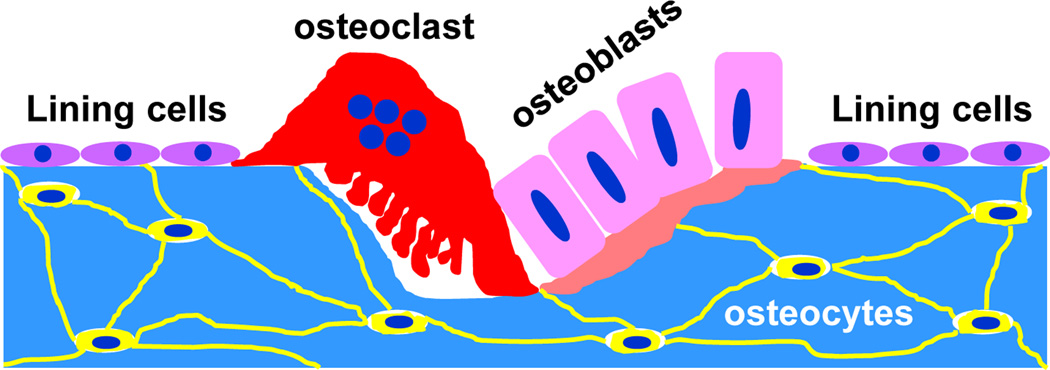
Bone surface is covered by lining cells. Osteocytes are the most abundant and long-lived bone cells. They are derived from osteoblasts that have embedded in lacunae in the bone matrix that they secrete. Osteocytes sense mechanical signals and/or micro-damages in bone, thereby sending signals to osteoclasts to initiate bone resorption, which is followed by bone matrix deposition by osteoblasts. Osteoblasts eventually become bone lining cells or osteocytes.
Osteoblasts arise from mesenchymal stem cells that can differentiate into fibroblasts, myoblasts, osteoblasts, or adipocytes. Mature osteoblasts synthesize and secrete large amounts of type I collagen, noncollagenous proteins, enzymes, and growth factors, which comprise major components of the organic matrix of bone (osteoid) that later becomes mineralized [3] (Figure 2). Osteoclasts are multinucleated cells of hematopoietic origin that degrade the bone matrix. They are formed by fusion of mononuclear precursors of the monocyte/macrophage lineage [4]. The discovery of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor k B (RANK) ligand (RANKL) as the two key cytokines in osteoclastogenesis has greatly advanced osteoclast cell biology research. With these cytokines, a large number of mature osteoclasts can be generated in vitro using bone marrow macrophages of rodents or human peripheral blood monocytes. When osteoclasts are cultured on glass or plastic, a unique feature of these polykaryons is the formation of podosomes, highly dynamic actin-containing structures that mediate the adhesion and migration of osteoclasts. The individual podosomes cluster and move to the periphery of osteoclasts to form a stable “podosome belt” [5]. When cultured onto bovine cortical bone slices or whale/elephant dentin slices, an in vitro osteoclast model system developed almost 30 years ago, osteoclasts can degrade the mineralized matrix, mimicking bone resorption in vivo [6, 7]. Under these conditions, podosomes become more concentrated and interconnected at the sealing zone, where the plasma membrane forms a tight attachment to bone surface [8]. Protons and acidic proteases are secreted into the resorption lacuna through the ruffled border to solubilize bone mineral and digest the organic matrix, respectively [9, 10] (Figure 3).
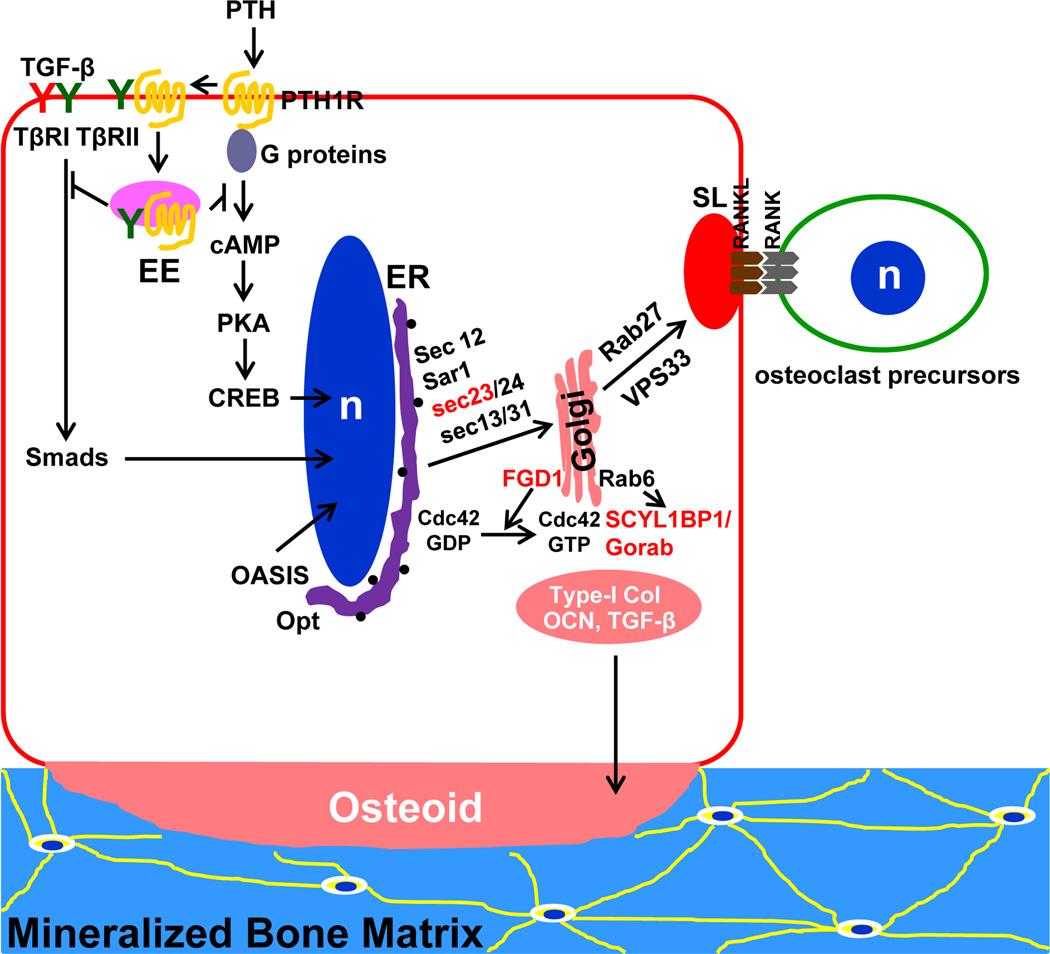
Figure 2. Endocytic and secretory pathways in osteoblasts.
In addition to binding to PTH receptor (PTH1R), PTH has been recently shown to induce the endocytosis of TGF-β receptor II (TβRII) and PTH1R into early endosomes (EE), which attenuates the cognate signaling pathways of both receptors. OASIS is an endoplasmic reticulum (ER)-residing transcription factor of CREB/ATF family. It is truncated and translocated into the nucleus to promote type I collage expression in response to ER stress. Opt, osteopotentia, is a newly identified protein that regulates ER volume during osteoblast activation. Sec23, FGD1, and SCYL1BP1/Gorab in red color are proteins mutated in human genetic skeleton diseases. They regulate bone matrix secretion at ER and Golgi, respectively. RANKL, an osteoclast differentiation cytokine, is released from osteoblast secretory lysosomes (SL) regulated by Vps33 and Rab27.
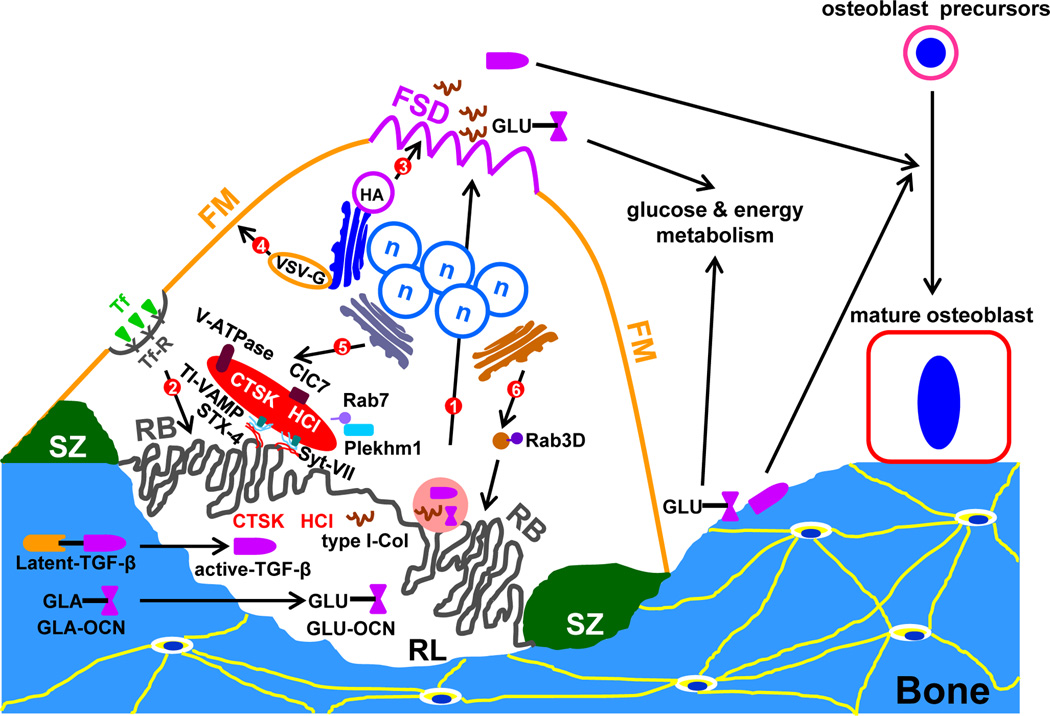
Figure 3. Major intracellular vesicular trafficking pathways in resorbing osteoclasts.
The plasma membrane of a resorbing osteoclast is highly polarized. It has four distinct domains: the functional secretory domain (FSD), the free membrane domain (FM), the sealing zone (SZ), and the ruffled border (RB). Six intracellular membrane trafficking pathways have been identified in resorbing osteoclasts so far (they are numbered in the sequence of their appearance in the text). Route 1 is the transcytotic pathway in which type I collagen (type I-Col), active TGF-β, and undercarboxylated osteocalcin (GLU-OCN), an active form of osteocalcin, are transported from the resorption lacunae (RL) to FSD. A fraction of active TGF-β and GLU-OCN are also released from a previous RL when osteoclast migrates to a new resorption site. GLA-OCN (γ-carboxylated osteocalcin) is the mature form of osteocalcin, which is stored in the bone matrix. Route 2 represents a trafficking pathway from the FM to the RB, which has been revealed by transferrin (Tf) and transferrin receptor (TfR). Route 3 and 4 are post-Golgi constitutive secretory pathways that connect Golgi to FM and FSD, respectively. The two pathways can be traced by virus envelop proteins, haemagglutinin (HA) and vesicular stomatitis virus G-protein (VSV-G). Route 5 is a secretory lysosome pathway which delivers cathepsin K (CTSK) and HCl to RL. This pathway is regulated by Rab7 and Plekhm1. The fusion of secretory lysosomes with RB is mediated by tetanus neurotoxin-insensitive vesicular-associated membrane protein (TI-VAMP), Syntaxin 4, and Synaptotagmin VII. Route 6 is a post-Golgi non-lysosomal vesicular pathway which is regulated by Rab3D and is also functional important for the ruffled border formation and bone resorption.
As in other eukaryotic cells, increasing evidence indicates that vesicular trafficking pathways play an important role in regulating osteoclasts and osteoblasts, and thus impact bone remodeling. The findings in this area of bone biology research have provided new insights into the cellular and molecular mechanisms that regulate skeletal development and homeostasis, and may reveal new therapeutic targets for metabolic disorders of bone.
Participation of the endosomal system in osteoblast and osteoclast regulation
Endocytosis, in eukaryotic cells, involves the formation of membrane vesicles at the plasma membrane. Endocytic pathways participate in and regulate a variety of cellular processes, including signal transduction through the plasma membrane [11]. This section focuses on how endocytic pathways regulate: (I) parathyroid hormone (PTH) and transforming growth factor-β (TGF-β) receptor signaling in osteoblasts, (II) removal of degraded bone matrix from the resorption lacunae by osteoclasts, and (III) the ruffled border formation in active osteoclasts.
Endosomes control signal transduction in osteoblasts
TGF-β, an abundant growth factor embedded in bone matrix, regulates proliferation and differentiation of osteoblast precursors through direct binding to its type II receptor (TβRII), which then recruits and phosphorylates the type I receptor. PTH regulates calcium homeostasis and bone metabolism by activating the PTH receptor (PTHR) in osteoblasts. The PTHR is a seven transmembrane G protein-coupled receptor (GPCR) which associates with heterotrimeric G proteins at the plasma membrane. Activated G proteins recruit and stimulate adenylyl cyclase leading to cyclic adenosine monophosphate (cAMP) production and protein kinase A (PKA) activation (Figure 2). Recent work by Qiu et al [12] revealed a novel mechanism by which PTH and TGF-β signaling are integrated through endocytosis of their cognate receptors. This work demonstrates that TGF-β receptor II is recruited to PTH-PTHR complex upon PTH stimulation. TβRII directly phosphorylates the cytoplasmic domain of PTHR which triggers the endocytosis of both receptors into the same endosome. This event occurs with the assistance of β-arrestins, adaptor proteins that promote GPCR endocytosis and desensitization. Removal of both receptors from the cell surface halts PTH and TGF-β signaling. Mirroring these in vitro findings, cells from osteoblast-specific TβRII-deficient mice display increased abundance of PTHR. These animals have high skeletal mass with increased trabecular, but decreased cortical, bone formation, recapitulating the phenotypes of constitutively active PTHR transgenic mice.
Another paradigm of endosomal regulation of PTHR signaling is provided by the recent work from Ferrandon et al, using fluorescence resonance energy transfer (FRET) and spinning disk confocal microscopy in live cells. These investigators have shown that although PTH and PTH-related protein (PTHrP) recognize the same PTHR, they induce different conformational changes of the receptor and its effector signaling [13]. PTH-binding translocates PTHR from the plasma membrane to Rab5-positive early endosomes. The endosome-residing PTH-PTHR complex remains associated with G proteins and adenylyl cyclase, and thus continues to sustain cAMP production. In contrast, PTHrP-PTHR remains at the cell surface where it induces only a transient cAMP increase. Given the fact that continuous administration of PTH results in catabolic effects on bone while intermittent PTH or PTHrP administration is anabolic [14, 15], the different durations of the signaling responses, induced by distinct PTHR conformations, may provide a mechanistic explanation of the complicated effects of PTH on bone remodeling.
Wnt receptors are also targets of endocytic pathways [16] and recruitment to endosomes is an essential step in Notch activation [17, 18]. Since both Wnt and Notch signaling are critical for osteoblast differentiation, survival, and function [19, 20], it will be of interest to determine if these signaling pathways are also regulated by endocytosis in osteoblasts.
Endocytic and transcytotic pathways in osteoclastic bone resorption
Two endocytotic pathways participate directly in osteoclastic bone resorption. The first involves transcytosis of degraded products from the resorption lacuna. Dissolution of bone minerals and the digestion of organic bone matrix during bone resorption release large amounts of calcium, phosphate, and collagen fragments, which need to be removed from the resorption lacuna to prevent their accumulation to levels that would be toxic to osteoclasts. Light and electron microscopic studies have shown that collagen fragments and calcium are taken up at the ruffled border and released from the functional secretory domain (FSD) at the center of the bone opposing surface (route 1 in Figure 3) [21–23]. Due to the diverse size of transcytotic bone matrix vesicles, both endocytosis and macropinocytosis have been proposed as candidate mechanisms for incorporation of degraded bone products into osteoclasts [24, 25]. Clathrin, its adaptor protein AP-2, and the large GTPase dynamin (a molecular “pinchase” that helps to mediate the scission of clathrin-coated vesicles from the plasma membrane) are localized at the central area of the ruffled border, suggesting clathrin-mediated budding is important for the initial formation of the transcytotic vesicles [24]. In line with this notion, a combination of cell-shearing and quick-freezing/rotary replication techniques have demonstrated that clathrin-coated patches and pits do exist at the ruffled border [26]. It should be noted that dynamin is also localized at the podosome belt and the sealing zone of osteoclasts cultured on plastic and bone matrix, respectively, where it regulates podosome dynamics during osteoclast adhesion and migration [27]. Dynamin executes this function through its direct interaction with signaling complexes downstream of Src, a non-receptor tyrosine kinase critical for podosome organization, sealing zone formation, and osteoclast function [28, 29]. Src is also associated with intracellular vesicular membranes and the ruffled border [30, 31], where it regulates acid and lysosomal enzyme secretion into the resorption lacuna [32–34].
During transportation of transcytotic vesicles, engulfed bone matrix is further digested by tartrate-resistant acidic phosphatase (TRAP) and cathepsin K, both of which are present in transcytotic vesicles. TRAP5b, an osteoclast-specific isoform of TRAP and a cellular marker for osteoclasts, has two distinct enzymatic activities: one as a phosphatase and the other as a generator of reactive oxygen species (ROS). The ROS-generating activity of TRAP5b facilitates collagen degradation and may have a role in the final degradation of internalized resorption products [35]. Thus, this transcytotic pathway may be used by osteoclasts to process and release bone matrix-embedded growth factors such as TGF-β and insulin-like growth factors (IGFs) to stimulate osteoblasts as part of the mechanism that couples bone resorption to bone formation during remodeling [36]. Alternatively, the acidic microenvironment in resorption lacuna and transcytotic vesicles activate the bone-derived hormone osteocalcin, which may modulate glucose homeostasis, energy metabolism, and male fertility [37, 38]. Besides these paracrine functions, the transcytotic pathway may exert autocrine effects on the osteoclast itself. L-glutamate, an excitatory neurotransmitter of the central nervous system, is secreted from osteoclasts through the same transcytotic pathway as degraded bone matrix. Secreted L-glutamate, in turn, inhibits osteoclast transcytosis and bone resorption. Consistent with this model, mice which lack the vesicular glutamate transporter 1 develop osteoporosis [39].
The second important endocytic pathway in osteoclasts is the endocytic trafficking from the basal membrane to the ruffled border, which participates in turnover of the ruffled border [40]. When added to culture medium, both fluid, such as horseradish peroxidase (HRP), and receptor-mediated (transferrin receptor) endocytic tracing markers accumulate at the ruffled border (route 2 in Figure 3). Moreover, inhibition of lysosome biogenesis or transportation impairs the targeting of endocytosed materials towards the ruffled border, indicating the interaction of these two pathways [41].
Secretory pathways in osteoblast and osteoclast function
The second part of this article focuses on osteoblast and osteoclast secretion.
The regulatory mechanisms of bone matrix protein and mineral crystal secretion by osteoblasts
In osteoblasts, the precursors of type I collagen and other bone matrix components are processed along the rough ER (rER)-Golgi-secretory granule pathway in the same manner as secretory proteins [42] (Figure 2). The high metabolic demand of skeletal development or that imposed by high remodeling metabolic disorders such as hyperparathyroidism, stimulates quality control mechanisms at the ER, referred to as the ER stress response [43, 44]. To cope with this challenge, osteoblasts employ several strategies. First, they increase synthesis of bone matrix proteins, mainly type I collagen, by transcriptional and posttranscriptional mechanisms regulated by two newly identified proteins, old astrocyte specifically-induced substance (OASIS) and osteopotentia (Opt). OASIS, a member of the CREB/ATF family of transcription factors, is highly expressed in osteoblasts and is induced by BMP2 (bone morphogenetic protein 2), an important cytokine required for bone formation. OASIS is a transmembrane ER protein and is processed by regulated intramembrane proteolysis in response to ER stress. The N-terminal fragment of OASIS translocates to nucleus, where it activates transcription of collagen a1 (Col1a1) through an unfolded protein response (UPR) element in the promoter region of Col1a1 gene. OASIS−/− mice are osteoporotic due to diminished type I collagen synthesis by osteoblasts [45]. Opt is a widely expressed rER-localized integral membrane protein which stimulates rER expansion and increases type I collagen synthesis in osteoblasts. Mice lacking Opt have impaired bone formation and experience spontaneous fractures [46]. Since the phenotypes in both OASIS and Opt deficient mice are similar to osteogenesis imperfecta (OI), a genetic disorder characterized by a brittle, fracture-prone skeleton, it will be interesting to determine if these genes are mutated in a subset of affected patients.
Second, osteoblasts ensure the correct folding and translocation of increased amounts of bone matrix proteins via ER molecular chaperones. In this regard, BiP/Grp78 and PDI (protein disulfide isomerase), two ER chaperones, are down-regulated in osteoblasts of osteoporotic patients. Moreover, treatment of ovariectomized mice, an animal model of postmenopausal osteoporosis, with a Bip activator increases bone formation through the induction of folding and secretion of bone matrix proteins [47].
Third, osteoblasts efficiently transport proteins from ER/Golgi to the cell surface, a process which, when disturbed, has pathological consequences. Cranio-lenticulo-suture dysplasia (CLSD) is a syndrome characterized by facial dysmorphisms, skeletal defects, late-closing fontanels, and cataracts [48]. CLSD is caused by a missense mutation in the sec23A subunit of the COP II complex, resulting in disturbed COPII coat assembly at ER and defective ER-to-Golgi trafficking [49, 50]. Gerodermia osteodysplastica (GO) is an autosomal recessive disorder causing wrinkled skin and osteoporosis [51]. GO is caused by loss-of-function mutations in SCYL1BP1/Gorab, which is highly expressed in the epidermis and osteoblasts. SCYL1BP1/Gorab is localized at the Golgi apparatus and interacts with Rab6, a small GTPase regulating vesicular trafficking among intra-stacks of Golgi and at the trans-Golgi network (TGN) [52]. Faciogenital dysplasia (FGDY)/Aarskog Syndrome is an X-linked developmental disorder characterized by short stature and facial, skeletal, cardiac, ocular, and urogenital anomalies [53, 54]. Mutations in FGD1 gene cause this disease [55]. FGD1 is a TGN-associated guanine nucleotide exchange factor (GEF) that specifically activates Cdc42 and is involved in the formation of the post-Golgi transport vesicles directed to the cell surface [56]. The trafficking pathways regulating collagen secretion by osteoblasts are summarized in Figure 2.
The mechanisms whereby post-Golgi vesicles reach and fuse with the plasma membrane in osteoblasts are less clear. Bone matrix-containing vesicles move along microtubules, probably driven by cytoplasmic dynein and/or kinesin family members, or both [57]. These vesicles are partially associated with lysosomes in osteoblasts as demonstrated by immunofluorescent colocalization studies and subcellular fractionation [57, 58]. Several components of the membrane fusion machinery, including NSF (N-ethylmaleimide-sensitive factor), VAMP1 (vesicle-associated membrane protein 1), SNAPs (soluble NSF attachment proteins), syntaxin 4, munc-18, and the mammalian homolog of the yeast exocyst, Sec6/Sec8 complex, are expressed in osteoblasts and may mediate fusion of their secretory vesicles with the plasma membrane [59, 60]. Synaptotagmin VII (Syt VII), which promotes lysosome/plasmalemma fusion in fibroblasts, permits fusion of matrix vesicles with the plasma membrane in osteoblasts. Syt VII−/− mice exhibit impaired bone formation without changes in osteoblast differentiation [58]. Amorphous calcium/phosphate crystals are also directly secreted via an exocytotic process by osteoblast lysosomes [61]. Interestingly, the key osteoclastogenic cytokine, RANKL, is stored in secretory lysosomes of osteoblastic cells [62] and its secretion is regulated by Rab27, a small GTPase that regulaties secretory lysosome exocytosis in several cell types [63–66] and VPS33A (vesicle protein sorting 33A) [67], a component of VPS class-C complexes that are essential for late endosome/lysosome assembly and sorting [68]. A similar mechanism may also regulate osteocyte-derived RANKL, which has recently been shown to control bone remodeling and homeostasis [69, 70]. Thus, the endosomal/lysosomal pathway in osteoblastic cells may not only facilitate bone matrix deposition, but it may also regulate osteoclastogenesis.
Secretory pathways regulate osteoclastic bone resorption
Using enveloped viral glycoproteins to investigate molecular sorting and trafficking in osteoclasts, Väänänen and colleagues showed that influenza haemagglutinin (HA), which is apically targeted in epithelial cells, is localized at FSD of resorbing osteoclasts (route3 in Figure 3). On the other hand, vesicular stomatitis virus G-protein (VSV-G), which is basolaterally targeted in epithelium, occupies the rest of the bone opposing membrane in resorbing osteoclasts (route 4 in Figure 3). Neither of these viral glycoproteins accumulates at the ruffled border [71]. Replacing the cytoplasmic tail of the VSV-G protein with that of CD4 results in its localization to lipid rafts and retargeting VSV-G to FSD [72]. Accumulating evidence indicates that the lysosomal/autophagy pathway is involved in osteoclast secretion and ruffled border formation (route 5 in Figure 3). This is not necessarily surprising since the lysosomes are the most acidic organelles within eukaryotic cells and contain an abundance of different kinds of hydrolases. It appears that osteoclasts have evolved to utilize lysosomes to carry out one of the most difficult jobs in our body, namely, to excavate the mineralized skeleton.
The key components of the osteoclast acid-secreting machinery, including the a3 subunit of vacuolar proton pumps, the Clc-7 chloride channel and its β-subunit OSTM1 (osteopetrosis associated transmembrane protein 1), are all associated with lysosomes and in active osteoclasts they translocate to the ruffled border membrane [73, 74]. Cathepsin K, which is the principal lysosomal acidic hydrolase degrading the organic matrix of bone, is also secreted into the resorption lacuna in resorbing osteoclasts [41], indicating that lysosome secretion is a major pathway of osteoclastic bone resorption. Cathepsin K contains a mannose-6-phosphate (Man-6-P) moiety [75] which binds Man-6-P receptors (MPRs) that help to transport soluble lysosomal enzymes to endo-lysosomal compartments [76]. Osteoclasts express high levels of MPRs [77] which mediate sorting of cathepsin K from the TGN to secretory lysosomes [78]. Lysosomal enzymes and MPRs are co-distributed in the ER, the Golgi stacks, and numerous transport vesicles that fuse with the ruffled border [77]. In addition to secretory lysosomes, osteoclasts also contain conventional lysosomes bearing ubiquitously expressed acidic hydrolase cathepsin D, but not Cathepsin K or TRAP. The targeting of Cathepsin D to lysosomes is, instead, Man-6-P independent [78]. Thus, osteoclasts employ two different lysosomal systems for bone resorption and cellular homeostasis, respectively.
Rab7, a small GTPase that specifically regulates late endosomal/lysosomal vesicular trafficking [79], is predominantly localized at the ruffled border membrane [40]. Inhibition of Rab7 expression impairs osteoclast secretion and ruffled border formation [80]. Rab7 may govern osteoclast lysosome biogenesis, as it does in other cell types [81]. Rab7 also modulates local actin organization through its interaction with Rac1, a small GTPase of the Rho family, which is essential for osteoclast actin organization and whose absence causes severe osteopetrosis [82]. In this manner, Rab7 regulates the movement of osteoclast lysosomes to the ruffled border through cytoskeletal organization [83]. Rab7 has also been shown to associate with osteoclast phagosomes which contain collagen fibers [84]. Thus, Rab7 may be involved in processing and degradation of intracellular collagen along the transcytotic pathway. More recently, mutations of the PLEKHM1 gene have been identified as the cause of osteopetrosis in ia/ia (incisors absent) rat as well as a subset of patients with intermediate osteopetrosis [85]. Osteoclast formation in ia rats and human patients is normal, but Plekhm1 deficient osteoclasts have impaired resorptive activity [85, 86]. It has been shown that Plekhm1 is associated with lysosomes, probably through its interaction with Rab7 [87]. Plekhm1 regulates cathepsin K secretion and bone resorption in osteoclasts [88]. Thus, Plekhm1 collaborates with Rab7 in lysosome biogenesis and trafficking in osteoclasts. A new piece of evidence added to this model comes from the recent finding that autophagy proteins participate in polarized secretion of lysosomal contents into the extracellular space by directing lysosomes to fuse with the plasma membrane in osteoclasts [89].
In addition to the lysosomal pathway, a non-endosomal/lysosomal secretory pathway of small post-TGN vesicles has been shown to participate in the ruffled border formation and osteoclastic bone resorption (route 6 in Figure 3). This pathway is governed by Rab3D, an isoform of Rab3 known to regulate exocytosis in neurons and other eukaryotic cells. Rab3D−/− mice are osteosclerotic due to decreased osteoclast activity [90]. Interestingly, lack of the Man-6-P targeting pathway in osteoclasts, due to the deletion of Gnptab (encoding GlcNAc-1-phosphotransferase α, β-subunits), impairs secretory lysosome formation. Cathepsin K and TRAP in Gnptab−/− osteoclasts are, instead, sorted into small post-TGN vesicles and their secretion is increased as compared to that in wild type osteoclasts [78]. Whether this secretory activity is regulated by Rab3D needs to be further characterized.
Extensive membrane fusion occurs at the ruffled border, generating the highly convoluted morphology of this membrane domain. The machinery mediating this event has just begun to be characterized. Syt VII is a lysosome-associated protein in osteoclasts [58] that [91–94] colocalizes with Cathepsin K and lysosomal membrane protein, LAMP2. Density gradient analysis shows that SytVII is in a lysosome-enriched fraction containing Rab7 and sec6/sec8, two components of the exocyst complex mediating vesicular secretion [95]. Each of these proteins translocate to the ruffled border in resorbing osteoclasts [58]. As in other cell types, Syt VII interacts with the lysosome-specific SNARE (SNAP receptor) TI-VAMP (tetanus neurotoxin-insensitive vesicular-associated membrane protein) in osteoclasts [96]. A likely binding partner of the SytVII/TI-VAMP complex at the plasma membrane is syntaxin 4, a plasma membrane SNARE protein [96] which also localizes to the ruffled border [74] (Figure 3). Taken together, these results indicate that Syt VII promotes the fusion of lysosomes containing proton pumps, chloride channels, and acidic hydrolases with the ruffled border.
Conclusions and perspectives
Significant progress has been made during the past few years in our understanding of how intracellular vesicular trafficking pathways regulate osteoblasts and osteoclasts. The findings have provided new insights into the mechanisms of bone formation and bone resorption in skeletal health and diseases. It is clear that endosomes play an important role in the fine-tuning regulation of signal transduction in osteoblasts. The secretory pathways in osteoblasts and osteoclasts not only participate in bone matrix deposition/degradation processes, but also provide the means by which these two bone cells communicate with each other during bone remodeling. However, our knowledge of the molecular mechanisms of intracellular vesicular trafficking pathways in osteoblasts and osteoclasts is still limited relative to what is known about these in neurons and epithelial cells. More importantly, osteocytes are the most abundant bone cells that play an essential role in regulating bone remodeling. These cells, like neurons, have long processes through which regulatory molecules are transported to the neighboring osteocytes or bone surface. Therefore, studies of membrane trafficking in osteocytes may become an important area of skeletal research. Future work using both in vitro cell culture systems and in vivo genetically-modified mouse models will be needed to uncover more specific functions of trafficking-associated proteins in bone physiology and pathology. Application of state-of-the-art biochemical and biophysical techniques and assays, especially organelle-specific tracing markers and multiple tags of fluorescent proteins in live cell imaging systems, should provide new avenues and platforms for elucidation of the molecular mechanisms of skeletal tropic hormones, growth factors and cytokines. The discoveries may offer new strategies and drug targets for the prevention and treatment of skeletal diseases.
Acknowledgements
The work in author’s laboratory was supported by National Institutes of Health grant AR055694, AR062012, P01 AG13918 and UAMS Tobacco Settlement Funds provided by Arkansas Biosciences Institute. I would like to thank Drs Maria Almeida, Robert L Jilka, Vladimir V Lupashin, Stavros C Manolagas, Michael S. Marks, Roy Morello, Charles A Obrien, Brian Storrie, Steve L Teitelbaum, Robert S Weinstein and two anonymous reviewers for their critics and comments on the manuscript.
References
- 1.Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH. Bone remodelling at a glance. J Cell Sci. 2011;124:991–998. doi: 10.1242/jcs.063032. [DOI] [PubMed] [Google Scholar]
- 2.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 4.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 5.Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyde A, Ali NN, Jones SJ. Resorption of dentine by isolated osteoclasts in vitro. Br Dent J. 1984;156:216–220. doi: 10.1038/sj.bdj.4805313. [DOI] [PubMed] [Google Scholar]
- 7.Chambers TJ, Revell PA, Fuller K, Athanasou NA. Resorption of bone by isolated rabbit osteoclasts. J Cell Sci. 1984;66:383–399. doi: 10.1242/jcs.66.1.383. [DOI] [PubMed] [Google Scholar]
- 8.Luxenburg C, Geblinger D, Klein E, Anderson K, Hanein D, Geiger B, Addadi L. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 10.Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113(Pt 3):377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 11.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 12.Qiu T, Wu X, Zhang F, Clemens TL, Wan M, Cao X. TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat Cell Biol. 2010;12:224–234. doi: 10.1038/ncb2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982;110:506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- 15.Stewart AF. PTHrP(1–36) as a skeletal anabolic agent for the treatment of osteoporosis. Bone. 1996;19:303–306. doi: 10.1016/s8756-3282(96)00221-9. [DOI] [PubMed] [Google Scholar]
- 16.Gagliardi M, Piddini E, Vincent JP. Endocytosis: a positive or a negative influence on Wnt signalling? Traffic. 2008;9:1–9. doi: 10.1111/j.1600-0854.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- 17.Gupta-Rossi N, Six E, LeBail O, Logeat F, Chastagner P, Olry A, Israël A, Brou C. Monoubiquitination and endocytosis direct gamma-secretase cleavage of activated Notch receptor. J Cell Biol. 2004;166:73–83. doi: 10.1083/jcb.200310098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;180:755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007;21:2605–2614. doi: 10.1210/me.2007-0259. [DOI] [PubMed] [Google Scholar]
- 21.Nesbitt SA, Horton MA. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science. 1997;276:266–269. doi: 10.1126/science.276.5310.266. [DOI] [PubMed] [Google Scholar]
- 22.Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 23.Yamaki M, Nakamura H, Takahashi N, Udagawa N, Ozawa H. Transcytosis of calcium from bone by osteoclast-like cells evidenced by direct visualization of calcium in cells. Arch Biochem Biophys. 2005;440:10–17. doi: 10.1016/j.abb.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Mulari MT, Zhao H, Lakkakorpi PT, Vaananen HK. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic. 2003;4:113–125. doi: 10.1034/j.1600-0854.2003.40206.x. [DOI] [PubMed] [Google Scholar]
- 25.Stenbeck G, Horton MA. A new specialized cell-matrix interaction in actively resorbing osteoclasts. J Cell Sci. 2000;113(Pt 9):1577–1587. doi: 10.1242/jcs.113.9.1577. [DOI] [PubMed] [Google Scholar]
- 26.Akisaka T, Yoshida H, Suzuki R. The ruffled border and attachment regions of the apposing membrane of resorbing osteoclasts as visualized from the cytoplasmic face of the membrane. J Electron Microsc (Tokyo) 2006;55:53–61. doi: 10.1093/jmicro/dfl012. [DOI] [PubMed] [Google Scholar]
- 27.Bruzzaniti A, Neff L, Sanjay A, Horne WC, De CP, Baron R. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol Biol Cell. 2005;16:3301–3313. doi: 10.1091/mbc.E04-12-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe C, Yoneda T, Boyce BF, Chen H, Mundy GR, Soriano P. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc Natl Acad Sci U S A. 1993;90:4485–4489. doi: 10.1073/pnas.90.10.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 30.Horne WC, Neff L, Chatterjee D, Lomri A, Levy JB, Baron R. Osteoclasts express high levels of pp60c-src in association with intracellular membranes. J Cell Biol. 1992;119:1003–1013. doi: 10.1083/jcb.119.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka S, Takahashi N, Udagawa N, Sasaki T, Fukui Y, Kurokawa T, Suda T. Osteoclasts express high levels of p60c-src, preferentially on ruffled border membranes. FEBS Lett. 1992;313:85–89. doi: 10.1016/0014-5793(92)81190-w. [DOI] [PubMed] [Google Scholar]
- 32.Edwards JC, Cohen C, Xu W, Schlesinger PH. c-Src control of chloride channel support for osteoclast HCl transport and bone resorption. J Biol Chem. 2006;281:28011–28022. doi: 10.1074/jbc.M605865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuyama N, Fujisawa Y. Regulation of collagenolytic protease secretion through c-Src in osteoclasts. Biochem Biophys Res Commun. 2000;272:116–124. doi: 10.1006/bbrc.2000.2698. [DOI] [PubMed] [Google Scholar]
- 34.Pascoe D, Oursler MJ. The Src signaling pathway regulates osteoclast lysosomal enzyme secretion and is rapidly modulated by estrogen. J Bone Miner Res. 2001;16:1028–1036. doi: 10.1359/jbmr.2001.16.6.1028. [DOI] [PubMed] [Google Scholar]
- 35.Halleen JM, Raisanen S, Salo JJ, Reddy SV, Roodman GD, Hentunen TA, Lehenkari PP, Kaija H, Vihko P, Väänänen HK. Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. J Biol Chem. 1999;274:22907–22910. doi: 10.1074/jbc.274.33.22907. [DOI] [PubMed] [Google Scholar]
- 36.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferron M, Wei J, Yoshizawa T, Del FA, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, Karsenty G. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morimoto R, Uehara S, Yatsushiro S, Juge N, Hua Z, Senoh S, Echigo N, Hayashi M, Mizoguchi T, Ninomiya T, Udagawa N, Omote H, Yamamoto A, Edwards RH, Moriyama Y. Secretion of L-glutamate from osteoclasts through transcytosis. EMBO J. 2006;25:4175–4186. doi: 10.1038/sj.emboj.7601317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palokangas H, Mulari M, Vaananen HK. Endocytic pathway from the basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. J Cell Sci. 1997;110(Pt 15):1767–1780. doi: 10.1242/jcs.110.15.1767. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Vaananen HK. Pharmacological sequestration of intracellular cholesterol in late endosomes disrupts ruffled border formation in osteoclasts. J Bone Miner Res. 2006;21:456–465. doi: 10.1359/JBMR.051204. [DOI] [PubMed] [Google Scholar]
- 42.Leblond CP. Synthesis and secretion of collagen by cells of connective tissue, bone, and dentin. Anat Rec. 1989;224:123–138. doi: 10.1002/ar.1092240204. [DOI] [PubMed] [Google Scholar]
- 43.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 44.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, Sekiya H, Tsumagari K, Ochiai K, Yoshinaga K, Saitoh M, Nishimura R, Yoneda T, Kou I, Furuichi T, et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- 46.Sohaskey ML, Jiang Y, Zhao JJ, Mohr A, Roemer F, Harland RM. Osteopotentia regulates osteoblast maturation, bone formation, and skeletal integrity in mice. J Cell Biol. 2010;189:511–25. doi: 10.1083/jcb.201003006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hino S, Kondo S, Yoshinaga K, Saito A, Murakami T, Kanemoto S, Sekiya H, Chihara K, Aikawa Y, Hara H, Kudo T, Sekimoto T, Funamoto T, Chosa E, Imaizumi K. Regulation of ER molecular chaperone prevents bone loss in a murine model for osteoporosis. J Bone Miner Metab. 2010;28:131–138. doi: 10.1007/s00774-009-0117-z. [DOI] [PubMed] [Google Scholar]
- 48.Boyadjiev SA, Justice CM, Eyaid W, McKusick VA, Lachman RS, Chowdry AB, Jabak M, Zwaan J, Wilson AF, Jabs EW. A novel dysmorphic syndrome with open calvarial sutures and sutural cataracts maps to chromosome 14q13-q21. Hum Genet. 2003;113:1–9. doi: 10.1007/s00439-003-0932-6. [DOI] [PubMed] [Google Scholar]
- 49.Boyadjiev SA, Fromme JC, Ben J, Chong SS, Nauta C, Hur DJ, Zhang G, Hamamoto S, Schekman R, Ravazzola M, Orci L, Eyaid W. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38:1192–1197. doi: 10.1038/ng1876. [DOI] [PubMed] [Google Scholar]
- 50.Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13:623–634. doi: 10.1016/j.devcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter AG. Is geroderma osteodysplastica underdiagnosed? J Med Genet. 1988;25:854–857. doi: 10.1136/jmg.25.12.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hennies HC, Kornak U, Zhang H, Egerer J, Zhang X, Seifert W, Kühnisch J, Budde B, Nätebus M, Brancati F, Wilcox WR, Müller D, Kaplan PB, Rajab A, Zampino G, et al. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet. 2008;40:1410–1412. doi: 10.1038/ng.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aarskog D. A familial syndrome of short stature associated with facial dysplasia and genital anomalies. J Pediatr. 1970;77:856–861. doi: 10.1016/s0022-3476(70)80247-5. [DOI] [PubMed] [Google Scholar]
- 54.Scott CI. Chondrodystrophia calcificans congenita (Conradi's disease) with cutaneous changes. Birth Defects Orig Artic Ser. 1971;7:309. [PubMed] [Google Scholar]
- 55.Pasteris NG, Cadle A, Logie LJ, Porteous ME, Schwartz CE, Stevenson RE, Glover TW, Wilroy RS, Gorski JL. Isolation and characterization of the faciogenital dysplasia (Aarskog-Scott syndrome) gene: a putative Rho/Rac guanine nucleotide exchange factor. Cell. 1994;79:669–678. doi: 10.1016/0092-8674(94)90552-5. [DOI] [PubMed] [Google Scholar]
- 56.Egorov MV, Capestrano M, Vorontsova OA, Di PA, Egorova AV, Mariggio S, Ayala MI, Tetè S, Gorski JL, Luini A, Buccione R, Polishchuk RS. Faciogenital dysplasia protein (FGD1) regulates export of cargo proteins from the golgi complex via Cdc42 activation. Mol Biol Cell. 2009;20:2413–2427. doi: 10.1091/mbc.E08-11-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nabavi N, Urukova Y, Cardelli M, Aubin JE, Harrison RE. Lysosome dispersion in osteoblasts accommodates enhanced collagen production during differentiation. J Biol Chem. 2008;283:19678–19690. doi: 10.1074/jbc.M802517200. [DOI] [PubMed] [Google Scholar]
- 58.Zhao H, Ito Y, Chappel J, Andrews NW, Teitelbaum SL, Ross FP. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev Cell. 2008;14:914–925. doi: 10.1016/j.devcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhangu PS, Genever PG, Spencer GJ, Grewal TS, Skerry TM. Evidence for targeted vesicular glutamate exocytosis in osteoblasts. Bone. 2001;29:16–23. doi: 10.1016/s8756-3282(01)00482-3. [DOI] [PubMed] [Google Scholar]
- 60.Prele CM, Horton MA, Caterina P, Stenbeck G. Identification of the molecular mechanisms contributing to polarized trafficking in osteoblasts. Exp Cell Res. 2003;282:24–34. doi: 10.1006/excr.2002.5668. [DOI] [PubMed] [Google Scholar]
- 61.Rohde M, Mayer H. Exocytotic process as a novel model for mineralization by osteoblasts in vitro and in vivo determined by electron microscopic analysis. Calcif Tissue Int. 2007;80:323–336. doi: 10.1007/s00223-007-9013-5. [DOI] [PubMed] [Google Scholar]
- 62.Kariya Y, Honma M, Aoki S, Chiba A, Suzuki H. Vps33a mediates RANKL storage in secretory lysosomes in osteoblastic cells. J Bone Miner Res. 2009;24:1741–1752. doi: 10.1359/jbmr.090409. [DOI] [PubMed] [Google Scholar]
- 63.Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, Seabra MC. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol. 2001;152:795–808. doi: 10.1083/jcb.152.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bahadoran P, Aberdam E, Mantoux F, Busca R, Bille K, Yalman N, de Saint-Basile G, Casaroli-Marano R, Ortonne JP, Ballotti P. Rab27a: A key to melanosome transport in human melanocytes. J Cell Biol. 2001;152:843–850. doi: 10.1083/jcb.152.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haddad EK, Wu X, Hammer JA, III, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J Cell Biol. 2001;152:835–842. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kariya Y, Honma M, Hanamura A, Aoki S, Ninomiya T, Nakamichi Y, Udagawa N, Suzuki H. Rab27a and Rab27b are involved in stimulation-dependent RANKL release from secretory lysosomes in osteoblastic cells. J Bone Miner Res. 2011;26:689–703. doi: 10.1002/jbmr.268. [DOI] [PubMed] [Google Scholar]
- 68.Nickerson DP, Brett CL, Merz AJ. Vps-C complexes: gatekeepers of endolysosomal traffic. Curr Opin Cell Biol. 2009;21:543–551. doi: 10.1016/j.ceb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 71.Salo J, Metsikko K, Palokangas H, Lehenkari P, Vaananen HK. Bone-resorbing osteoclasts reveal a dynamic division of basal plasma membrane into two different domains. J Cell Sci. 1996;109(Pt 2):301–307. doi: 10.1242/jcs.109.2.301. [DOI] [PubMed] [Google Scholar]
- 72.Mulari MT, Nars M, Laitala-Leinonen T, Kaisto T, Metsikko K, Sun Y, Väänänen HK. Recombinant VSV G proteins reveal a novel raft-dependent endocytic pathway in resorbing osteoclasts. Exp Cell Res. 2008;314:1641–1651. doi: 10.1016/j.yexcr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 73.Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature. 2006;440:220–223. doi: 10.1038/nature04535. [DOI] [PubMed] [Google Scholar]
- 74.Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, Futai M. From lysosomes to the plasma membrane: localization of vacuolar-type H+ -ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem. 2003;278:22023–22030. doi: 10.1074/jbc.M302436200. [DOI] [PubMed] [Google Scholar]
- 75.Czupalla C, Mansukoski H, Riedl T, Thiel D, Krause E, Hoflack B. Proteomic analysis of lysosomal acid hydrolases secreted by osteoclasts: implications for lytic enzyme transport and bone metabolism. Mol Cell Proteomics. 2006;5:134–143. doi: 10.1074/mcp.M500291-MCP200. [DOI] [PubMed] [Google Scholar]
- 76.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 77.Baron R, Neff L, Brown W, Courtoy PJ, Louvard D, Farquhar MG. Polarized secretion of lysosomal enzymes: co-distribution of cation-independent mannose-6-phosphate receptors and lysosomal enzymes along the osteoclast exocytic pathway. J Cell Biol. 1988;106:1863–1872. doi: 10.1083/jcb.106.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Meel E, Boonen M, Zhao H, Oorschot V, Ross FP, Kornfeld S, Klumperman J. Disruption of the Man-6-P Targeting Pathway in Mice Impairs Osteoclast Secretory Lysosome Biogenesis. Traffic. 2011;12:912–924. doi: 10.1111/j.1600-0854.2011.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao H, Laitala-Leinonen T, Parikka V, Vaananen HK. Downregulation of small GTPase Rab7 impairs osteoclast polarization and bone resorption. J Biol Chem. 2001;276:39295–39302. doi: 10.1074/jbc.M010999200. [DOI] [PubMed] [Google Scholar]
- 81.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van DB. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Croke M, Ross FP, Korhonen M, Williams DA, Zou W, Teitelbaum SL. Rac deletion in osteoclasts causes severe osteopetrosis. J Cell Sci. 2011;124:3811–3821. doi: 10.1242/jcs.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Y, Buki KG, Ettala O, Vaaraniemi JP, Vaananen HK. Possible role of direct Rac1-Rab7 interaction in ruffled border formation of osteoclasts. J Biol Chem. 2005;280:32356–32361. doi: 10.1074/jbc.M414213200. [DOI] [PubMed] [Google Scholar]
- 84.Sakai E, Miyamoto H, Okamoto K, Kato Y, Yamamoto K, Sakai H. Characterization of phagosomal subpopulations along endocytic routes in osteoclasts and macrophages. J Biochem. 2001;130:823–831. doi: 10.1093/oxfordjournals.jbchem.a003054. [DOI] [PubMed] [Google Scholar]
- 85.Van wesenbeeck L, Odgren PR, Coxon FP, Frattini A, Moens P, Perdu B, MacKay CA, Van Hul E, Timmermans JP, Vanhoenacker F, Jacobs R, Peruzzi B, Teti A, Helfrich MH, Rogers MJ, Villa A. Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J Clin Invest. 2007;117:919–930. doi: 10.1172/JCI30328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reinholt FP, Hultenby K, Heinegard D, Marks SC, Jr., Norgard M, Anderson G. Extensive clear zone and defective ruffled border formation in osteoclasts of osteopetrotic (ia/ia) rats: implications for secretory function. Exp Cell Res. 1999;251:477–491. doi: 10.1006/excr.1999.4585. [DOI] [PubMed] [Google Scholar]
- 87.Tabata K, Matsunaga K, Sakane A, Sasaki T, Noda T, Yoshimori T. Rubicon and PLEKHM1 Negatively Regulate the Endocytic/Autophagic Pathway via a Novel Rab7-binding Domain. Mol Biol Cell. 2010;21:4162–4172. doi: 10.1091/mbc.E10-06-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye S, Fowler TW, Pavlos NJ, Ng PY, Liang K, Feng Y, Zheng M, Kurten R, Manolagas SC, Zhao H. LIS1 regulates osteoclast formation and function through its interactions with dynein/dynactin and Plekhm1. PLoS One. 2011;6:e27285. doi: 10.1371/journal.pone.0027285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeSelm CJ, Miller BC, Zou W, Beatty WL, van Meel E, Klumperman J, Tooze SA, teitelbaum SL, Virgin HW. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell. 2011;21:966–974. doi: 10.1016/j.devcel.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pavlos NJ, Xu J, Riedel D, Yeoh JS, Teitelbaum SL, Papadimitriou JM, Jahn R, Ross FP, Zheng MH. Rab3D regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol Cell Biol. 2005;25:5253–5269. doi: 10.1128/MCB.25.12.5253-5269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Z, Reavey-Cantwell J, Young RA, Jegier P, Wolf BA. Synaptotagmin III/VII isoforms mediate Ca2+-induced insulin secretion in pancreatic islet beta -cells. J Biol Chem. 2000;275:36079–36085. doi: 10.1074/jbc.M004284200. [DOI] [PubMed] [Google Scholar]
- 92.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsuboi T, Fukuda M. Synaptotagmin VII modulates the kinetics of dense-core vesicle exocytosis in PC12 cells. Genes Cells. 2007;12:511–519. doi: 10.1111/j.1365-2443.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 94.Wang P, Chicka MC, Bhalla A, Richards DA, Chapman ER. Synaptotagmin VII is targeted to secretory organelles in PC12 cells, where it functions as a high-affinity calcium sensor. Mol Cell Biol. 2005;25:8693–8702. doi: 10.1128/MCB.25.19.8693-8702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. Int Rev Cytol. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- 96.Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem. 2004;279:20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]