Abstract
CD47 or integrin-associated protein promotes cell death in blood and tumor cells. Recently, CD47 signaling has been identified in neurons as well. In this study, we investigated the role of CD47 in neuronal cell death. Exposure of primary mouse cortical neurons to the CD47 ligand thrombospondin-1 or the specific CD47-activating peptide 4N1K induced cell death. Activation of CD47 elevated levels of active caspase 3 and increased the generation of reactive oxygen species (ROS) in a time-dependent manner. Both ROS scavengers and caspase inhibitors attenuated cell death. But ROS scavenging did not reduce the activation of caspase 3, and combination treatments with a caspase inhibitor plus free radical scavenger did not yield additive protection. Taken together, these data suggest that parallel and redundant pathways of oxidative stress and caspase-mediated cell death are involved. We conclude that CD47 mediates neuronal cell death through caspase-dependent and caspase-independent pathways.
Keywords: caspase 3, CD47, cell death, reactive oxygen species, thrombospondin
CD47 (also known as integrin-associated protein) is a member of the immunoglobulin superfamily, and consists of an extracellular IgV domain followed by five transmembrane segments and a short cytoplasmic tail (Brown and Frazier 2001). Depending on the activation of heterotrimeric G protein, CD47 can associate with β1, β2, and β3 integrins families, and modulate cell motility, leukocyte adhesion and migration, phagocytosis, and platelet activation (Brown and Frazier 2001). Overall, a prominent role for CD47 has been documented in leukocyte and neutrophil infiltration during tissue inflammation.
In addition to these functions associated with integrins, CD47 plays important roles in other cellular regulation through binding with its two natural ligands, thrombospondins (TSPs) and signal regulatory protein-α. Activation of CD47 by these ligands are now thought to trigger cell death in a wide range of cells including T cells (Manna and Frazier 2003), monocytes and dendritic cells (Johansson et al. 2004), and leukemia cells (Mateo et al. 1999, 2002; Saumet et al. 2005; Bras et al. 2007). Because many fundamental mechanisms of cell death are known to be highly conserved between multiple cell types, it is possible that CD47 may possess neurotoxic actions as well. CD47 is present in neuronal cells, and one study showed that viral over-expression of CD47 in neuron induced apoptosis (Koshimizu et al. 2002).
In this study, we used primary mouse cortical neurons to investigate the mechanisms of CD47-induced neuronal death. Specifically, we asked whether ligand-mediated activation of CD47 is neurotoxic, and if so, whether downstream pathways of oxidative stress and caspases are involved.
Materials and methods
Reagents
Neurobasal media, B27 supplement, 0.05% trypsin–EDTA, L-glutamine, antibiotics, and fetal bovine serum for cell culture were from Gibco (Rockville, MD, USA). TSP and U83836E were purchased from Calbiochem (San Diego, CA, USA). 4N1K (KRFYVVMWKK) was from Sigma Genosys (The Woodlands, TX, USA) and was dissolved in sterile ddH2O at a concentration of 100 mg/mL as a stock solution. This stock was aliquoted and stored at −80°C. 5-(and-6)-Chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) was purchased from Molecular Probes (Eugene, OR, USA). Caspase inhibitors (z-VAD-fmk and z-DEVD-fmk) were from R&D (Minneapolis, MN, USA), and were dissolved in dimethyl sulfoxide at a concentration of 20 mM as a stock solution. Rabbit anti-caspase 3 primary antibody was purchased from Cell signaling (Danvers, MA, USA). Anti-mouse CD47 monoclonal antibody (Clone miap301) was from BD Pharmingen (San Jose, CA, USA). Normal mouse IgG was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Primary mouse cortical neuron culture and experimental conditions
Mice were housed in community cages under a 12 h light/dark cycle at 20–22°C and fed ad libitum. Pregnant mice were anesthetized and the embryos were delivered by cesarean section. The cortex were dissected out from the embryonic days 15–17 mouse fetuses, then dissociated, both enzymatically (0.05% trypsin–EDTA) for 15 min at 37°C and mechanically. The single cell suspension was diluted in serum-free neurobasal medium containing 2% B27 supplement and 0.3 mM L-glutamine, and then plated into 96-well plate, 60 mm dish, or 35 mm dish pre-coated with poly-D-lysine at a different cell density (2.5, 5, or 3 × 105 cells/mL, respectively). Cells were maintained at 37°C in a humidified incubator with 5% CO2. The medium was half renewal every 3 days. Cultures were used for experiments on 8–10 days after plating. To determine the effects of TSP and 4N1K in primary cultured neurons, the cortical neurons were incubated with different doses of TSP (0.5, 1, 5, and 10 μg/mL) and 4N1K (12.5, 25, 50, and 100 μg/mL) for 24 h, or with 10 μg/mL TSP or 100 μg/mL 4N1K for various times (0, 3, 6, 12, and 24 h). Although we did not use a scrambled peptide, the specificity of 4N1K for activating CD47 has been extensively confirmed in a wide variety of cells in previous studies (Manna and Frazier 2003, 2004; Johansson et al. 2004). In the inhibitors experiment, cultures were pre-treated with inhibitors for 1 h prior to addition of 100 μg/mL 4N1K for 24 h. Neurotoxicity was evaluated by the standard lactate dehydrogenase (LDH) release assay (Roche Diagnostics, Mannheim, Germany) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, St Louis, MO, USA). All LDH and MTT assays were repeated at least three times in triplicate.
Reactive oxygen species measurement
Levels of cellular reactive oxygen species (ROS) were measured using CM-H2DCFDA. Briefly, after the neurons were treated with 10 μg/mL TSP or 100 μg/mL 4N1K for various times (0, 3, 6, 12, and 24 h), CM-H2DCFDA was added to the neuron cultures to a final concentration of 1.25 μM, and incubated for 30 min at 37°C. The amount of intracellular oxidants is proportional to the intensity of fluorescence. The fluorescence intensity of the cells was used as an indicator of the production of ROS, and analyzed by flow cytometry (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ, USA).
Western blotting analysis
Caspase 3 activation was determined by western blot. Cells were lysed in cell lysis buffer (Cell signaling) in the presence of protease inhibitors. Insoluble materials were removed by centrifugation (20 800 g, 20 min). The protein concentrations of the lysates were determined by Bio-Rad protein assay (Hercules, CA, USA). Equal amount of protein were separated in a 4–20% Tris–glycine gel (Invitrogen, Carlsbad, CA, USA) (30 μg/lane) for approximately 1.5 h at 110 V, and then transferred onto polyvinylidene difluoride membrane (0.2 μm; Invitrogen) at constant voltage (40 V) for 2 h. The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline (20 mM Tris–HCl, pH 7.4, 150 mM NaCl, and 0.05% Tween 20) and then probed with rabbit anti-caspase 3 primary antibody at 4°C overnight. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at 25°C, followed by an enhanced chemiluminescent substrate for detection of horseradish peroxidase (Pierce, Rockford, IL, USA).
Statistical analysis
Data were expressed as mean ± SD. Three to five separate experiments were performed. Data were analyzed using ANOVA with Tukey post hoc tests (SPSS version 11.5, SPSS Inc., Chicago, IL, USA). Statistical significance was at p < 0.05.
Results
Activation of CD47 is neurotoxic
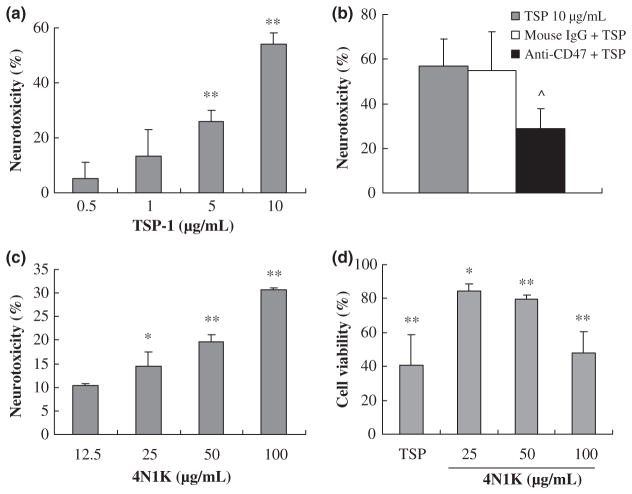
Neurotoxic effects of CD47 were evaluated using a standard LDH release assay. Exposure to the CD47 ligand TSP (0.5–10 μg/mL) for 24 h induced a dose-dependent cell death in primary cortical mouse neurons (Fig. 1a). Pre-treatment with a CD47 blocking antibody for 1 h significantly decreased TSP-induced neuronal death (Fig. 1b). The specificity of this pathway was further confirmed using 4N1K, a CD47-specific activating peptide. Exposure to 4N1K (12.5–100 μg/mL) induced a similar neurotoxic response (Fig. 1c). To further confirm these LDH neurotoxicity findings, we also measured cell viability using an MTT technique. Neuronal cell viability was significantly reduced after exposure to TSP (10 μg/mL) or 4N1K (25–100 μg/mL) for 24 h (Fig. 1d).
Fig. 1.
Neurotoxic effects of CD47 in primary mouse cortical neurons. (a) Exposure to CD47 ligand TSP for 24 h induced a dose-dependent neurotoxic response; (b) Pre-treatment with the CD47 blocking antibody for 1 h reduced TSP-induced cell death; (c) The CD47-specific activating peptide 4N1K induced a similar neurotoxic response; n = 3 independent experiments performed in triplicate by the LDH assay; (d) Cell viability reduced after exposure to TSP (10 μg/mL) and 4N1K (25–100 μg/mL) for 24 h; n = 3 independent experiments performed in triplicate by the MTT assay; *p < 0.05 and **p < 0.01 compared with the control group; ^p < 0.05 compared with TSP-treated group (10 μg/mL).
CD47-mediated neuronal death is partially caspase dependent
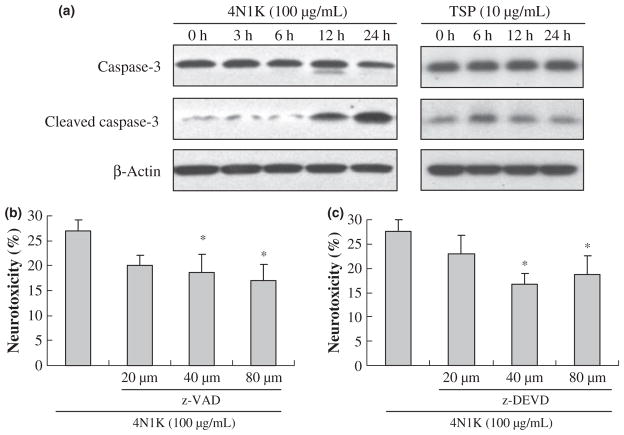
Exposure of neurons to 100 μg/mL of the CD47-activating peptide 4N1K over 24 h induced a clear activation of caspase 3. By 12 and 24 h, levels of cleaved 17 kDa caspase 3 were significantly elevated (Fig. 2a). Similarly, 10 μg/mL of TSP induced caspase 3 activation at 6 and 12 h (Fig. 2a). The neurotoxic role of caspases was confirmed with a pharmacologic approach. Treatment with either a broad-spectrum caspase inhibitor z-VAD-fmk or the caspase 3 inhibitor z-DEVD-fmk both significantly reduced CD47-mediated neuronal cell death (Fig. 2b and c). The specificity of these caspase pathways were further assessed by testing other protease inhibitors. In contrast to the neuroprotection obtained with caspase inhibition, neither calpain inhibitors nor necroptosis inhibitors provided any significant benefit (Fig. 3a and b).
Fig. 2.
CD47-mediated neuronal death is partially caspase-dependent. (a) Western blot detection of caspase 3 activation after treatment with 100 μg/mL 4N1K or 10 μg/mL TSP for various times. (b and c) Pre-treatment with caspase inhibitors (b, z-VAD-fmk and c, z-DEVD-fmk) significantly reduced 4N1K-induced cell death; n = 3 independent experiments performed in triplicate and *p < 0.05 compared with 4N1K-treated group.
Fig. 3.
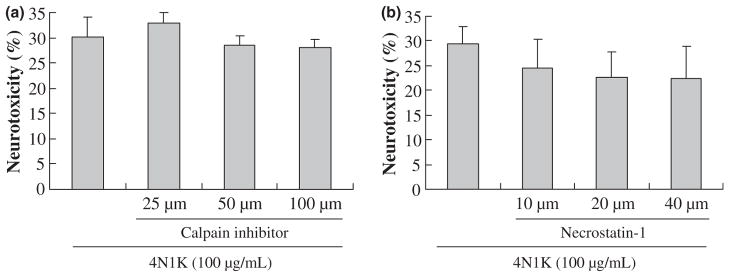
Calpain inhibitor III and necrostatin-1 cannot reduce 4N1K-induced cell death. (a) Calpain inhibitor III; (b) Necroptosis pathway inhibitor (Necrostatin-1); n = 3 independent experiments performed in triplicate. There is no significant difference among the groups.
CD47-mediated neuronal death activates oxidative stress in a caspase-independent pathway
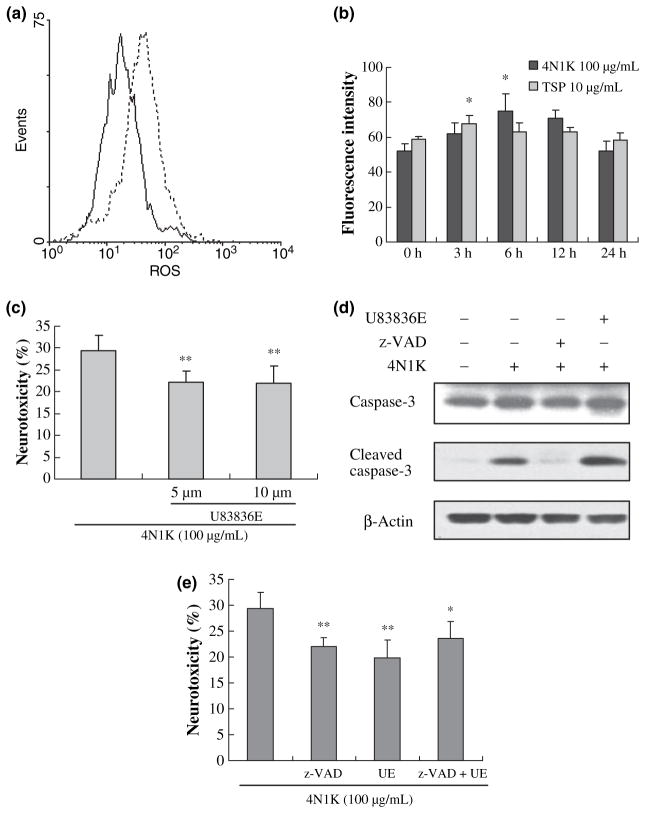
Although inhibition of caspases was neuroprotective, it did not completely prevent CD47-mediated neuronal death. Therefore, we asked whether other cell death mechanisms might be involved. Exposure of neurons to 10 μg/mL TSP or 100 μg/mL of the CD47-activating peptide 4N1K triggered the generation of ROS as measured with a dichlofluorescein assay (Fig. 4a). Quantitation of these measurements showed that ROS were generated in a transient manner, peaking at about 3 or 6 h after exposure to TSP or 4N1K (Fig. 4b), respectively. Accordingly, treatment with the potent ROS scavenger U83836E significantly reduced neuronal death (Fig. 4c). However, U83836E did not inhibit the cleavage/activation of caspase 3 (Fig. 4d), consistent the independence of the ROS versus caspase pathways. To further assess this idea of parallel and redundant pathways, we tested the efficacy of combination treatments of z-VAD-fmk (40 μM) plus U83836E (5 μM). Caspase inhibition plus radical scavenging did not yield additive neuroprotection (Fig. 4e).
Fig. 4.
CD47-mediated neuronal death activates oxidative stress in a caspase-independent pathway. After treatment with 10 μg/mL TSP or 100 μg/mL of 4N1K for different times, ROS was detected with the CM-H2DCFDA using the flow cytometry in primary mouse cortical neurons. (a) Representative fluorescence of ROS. Black line, 0 h group; dotted line, 6 h of 4N1K-treated group; (b) Bar graph of the fluorescence intensity of ROS (mean ± SD); n = 3 independent experiments; *p < 0.05 compared with 0 h group; (c) Pre-treatment with U83836E reduced 4N1K-induced cell death; n = 3 independent experiments performed in triplicate; **p < 0.01 compared with 4N1K-treated group. (d) After treatment with 100 μg/mL 4N1K for 24 h, cleavage of caspase 3 increased (lane 2). Pre-treatment with pan caspase inhibitor (z-VAD-fmk, 40 μM) inhibited the caspase 3 activation triggered by 4N1K (lane 3). However, ROS scavenger U83836E (5 μM) can not inhibit caspase 3 activation (lane 4); (e) Combination pre-treatments with z-VAD-fmk (40 μM) plus U83836E (UE, 5 μM) did not yield additive neuroprotection. n = 3 independent experiments performed in triplicate; *p < 0.05 and **p < 0.01 compared with 4N1K-treated group.
Discussion
Through its interactions with integrins, CD47 is perhaps best characterized as an inflammatory mediator that participates in the migration and infiltration of leukocytes and neutrophils (Cooper et al. 1995). More recently, however, a whole host of other extracellular ligands have been described including TSP and signal regulatory protein-α (Brown and Frazier 2001). Activation of CD47 receptor signaling through these external ligands is now known to trigger complex cell death pathways in a wide range of cell types (Mateo et al. 1999; Manna and Frazier 2003, 2004; Johansson et al. 2004; Saumet et al. 2005). Because CD47 can be expressed in neurons (Reinhold et al. 1995), it is conceivable that these pathways may also be operational in neurons. Our present study showed that activation of CD47 induced neuronal death via caspase and non-overlapping ROS pathways.
Others have reported that ligation of CD47 by TSP-1 or 4N1K induces a novel, caspase-independent apoptosis in other cell types such as T cells and leukemia cells (Mateo et al. 1999, 2002; Johansson et al. 2004; Saumet et al. 2005). However, our results were different. In our primary cortical neurons, a caspase-dependent component of cell death was detectable. Caspase 3 was cleaved and activated after activation of CD47 signaling. However, it was noted that caspase 3 activation induced by TSP seemed to take place earlier than 4N1K. It is possible that besides CD47, TSP may also bind with other receptors to induce apoptosis, e.g. CD36 (Jimenez et al. 2000; Li et al. 2003). Consistent with these caspase data, treatment with various caspase inhibitors (z-VAD-fmk and z-DEVD-fmk) was able to reduce CD47-mediated neuronal death. In contrast, calpain inhibitors were not neuroprotective. Furthermore, we eliminated a role for programmed necrosis or necroptosis (Degterev et al. 2005), since the potent necroptosis inhibitor necrostatin-1 also did not provide any protection against CD47-mediated death in our system.
Since CD47 is generally implicated in inflammation (Lindberg et al. 1996; Demeure et al. 2000; Hagnerud et al. 2006) and ROS is known to be involved in inflammation (Droge 2002), we also assessed the role of ROS in our experiments. Activation of CD47 signaling clearly elevated ROS levels in neurons, although the response appeared transient. The importance of ROS was confirmed by showing that the potent ROS scavenger U83836E significantly reduced CD47-mediated neuronal death. So it was possible that in our CD47-mediated neuronal death model, the transient ROS generation was an upstream trigger that up-regulated caspase 3. However, our results suggested that these two pathways may be independent. Whereas scavenging of ROS with U83836E significantly reduced neurotoxicity after CD47 signaling, there appeared to be no effects on caspase 3 activation. Taken together, these data suggest that caspase-dependent mechanisms as well as a caspase-independent ROS pathway is involved in CD47-mediated neuronal death. Furthermore, these pathways may be parallel and redundant because combining caspase inhibitors plus radical scavengers did not provide additional neuroprotection. The complexity of these mechanisms warrants further investigation.
A caveat in this study was our limited focus on caspase 3. So we cannot be sure about the role of intrinsic versus extrinsic caspase mechanisms. Caspase 8 can directly activate pro-caspase 3, which cleaves target proteins, leading to apoptosis. These death receptor signals are usually amplified in the mitochondria via Bid, Bax, and Bak processing (Schmitz et al. 1999). This results in mitochondrial outer membrane permeabilization and the release of pro-apoptotic proteins, including apoptosis-inducing factor, endonuclease G, cytochrome c, Omi/serine protease high temperature requirement protein A2, and second mitochondria-derived activator of caspase/direct integrin-associated protein binding protein with low pI, and provokes caspase-dependent and -independent cell death (Wang et al. 2004; Bras et al. 2005).
Independently of caspases, we also showed a central role for free radicals in CD47 signaling. Here, positive feedback loops of neuronal death may exist at the mitochondrial level (Leist and Jaattela 2001). Mitochondria are a major source of ROS generation and, at the same time, an important target for the damaging effects of ROS (Orrenius 2007). ROS induces DNA damage, oxidation of proteins, impairs mitochondrial respiration. High levels of ROS lead to overactivation of the enzyme poly(ADP-ribose) polymerase-1 in the nuclei and to NAD+ depletion, opening of mitochondrial transition pore in mitochondria, inducing translocation of apoptosis-inducing factor from the mitochondria to the nucleus, which is followed by DNA condensation, fragmentation, and cell death (Koh et al. 2005; van Wijk and Hageman 2005). Interactions between mitochondrial generation of ROS plus release of pro-apoptotic proteins with the activation of caspase-dependent and -independent apoptosis is highly complex (Ott et al. 2007). How these signals might provide crosstalk for CD47-mediated neuronal death requires careful validation. Ultimately perhaps, such pathways might be best targeted with agents that possess multiple effects on cell death and inflammation. Emerging findings in the field now suggest that such agents may exist, e.g. erythropoietin (Genc et al. 2004; Iwai et al. 2007), activated protein C (Mosnier et al. 2007), or even minocycline (Yong et al. 2004). The true utility of such agents will have to be validated in clinical trials of neuroprotection.
Our present findings may have relevance to CNS disease. It has been recently shown that CD47 is the key receptor that mediates the deleterious effects of TSP in ischemic tissues in vivo (Isenberg et al. 2007). Blockade of TSP-CD47 signaling alleviates tissue ischemia, and may provide a novel and accessible target for the treatment of cardiovascular disease (Isenberg et al. 2008). Insofar as similar cascades of tissue damage may be occurring in ischemic brain, CD47 signaling may underlie brain injury in stroke as well. Acute stroke patients with elevated TSP levels seemed to be more susceptible to further thrombosis and worsening (Legrand et al. 1991). If it is true that CD47 can mediate both inflammatory infiltration as well as direct neuronal cell death, then it might represent a novel neurovascular target for stroke therapy. Future studies, especially those using in vivo models, are warranted to validate the pathophysiologic role of CD47 in cerebral ischemia and neuronal injury.
Acknowledgments
This study is supported in part by R01-NS37074, R01-NS48422, R01-NS53560, P50-NS10828, P01-NS55104, and R01-AI064569.
Abbreviations used
- CM-H2DCFDA
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester
- LDH
lactate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ROS
reactive oxygen species
- TSP
thrombospondin
References
- Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) 2005;70:231–239. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- Bras M, Yuste VJ, Roue G, et al. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol. 2007;27:7073–7088. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc Natl Acad Sci USA. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol. 2000;164:2193–2199. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Genc S, Koroglu TF, Genc K. Erythropoietin as a novel neuroprotectant. Restor Neurol Neurosci. 2004;22:105–119. [PubMed] [Google Scholar]
- Hagnerud S, Manna PP, Cella M, Stenberg A, Frazier WA, Colonna M, Oldenborg PA. Deficit of CD47 results in a defect of marginal zone dendritic cells, blunted immune response to particulate antigen and impairment of skin dendritic cell migration. J Immunol. 2006;176:5772–5778. doi: 10.4049/jimmunol.176.10.5772. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Roberts DD, Frazier WA. CD47: a new target in cardiovascular therapy. Arterioscler Thromb Vasc Biol. 2008;28:615–621. doi: 10.1161/ATVBAHA.107.158154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- Johansson U, Higginbottom K, Londei M. CD47 ligation induces a rapid caspase-independent apoptosis-like cell death in human monocytes and dendritic cells. Scand J Immunol. 2004;59:40–49. doi: 10.1111/j.0300-9475.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- Koh DW, Dawson TM, Dawson VL. Mediation of cell death by poly(ADP-ribose) polymerase-1. Pharmacol Res. 2005;52:5–14. doi: 10.1016/j.phrs.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Araki T, Takai S, Yokomaku D, Ishikawa Y, Kubota M, Sano S, Hatanaka H, Yamada M. Expression of CD47/integrin-associated protein induces death of cultured cerebral cortical neurons. J Neurochem. 2002;82:249–257. doi: 10.1046/j.1471-4159.2002.00965.x. [DOI] [PubMed] [Google Scholar]
- Legrand C, Woimant F, Haguenau M, Caen J. Platelet surface glycoprotein changes in patients with cerebral ischemia. Nouv Rev Fr Hematol. 1991;33:497–499. [PubMed] [Google Scholar]
- Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- Li K, Yang M, Yuen PM, Chik KW, Li CK, Shing MM, Lam HK, Fok TF. Thrombospondin-1 induces apoptosis in primary leukemia and cell lines mediated by CD36 and caspase-3. Int J Mol Med. 2003;12:995–1001. [PubMed] [Google Scholar]
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- Manna PP, Frazier WA. The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J Immunol. 2003;170:3544–3553. doi: 10.4049/jimmunol.170.7.3544. [DOI] [PubMed] [Google Scholar]
- Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.can-03-1708. [DOI] [PubMed] [Google Scholar]
- Mateo V, Lagneaux L, Bron D, Biron G, Armant M, Delespesse G, Sarfati M. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med. 1999;5:1277–1284. doi: 10.1038/15233. [DOI] [PubMed] [Google Scholar]
- Mateo V, Brown EJ, Biron G, Rubio M, Fischer A, Deist FL, Sarfati M. Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells: link between phosphatidylserine exposure and cytoskeleton organization. Blood. 2002;100:2882–2890. doi: 10.1182/blood-2001-12-0217. [DOI] [PubMed] [Google Scholar]
- Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev. 2007;39:443–455. doi: 10.1080/03602530701468516. [DOI] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47) J Cell Sci. 1995;108:3419–3425. doi: 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- Saumet A, Slimane MB, Lanotte M, Lawler J, Dubernard V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphav-beta3 in promyelocytic leukemia NB4 cells. Blood. 2005;106:658–667. doi: 10.1182/blood-2004-09-3585. [DOI] [PubMed] [Google Scholar]
- Schmitz I, Walczak H, Krammer PH, Peter ME. Differences between CD95 type I and II cells detected with the CD95 ligand. Cell Death Differ. 1999;6:821–822. doi: 10.1038/sj.cdd.4400569. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death. J Neurosci. 2004;24:10963–10973. doi: 10.1523/JNEUROSCI.3461-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk SJ, Hageman GJ. Poly(ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion. Free Radic Biol Med. 2005;39:81–90. doi: 10.1016/j.freeradbiomed.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744–751. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]