Abstract
BACKGROUND
The dopaminergic and endothelin systems, by regulating sodium transport in the renal proximal tubule (RPT), participate in the control of blood pressure. The D3 and ETB receptors are expressed in RPTs, and D3 receptor function in RPTs is impaired in spontaneously hypertensive rats (SHRs). Therefore, we tested the hypothesis that D3 receptors can regulate ETB receptors, and that D3 receptor regulation of ETB receptors in RPTs is impaired in SHRs.
METHODS
ETB receptor expression in RPT cells was measured by immunoblotting and reverse transcriptase–PCR and ETB receptor function by measuring Na+–K+ ATPase activity. D3/ETB receptor interaction was studied by co-immunoprecipitation.
RESULTS
In Wistar-Kyoto (WKY) RPT cells, the D3 receptor agonist, PD128907, increased ETB receptor protein expression, effects that were blocked by removal of calcium in the culture medium. The stimulatory effect of D3 on ETB receptor mRNa and protein expression was also blocked by nicardipine. In contrast, in SHR RPT cells, PD128907 decreased ETB receptor expression. Basal D3/ETB receptor co-immunoprecipitation was three times greater in WKY than in SHRs. The absolute amount of D3/ETB receptor co-immunoprecipitation induced by a D3 receptor agonist was also greater in WKY than in SHRs. Stimulation of ETB receptors decreased Na+–K+ ATPase activity in WKY but not in SHR cells. Pretreatment with PD128907 augmented the inhibitory effect of BQ3020 on Na+–K+ ATPase activity in WKY but not in SHR cells.
CONCLUSIONS
D3 receptors regulate ETB receptors by physical receptor interaction and govern receptor expression and function. D3 receptor regulation of ETB receptors is aberrant in RPT cells from SHRs.
Endothelin, originally characterized in vascular endothelial cells, is now known to be secreted by renal tubules where it can regulate sodium transport in an autocrine and/or paracrine manner.1–4 Three endothelin isoforms (ET-1, ET-2, and ET-3) interact with two receptors, ETA and ETB. ETA receptors may contribute to the pathogenesis of hypertension by stimulating vasopressor centers in the brain, and increasing the secretion of aldosterone and the release of catecholamines, renal sodium transport, growth factors, reactive oxygen species, and vascular smooth muscle contractility.2–5 However, endothelins can also relax vascular smooth muscles by releasing endothelium-derived vasodilators.3 In addition, ETB receptors can lower blood pressure by decreasing ET-1 levels, and promoting renal loss of sodium and water.1,4 Indeed, in uninephrectomized rats given deoxycorticosterone acetate and a high-salt diet, spontaneously hypertensive rats (SHRs) and humans with essential hypertension, ETB receptor activation may be a counter regulatory mechanism to the increase in blood pressure.1,4,6,7 Naturally occurring or induced deletion of the ETB receptor gene and chronic pharmacological blockade of ETB receptors in rats result in salt-sensitive hypertension.1
The actions of dopamine on cardiovascular centers in the brain, renal hemodynamics, intestinal and renal epithelial transport, production of reactive oxygen species, and secretion of hormonal/humoral agents, such as aldosterone, catecholamines, endothelin, prolactin, pro-opiomelanocortin, renin, and vasopressin, place dopamine in a central homeostatic position for regulating extracellular fluid volume and blood pressure.8,9 There are two D1-like receptors, D1 and D5, and three D2-like receptors, D2, D3, and D4, expressed in mammalian kidneys. Renal dopamine receptors,8,9 like ETB receptors,7,10–14 inhibit sodium transport. Specifically, dopamine, via D1 and D3 receptors, decreases renal sodium transport in various segments of the nephron, including the renal proximal tubule (RPT).8,9 Disruption of either the D1 or D3 dopamine receptor gene in mice produces hypertension.9,15 The hypertension in the D3 receptor null (D3–/–) mouse is associated with a decreased ability to excrete a sodium load.15 In a previous study, we found that the natriuretic consequence of D3 receptor stimulation is attenuated by an ETB receptor antagonist in Wistar-Kyoto (WKY) rats.16 Because D3 receptors in the kidney are predominantly expressed in RPTs where ETB receptors are also expressed,17–24 we hypothesize that the ETB receptor is regulated by the D3 receptor. Renal D3 receptor function is impaired in SHRs;16,18,19 therefore, we also hypothesize that D3 receptor regulation of the ETB receptor may also be aberrant in SHRs. In order to test the above hypotheses, we studied D3 and ETB receptor interaction in immortalized RPT cells. These RPT cells behave similarly to nonimmortalized RPT cells, at least with regard to dopamine receptors and responses to G protein stimulation.25–27
METHODS
Cell culture
Immortalized RPT cells (passages 25–40) from the S1 segment of nephrons from 4- to 8-week-old WKY and SHRs were cultured at 37 °C in 95% air/5% CO2 atmosphere in Dulbecco's modified Eagle's medium/F-12 culture media, as previously described.18 The cells (80% confluent) were lysed in an ice-cold lysis buffer (phosphate-buffered saline with 1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mmol/l EDTA, 1 mmol/l EGTA, 1 mmol/l phenylmethylsulphonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ ml leupeptin), sonicated, kept on ice for 1 h, and centrifuged at 16,000 g for 30 min. The supernatants were stored at –70 °C until use for immunoblotting and/or immunoprecipitation.
Immunoblotting
Specific polyclonal D3 and ETB receptors were used.17–19,22 Rat RPT cells were treated with vehicle (dH2O), a D3 receptor agonist (PD128907; Sigma, St Louis, MO),28 or a D3 receptor antagonist (U99194A; Sigma)29 at the indicated concentrations and durations of incubation. D3 and ETB receptor antibodies were specific because no specific bands were observed when the antibodies were preadsorbed with their respective immunizing peptides (1:10 wt/wt) for 12 h at 4 °C.19,30
The densities of the bands were semiquantified by densitometry using Quantiscan (Ferguson, MO). When appropriate, the densities of the receptor bands were corrected by the densities of the α-actin bands. All immunoblot bands in one group (receptor of interest or actin) were given a value of 100%. The density of each sample was calculated as a fraction of 100%. The ordinates indicate the ratio of the density of the protein of interest as a fraction of 100% and the density of actin as a fraction of 100%.
Immunoprecipitation
RPT cells were incubated with vehicle or PD128907 (10–7 mol/l) for 24 h, as described earlier and immunoprecipitation was performed as reported, except that anti-D3 and anti-ETB receptor antibodies were used.17–19,22 In order to determine the specificity of the bands, normal rabbit IgG (negative control) and D3 receptor antibody (positive control) were used as the immunoprecipitants instead of the ETB receptor antibody. The densities of the bands were semiquantified by densitometry.17–19,25–27,31
Determination of the second messenger(s) involved in the regulation of D3 receptor on ETB receptor expression in WKY cells
To determine the second messenger(s) involved into the D3 receptor–mediated regulation of ETB receptor expression in WKY cells, several inhibitors or agonists were used: PKC inhibitor (19–31, 10–6 mol/l), PKA inhibitor (14–22, 10–6 mol/l), PKC activator (phorbol 12-myristate 13-acetate, 10–7 mol/l), PKA activator (Sp-cAMP-S, 10–7 mol/l), calcium channel blocker (nicardipine, 10–6 mol/l), and calcium channel agonist (BAY-K8644, 10–6 mol/l). PKC inhibitor 19–31, phorbol 12-myristate 13-acetate, Sp-cAMP-S, nicardipine, and BAY-K8644 were purchased from Sigma, PKA inhibitor 14–22 was purchased from Calbiochem (Darmstadt, Germany). The specificities of PKC inhibitor 19–31 and PKA inhibitor 14–22 were reported in our previous studies and those of others.32,33
Na+–K+ ATPase activity assay
Na+–K+ ATPase activity was measured as the rate of inorganic phosphate released in the presence or absence of ouabain.34–36 To prepare membranes for Na+–K+ ATPase activity assay, RPT cells cultured in 21-cm2 plastic culture dishes were washed twice with 5 ml chilled phosphate-free buffer, and centrifuged at 3,000 g for 10 min. The cells were then placed on ice and lysed in 2 ml lysis buffer (1 mmol/l NaHCO3, 2 mmol/l CaCl2, and 5 mmol/l MgCl2). The cell lysates were centrifuged at 3,000 g for 2 min to remove intact cells, debris, and nuclei. The resulting supernatant was suspended in an equal volume of 1 mol/l NaI, and the mixture was centrifuged at 48,000 g for 25 min. The pellet (membrane fraction) was washed two times and suspended in 10 mmol/l Tris and 1 mmol/l EDTA (pH 7.4). Protein concentrations were determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA) and adjusted to 1 mg/ml. The membranes were stored at –70 °C until use.
To measure Na+–K+ ATPase activity, 100-μl aliquots of membrane fraction were added to an 800 μl reaction mixture (75 mmol/l NaCl, 5 mmol/l KCl, 5 mmol/l MgCl2, 6 mmol/l sodium azide, 1 mmol/l Na4EGTA, 37.5 mmol/l imidazole, 75 mmol/l Tris–HCl, and 30 mmol/l histidine; pH 7.4) with or without 1 mmol/l ouabain (final volume = 1 ml) and preincubated for 5 min in a water bath at 37 °C. Reactions were initiated by adding Tris–ATP (4 mmol/l) and terminated after 15 min of incubation at 37 °C by adding 50 μl of 50% trichloroacetate. For determination of ouabain-insensitive ATPase activity, NaCl and KCl were omitted from the reaction mixtures containing ouabain. To quantify the amount of phosphate produced, 1 ml of coloring reagent (10% ammonium molybdate in 10 N sulfuric acid) was mixed thoroughly with the assay mix and centrifuged at 3,000 g for 10 min. The formation of phosphomolybdate was determined spectrophotometrically at 740 nm, against a standard curve prepared from K2HPO4. Na+–K+ ATPase activity was estimated as the difference between total and ouabain-insensitive ATPase activity and expressed as nmol phosphate released per mg protein per min.
Reverse transcriptase–PCR of ETB receptors
A total of 2–3 μg of total RNA extracted from WKY cells was used to synthesize cDNA which served as template for the amplification of ETB and β-actin (as housekeeping gene).37 For β-actin, the forward primer was 5′-GTGGGTATGGGTCAGAAGGA-3′ and the reverse primer was 5′-AGCGCGTAACCCTCATAGAT-3′ (GenBank accession no. NM031144). The amplification was performed with the following conditions: denaturation at 94 °C for 30 s, annealing for 30 s at 54 °C, and extension for 45 s at 72 °C for 35 cycles. For the ETB receptor, the forward primer was 5′-TTACAAGACAGCCAAAGACT-3′ and the reverse primer was 5′-CACGATGAGGACAATGAGAT-3′ (GenBank accession no. X57764). The amplification was performed with the following conditions: denaturation at 94 °C for 30 s, annealing for 30 s at 69 °C, and extension for 45 s at 72 °C for 35 cycles. The ETB receptor mRNA expression was normalized for β-actin mRNA.
Statistical analysis
The data are expressed as mean ± s.e.m. Comparison within groups was made by repeated measures analysis of variance with Duncan's test (or Student's t-test when only two groups were compared); comparison among groups was made by factorial analysis of variance with Duncan's test. A value of P < 0.05 was considered significant.
RESULTS
D3 receptors increase ETB receptor expression in WKY RPT cells, but decrease it in SHR cells
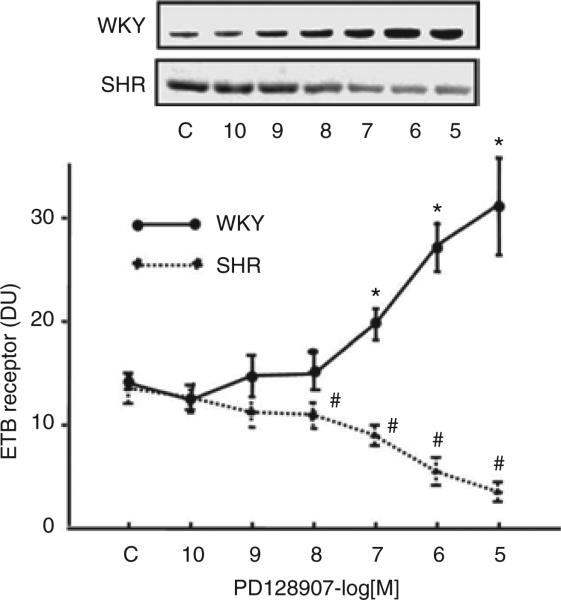
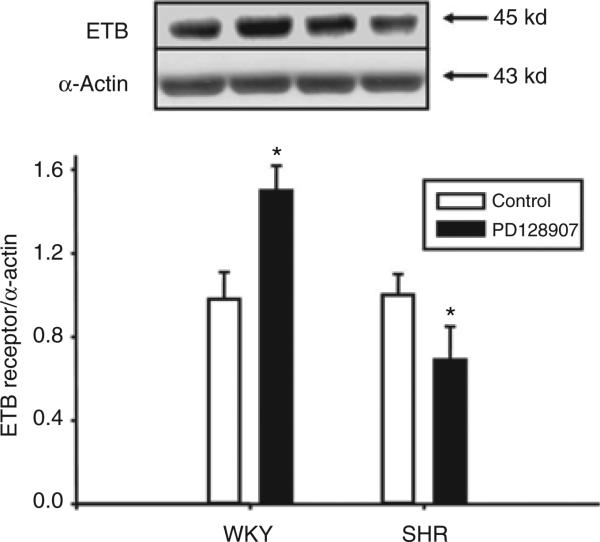
A D3 receptor agonist, PD128907, increased ETB receptor protein expression in a concentration- and time-dependent manner in WKY RPT cells. The stimulatory effect was evident at 10–7 mol/l (Figure 1), noted as early as 8 h, and maintained for at least 24 h (data not shown). In contrast, in SHR RPT cells, PD128907 (10–7 mol/l/24 h) decreased ETB receptor expression (WKY: control = 1.0 ± 0.1, PD128907 (10–7 mol/l) = 1.5 ± 0.1; SHR: control = 1.0 ± 0.1, PD128907 (10–7 mol/l) = 0.7 ± 0.2 density units (DU); n = 9/group) (Figure 2), that was also concentration dependent (Figure 1).
Figure 1.
Concentration response of ETB receptor protein expression to the D3 receptor agonist, PD128907 in renal proximal tubule cells from SHR and WKY rats. Immunoreactive ETB receptor band was quantified after 24-h incubation with the indicated concentrations of PD128907. Results are expressed as density units (DU) as a fraction of 100% and the seven samples from each rat strain set at 100% (WKY: n = 5, *P < 0.05 vs. control (C); SHR: n = 10, #P < 0.05 vs. C, analysis of variance, Duncan's test). SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto.
Figure 2.
Differential effect of a D3 receptor agonist, PD128907 (10–7 mol/l/24 h), on ETB receptor protein in WKY and SHR renal proximal tubule cells. The cells were incubated at the indicated time and concentration. Results are expressed as the ratio of ETB receptor to α-actin densities (n = 11, *P < 0.05 vs. control, analysis of variance, Duncan's test). SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto.
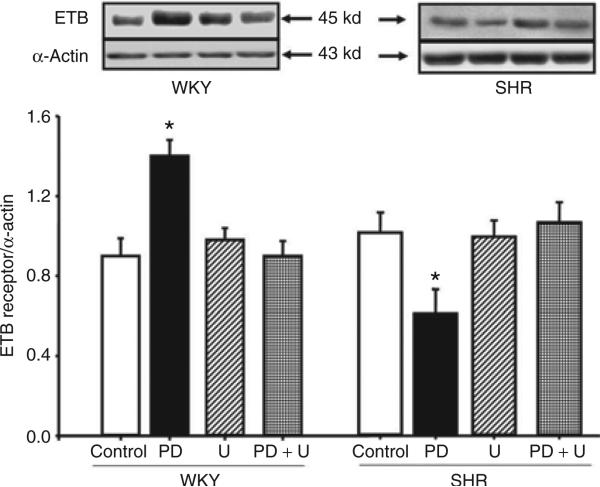
The specificity of PD128907 as a D3 receptor agonist was determined using the D3 receptor antagonist, U99194A, in WKY RPT cells. Consistent with the results shown in Figure 1, PD128907 (10–7 mol/l/24 h) increased ETB receptor expression (control = 0.9 ± 0.09, PD128907 = 1.4 ± 0.08 DU; n = 6, P < 0.05). U99194A (10–7 mol/l), by itself, had no effect on ETB receptor expression (0.98 ± 0.06 DU), but reversed the stimulatory effect of PD128907 on ETB receptor expression (0.9 ± 0.08 DU) (Figure 3) in WKY RPT cells. The inhibitory effect of D3 receptor on ETB receptor expression in SHR cells was also blocked by D3 receptor antagonist, U99194A (Figure 3).
Figure 3.
Effect of a D3 receptor agonist (PD128907) and a D3 receptor antagonist (U99194A) on ETB receptor protein in renal proximal tubule cells from SHR (n = 5) and WKY (n = 6) rats. The cells were incubated with the indicated reagents (PD128907, 10–7 mol/l; U99194A, 10–7 mol/l) for 24 h. Results are expressed as the ratio of ETB receptor to α-actin densities (*P < 0.05 vs. others, analysis of variance, Duncan's test). PD, PD128907; U, U99194A. SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto.
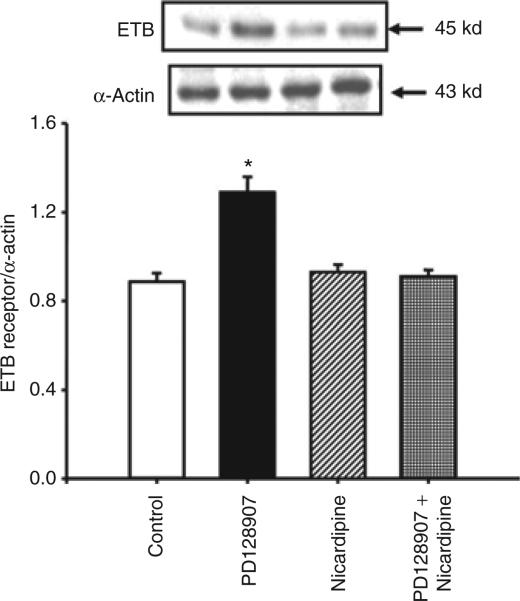
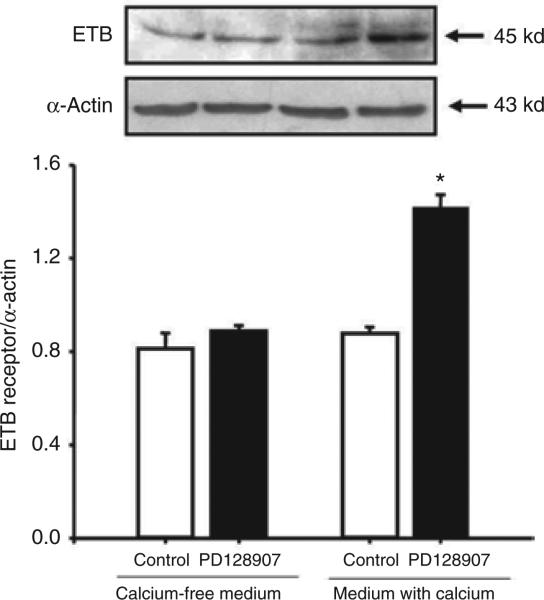
A calcium channel blocker (nicardipine, 10–6 mol/l), by itself, had no effect on ETB receptor expression, but blocked the stimulatory effect of PD128907 on ETB receptor expression in WKY cells (Figure 4). To determine, further, the importance of extracellular calcium entry in the D3 receptor–mediated upregulation of ETB receptor expression, we studied the effect of PD128907 on RPT cells grown in culture medium with or without calcium. The ability of PD128907 to upregulate the expression of ETB receptor protein was lost when the WKY RPT cells were maintained in calcium-free medium, indicating the requirement for calcium entry in this action (calcium-free medium: control = 0.87 ± 0.09, PD128907 = 0.9 ± 0.02 DU; regular medium with calcium: control = 0.9 ± 0.02, PD128907 = 1.4 ± 0.05 DU; n = 5, P < 0.05) (Figure 5).
Figure 4.
Effect of a D3 receptor agonist (PD128907) and a calcium channel blocker (nicardipine) on ETB receptor protein in Wistar-Kyoto renal proximal tubule cells. The cells were incubated with the indicated reagents (PD128907, 10–7 mol/l; nicardipine, 10–6 mol/l) for 24 h. Results are expressed as the ratio of ETB receptor to α-actin densities (n = 5/group, *P < 0.05 vs. others, analysis of variance, Duncan's test).
Figure 5.
Effect of a D3 receptor agonist, PD128907, on ETB receptor expression in Wistar-Kyoto cells grown in medium with or without calcium. The cells were incubated with PD128907 (10–7 mol/l) for 24 h. Results are expressed as the ratio of ETB receptor to α-actin densities (n = 5, *P < 0.05 vs. others, analysis of variance, Duncan's test).
We also studied the effect of other cell signaling reagents: PKC inhibitor (19–31, 10–6 mol/l); PKA inhibitor (14–22, 10–6 mol/l); PKC activator (phorbol 12-myristate 13-acetate, 10–7 mol/l), PKA activator (Sp-cAMP-S, 10–7 mol/l); and calcium channel agonist (BAY-K8644, 10–6 mol/l). None of those reagents could block the stimulatory effect of D3 receptor on ETB receptor expression (data not shown).
In additional studies, we measured ETB receptor mRNA expression by reverse transcriptase–PCR and found that the activation of D3 receptor with PD128907 (10–7 mol/l) for 24 h increased ETB receptor mRNA expression in WKY cells. However, in the presence of the calcium channel blocker, nicardipine (10–6 mol/l/24 h), the upregulation of D3 receptor on ETB receptor mRNA expression was lost (control = 0.82 ± 0.03, PD128907 = 1.0 ± 0.03 DU; nicardipine= 0.79 ± 0.04, nicardipine + PD128907 = 0.83 ± 0.02, n = 5, P < 0.05).
D3 receptor co-immunoprecipitates with the ETB receptor in rat RPT cells
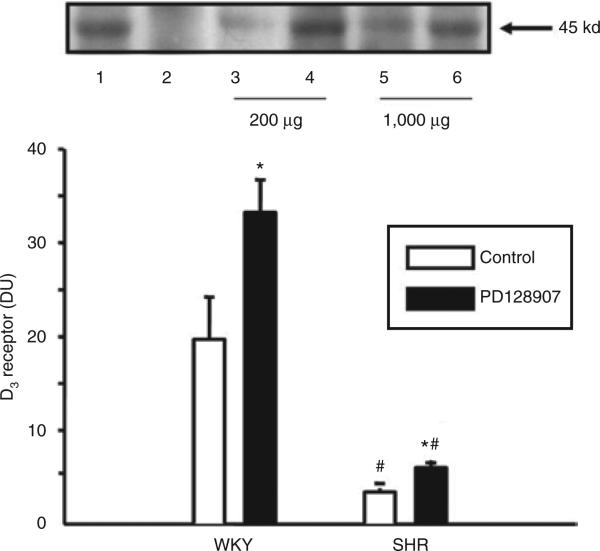
We next determined whether there is a physical interaction between the D3 and the ETB receptor. ETB receptors were first immunoprecipitated with anti-ETB receptor antibodies and then probed with anti-D3 receptor antibodies. As shown in Figure 6, the density of the 45 kd band, representing the co-immunoprecipitated D3 receptors with the ETB receptors, was increased by a 24-h treatment with PD28907 (10–7 mol/l) in WKY and SHR RPT cells (WKY: control = 20 ± 5, PD128907 = 33 ± 4 DU; SHRs: control = 3.4 ± 0.4, PD128907 = 6.0 ± 0.8 DU; n = 8, P < 0.05). The absolute increase in the amount of D3/ETB co-immunoprecipitation with PD128907 (10–7 mol/l/24 h) treatment was greater in RPT cells from WKY than from SHRs (WKY: 14 ± 6 DU vs. SHR: 2.6 ± 0.8 DU, n = 8, P < 0.05), although the percent increases were similar. However, basal co-immunoprecipitation was greater in RPT cells from WKY than those from SHRs.
Figure 6.
Effect of a D3 receptor agonist, PD128907, on the co-immunoprecipitation of D3 and ETB receptors in WKY and SHR RPT cells. The cells were incubated with PD128907 (10–7 mol/l) for 24 h. Thereafter, the samples were immunoprecipitated with an anti-ETB receptor antibody and immunoblotted with an anti-D3 antibody (*P < 0.05 vs. control, #P < 0.05 vs. WKY n = 8/group, analysis of variance, Duncan's test). One immunoblot (45 kd) is depicted in the upper panel: (lane 1, positive control; lane 2, negative control; lane 3, vehicle-treated RPT cells from WKY rats; lane 4, PD128907-treated RPT cells from WKY rats; lane 5, vehicle-treated RPT cells from SHRs; lane 6, PD128907-treated RPT cells from SHRs). For a positive control, anti-D3 antibodies (1 μg/ml) were used as the immunoprecipitant; for a negative control, normal rabbit IgG (1 μg/ml) was used as the immunoprecipitant, instead of the anti-ETB antibodies, and immunoblotted with anti-D3 antibodies as above. The amount of D3 receptor antibody needed to immunoprecipitate ETB receptors in SHR RPT cells (1,000 μg, lanes 5 and 6) is five times that used in WKY RPT cells (200 μg, lanes 3 and 4). RPT, renal proximal tubule; SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto.
Pretreatment with PD128907 increases the inhibitory effect of ETB receptor on Na+–K+ ATPase activity in WKY cells but not in SHR cells
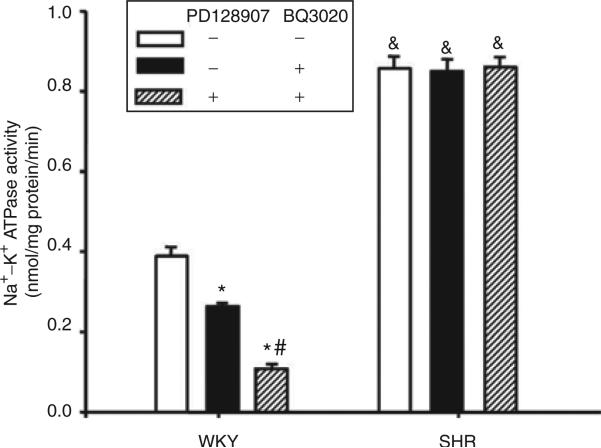
To investigate the physiological significance of the D3/ETB receptor interaction, the effects of D3 or/and ETB receptor stimulation on Na+–K+ ATPase activity were determined in WKY and SHR cells. Stimulation of ETB receptors by BQ3020 (10–8 mol/l/15 min; Sigma) decreased Na+–K+ ATPase activities in WKY cells, which could be blocked by the ETB receptor antagonist, BQ788 (10–8 mol/l/15 min; Sigma) (control = 0.39 ± 0.02, BQ3020 = 0.26 ± 0.03; BQ788 = 0.40 ± 0.06, BQ3020 + BQ788 = 0.37 ± 0.04 nmol/mg protein/min; n = 5, P < 0.05). In contrast, the inhibitory effect of ETB receptor on Na+–K+ ATPase activity was lost in SHR cells (Figure 7). Pretreatment with PD128907 (10–7 mol/l) for 24 h augmented the inhibitory effect of BQ3020 (10–8 mol/l/15 min) on Na+–K+ ATPase activity in WKY but not in SHR cells. The basal level of Na+–K+ ATPase activity was higher in SHR than in WKY cells (Figure 7).
Figure 7.
Effect of pretreatment with a D3 receptor agonist, PD128907, on the inhibitory effect of ETB receptor on Na+–K+ ATPase activity in WKY and SHR RPT cells. The cells were pretreated with PD128907 (10–7 mol/l) or vehicle (dH2O) for 24 h. After washing for 15 min, the cells were treated with the ETB receptor agonist, BQ3020 (10–8 mol/l), for 15 min. Results are expressed as nmol phosphate released per mg protein per min (*P < 0.05 vs. control, #P < 0.05 vs. BQ3020, &P < 0.05 vs. WKY, n = 6/group, analysis of variance, Duncan's test). RPT, renal proximal tubule; SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto.
DISCUSSION
ETB receptors, which are expressed in RPTs, medullary thick ascending limbs of Henle, and collecting ducts of the kidney have been shown to affect sodium transport in vivo and in vitro.20–22,38,39 An inhibitory effect has been found in collecting ducts and medullary thick ascending limbs of Henle.3,4,12,13 However, no effect, inhibitory, and stimulatory effects have been reported in the proximal tubule.10,13,14,39 Short-term stimulation of ETB receptors in opossum kidney cells, a proximal tubular cell line, activates the sodium hydrogen exchanger, NHE3.39 In contrast, a 6-h exposure of these opossum kidney cells to endothelin-1 inhibits NHE3 expression and activity via ETB receptors.14 It is of interest that the ability of the D3 receptor agonist to increase ETB expression also occurs within the same time frame, 8 h, as the ability of ETB to inhibit NHE3 expression and activity. In this study, we showed that activation of ETB receptors in the short term (15 min) could inhibit Na+–K+ ATPase activity in RPT cells from WKY rats.
The natriuretic effect of dopamine is exerted mainly via D1-like receptors.8,9 The effect of D2-like receptors, independent of Dl-like receptors, on sodium excretion has ranged from antinatriuresis, no effect, to natriuresis.8,9 It is possible that these discrepant effects are related to the use of drugs that have poor selectivity to the D2-like receptor subtypes.28,29,40,41 7-OH-DPAT and PD128907, which are D3 receptor agonists with preferential selectivity for D3 over D2 and D4 receptors,28,29 decrease sodium transport. The acute intravenous administration of 7-OH-DPAT in Dahl salt–resistant rats increases sodium and water excretion without affecting blood pressure.42 The selective intrarenal infusion of PD128907 in WKY rats also induces a natriuresis that is attenuated by a D3 receptor antagonist, GR103691.16 That D3 receptors can mediate natriuresis is supported by the decreased ability of D3 receptor deficient mice to excrete an acute saline load.15
The D3 receptor may also interact with other G protein–coupled receptors to increase sodium excretion. We have reported synergistic effects between renal D1 and D3 receptors,43 and antagonistic effects between D3 and AT1 receptors on their expressions18,31 and blood pressure regulation.15 Our previous study also showed that D3 and ETB receptors colocalize in RPTs of WKY rats similar to the colocalization observed in immortalized RPT cells in this study. The natriuretic effect of the D3 receptor agonist, PD128907 in WKY rats, is attenuated by an ETB receptor antagonist (BQ788).16 It is possible that the ability of the D3 receptor to affect the cellular trafficking of the ETB receptor participates in the acute interaction between D3 and ETB receptors to affect sodium transport acutely.
It is likely that D3 receptors can regulate ETB receptor expression in the long term. To determine the mechanisms by which the D3 receptor regulates ETB receptor, we studied the influence of a D3 receptor agonist (PD128907) on ETB receptor expression in RPT cells. We found that stimulation of the D3 receptor increases ETB receptor protein in WKY RPT cells. The D3 receptor regulation of ETB receptor expression cannot be ascribed to increased endothelin synthesis, because stimulation of D3 receptors with PD128907 for 24 or 48 h has no effect on endothelin production in WKY RPT cells (data not shown). However, pretreatment of WKY cells with a D3 receptor agonist for 24 h augments the ETB receptor–mediated inhibitory effect on Na+–K+ ATPase activity in WKY cells. Therefore, it is possible that the long-term natriuretic effect of D3 receptors may also be, in part, mediated by ETB receptors.
The D3 receptor has been shown to increase calcium flux in isolated nerve terminals and human embryonic kidney 293 cells stably expressing human D3 receptors and Gα(qo5).44,45 It has also been reported that activation of the L-type calcium channel stimulates protein synthesis in peripheral blood mononuclear cells.46 We find in the current study that the stimulatory effect of D3 receptor on ETB receptor expression is blocked by a calcium channel blocker and the removal of calcium in the cell culture medium. Therefore, the D3 receptor stimulates ETB receptor expression by increasing intracellular calcium concentrations by activation of calcium channels. Furthermore, the regulation may occur at the transcriptional/post-transcriptional level. The detailed mechanisms remain to be elucidated in the future.
The interaction between D3 and ETB receptors is different between WKY and SHRs. D3 receptor stimulation does not elicit a natriuresis in SHRs,16 and pretreatment of SHR cells with a D3 receptor agonist for 24 h does not augment the ETB receptor–mediated inhibitory effect on Na+–K+ ATPase that is observed in WKY cells. In contrast to the stimulatory effect of a D3 receptor agonist on ETB expression in WKY rats, the opposite occurs in SHRs. The aberrant interaction between D3 and ETB receptor might be involved into the pathogenesis of hypertension.
We also find that D3 and ETB receptors can physically interact, as determined by their co-immunoprecipitation. D3 receptor stimulation results in an increase in the interaction co-immunoprecipitation of D3 and ETB receptors in WKY RPT cells. The increase in D3/ETB receptor co-immunoprecipitation in RPT cells following D3 receptor agonist stimulation could have been caused by the increase in the expression of either the ETB or D3 receptors. We have reported that D3 receptor stimulation increases its own receptor expression in RPT cells from WKY rats.31 It is also possible that the D3 receptor agonist could have increased the physical interaction between the D3 and ETB receptors. However, further studies are needed to determine whether the increased interaction between these two receptors in WKY rats is a direct or an indirect mechanism, possibly by the alteration of an adapter gene or adapter proteins.
In the basal state, there is less D3/ETB receptor co-immunoprecipitation in SHR RPT cells compared to WKY RPT cells. This could be due to the decreased D3 receptor expression in SHR cells relative to WKY cells, but could not be caused by differences in ETB receptor expression, because the expression of renal ETB receptor is the same in WKY and SHRs. Moreover, the percent increase in D3/ETB co-immunoprecipitation with the D3 receptor agonist is similar in the two rat strains. The mechanism for the decreased basal D3/ETB co-immunoprecipitation in SHR cells remains to be determined.
In summary, we have demonstrated that D3 receptors regulate the expression and function of ETB receptors in immortalized rat RPT cells. Furthermore, D3 and ETB receptors co-immunoprecipitate in RPT cells and D3 receptor agonist stimulation enhances the interaction between these two G protein–coupled receptors. In RPT cells from SHRs, this interaction between D3 and ETB receptors is aberrant, which may participate in the increased renal sodium transport in spontaneous hypertension.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health, HL23081, DK39308, HL68686, HL62211, HL41618, HL074940, HL092196, the National Natural Science Foundation of China 30470728, 30672199, 30570764, 30772281, and the National Basic Research Program of China (973 Program, 2008CB517308).
Footnotes
The first two authors contributed equally to this work.
Disclosure: The authors declared no conflict of interest.
References
- 1.Ohuchi T, Yanagisawa M, Gariepy CE. Renal tubular effects of endothelin-B receptor signaling: its role in cardiovascular homeostasis and extracellular volume regulation. Curr Opin Nephrol Hypertens. 2000;9:435–439. doi: 10.1097/00041552-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Todd-Turla K, Wang WH, Cao X, Smart A, Brosius FC, Killen PD, Keiser JA, Briggs JP, Schnermann J. Endothelin-1 mRNA in glomerular and epithelial cells of kidney. Am J Physiol. 1993;265:F542–F550. doi: 10.1152/ajprenal.1993.265.4.F542. [DOI] [PubMed] [Google Scholar]
- 3.Pollock DM. Endothelin, angiotensin, and oxidative stress in hypertension. Hypertension. 2005;45:477–480. doi: 10.1161/01.HYP.0000158262.11935.d0. [DOI] [PubMed] [Google Scholar]
- 4.Dai X, Galligan JJ, Watts SW, Fink GD, Kreulen DL. Increased O2*-production and upregulation of ETB receptors by sympathetic neurons in DOCA-salt hypertensive rats. Hypertension. 2004;43:1048–1054. doi: 10.1161/01.HYP.0000126068.27125.42. [DOI] [PubMed] [Google Scholar]
- 5.Lange DL, Haywood JR, Hinojosa-Laborde C. Endothelin enhances and inhibits adrenal catecholamine release in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 2000;35:385–390. doi: 10.1161/01.hyp.35.1.385. [DOI] [PubMed] [Google Scholar]
- 6.Miki S, Takeda K, Kiyama M, Hatta T, Morimoto S, Kawa T, Itoh H, Nakata T, Sasaki S, Nakagawa M. Augmented response of endothelin-A and endothelin-B receptor stimulation in coronary arteries of hypertensive hearts. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S94–S98. doi: 10.1097/00005344-199800001-00029. [DOI] [PubMed] [Google Scholar]
- 7.Pollock DM, Allcock GH, Krishnan A, Dayton BD, Pollock JS. Upregulation of endothelin B receptors in kidneys of DOCA-salt hypertensive rats. Am J Physiol Renal Physiol. 2000;278:F279–F286. doi: 10.1152/ajprenal.2000.278.2.F279. [DOI] [PubMed] [Google Scholar]
- 8.Hussain T, Lokhandwala MF. Renal dopamine receptor function in hypertension. Hypertension. 1998;32:187–197. doi: 10.1161/01.hyp.32.2.187. [DOI] [PubMed] [Google Scholar]
- 9.Zeng C, Felder RA, Jose PA. A new approach for treatment of hypertension: modifying D1 dopamine receptor function. Cardiovasc Hematol Agents Med Chem. 2006;4:369–377. doi: 10.2174/187152506778520727. [DOI] [PubMed] [Google Scholar]
- 10.Garcia NH, Garvin JL. Endothelin's biphasic effect on fluid absorption in the proximal straight tubule and its inhibitory cascade. J Clin Invest. 1994;93:2572–2577. doi: 10.1172/JCI117268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garvin J, Sanders K. Endothelin inhibits fluid and bicarbonate transport in part by reducing Na+/K+ ATPase activity in the rat proximal straight tubule. J Am Soc Nephrol. 1991;2:976–982. doi: 10.1681/ASN.V25976. [DOI] [PubMed] [Google Scholar]
- 12.Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ET(B) receptor-mediated NO release. Am J Physiol Renal Physiol. 2000;279:F326–F333. doi: 10.1152/ajprenal.2000.279.2.F326. [DOI] [PubMed] [Google Scholar]
- 13.Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na(+)-K(+)-ATPase in intact renaltubular epithelial cells. Am J Physiol. 1989;257:C1101–C1107. doi: 10.1152/ajpcell.1989.257.6.C1101. [DOI] [PubMed] [Google Scholar]
- 14.Chu TS, Wu KD, Wu MS, Hsieh BS. Endothelin-1 chronically inhibits Na/H exchanger-3 in ET(B)-overexpressing OKP cells. Biochem Biophys Res Commun. 2000;271:807–811. doi: 10.1006/bbrc.2000.2724. [DOI] [PubMed] [Google Scholar]
- 15.Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest. 1998;102:493–498. doi: 10.1172/JCI3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int. 2008;74:750–759. doi: 10.1038/ki.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XX, Bek M, Asico LD, Yang Z, Grandy DK, Goldstein DS, Rubinstein M, Eisner GM, Jose PA. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension. 2001;38:303–308. doi: 10.1161/01.hyp.38.3.303. [DOI] [PubMed] [Google Scholar]
- 18.Zeng C, Asico LD, Wang X, Hopfer U, Eisner GM, Felder RA, Jose PA. Angiotensin II regulation of AT1 and D3 dopamine receptors in renal proximal tubule cells of SHR. Hypertension. 2003;41:724–729. doi: 10.1161/01.HYP.0000047880.78462.0E. [DOI] [PubMed] [Google Scholar]
- 19.Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D(1)-like and D(2)-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1071–R1078. doi: 10.1152/ajpregu.2001.281.4.R1071. [DOI] [PubMed] [Google Scholar]
- 20.Kohzuki M, Johnston CI, Chai SY, Casley DJ, Mendelsohn FA. Localization of endothelin receptors in rat kidney. Eur J Pharmacol. 1989;160:193–194. doi: 10.1016/0014-2999(89)90673-0. [DOI] [PubMed] [Google Scholar]
- 21.Chu TS, Tsuganezawa H, Peng Y, Cano A, Yanagisawa M, Alpern RJ. Role of tyrosine kinase pathways in ETB receptor activation of NHE3. Am J Physiol. 1996;271:C763–C771. doi: 10.1152/ajpcell.1996.271.3.C763. [DOI] [PubMed] [Google Scholar]
- 22.Moridaira K, Morrissey J, Fitzgerald M, Guo G, McCracken R, Tolley T, Klahr S. ACE inhibition increases expression of the ETB receptor in kidneys of mice with unilateral obstruction. Am J Physiol Renal Physiol. 2003;284:F209–F217. doi: 10.1152/ajprenal.00352.2001. [DOI] [PubMed] [Google Scholar]
- 23.O'Connell DP, Vaughan CJ, Aherne AM, Botkin SJ, Wang ZQ, Felder RA, Carey RM. Expression of the dopamine D3 receptor protein in the rat kidney. Hypertension. 1998;32:886–895. doi: 10.1161/01.hyp.32.5.886. [DOI] [PubMed] [Google Scholar]
- 24.Wendel M, Knels L, Kummer W, Koch T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J Histochem Cytochem. 2006;54:1193–1203. doi: 10.1369/jhc.5A6888.2006. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Li XX, Albrecht FE, Hopfer U, Carey RM, Jose PA. Dopamine(1) receptor, G(salpha), and Na(+)-H(+) exchanger interactions in the kidney in hypertension. Hypertension. 2000;36:395–399. doi: 10.1161/01.hyp.36.3.395. [DOI] [PubMed] [Google Scholar]
- 26.Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, Felder RA, Jose PA. Galpha12- and Galpha13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–610. doi: 10.1161/01.HYP.0000057422.75590.D7. [DOI] [PubMed] [Google Scholar]
- 27.Bek MJ, Zheng S, Xu J, Yamaguchi I, Asico LD, Sun XG, Jose PA. Differential expression of adenylyl cyclases in the rat nephron. Kidney Int. 2001;60:890–899. doi: 10.1046/j.1523-1755.2001.060003890.x. [DOI] [PubMed] [Google Scholar]
- 28.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 29.Kling-Petersen T, Ljung E, Svensson K. Effects on locomotor activity after local application of D3 preferring compounds in discrete areas of the rat brain. J Neural Transm Gen Sect. 1995;102:209–220. doi: 10.1007/BF01281155. [DOI] [PubMed] [Google Scholar]
- 30.Zeng C, Hopfer U, Asico LD, Eisner GM, Felder RA, Jose PA. Altered AT1 receptor regulation of ETB receptors in renal proximal tubule cells of spontaneously hypertensive rats. Hypertension. 2005;46:926–931. doi: 10.1161/01.HYP.0000174595.41637.13. [DOI] [PubMed] [Google Scholar]
- 31.Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Yu C, Han Y, Ren H, Shi W, Fu C, He D, Huang L, Yang C, Wang X, Zhou L, Asico LD, Zeng C, Jose PA. Inhibitory effect of D1-like and D3 dopamine receptors on norepinephrine-induced proliferation in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2008;294:H2761–H2768. doi: 10.1152/ajpheart.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 34.Kotlo K, Shukla S, Tawar U, Skidgel RA, Danziger RS. Aminopeptidase N reduces basolateral Na+-K+-ATPase in proximal tubule cells. Am J Physiol Renal Physiol. 2007;293:F1047–F1053. doi: 10.1152/ajprenal.00074.2007. [DOI] [PubMed] [Google Scholar]
- 35.Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+, K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens. 2006;28:29–40. doi: 10.1080/10641960500386650. [DOI] [PubMed] [Google Scholar]
- 36.Silva E, Gomes P, Soares-da-Silva P. Overexpression of Na(+)/K(+)-ATPase parallels the increase in sodium transport and potassium recycling in an in vitro model of proximal tubule cellular ageing. J Membr Biol. 2006;212:163–175. doi: 10.1007/s00232-005-7017-5. [DOI] [PubMed] [Google Scholar]
- 37.Faisy C, Pinto F, Danel C, Naline E, Risse PA, Leroy I, Israel-Biet D, Fagon JY, Candenas ML, Advenier C. beta2-Adrenoceptor agonist modulates endothelin-1 receptors in human isolated bronchi. Am J Respir Cell Mol Biol. 2006;34:410–416. doi: 10.1165/rcmb.2005-0091OC. [DOI] [PubMed] [Google Scholar]
- 38.Hocher B, Dembowski C, Slowinski T, Friese ST, Schwarz A, Siren AL, Neumayer HH, Thöne-Reineke C, Bauer C, Nafz B, Ehrenreich H. Impaired sodium excretion, decreased glomerular filtration rate and elevated blood pressure in endothelin receptor type B deficient rats. J Mol Med. 2001;78:633–641. doi: 10.1007/s001090000158. [DOI] [PubMed] [Google Scholar]
- 39.Peng Y, Moe OW, Chu T, Preisig PA, Yanagisawa M, Alpern RJ. ETB receptor activation leads to activation and phosphorylation of NHE3. Am J Physiol. 1999;276:C938–C945. doi: 10.1152/ajpcell.1999.276.4.C938. [DOI] [PubMed] [Google Scholar]
- 40.Hussain T, Abdul-Wahab R, Lokhandwala MF. Bromocriptine stimulates Na+, K(+)-ATPase in renal proximal tubules via the cAMP pathway. Eur J Pharmacol. 1997;321:259–263. doi: 10.1016/s0014-2999(97)00039-3. [DOI] [PubMed] [Google Scholar]
- 41.Lévesque D. Aminotetralin drugs and D3 receptor functions. What may partially selective D3 receptor ligands tell us about dopamine D3 receptor functions? Biochem Pharmacol. 1996;52:511–518. doi: 10.1016/0006-2952(96)00239-0. [DOI] [PubMed] [Google Scholar]
- 42.Luippold G, Küster E, Joos TO, Mühlbauer B. Dopamine D3 receptor activation modulates renal function in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:690–693. doi: 10.1007/pl00005314. [DOI] [PubMed] [Google Scholar]
- 43.Zeng C, Wang Z, Li H, Yu P, Zheng S, Wu L, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. D3 dopamine receptor directly interacts with D1 dopamine receptor in immortalized renal proximal tubule cells. Hypertension. 2006;47:573–579. doi: 10.1161/01.HYP.0000199983.24674.83. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Dougherty JJ, Nichols RA. Dopamine receptor regulation of Ca2+ levels in individual isolated nerve terminals from rat striatum: comparison of presynaptic D1-like and D2-like receptors. J Neurochem. 2006;98:481–494. doi: 10.1111/j.1471-4159.2006.03901.x. [DOI] [PubMed] [Google Scholar]
- 45.Moreland RB, Nakane M, Donnelly-Roberts DL, Miller LN, Chang R, Uchic ME, Terranova MA, Gubbins EJ, Helfrich RJ, Namovic MT, El-Kouhen OF, Masters JN, Brioni JD. Comparative pharmacology of human dopamine D(2)-like receptor stable cell lines coupled to calcium flux through Galpha(qo5). Biochem Pharmacol. 2004;68:761–772. doi: 10.1016/j.bcp.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Lijnen P, Fagard R, Petrov V. Proliferation of human peripheral blood mononuclear cells during calcium channel blockade. Am J Hypertens. 1998;11:1461–1468. doi: 10.1016/s0895-7061(98)00179-4. [DOI] [PubMed] [Google Scholar]