Abstract
BACKGROUND
Ion transport in the renal proximal tubule (RPT) is regulated by numerous hormones and humoral factors, including insulin and dopamine. Previous studies show an interaction between insulin and the D1 receptor. Because both D1 and D5 receptors belong to the D1-like receptor subfamily, it is possible that an interaction between insulin and the D5 dopamine receptor exists in RPT cells from normotensive Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs).
METHODS
D5 receptor expression in immortalized RPT cells from WKY and SHRs was quantified by immunoblotting and D5 receptor function by measuring Na+–K+ ATPase activity.
RESULTS
Insulin increased the expression of the D5 receptor. Stimulation with insulin (10−7 mol/l) for 24 h increased D5 receptor expression in RPT cells from WKY rats. This effect of insulin on D5 receptor expression was aberrant in RPT cells from SHRs. The stimulatory effect of insulin on D5 receptor expression in RPT cells from WKY rats was inhibited by a protein kinase C (PKC) inhibitor (PKC inhibitor peptide 19–31, 10−6 mol/l) or a phosphatidylinositol 3 (PI3) kinase inhibitor (wortmannin, 10−6 mol/l), indicating that both PKC and PI3 kinase were involved in the signaling pathway. Stimulation of the D5 receptor heterologously expressed in HEK293 cells with fenoldopam (10−7 mol/l/15 min) inhibited Na+–K+ ATPase activity, whereas pretreatment with insulin (10−7 mol/l/24 h) increased the D5 receptor-mediated inhibition.
CONCLUSIONS
Insulin and D5 receptors interact to regulate renal sodium transport; an aberrant interaction between insulin and D5 receptor may participate in the pathogenesis of hypertension.
Essential hypertension is one of the most common risk factors in developed and developing countries, affecting ~25% of the middle-aged adult population. The long-term regulation of blood pressure rests on renal and nonrenal mechanisms.1 Ion transport in the renal proximal tubule (RPT) and thick ascending limb of Henle, which is increased in essential hypertension, is regulated by numerous hormones and humoral factors, including insulin and dopamine. Activation of dopamine receptor inhibits sodium reabsorption, whereas activation of insulin receptor increases sodium reabsorption in RPTs.2–5
Insulin is an important regulator of renal salt and water excretion, and hyperinsulinemia has been implicated to play a role in hypertension.3–5 Diabetes mellitus is associated with Na+ and water retention and extracellular fluid volume expansion.4 There is evidence that insulin directly stimulates fluid absorption in rabbit proximal convoluted tubules.5 This stimulatory effect on the proximal tubule is associated with increased Na+/H+ exchanger and Na+–K+ ATPase activities.
Dopamine is an endogenous catecholamine that regulates/modulates many cellular activities, including blood pressure and transmembrane ion transport. Dopamine receptors are classified into the D1- and D2-like subtypes based on their structure and pharmacology. D1-like receptors, consisted of D1 and D5 receptors, stimulate adenylyl cyclase activity, whereas D2-like receptors, consisted of D2, D3, and D4 receptors, inhibit adenylyl cyclase activity and regulate/modulate the activity of several ion channels.2
Increasing pieces of evidence show an interaction between insulin and dopamine receptors.3,6,7 Hyperinsulinemic animals and patients with noninsulin-dependent diabetes have defective renal dopaminergic system.6 In obese Zucker rats, a model of type 2 diabetes, renal D1 receptor is downregulated and dopamine fails to produce both diuresis and natriuresis. Treatment with the insulin sensitizer rosiglitazone, a peroxisome proliferator–activated receptor-γ agonist, decreases plasma insulin levels and restores renal D1-receptor function. Chronic exposure of cells to insulin causes both a reduction in D1-receptor abundance, and uncoupling of D1 receptor from G protein/effector complex;3 there is also a diminution of the inhibitory effect of dopamine on Na+–K+ ATPase activity in hyperinsulinemic rats.7 As a major receptor subtype, the D5 receptor, like the Dl receptor, is expressed in RPTs.2,8 Disruption of the D5 receptor gene produces hypertension in mice, and a high salt diet increases their blood pressure further.9,10 Activation of the D5 receptor stimulates cyclic adenosine monophosphate (cAMP) production.9,11 Therefore, it is possible that the natriuretic effect of dopamine is partially mediated by the D5 receptor. Hence, we hypothesized that insulin may increase D5 receptor expression and function, which may be aberrant in hypertensive states. To test the hypothesis mentioned earlier, we evaluated insulin and D5 receptor interaction in immortalized RPT cells from Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs), and the physiological significance of any interaction in D5 receptor cDNA-transfected HEK293 (HEK293-D5) cells. These RPT cells behave similarly to freshly obtained RPT cells, at least with regard to dopamine receptors and responses to G protein stimulation.12,13
METHODS
Cell culture
Immortalized RPT cells from WKY and SHRs were cultured at 37 °C in 95% air and 5% CO2 atmosphere in DMEM/F–12 culture media, as previously described.14 HEK293 cells were transfected with human D5 receptor fused to a V5-His tag at the C-terminus or empty vector (pcDNA6/V5-His),13 which served as a control. The cells (80% confluence) were lysed in ice–cold lysis buffer (PBS with 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mmol/l EDTA, 1 mmol/l EGTA, 1 mmol/l PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin), sonicated, kept on ice for 1 h, and centrifuged at 16,000g for 30 min. The supernatants were stored at −70 °C until use for immunoblotting.
Immunoblotting
Rat RPT cells were treated with vehicle (dH2O) or insulin (Sigma, St Louis, MO) at the indicated concentrations and time points. Immunoblotting was performed as previously reported. The transblots were probed using anti-D5 receptor antibody (1:500; Research Genetics, Huntsville, AL) or human anti-rabbit insulin receptor antibody (β-subunit; Santa Cruz Biotechnology, Santa Cruz, CA). The epitope recognized by the rabbit anti-human D5 receptor antibody corresponds to the third intracellular loop of the receptor. We have reported the specificity of this D5 receptor antibody.13 The relative amount of protein transferred onto the membranes was determined by immunoblotting for α-actin. The receptor densities were normalized for α-actin.14
Determination of the second messenger(s) involved in the regulation of insulin on D5 receptor expression in RPT cells from WKY rats
To determine the second messenger(s) involved in the regulation by insulin of D5 receptor expression in RPT cells from WKY rats, several inhibitors or agonists were used: protein kinase C (PKC) inhibitor (PKC inhibitor 19–31, 10−6 mol/l),15 protein kinase A (PKA) inhibitor (PKA inhibitor 14–22, 10−6 mol/l),16 PKC activator (phorbol 12-myristate 13-acetate, PMA, 10−7 mol/l),17 PKA activator (Sp-cAMP-S, 10−7 mol/l), calcium channel blocker (nicardipine, 10−6 mol/l), calcium channel agonist (BAY-K8644, 10−6 mol/l)18 and phosphatidylinositol 3 (PI3) kinase inhibitor wortmannin (10−6 mol/l).19
PKC inhibitor 19–31, PMA, Sp-cAMP-S, nicardipine, BAY-K8644 and wortmannin were purchased from Sigma; PKA inhibitor 14–22 was purchased from Calbiochem (Darmstadt, Germany).
Na+–K+ ATPase activity assay
Na+–K+ ATPase activity was determined as the rate of inorganic phosphate released in the presence or absence of ouabain.20 To prepare membranes for Na+–K+ ATPase activity assay, HEK293 cells cultured in 21-cm2 plastic culture dishes were washed twice with 5 ml chilled phosphate-free buffer (2.36 mmol/l NaCl, 0.54 mmol/l NaHCO3, 0.4 mmol/l KCl, and 0.12 mmol/l MgCl2 scraped in phosphate-free buffer), and centrifuged at 3,000g for 10 min. The cells were then placed on ice and lysed in 2 ml of lysis buffer (1 mmol/l NaHCO3, 2 mmol/l CaCl2 and 5 mmol/l MgCl2). Cellular lysates were centrifuged at 3,000g for 2 min to remove the insoluble debris. The supernatant was suspended in an equal volume of 1 mol/l NaI, and the mixture was centrifuged at 48,000g for 25 min. The pellet (membrane fraction) was washed twice and re-suspended in 10 mmol/l Tris with 1 mmol/l EDTA (pH 7.4). Protein concentrations were determined via Bradford assay (Bio-Rad Laboratories, Hercules, CA) and adjusted to 1 mg/ml. The membranes were stored at –70 °C until further use.
To measure Na+–K+ ATPase activity, 100-μl aliquots of the membrane fraction were added to 800-μl reaction mixture (75 mmol/l NaCl, 5 mmol/l KCl, 5 mmol/l MgCl2, 6 mmol/l sodium azide, 1 mmol/l Na4EGTA, 37.5 mmol/l imidazole, 75 mmol/l Tris HCl, and 30 mmol/l histidine; pH 7.4), with or without 1 mmol/l ouabain (final volume = 1 ml), and pre-incubated for 5 min in a water bath at 37 °C. Reactions were initiated by adding Tris ATP (4 mmol/l) and terminated after 15 min of incubation at 37 °C by adding 50 μl of 50% trichloracetate. For the determination of ouabain-insensitive ATPase activity, NaCl and KCl were omitted from the reaction mixtures containing ouabain. To quantify the amount of phosphate produced, 1 ml of coloring reagent (10% ammonium molybdate in 10 N sulfuric acid + ferrous sulfate) was added to the reaction mixture. The mixture was then combined thoroughly and centrifuged at 3,000g for 10 min. The formation of phosphomolybdate was determined spectrophotometrically at 740 nm against a standard curve prepared from K2HPO4. Na+–K+ ATPase activity was estimated as the difference between total and ouabain-insensitive ATPase activity.20 The results in these experiments were expressed as percent change from control.
Statistical analysis
The data are expressed as mean ± s.e.m. Comparison within groups was made by repeated measures analysis of variance with Duncan's test (or paired t-test when only two groups were compared); comparison among groups was made by factorial analysis of variance with Duncan's test. A value of P < 0.05 was considered significant.
RESULTS
Insulin receptor localization in HEK293 cells
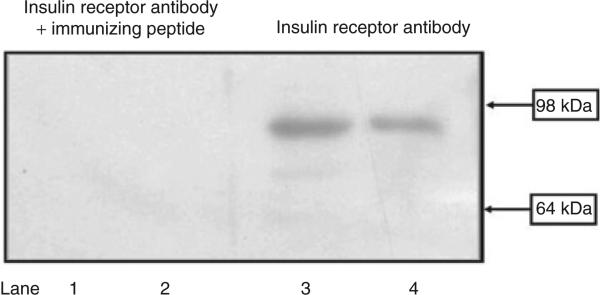
Specific insulin receptor (≈95 kDa) bands were found in HEK293 cells (Figure 1, lanes 3 and 4), which were no longer visible when the antibodies were preadsorbed by the immunizing peptide. The immunoblotting pattern of the insulin receptor was similar to previous report.21
Figure 1.
Insulin receptor expression in HEK293 cells. Cell lysate proteins (50 μg) from RPT cells from WKY rats (lanes 1 and 3) and HEK293 cells (lanes 2 and 4) were subjected to immunoblotting with anti-insulin receptor antibody (1:250). In HEK293 cells and RPT cells from WKY rats, the 95-kDa band was no longer visible when the antibody was preadsorbed with the immunizing peptide (1:10 wt/wt incubation for 12 h). The molecular sizes are indicated. RPT, renal proximal tubule; WKY, Wistar-Kyoto.
Insulin increases D5 receptor expression in RPT cells from WKY rats, but decreases it in RPT cells from SHRs
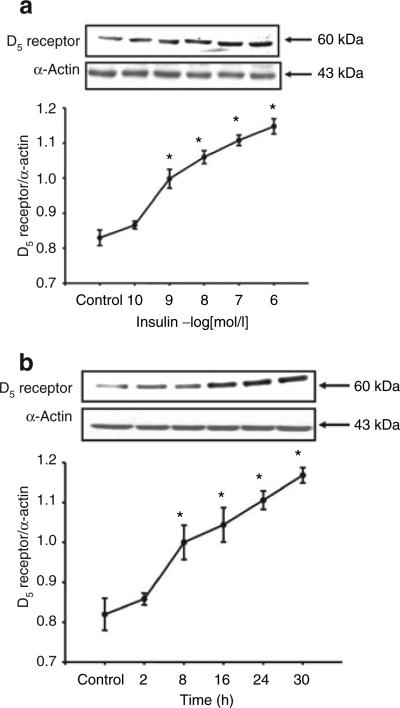
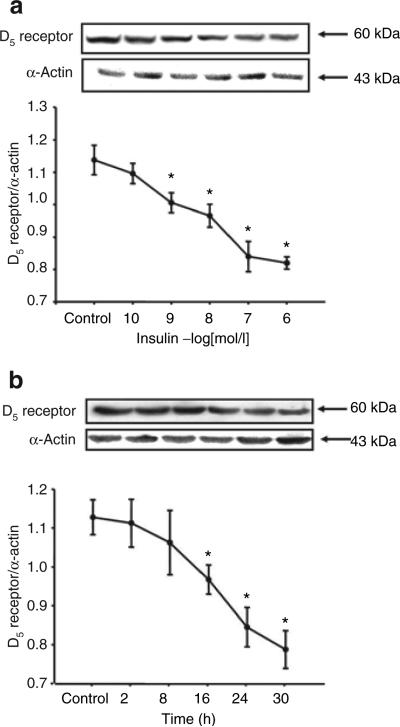
Insulin increased D5 receptor expression in a concentration- and time-dependent manner in RPT cells from WKY rats. The stimulatory effect was evident at 10−9 mol/l, noted as early as 8 h, and maintained for at least 30 h (Figure 2a,b). In contrast to the stimulatory effect of insulin on D5 receptor in RPT cells from WKY rats, in RPT cells from SHRs, insulin decreased D5 receptor expression (WKY: control = 1.1 ± 0.05 density units (DUs), 10−7 mol/l/24 h insulin = 1.5 ± 0.1 DU; SHR: control = 0.8 ± 0.03 DU, 10−7 mol/l/24 h insulin = 0.6 ± 0.06 DU, P < 0.05, n = 5), with the inhibition occurring at 10−9 mol/l (Figure 3a) and beginning at 16 h (Figure 3b).
Figure 2.
Effect of insulin on D5 receptor expression in RPT cells from WKY rats. (a) Concentration-response of D5 receptor protein expression in RPT cells from WKY rats treated with varying concentrations of insulin for 24 h. Results are expressed as the ratio of D5 receptor and α-actin densities (n = 5, *P < 0.05 vs. control, ANOVA, Duncan's test). (b) Time-course of D5 receptor protein expression in RPT cells from WKY rats treated with insulin (10−7 mol/l). Results are expressed as the ratio of D5 receptor and α-actin densities (n = 5, *P < 0.05 vs. control, ANOVA, Duncan's test). ANOVA, analysis of variance; RPT, renal proximal tubule; WKY, Wistar-Kyoto.
Figure 3.
Effect of insulin on D5 receptor expression in RPT cells from SHRs. (a) Concentration-dependent response of D5 receptor protein expression in RPT cells from SHRs treated with varying concentrations of insulin for 24 h. Results are expressed as the ratio of D5 receptor and α-actin densities (n = 6, *P < 0.05 vs. control, ANOVA, Duncan's test). (b) Time-dependent response of D5 receptor protein expression in RPT cells from SHRs treated with insulin (10−7 mol/l). Results are expressed as the ratio of D5 receptor and α-actin densities (n = 4, *P < 0.05 vs. control, ANOVA, Duncan's test). ANOVA, analysis of variance; RPT, renal proximal tubule; SHRs, spontaneously hypertensive rats.
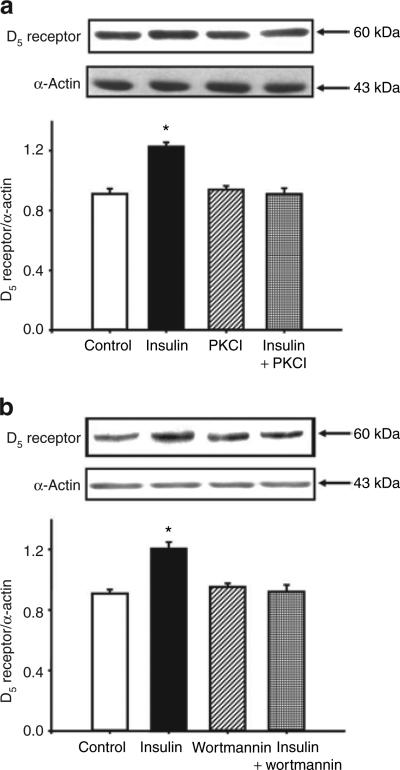
To investigate a mechanism for the insulin induced upregulation of D5 receptor, the RPT cells from WKY were treated with different agonists or antagonists. Treatment with the PKC inhibitor 19–31 (10−6 mol/l) or the PI3 kinase inhibitor wortmannin (10−6 mol/l), which by themselves had no effect on D5 receptor expression, blocked the stimulatory effect of insulin on D5 receptor expression in RPT cells from WKY rats (Figure 4a,b), indicating that both PKC and PI3 kinase were involved in the signal transduction pathway activated by insulin. We also evaluated the involvement of other key cell signaling proteins with the use of a PKA inhibitor (PKA inhibitor 14–22, 10−6 mol/l), PKC activator (phorbol 12-myristate 13-acetate, PMA, 10−7 mol/l), PKA activator (Sp-cAMP-S, 10−7 mol/l), calcium channel blocker (nicardipine, 10−6 mol/l), and calcium channel agonist (BAY-K8644, 10−6 mol/l). None of these reagents was able to block the stimulatory effect of insulin on D5 receptor expression (data not shown).
Figure 4.
Role of PKC and PI3 kinase in the effect of insulin on D5 receptor expression in RPT cells from WKY rats. (a) Effect of insulin and the PKC inhibitor 19–31 on D5 receptor protein in RPT cells from WKY rats. The cells were incubated with the indicated reagents (insulin, 10−7 mol/l; PKC inhibitor 19–31 (PKCI), 10−6 mol/l) for 24 h. Results are expressed as the ratio of D5 receptor to α-actin densities (n = 5, *P < 0.05 vs. others, ANOVA, Duncan's test). (b) Effect of insulin and the PI3 kinase inhibitor wortmannin (10−6 mol/l) on D5 receptor protein in RPT cells from WKY rats. The cells were incubated with the indicated reagents (insulin, 10−7 mol/l; wortmannin, 10−6 mol/l) for 24 h. Results are expressed as the ratio of D5 receptor to α-actin densities (n = 5, *P < 0.05 vs. others, ANOVA, Duncan's test). ANOVA, analysis of variance; PI3, phosphatidylinositol 3; PKC, protein kinase C; RPT, renal proximal tubule; WKY, Wistar-Kyoto.
We also found that, consistent with the results in RPT cells from WKY rats, insulin (10−7 mol/l/24 h) increased D5 receptor expression in D5 receptor cDNA-transfected HEK293 cells (control = 0.9 ± 0.04 DU, insulin = 1.1 ± 0.04 DU; P < 0.05, n = 4).
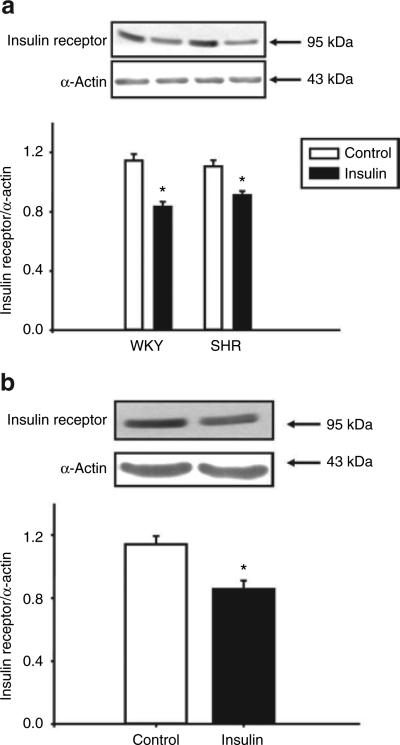
Insulin decreases insulin receptor expression in RPT cells from SHR and WKY rats
To investigate the effect of insulin on its own receptor, RPT cells from SHR and WKY rats were incubated with insulin (10−7 mol/l) for 24 h. Immunoblots showed that insulin decreased its own receptor expression in RPT cells from SHR and WKY rats (Figure 5a). Consistent with the finding in RPT cells from WKY rats, stimulation with insulin (10−7 mol/l/24 h) decreased insulin receptor expression in D5-HEK293 cells (Figure 5b).
Figure 5.
Effects of insulin on insulin receptor expression in renal proximal tubule cells from (a) SHR and WKY rats and (b) HEK293 cells heterologously expressing the D5 receptor. The cells were incubated with the insulin (10−7 mol/l) for 24 h. Results are expressed as the ratio of insulin receptor to α-actin density (n = 5 in a, or n = 4 in b, *P < 0.05 vs. control, t-test). SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto.
Pretreatment with insulin increases the inhibitory effect of D5 receptor on Na+–K+ ATPase activity in D5 receptor cDNA-transfected HEK293 cells
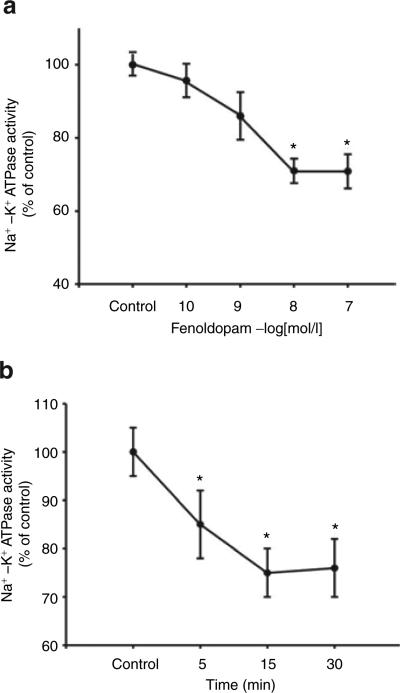
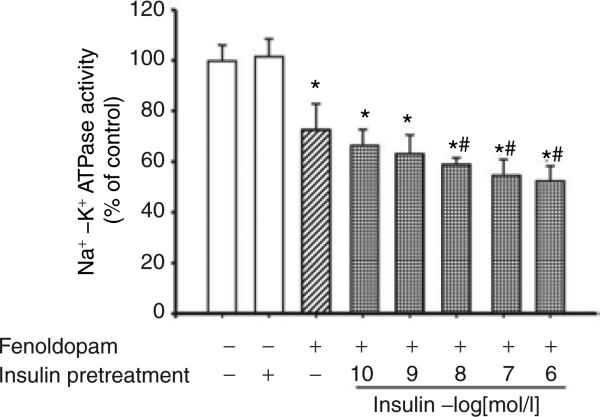
A D1-like receptor agonist, fenoldopam, inhibited Na+–K+ ATPase activity in a concentration- and time-dependent manner (Figure 6a,b). As there is no D1-receptor mRNA expression in HEK293 cells (data not shown), the inhibitory effect of fenoldopam on Na+–K+ ATPase activity was via the D5 receptor. To investigate the physiological significance of insulin and D5 receptor interaction, the stimulatory effect of insulin on D5 receptor function was also determined in D5 receptor cDNA-transfected HEK293 cells. We found that insulin pretreatment (10−10–10−6 mol/l), augmented the fenoldopam-mediated inhibition (via the D5 receptor) of Na+–K+ ATPase activity in a concentration-dependent manner (Figure 7). Although insulin (10−7 mol/l/15 min, n = 8, P < 0.01) acutely stimulates Na+–K+ ATPase activity (control = 100 ± 7.6%, insulin = 124 ± 8.3% of control), insulin (10−7 mol/l) pretreatment for 24 h had no effect on Na+–K+ ATPase activity in D5 cDNA-transfected HEK293 cells, which is consistent with the report of Banday et al.7 The cells pretreated with insulin for 24 h were washed three times (15 min/wash) with serum-free culture medium to remove all the added insulin, kept in serum-free culture medium for 2 h, and then treated with vehicle or fenoldopam for 15 min.
Figure 6.
Effect of the D5 receptor on Na+–K+ ATPase activity in HEK293 cells heterologously expressing the D5 receptor. (a) Concentration-response of Na+–K+ ATPase activity caused by fenoldopam (10−10–10−6 mol/l), a D1-like receptor agonist, for 15 min. Results in this experiment are expressed as percent change from control (n = 6, *P < 0.01 vs. control, ANOVA, Duncan's test). (b) Time-dependent response (5–30 min) of Na+–K+ ATPase activity caused by fenoldopam (10−7 mol/l). Results in this experiment are expressed as percent change from control (n = 7, *P < 0.01 vs. control, ANOVA, Duncan's test). ANOVA, analysis of variance.
Figure 7.
Effect of pretreatment with insulin on the inhibitory effect of the D5 receptor on Na+–K+ ATPase activity in HEK293 cells heterologously expressing the D5 receptor. The cells were pretreated with varying concentrations of insulin (10−10–10−6 mol/l) or vehicle (dH2O) for 24 h. After insulin pretreatment, the cells were washed three times (15 min/times) with serum-free culture medium to remove all the added insulin, kept in serum-free culture medium for 2 h, and then treated with the D1-like receptor agonist, fenoldopam (10−7 mol/l), for 15 min. Results are expressed as percent change from control (*P < 0.05 vs. control, #P < 0.05 vs. fenoldopam, n = 5, analysis of variance, Duncan's test).
DISCUSSION
There are several novel observations in our study. First, we showed that insulin increased D5 receptor expression in rat RPT cells from WKY rats, but not in RPT cells from SHRs. Second, the stimulatory effect of insulin on D5 receptor involves PI3 kinase and PKC. Third, the interaction of insulin and D5 receptors has physiological significance since pretreatment with insulin for 24 h augmented the inhibitory effect of fenoldopam on Na+–K+ ATPase activity in D5 receptor cDNA-transfected HEK293 cells.
Insulin receptors exist in RPTs and their activation results in antinatriuresis, shown by numerous studies. Early studies by Atchley et al. demonstrated that discontinuation of an insulin infusion to diabetic patient results in a brisk natriuresis.22 Miller and Bogdonoff showed that infusion of insulin into normal subjects results in a reduction in urinary sodium excretion.23 We found that fenoldopam (10−7 mol/l/15 min), a D1-like receptor agonist, decreased Na+–K+ ATPase activity, which was augmented by pretreatment with insulin (10−7 mol/l/24 h) in D5 receptor cDNA-transfected HEK293 cells.
The D5 receptor, like the Dl receptor, is expressed in the RPTs.2,8,9,13 However, there is no ligand that can distinguish the D5 receptor from the Dl receptor. While most of the knowledge on D1-like receptor activity outside the nervous system has come from studies of the D1 receptor, there is now increasing evidence showing the importance of the D5 receptor in regulating blood pressure. The D5 receptor gene locus (chromosome 4p15.1–16.1) is linked to essential hypertension24 and the human D5 receptor gene contains polymorphisms that encode for receptors with abnormal coupling to adenylyl cyclase.25 Moreover, D5 receptor gene deficient mice exhibit hypertension, which is aggravated by a high salt diet, suggesting that renal D5 receptors play an important role in the control of blood pressure by regulating renal sodium chloride transport.8 Activation of D5 receptor stimulates cAMP production, which, in turn, mediates the D1-like receptor-mediated inhibition of ion transport. Thus, it is possible that the natriuretic effect of dopamine is partially mediated by the D5 receptor.8,11
The stimulatory effect of the D5 receptor on cAMP production is of ~25%11 of the response to the D1-like receptor agonist, fenoldopam, which stimulates both the D1-like receptors (D1 receptor and D5 receptor). D1-like receptor inhibition of Na+–K+ ATPase in RPTs is ~25%.26 However, D1-like receptors inhibit several ion transporters in the apical membrane (NHE3, HCO3/Cl exchanger, Na/Pi cotransporter) and basolateral membrane (Na/HCO3 cotransporter), including Na+–K+ ATPase.2 The inhibitory effect of 25% of sodium transport in the proximal tubule is physiologically relevant because knockout of the enzyme that produces dopamine (aromatic amino acid decarboxylase) in the proximal tubule results in hypertension.27 The effect of dopamine on D1-like receptors is enhanced by actions not only on other dopamine receptors but also by modulation of COX2 activity and renin secretion,28 inhibition of AT1 receptor function,29 stimulation of the AT2 receptor, and interaction with atrial natriuretic polypeptide.30 D1-like receptors also interact with D2-like receptors (e.g., D3 receptors) to inhibit ion transport.2 Furthermore, dopamine receptors also decrease transport in the thick ascending limb of Henle, distal convoluted tubule and cortical collecting duct, responsible for at least 50% regulation of ion transport under conditions of moderate sodium excess.2 Therefore, a modest effect on several ion transporters/pump/channel in several segments of the nephron can have a considerable effect on overall sodium balance.
There are increasing pieces of evidence for the interaction between insulin and dopamine receptors. D2-like dopamine receptors in pancreatic beta cells inhibit insulin secretion.31 Hyperinsulinemic animals and patients with noninsulin-dependent diabetes have defective renal dopaminergic system.6 In obese Zucker rats, a model of type 2 diabetes, renal D1 receptors are downregulated and dopamine fails to produce diuresis and natriuresis. Treatment with rosiglitazone, a peroxisome proliferator–activated receptor-γ agonist which increases the sensitivity of insulin receptors, decreases plasma insulin levels and restores renal D1-receptor function. Chronic exposure of cells to insulin causes both the reduction in D1 receptor abundance and its uncoupling from G proteins, and diminishes the inhibitory effect of dopamine on Na+–K+ ATPase activity in hyperinsulinemic rats.7 Activation of the D2 receptor with bromocriptine simultaneously ameliorates various metabolic features of obese women.32 Fenoldopam treatment improves peripheral insulin sensitivity and renal function in streptozotocin-induced type 1 diabetic rats.33 Our current study shows that insulin can modulate D5 receptor expression since treatment with insulin stimulates D5 receptor expression in RPT cells from WKY rats, but not in RPT cells from SHRs. The stimulatory effect of insulin on the D5 receptor involves both PKC and PI3 kinase. The effect of insulin on D5 receptor is functionally relevant; pretreatment with insulin for 24 h enhances the inhibitory effect of fenoldopam on Na+–K+ ATPase activity in D5 receptor expressing HEK293 cells. Because insulin increases renal tubular sodium reabsorption, the increased D5 receptor expression following insulin treatment could counter-regulate the stimulatory effect of insulin on Na+–K+ ATPase activity in RPTs. Hence, a balance between the expression and activity of both insulin and D5 receptors would be an important mechanism to keep sodium reabsorption in the proximal tubule within the normal range. However, the compensatory increase in D5 receptor expression following insulin treatment is lost in RPT cells from SHRs, and this aberrant regulation of insulin on D5 receptor in the SHR might provide a mechanism for the pathogenesis of hypertension in this animal model.
Insulin interacts with G protein-coupled receptor other than the dopamine receptors. Thus, insulin also interacts with the AT1 receptor: insulin increases AT1 receptor expression and function in kidney34 that could result in an additive antinatriuretic effect. In contrast, we have reported that the stimulation of heterologously35 and endogenously36 expressed D5 receptors in kidney cells decreases AT1 receptor expression. This negative interaction could presumably decrease the antinatriuretic effect of angiotensin II. In the present study we found that insulin increases D5 receptor expression in RPT cells from normotensive WKY rats but decreases it in RPT cells from SHRs. It is possible that the stimulation of D5 receptor expression by insulin treatment counter-regulates the stimulatory effect of insulin and/or AT1 receptor on sodium transport in cells from normotensive rats, but not in cells from hypertensive rats. Hence, net sodium excretion and blood pressure level may be the result of the balance of the interaction among insulin, the D5 receptor, and the AT1 receptor, among others.
In summary, we have demonstrated that insulin regulates the expression and function of D5 receptors in immortalized rat RPT cells. In RPT cells from SHRs, this interaction between insulin and D5 receptors is aberrant, which may contribute to the increased renal sodium transport in spontaneous hypertension.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health, HL23081, DK39308, HL68686, HL074940, HL092196, the National Natural Science Foundation of China 30470728, 30672199, 30570764, 30772281, and the National Basic Research Program of China (973 Program, 2008CB517308).
Footnotes
The first two authors contributed equally to this work.
Disclosure: The authors declared no conflict of interest.
References
- 1.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19:233–246. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
- 3.Banday AA, Fazili FR, Lokhandwala MF. Insulin causes renal dopamine D1 receptor desensitization via GRK2-mediated receptor phosphorylation involving phosphatidylinositol 3-kinase and protein kinase C. Am J Physiol Renal Physiol. 2007;293:F877–F884. doi: 10.1152/ajprenal.00184.2007. [DOI] [PubMed] [Google Scholar]
- 4.Boden G, Homko C, Mozzoli M, Zhang M, Kresge K, Cheung P. Combined use of rosiglitazone and fenofibrate in patients with type 2 diabetes: prevention of fluid retention. Diabetes. 2007;56:248–255. doi: 10.2337/db06-0481. [DOI] [PubMed] [Google Scholar]
- 5.Baum M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest. 1987;79:1104–1109. doi: 10.1172/JCI112925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchida H, Imai G, Shima Y, Satoh T, Owada S. Mechanism of sodium load-induced hypertension in non-insulin dependent diabetes mellitus model rats: defective dopaminergic system to inhibit Na-K-ATPase activity in renal epithelial cells. Hypertens Res. 2001;24:127–135. doi: 10.1291/hypres.24.127. [DOI] [PubMed] [Google Scholar]
- 7.Banday AA, Asghar M, Hussain T, Lokhandwala MF. Dopamine-mediated inhibition of renal Na,K-ATPase is reduced by insulin. Hypertension. 2003;41:1353–1358. doi: 10.1161/01.HYP.0000069260.11830.CD. [DOI] [PubMed] [Google Scholar]
- 8.Zeng C, Yang Z, Asico LD, Jose PA. Regulation of blood pressure by D5 dopamine receptors. Cardiovasc Hematol Agents Med Chem. 2007;5:241–248. doi: 10.2174/187152507781058708. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Sibley DR, Jose PA. D5 dopamine receptor knockout mice and hypertension. J Recept Signal Transduct Res. 2004;24:149–164. doi: 10.1081/rrs-200029971. [DOI] [PubMed] [Google Scholar]
- 10.Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young WS, 3rd, Westphal H, Jose PA, Sibley DR. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci. 2002;22:10801–10810. doi: 10.1523/JNEUROSCI.22-24-10801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanada H, Xu J, Watanabe H, Jose PA, Felder RA. Differential expression and regulation of dopamine-1 (D-1) and dopamine-5 (D-5) receptor function in human kidney. Am J Hypertens. 2000;13:156A. [Google Scholar]
- 12.Xu J, Li XX, Albrecht FE, Hopfer U, Carey RM, Jose PA. Dopamine(1) receptor, G(salpha), and Na(+)-H(+) exchanger interactions in the kidney in hypertension. Hypertension. 2000;36:395–399. doi: 10.1161/01.hyp.36.3.395. [DOI] [PubMed] [Google Scholar]
- 13.Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, Felder RA, Jose PA. Galpha12-and Galpha13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–610. doi: 10.1161/01.HYP.0000057422.75590.D7. [DOI] [PubMed] [Google Scholar]
- 14.Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- 15.Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol. 2006;70:676–685. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- 16.Bobalova J, Mutafova-Yambolieva VN. Activation of the adenylyl cyclase/protein kinase A pathway facilitates neural release of beta-nicotinamide adenine dinucleotide in canine mesenteric artery. Eur J Pharmacol. 2006;536:128–132. doi: 10.1016/j.ejphar.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 17.Lei J, Mariash CN, Bhargava M, Wattenberg EV, Ingbar DH. T3 increases Na-K-ATPase activity via a MAPK/ERK1/2-dependent pathway in rat adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L749–L754. doi: 10.1152/ajplung.00335.2007. [DOI] [PubMed] [Google Scholar]
- 18.Inui T, Mori Y, Watanabe M, Takamaki A, Yamaji J, Sohma Y, Yoshida R, Takenaka H, Kubota T. Physiological role of L-type Ca2+ channels in marginal cells in the stria vascularis of guinea pigs. J Physiol Sci. 2007;57:287–298. doi: 10.2170/physiolsci.RP006807. [DOI] [PubMed] [Google Scholar]
- 19.Ren Z, Raucci FJ, Jr, Browe DM, Baumgarten CM. Regulation of swelling-activated Cl(-) current by angiotensin II signalling and NADPH oxidase in rabbit ventricle. Cardiovasc Res. 2008;77:73–80. doi: 10.1093/cvr/cvm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva E, Gomes P, Soares-da-Silva P. Overexpression of Na(+)/K (+)-ATPase parallels the increase in sodium transport and potassium recycling in an in vitro model of proximal tubule cellular ageing. J Membr Biol. 2006;212:163–175. doi: 10.1007/s00232-005-7017-5. [DOI] [PubMed] [Google Scholar]
- 21.Chisalita SI, Arnqvist HJ. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E896–E901. doi: 10.1152/ajpendo.00327.2003. [DOI] [PubMed] [Google Scholar]
- 22.Atchley DW, Loeb RF, Richards DW, Benedict EM, Driscoll ME. On diabetic acidosis: a detailed study of electrolyte balances following the withdrawal and reestablishment of insulin therapy. J Clin Invest. 1933;12:297–326. doi: 10.1172/JCI100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JH, Bogdonoff MD. Antidiuresis associated with administration of insulin. J Appl Physiol. 1954;6:509–512. doi: 10.1152/jappl.1954.6.8.509. [DOI] [PubMed] [Google Scholar]
- 24.Allayee H, de Bruin TW, Michelle Dominguez K, Cheng LS, Ipp E, Cantor RM, Krass KL, Keulen ET, Aouizerat BE, Lusis AJ, Rotter JI. Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension. 2001;38:773–778. doi: 10.1161/hy1001.092617. [DOI] [PubMed] [Google Scholar]
- 25.Cravchik A, Gejman PV. Functional analysis of the human D5 dopamine receptor missense and nonsense variants: differences in dopamine binding affinities. Pharmacogenetics. 1999;9:199–206. [PubMed] [Google Scholar]
- 26.Horiuchi A, Takeyasu K, Mouradian MM, Jose PA, Felder RA. D1A dopamine receptor stimulation inhibits Na+/K(+)-ATPase activity through protein kinase A. Mol Pharmacol. 1993;43:281–285. [PubMed] [Google Scholar]
- 27.Zhang MZ, Wang S, Yao B, Fan X, Xu J, Wu G, Harris RC. Inhibition of intrarenal dopamine synthesis predisposes to salt-sensitive hypertension. J Am Soc Nephrol. 2007;18:21A. [Google Scholar]
- 28.Zhang MZ, Yao B, McKanna JA, Harris RC. Cross talk between the intrarenal dopaminergic and cyclooxygenase-2 systems. Am J Physiol Renal Physiol. 2005;288:F840–F845. doi: 10.1152/ajprenal.00240.2004. [DOI] [PubMed] [Google Scholar]
- 29.Zeng C, Luo Y, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Perturbation of D1 dopamine and AT1 receptor interaction in spontaneously hypertensive rats. Hypertension. 2003;42:787–792. doi: 10.1161/01.HYP.0000085334.34963.4E. [DOI] [PubMed] [Google Scholar]
- 30.Hegde SS, Chen CJ, Lokhandwala MF. Involvement of endogenous dopamine and DA-1 receptors in the renal effects of atrial natriuretic factor in rats. Clin Exp Hypertens A. 1991;13:357–369. doi: 10.3109/10641969109045056. [DOI] [PubMed] [Google Scholar]
- 31.Rubí B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280:36824–36832. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- 32.Kok P, Roelfsema F, Frölich M, van Pelt J, Stokkel MP, Meinders AE, Pijl H. Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese women. Am J Physiol Endocrinol Metab. 2006;291:E1038–E1043. doi: 10.1152/ajpendo.00567.2005. [DOI] [PubMed] [Google Scholar]
- 33.Umrani DN, Goyal RK. Fenoldopam treatment improves peripheral insulin sensitivity and renal function in STZ-induced type 2 diabetic rats. Clin Exp Hypertens. 2003;25:221–233. doi: 10.1081/ceh-120020392. [DOI] [PubMed] [Google Scholar]
- 34.Banday AA, Siddiqui AH, Menezes MM, Hussain T. Insulin treatment enhances AT1 receptor function in OK cells. Am J Physiol Renal Physiol. 2005;288:F1213–F1219. doi: 10.1152/ajprenal.00361.2003. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–2189. doi: 10.1172/JCI33637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gildea JJ, Wang X, Jose PA, Felder RA. Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension. 2008;51:360–366. doi: 10.1161/HYPERTENSIONAHA.107.100099. [DOI] [PubMed] [Google Scholar]