Abstract
Acute encephalitis, encephalopathy, and seizures are known rare neurologic sequelae of respiratory tract infection with seasonal influenza A and B virus, but the neurological complications of the pandemic 2009 swine influenza A (H1N1) virus, particularly in adults, are ill-defined. We document two young adults suffering from H1N1-associated acute respiratory distress syndrome and renal failure who developed cerebral edema. The patients acutely developed a transtentorial brain herniation syndrome including a unilateral third nerve palsy (dilated and unresponsive pupils), elevated intracranial pressure, coma, and radiological evidence of diffuse cerebral edema. In both patients, neurological deterioration occurred in the context of hyponatremia and a systemic inflammatory state. These patients illustrate that severe neurologic complications, including malignant cerebral edema, can occur in adults infected with H1N1 virus, and illustrate the need for close neurological monitoring of potential neurological morbidities in future pandemics.
Keywords: Brain edema, H1N1 subtype, Inappropriate ADH syndrome, Influenza A virus, Intracranial pressure, Respiratory distress syndrome, Systemic inflammatory response syndrome
1. Introduction
Respiratory tract infection with seasonal influenza A or B viruses cause substantial morbidity and mortality annually world-wide. Neurologic complications, including encephalitis, encephalopathy, and Reye’s syndrome, are relatively uncommon but likely under recognized manifestations of infection with seasonal influenza, accounting for nearly 5% of patients with acute childhood encephalitis or encephalopathy, and up to 6% of influenza-associated deaths among children each flu season.1 While the pathophysiology of influenza-associated encephalopathy is unknown and likely multifactorial, it often occurs in the context of a systemic inflammatory state,2–4 and is associated with the radiographic appearance of cerebral edema.5 Since its outbreak in the spring of 2009, an estimated 59 million illnesses and 12,000 deaths have been caused by influenza A (H1N1) virus.6 Infection most often results in an acute and self-limited febrile upper respiratory tract illness that includes rhinorrhea, cough, and sore throat. However, rapid progression to a fulminant systemic syndrome characterized by a diffuse viral pneumonitis associated with severe hypoxemia, acute respiratory distress syndrome (ARDS), renal failure, and shock can occur.7
The neurologic complications associated with the pandemic 2009 influenza A virus (H1N1) have only recently been recognized. 2,5 Seizures, encephalopathy, and status epilepticus are typical presentations, and appear to be more common in children than adults.8 Several case reports have also documented neurologic complications in adults with H1N1 influenza infection, with a subset of patients displaying a particularly malignant clinical course with high morbidity and mortality.5 Here, we describe two young adults who presented during the fall of 2009 with H1N1 infection, each rapidly progressing to ARDS, renal failure, and cerebral edema that resulted in transtentorial brain herniation.
2. Patients
2.1. Patient 1
In early December 2009, a 26-year-old morbidly obese Hispanic female presented with dyspnea and tachypnea. Her medical history was significant for asthma with several previous hospitalizations for flare-ups, and a 20 pack-year history of smoking (Table 1). She became confused, cyanotic, and short of breath. Her temperature was 101 °F (38.3 °C) and her oxygen saturation was 65% on room air. A chest radiograph obtained in the emergency ward (EW) demonstrated diffuse bilateral airspace opacities. The patient was admitted for pneumonia. On hospital day 1, she required intubation and mechanical ventilation. Broad spectrum antibiotic coverage was initiated and soon she developed oliguric acute renal failure. A nasal swab reverse transcriptase–polymerase chain reaction (rt–PCR) testing for H1N1 was positive. Over the next 2 days, she became anuric and continuous veno-venous hemofiltration (CVVH) was initiated. Nitric oxide nebulizer treatments were started for difficulty with oxygenation on ARDS ventilator settings. On hospital day 4, the patient had a new fixed (non-reactive) and dilated left pupil, measuring 8 mm. Neurology was emergently consulted for presumed transtentorial brain herniation in the setting of hyponatremia (serum Na, 126 mmol/L) and possible brain edema. Mannitol (100 g) and hypertonic saline (30 mL of 23% sodium chloride) boluses were immediately administered en route to the CT scanner. Imaging revealed diffuse effacement of the cortical sulci, a left uncus tightly abutting the midbrain without frank transtentorial herniation, and subtle loss of gray– white matter differentiation, particularly in the bilateral corticies (left greater than right), indicative of diffuse cerebral edema. An external ventricular drain (EVD) was emergently placed with an opening pressure of 26 cm H2O. Hypertonic saline boluses (23%) and a continuous 3% hypertonic saline infusion with a rate up to 60 mL/hour were administered. In addition, intermittent boluses of mannitol were given and cerebrospinal fluid (CSF) drainage from the EVD (averaging 280 mL of CSF per day) were required over the next 2 days to maintain intracranial pressure (ICP) below 20 cm H2O, and serum Na at a goal of >145 mmol/L (Fig. 1). Her pupils returned to normal size and function 7 hours following the placement of the EVD and initiation of hyperosmolar therapy. CSF culture, chemistries, and cell counts sampled from the EVD catheter remained unremarkable. After 14 days of aggressive therapy without signs of recovery, her family decided to transition her care to comfort measures only, and she died soon thereafter.
Table 1.
Selected characteristics and laboratory, radiologic, and neurodiagnostic results for two patients with acute neurologic complications associated with novel influenza A (H1N1)
| Patient 1 | Patient 2 | |
|---|---|---|
| Demographics | ||
| Age (years) | 26 | 29 |
| Sex | F | M |
| Ethnicity | Hispanic | Hispanic |
| Underlying medical conditions | ||
| Asthma | Yes | No |
| Diabetes | No | No |
| Immunocompromised | No | No |
| Chronic cardiovascular disease | No | No |
| Chronic renal disease | No | No |
| Neurocognitive disorder | No | No |
| Neuromuscular disorder | No | No |
| Other prior neurological conditions | No | No |
| Pregnancy | No | N/A |
| Seizure disorder | No | No |
| Other prior medical conditions | Gastroesophageal reflux | Malaria |
| Prior medications | Fluticasone, salmeterol, montelukast, nabumetone, omeprazole, albuterol, prednisone taper | None |
| Allergies | NKDA | NKDA |
| Ever a smoker | Yes (current) | No |
| BMI | 66 | 31 |
| ASA therapy | No | No |
| Clinical symptoms at presenting admission | ||
| Fever | Yes | Yes |
| Cough | Yes | Yes |
| Shortness of breath | Yes | Yes |
| Fatigue/weakness | Yes | Yes |
| Chills | Yes | No |
| Rhinorrhea | Yes | Yes |
| Myalgias | Yes | Yes |
| Headache | No | Yes |
| Sore throat | Yes | Yes |
| Vomiting | No | Yes |
| Wheezing | Yes | No |
| Diarrhea | No | No |
| Presence of neurological signs/symptoms at admission | No | No |
| Presenting admission laboratory data | ||
| Serum sodium (mmol/L) | 134 | 136 |
| Liver function tests | Elevated | Elevated |
| Blood bacterial culture | Negative | Negative |
| Urine bacterial culture | Negative | Negative |
| Viral testing and antiviral therapy | ||
| H1N1 reverse transcriptase–polymerase chain reaction positive | Yes | Yes |
| Hospitalization results | ||
| Length of hospitalization (days) | 11 | 8 days |
| Selected laboratory abnormalities during hospitalization | ||
| Sodium (Na) nadir (mmol/L) | 123 | 123 |
| Na at time of neurological presentation (mmol/L) | 126 | 126 |
| Neurologic presentation | ||
| Neurologic complication(s) diagnosed | Cerebral edema | Cerebral edema |
| Interval from respiratory illness onset to neurologic symptoms (days) | 3 | >1 week |
| Intracranial pressure at time of neurologic presentation (cm CSF) | 26 | N/A |
| Plasma creatinine at time of neurological presentation (mg/dL) | 3.93 | 5.81 |
| Creatine kinase at time of neurological presentation (U/L) | 4868 | 2935 |
| Neurodiagnostic testing | ||
| Head CT scan | Cerebral edema | Cerebral edema |
| Brain MRI | No (obese – unable to fit in scanner) | No (patient not stable for travel) |
| Placement of ICP monitoring device | EVD | None |
ASA = aminosalicylic acid, BMI = body mass index, CSF = cerebrospinal fluid, EVD = external ventricular drain, N/A = not applicable, NKDA = no known drug allergies.
Fig. 1.
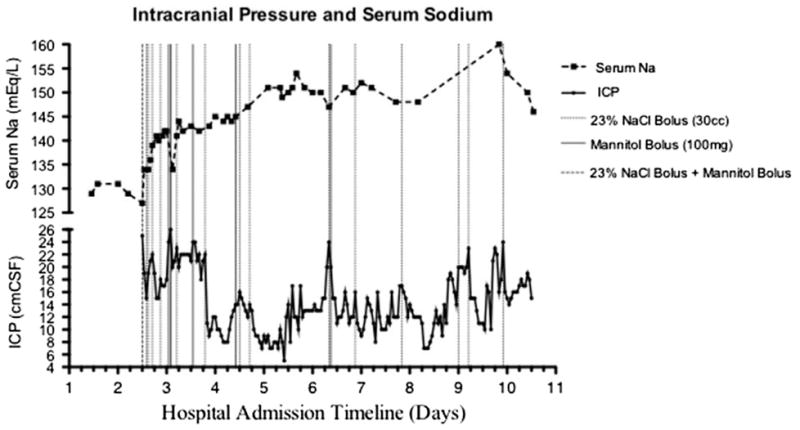
Patient 1: graph showing timeline of serum sodium (Na) and intracranial pressure (ICP).
2.2. Patient 2
In mid-August 2009, a 29-year-old, previously healthy man originally from Brazil developed progressive myalgias, dry cough, and headache (Table 1), along with chest pain on inspiration. A chest radiograph showed incomplete segmental consolidation of the apical posterior segment of the right upper lobe. Levofloxacin was started for presumed community-acquired pneumonia, and the patient was discharged home. The next day the patient developed nausea, vomiting, and blood-tinged emesis; he returned to his local hospital EW. On presentation, he was afebrile with a respiratory rate of 30/minute and an oxygen saturation of 94% on room air. A chest radiograph obtained in the EW demonstrated multifocal pneumonia. The patient was admitted to the hospital and started on broad-spectrum antibiotics. Later that day the patient was intubated for increasing respiratory distress and hypoxia; then transferred to our hospital.
The neurological exam at the time of transfer was limited secondary to the use of sedation and paralytics necessary for ventilation and oxygenation. Laboratory values revealed a serum sodium of 123 mmol/L (from 136 mmol/L on presentation 1 day earlier) confirming the diagnosis of acute hyponatremia. The patient soon developed oliguric renal failure. On hospital day 3, he was initiated on CVVH. In the early evening on hospital day 4, he was found to have an unreactive 8-mm right pupil. Serum Na was 126 mmol/L. Mannitol (100 g) and hypertonic saline (23%) boluses were immediately administered empirically. Continuous 3% hypertonic saline infusion was initiated. Chemical paralysis continued to be required and the patient was too unstable to undergo a portable CT scan. Given the concern for increased ICP and the inability to obtain routine neurological exams secondary to sedation and paralysis, an ICP monitor was indicated. However, the patient had an iatrogenic coagulopathy (therapeutic heparin infusion necessary to maintain vascular access for CVVH).
By the morning of hospital day 5, the patient’s pupils equalized in size at 4.5 mm. The serum Na was now 148 mmol/L. The patient continued on maximal ventilator settings. On hospital day 6, the patient’s condition stabilized such that a head CT scan could be attained. A non-contrast CT scan of the head demonstrated bilateral effacement of the cortical sulci, tight basal cisterns, and mild loss of gray/white cortical differentiation, consistent with diffuse cerebral edema (Fig. 2). Nasal swab rt–PCR testing for H1N1 was positive. On hospital day 7, the patient’s hemodynamics became increasingly unstable. The patient was unable to lay flat without developing increasing right pupillary diameter (indicating elevated ICP and third nerve compression from the adjacent temporal lobe). On the hospital day 8, the patient became increasingly hypoxic and acidotic. Hemodynamic instability developed into cardiac arrest and the patient expired despite extreme resuscitative efforts.
Fig. 2.
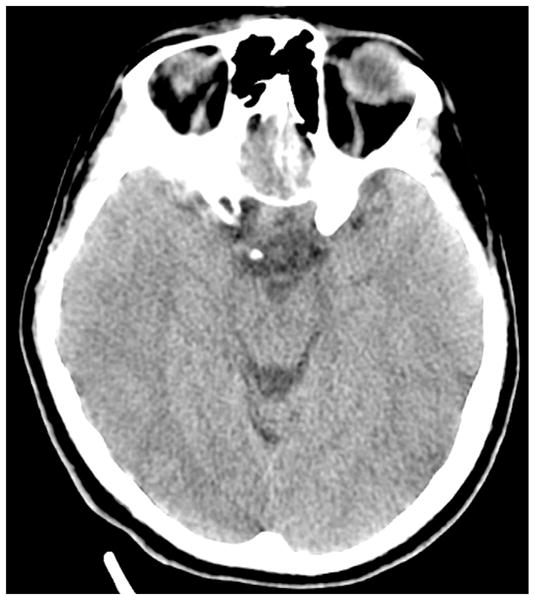
Patient 2: axial head CT scan showing diffuse cerebral edema.
3. Discussion
During the global pandemic of 2009, 33 adults diagnosed with influenza A (H1N1) were hospitalized at our institution; of these, 22 required admission to the intensive care unit. Both of our patients were obese, and developed ARDS, renal failure, and malignant cerebral edema. ARDS and renal failure are common in a severe and progressive syndrome associated with H1N1 infection,9 which can lead to shock and death in previously healthy persons who are young to middle-aged.7
Although several adult patients have been described,5 most cases of H1N1-associated neurological complications have been reported among children.8,10–12 In a recent study, H1N1 appears to be associated with heightened neurological complications compared to seasonal influenza: 18 of 303 hospitalized patients with 2009 H1N1 influenza developed a neurological complication, with seizures (67%) and/or encephalopathy (50%) being the most common, and focal neurological symptoms persisted in 22% of patients at the time of discharge.8
Both of our patients exhibited a transtentorial brain herniation syndrome, including a unilateral third nerve palsy (dilated and “fixed” unresponsive pupils), elevated ICP, and subsequent coma secondary to the development of acute, diffuse cerebral edema. The etiology of our patients’ brain swelling is unknown, but likely is multifactorial with contributions from both cytotoxic edema (related to hypoxic-ischemic injury and hyponatremia) and vasogenic edema (perhaps due to a systemic inflammatory state, which via cytokinemia can impact the permeability of the blood–brain-barrier). 3 Indeed, infection with influenza A virus increases the level of inflammatory cytokines in the blood and increases blood-brain barrier (BBB) permeability.13 Moreover, a pro-inflammatory systemic immune response has been documented with H1N1 viral infection.4 Exacerbating factors such as hypoxia may have occurred during episodes of oxygen desaturation before intubation, or with the progression of ARDS; hyponatremia was likely hypervolemic in nature, and secondary to anuric or oliguric renal failure.14
4. Conclusion
Severe neurologic complications, including malignant cerebral edema, can occur in adults infected with H1N1 virus, and illustrate the need for close neurological monitoring of potential neurological morbidities in patients during future pandemics. Severe infection with H1N1 virus, because of its ability to alter serum Na homeostasis and brain oxygenation via its effect on the kidney and lung, respectively, poses a risk for the development of cerebral edema, especially in the presence of potentially altered blood– brain barrier function. Therefore, hyponatremia should be aggressively treated and hypoxia should be avoided. When chemical paralysis and/or sedation for mechanical ventilation is needed, patients may benefit from having their medication lightened for frequent neurological exams, and a low threshold should be used by physicians to obtain neuroimaging, such as a non-contrast CT scan of the head.
References
- 1.Amin R, Ford-Jones E, Richardson SE, et al. Acute childhood encephalitis and encephalopathy associated with influenza: a prospective 11-year review. Pediatr Infect Dis J. 2008;27:390–5. doi: 10.1097/INF.0b013e31816507b2. [DOI] [PubMed] [Google Scholar]
- 2.Noriega LM, Verdugo RJ, Araos R, et al. Pandemic influenza A (H1N1) 2009 with neurological manifestations, a case series. Influenza Other Resp Viruses. 2010;4:117–20. doi: 10.1111/j.1750-2659.2010.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee N, Wong CK, Chan PKS, et al. Hypercytokinemia and hyperactivation of phospho-p38 mitogen-activated protein kinase in severe human influenza A virus infection. Clin Infect Dis. 2007;45:723–31. doi: 10.1086/520981. [DOI] [PubMed] [Google Scholar]
- 4.de Castro IF, Guzmán-Fulgencio M, García-Álvarez M, et al. First evidence of a pro-inflammatory response to severe infection with influenza virus H1N1. Crit Care. 2010;14:115. doi: 10.1186/cc8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akins PT, Belko J, Uyeki TM, et al. H1N1 Encephalitis with Malignant Edema and Review of Neurologic Complications from Influenza. Neurocrit Care. 2010;13:396–406. doi: 10.1007/s12028-010-9436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–19. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrand JJ, Herbener A, Rawlings J, et al. Heightened neurologic complications in children with pandemic H1N1 influenza. Ann Neurol. 2010;68:762–6. doi: 10.1002/ana.22184. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–7. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 10.Neurologic complications associated with novel influenza A (H1N1) virus infection in children – Dallas, Texas, May 2009. MMWR Morb. Mortal Wkly Rep. 2009;58:773–8. [PubMed] [Google Scholar]
- 11.Rellosa N, Bloch KC, Shane AL, et al. Neurologic Manifestations of Pediatric Novel H1N1 Influenza Infection. Pediatr Infect Dis J. 2011;30:165–7. doi: 10.1097/INF.0b013e3181f2de6f. [DOI] [PubMed] [Google Scholar]
- 12.Davis LE. Neurologic and muscular complications of the 2009 influenza A (H1N1) pandemic. Curr Neurol Neurosci Rep. 2010;10:476–83. doi: 10.1007/s11910-010-0135-1. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Sunden Y, Sakoda Y, et al. Lipopolysaccharide treatment and inoculation of influenza A virus results in influenza virus-associated encephalopathy-like changes in neonatal mice. J Neurovirol. 2010;16:125–32. doi: 10.3109/13550281003682521. [DOI] [PubMed] [Google Scholar]
- 14.Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356:2064–72. doi: 10.1016/j.jocn.2011.01.014. [DOI] [PubMed] [Google Scholar]


