Abstract
Health professionals and the public need to understand the natural history of human papillomavirus (HPV) infections of the cervix to best use the information provided by new molecular screening tests. We investigated outcomes of 800 carcinogenic HPV infections detected in 599 women at enrollment into a population-based cohort (Guanacaste, Costa Rica). For individual infections, we calculated cumulative proportions of three outcomes (viral clearance, persistence without cervical intraepithelial neoplasia grade 2 or worse [CIN2+], or persistence with new diagnosis of CIN2+) at successive 6-month time points for the first 30 months of follow-up. Cervical specimens were tested for carcinogenic HPV genotypes using an L1 degenerate-primer polymerase chain reaction method. Infections typically cleared rapidly, with 67% (95% confidence interval [CI] = 63% to 70%) clearing by 12 months. However, among infections that persisted at least 12 months, the risk of CIN2+ diagnosis by 30 months was 21% (95% CI = 15% to 28%). The risk of CIN2+ diagnosis was highest among women younger than 30 years with HPV-16 infections that persisted for at least 12 months (53%; 95% CI = 29% to 76%). These findings suggest that the medical community should emphasize persistence of cervical HPV infection, not single-time detection of HPV, in management strategies and health messages.
Human papillomavirus (HPV) assays are increasingly used in clinical practice, and there is increasing awareness that HPV causes cervical cancer. Indeed, some clinicians and members of the public become excessively alarmed at a finding of HPV detection (1-5). Cervical HPV infection is not a disease per se; infections with carcinogenic HPV types are extremely common, and the risk of cancer following single-time HPV detection (ie, its predictive value) is accordingly low, especially in young women (6-10).
On the other hand, persistent infections with a carcinogenic HPV type substantially increases the risk for cervical cancer, and the longer the carcinogenic infection lasts, the greater the risk (6,11-13). Distinguishing the risk associated with persistent vs transient HPV infection is especially important, given the application of HPV testing to screening. We present the outcomes (viral clearance, persistence without cervical intraepithelial neoplasia grade 2 or worse [CIN2+], or persistence with new diagnosis of CIN2+) during 30 months of follow-up of prevalent carcinogenic HPV infections detected at enrollment into a population-based cohort in Guanacaste, Costa Rica.
During 1993–1994, 10 049 randomly selected women, 18 years of age or older, living in Guanacaste were invited to participate in a longitudinal study of HPV infection and cervical neoplasia. The participation rate was 93.6%, and subsequent loss to follow-up was less than 10% (14). Baseline prevalence of carcinogenic HPV infection was similar to that in the United States; prevalence was 13.7% overall and highest (24.4%) in women 18–24 years old (8). All women signed an informed consent form at enrollment, and the study protocol was reviewed and reapproved annually by institutional review boards at the National Cancer Institute and in Costa Rica.
After enrollment, at which time all women with prevalent CIN2+ were treated by cold-knife cone, hysterectomy, or radio/chemotherapy, as appropriate, we targeted a risk-stratified subset of 2655 sexually active women to investigate the origins of incident CIN2+ (we also followed 410 non–sexually active women, but because they could not contribute prevalent HPV infections, they were excluded from this analysis) (15). Among the 2655 women, 1013 (38.2%) were HPV positive at enrollment and 657 (24.7%) had at least one carcinogenic HPV type. Women were followed with conventional and liquid-based cytology; samples for HPV testing were collected at each screening visit. Initially, 2163 women were followed annually and 492 women with low-grade intraepithelial lesion (LSIL) or CIN1 at baseline were followed at 6-month intervals. During the course of the study, as in regular clinical practice, women were shifted to an accelerated (every 6 month) screening if they presented with LSIL or CIN1. Approximately 3 months after enrollment, 1578 women (59.4%) underwent a colposcopy visit because of minor or equivocal baseline screening abnormalities or as normal control subjects without screening abnormalities. Cervical specimens collected at each visit were tested for the presence and type of HPV using an L1 degenerate-primer polymerase chain reaction–based method (8,16). The cohort study and ancillary projects continued for 10 years. New CIN2+ outcomes were defined by independent multiple reviews of histopathology by pathologists blinded to HPV results. Women with confirmed CIN2+ were treated with loop electrosurgical excision procedure, cold-knife cone, hysterectomy, or radio/chemotherapy, as appropriate (15). We optimized HPV typing by replicate testing and use of three different sets of primers (16-18) to adjudicate equivocal test results.
To standardize time among women, each clinic visit was assigned to one of seven time bins (0, 3, 6, 12, 18, 24, 30 months), with enrollment as 0. This analysis initially included 890 carcinogenic HPV infections detected in enrollment specimens among 657 women (180 of whom [27%] had infections with two or more carcinogenic types) who were actively followed and did not already have CIN2+ at enrollment. We considered persistence of each carcinogenic HPV genotype (HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, 82, 82v) independently (13).
We concentrated on the first 30 months of follow-up because clinicians are considering how to integrate HPV testing results into management of women during this time frame (19,20). The first 2 years or so after detection of carcinogenic HPV represents a clinically important decision period for whether to treat or not, during which the chance of clearance must be weighed against the risk of CIN2+.
Because of uncertain outcome, infections that were lost to follow-up before the end of the 30 months but whose infections were still persisting at the time of the last observation (n = 55, 6.2%) were excluded from the analysis. To further reduce un-certainty, we also excluded 35 (3.9%) infections with two or more consecutive intervening negative HPV results (including one CIN2).
For each infection in the final analysis (n = 800 infections detected among 599 women), we recategorized as positive (ie, as a false-negative test that day) any single intervening negative HPV result between positive tests (7.5%, 95% confidence interval [CI] = 5.8% to 9.5%) (11). After these recategorizations, infections with negative results during follow-up were considered cleared at the visit that yielded the first negative result. When there were missing results between the last positive and first negative HPV result (61%, 95% CI = 58% to 64%), we agnostically assigned clearance to the midpoint bin. Overall we imputed positive results at more than two bins for 168 (21%) of the 800 infections. We assigned “persistence with CIN2+” status to single or multiple persisting type-specific infections that were present from enrollment until the time bin that yielded CIN2+ histology.
We calculated cumulative proportions of three possible outcomes (clearance, persistence without CIN2+, or persistence with CIN2+) at successive time-bin points for all age groups combined, for women younger than 30 years (median age = 23 years), and for women 30 years of age and older (median age = 43 years), for all carcinogenic HPV types and for HPV-16 alone. We tested equality of proportions using large-sample statistics (α = .05, two-tailed).
Clearance of most of the 800 carcinogenic HPV infections was very rapid, with 55% (95% CI = 52% to 59%) and 67% (95% CI = 63% to 70%) clearing by 6 and 12 months, respectively (Figure 1, A). Persistence of these prevalently detected HPV infections at 30 months was less common in women younger than 30 years (35 of 393; 9%, 95% CI = 6% to 12%) (Figure 1, B) than among women 30 years and older (86 of 407; 21%, 95% CI = 17% to 25%) (Figure 1, C) (P < .001). Only 4% (95% CI = 3% to 6%) of carcinogenic HPV infections were linked to newly diagnosed CIN2+ by 30 months, and 7% (95% CI = 6% to 9%) were linked to it by year 7 (data not shown). However, viewed as a percentage of infections that persisted for at least 12 months (n = 153), the cumulative risk of a diagnosis of CIN2+ rose steadily after 12 months, reaching 21% (95% CI = 15% to 28%) by 30 months (32 of 153 persisting infections).
Figure 1.
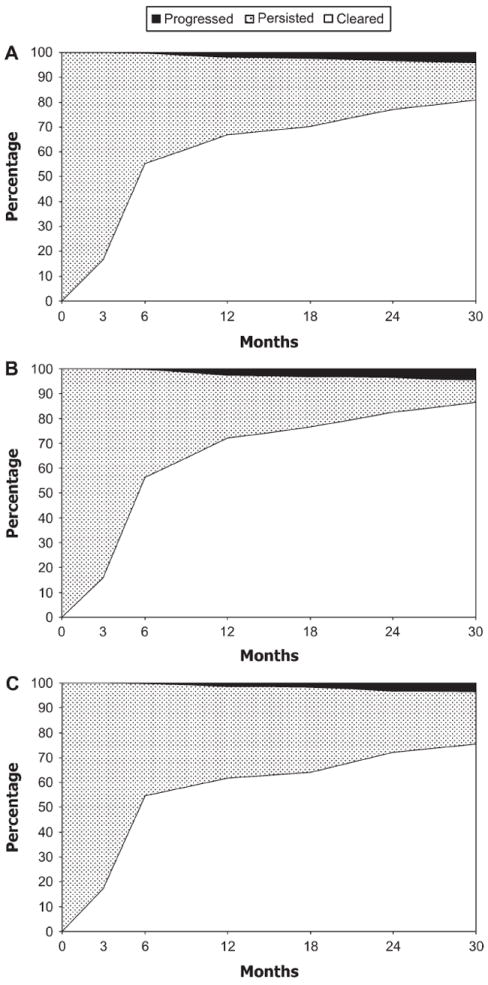
Clearance of carcinogenic human papillomavirus (HPV) infections during 30 months of follow-up. (A) All ages combined (n = 800 infections). (B) Women younger than 30 years (n = 393 infections). (C) Women aged 30 years or older (n = 407 infections). Carcinogenic types include HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, 82, 82v. The x-axes represent months of follow-up, and the y-axes represent the percentage of infections that cleared (unshaded), persisted without evidence of cervical intraepithelial neoplasia grade 2 or worse (CIN2+; stippled), or persisted with evidence of CIN2+ (solid shading). The 3-month data represent nonrandom samples (36% for A, 38% for B, and 34% for C).
For HPV-16, 45 of the 115 infections detected at enrollment persisted to 30 months; of these, 13 (29%; 95% CI = 16% to 44%) led to a CIN2+ diagnosis (Figure 2, A). The corresponding percentage was 53% (95% CI = 29% to 76%) among women younger than 30 years and 12% (95% CI = 2.4% to 30%) for women 30 years or older (Figure 2, B and C).
Figure 2.
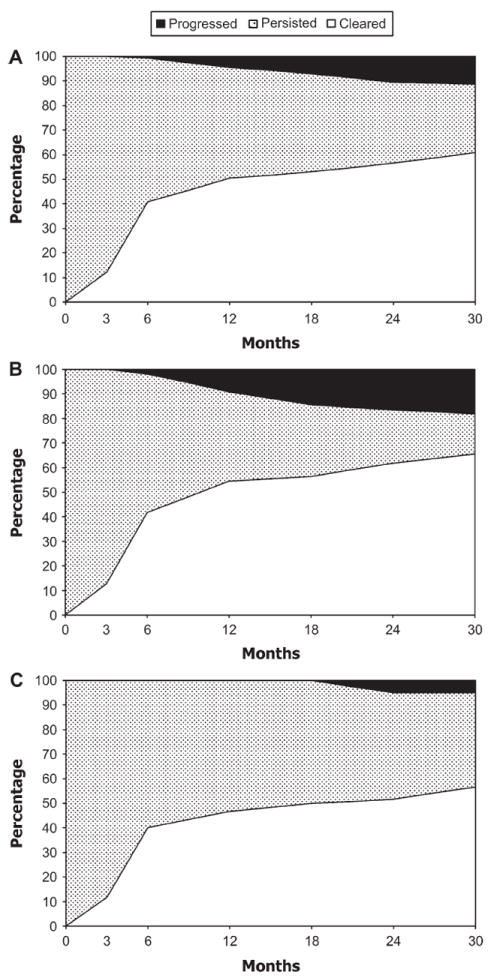
Clearance of human papillomavirus type 16 infections during 30 months of follow-up. (A) All ages combined (n = 115 infections). (B) Women younger than 30 years (n = 55 infections). (C) Women aged 30 years or older (n = 60 infections). The x-axes represent months of follow-up, and the y-axes represent the percentage of infections that cleared (unshaded), persisted without evidence of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) (stippled), or persisted with evidence of CIN2+ (solid shading). The 3-month data represent nonrandom samples (38% for A, 44% for B, and 33% for C).
These population-based, longitudinal data confirm that detection of carcinogenic HPV types is extremely common on a cross-sectional screening (8,13) but that the large majority of infections clear quickly (13). Clearance curves varied substantially by HPV type but were less affected by the presence of minor cytologic abnormalities at enrollment (data not shown). With greater duration of persistence, the chance for subsequent clearance declined and the risk of CIN2+ diagnosis among persisting infections rose (Figures 1 and 2). Interestingly, in our intensively screened cohort, most incident cases of CIN2+ associated with HPV-16 were diagnosed in women younger than 30 years.
Our clearance estimates are probably affected by screening intervals that were long compared with the rapid clearance of many HPV infections. Early clearance actually occurs faster than we could measure, especially for imputed midpoints when we had missing results between the last positive and first negative HPV result (13). At the time of CIN2+ diagnosis, four women out of 28 had two persistent infections with carcinogenic HPV; consequently, if we assume that each CIN2+ is caused by a single HPV type, our estimate of progression to CIN2+ is slightly overestimated. Two considerations must be taken into account when extrapolating the reported predominance of HPV-16 CIN2+ lesions in women younger than 30 years to other populations. First, our intensive screening strategy allowed detection of early CIN2+ lesions, lowering the average age of diagnosis (21); second, 50% of these lesions were CIN2 lesions, which have a higher regression rate than CIN3+ lesions and might have gone undetected with a less intensive screening strategy (22).
These data suggest that, when possible, a patient with a normal cytology and initial positive HPV result should be managed with watchful waiting because a 12-month follow-up can safely exclude more than 50% of infections as transient. Treatment of young women who have less than CIN3+ histopathology risks complications with uncertain benefit (23,24). Once type-specific HPV infections can be reliably detected by well-validated assays, new screening and management guidelines will need to be developed that incorporate these natural history data.
CONTEXT AND CAVEATS.
Prior knowledge
Cervical infections with carcinogenic human papillomavirus (HPV) types are common, but most infections are transient and do not increase a woman’s risk for cervical cancer. However, if the infection is persistent, the risk of cervical cancer is increased substantially. Understanding the natural history of HPV infection is important as molecular tests for HPV come into widespread use.
Study design
Longitudinal analysis of data from women enrolled in a population-based cohort in Guanacaste, Costa Rica, and screened regularly for HPV and cervical cytology. Viral clearance, viral persistence without cervical intraepithelial neoplasia grade 2 or worse (CIN2+), and viral persistence with CIN2+ were assessed over time for carcinogenic HPV infections detected at enrollment.
Contributions
Most infections cleared rapidly, with two-thirds clearing by 12 months. Among infections that persisted for at least 12 months, 21% were associated with a CIN2+ diagnosis by 30 months. The risk was highest in women younger than 30 years with persistent HPV-16 infection, with 53% of such infections leading to a diagnosis of CIN2+ by 30 months.
Implications
Patients with normal cytology but with cervical infection with carcinogenic HPV should be managed with watchful waiting because of the high likelihood that their infection will clear. Persistent infection calls for more active treatment.
Limitations
The intensive screening of women in this study could have led to overestimates of CIN2+ lesions because these have a relatively high regression rate. Similarly, the clearance estimates could have been affected by the screening intervals, with clearance likely occurring faster than could be measured.
Acknowledgments
Funding
National Institutes of Health (N01-CP21081, N01-CP33061, N01-CP40542, N01-CP50535, N01-CP81023, intramural program, CA78527 to R.B.).
Footnotes
The Guanacaste cohort (design and conduct of the study, sample collection, management, analysis and interpretation of the data) for the enrollment and follow-up phases was supported by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services. A. C. Rodríguez was supported by an appointment to the Senior Fellowship Program at the National Institutes of Health for analysis and manuscript preparation. The program is administered by the Oak Ridge Institute for Science and Education through an inter-agency agreement between the U.S. Department of Energy and the National Institutes of Health.
The authors take full responsibility for the design and conduct of the study, the analysis and interpretation of the data, the content of the manuscript, and the decision to submit it for publication.
References
- 1.Goldsmith MR, Bankhead CR, Kehoe ST, Marsh G, Austoker J. Information and cervical screening: a qualitative study of women’s awareness, understanding and information needs about HPV. J Med Screen. 2007;14(1):29–33. doi: 10.1258/096914107780154459. [DOI] [PubMed] [Google Scholar]
- 2.Jain N, Irwin K, Carlin L, Freeman C, Montano D, Kasprzyk D. Use of DNA tests for human papillomavirus infection by US clinicians, 2004. J Infect Dis. 2007;196(1):76–81. doi: 10.1086/518439. [DOI] [PubMed] [Google Scholar]
- 3.Tiro JA, Meissner HI, Kobrin S, Chollette V. What do women in the U.S. know about human papillomavirus and cervical cancer? Cancer Epidemiol Biomarkers Prev. 2007;16(2):288–294. doi: 10.1158/1055-9965.EPI-06-0756. [DOI] [PubMed] [Google Scholar]
- 4.Waller J, Marlow LA, Wardle J. The association between knowledge of HPV and feelings of stigma, shame and anxiety. Sex Transm Infect. 2007;83(2):155–159. doi: 10.1136/sti.2006.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D. Millions in U.S. infected with HPV. Washington Post. 2007 Feb 28;:A.1. [Google Scholar]
- 6.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(suppl 1):S1–S15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 7.Cuschieri KS, Cubie HA, Whitley MW, et al. Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. J Clin Pathol. 2004;57(1):68–72. doi: 10.1136/jcp.57.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrero R, Castle PE, Schiffman M, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191(11):1796–1807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 9.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 10.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papilloma-virus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97(14):1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 11.Schiffman M, Herrero R, Desalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337(1):76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191(2):182–192. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–1589. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 14.Herrero R, Schiffman MH, Bratti C, et al. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste Project. Rev Panam Salud Publica. 1997;1(5):362–375. doi: 10.1590/s1020-49891997000500005. [DOI] [PubMed] [Google Scholar]
- 15.Bratti MC, Rodriguez AC, Schiffman M, et al. Description of a seven-year prospective study of human papillomavirus infection and cervical neoplasia among 10000 women in Guanacaste, Costa Rica. Rev Panam Salud Publica. 2004;15(2):75–89. doi: 10.1590/s1020-49892004000200002. [DOI] [PubMed] [Google Scholar]
- 16.Castle PE, Schiffman M, Gravitt PE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68(3):417–423. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 17.Qu W, Jiang G, Cruz Y, et al. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol. 1997;35(6):1304–1310. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forslund O, Antonsson A, Nordin P, Stenquist B, Hansson BG. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J Gen Virol. 1999;80(pt 9):2437–2443. doi: 10.1099/0022-1317-80-9-2437. [DOI] [PubMed] [Google Scholar]
- 19.Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. Am J Obstet Gynecol. 2007;197(4):356.e1–6. doi: 10.1016/j.ajog.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2006;197(4):346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 21.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 22.Rousseau MC, Villa LL, Costa MC, Abrahamowicz M, Rohan TE, Franco E. Occurrence of cervical infection with multiple human papillomavirus types is associated with age and cytologic abnormalities. Sex Transm Dis. 2003;30(7):581–587. doi: 10.1097/00007435-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervicallesions: systematic review and meta-analysis. Lancet. 2006;367(9509):489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 24.Nohr B, Tabor A, Frederiksen K, Kjaer SK. Loop electrosurgical excision of the cervix and the subsequent risk of preterm delivery. Acta Obstet Gynecol Scand. 2007;86(5):596–603. doi: 10.1080/00016340701279145. [DOI] [PubMed] [Google Scholar]


