Several studies examined the mechanism responsible for T-cell death during the contraction phase of an antiviral immune response focusing on the relative contribution of different death receptors and individual BH3-only proteins. These studies identified a dominant role for Bim1 with auxiliary roles for Puma2 in acute infection. In the case of chronic infections, Bim cooperates with Bid to constrain the T-cell response3 in concert with death receptor signals to control unrestrained inflammation and to preclude autoimmunity.4
Frequently overlooked, apoptosis also has a role in the early immune response, facilitating the selection of T-cell clones with highest affinity for pathogen-associated antigens. The deletion of low-affinity T-cell clones upon T-cell receptor (TCR) stimulation is correlated with the induction of several pro-apoptotic proteins including Noxa,5 Bim and Bax,6 which are antagonized effectively by pro-survival Bcl-2 family proteins in those T cells expressing a TCR with high affinity, thus leading to their clonal dominance (Figure 1).
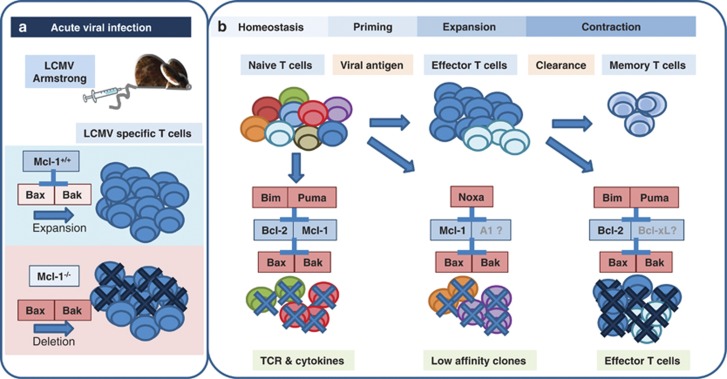
Figure 1.
(a) Mcl-1 prevents Bax/Bak activation in virus-specific T cells. Upon antigen encounter Mcl-1 protein levels increase in virus-specific T cells inhibiting Bax/Bak-dependent induction of apoptosis. Mcl-1 deficiency during an acute viral infection leads to massive deletion of antigen-specific T cells. (b) Bcl-2 family proteins involved in shaping the CD8 T-cell response during acute viral infection. Peripheral T cells receiving insufficient signaling through their TCR and IL2 or IL7 receptors activate Bim and Puma, which in turn leads to Bax or Bak activation, for example, by sequestration of Bcl-2 and Mcl-1. Upon antigen encounter and inflammation-driven co-stimulation, high affinity T-cell clones are selected to survive, whereas low-affinity TCR signaling facilitates Noxa-dependent death of sub-dominant clones that fail to stabilize or upregulate Mcl-1 and, possibly, A1. The contraction phase after acute infection is triggered by loss of pro-survival cytokines after antigen-clearance and is driven by Bim and Puma that neutralize Bcl-2 (and possibly Bcl-xL) for Bax/Bak activation
Until recently, the nature of the pro-survival Bcl-2 family proteins and the signaling pathways contributing to these effects remained rather ill-defined, as numerous studies proposed roles for Bcl-2, Bcl-xL, Mcl-1 and/or A1/Bfl-1 in different settings.5, 6, 7, 8, 9 Understanding individual contributions, however, is important in the development of intervention strategies for the treatment of infections and age-related immune-deficits.
Studies using adoptive transfer of Bcl-2 or Bcl-xL transgenic T cells into wild-type hosts, subsequently infected with lymphocytic choriomeningitis virus (LCMV), suggested that overexpression of neither protein had an impact on T-cell dynamics during infection in vivo, but saved virus-specific T cells from death in culture.8 However, it seems problematic to extrapolate from such results to loss-of-function phenotypes. Unfortunately, studies in Bcl-2−/− mice are hampered by the rapid loss of mature lymphocytes seen even in the absence of infection.10 Notably, although Bcl-xL is strongly induced upon T-cell activation, Lck-driven conditional ablation of this gene in T cells appears to have minimal effects on T-cell responses.11 Similarly, the relevance of the strong induction of A1/Bfl-1 noted after TCR engagement5, 6 still awaits elucidation, but A1 knockdown does not seem to affect T-cell homeostasis.12 Mcl-1, on the other hand, seems to be the key for T-cell development and survival, as Mx-Cre-mediated ablation leads to rapid loss of haematopoietic cells, whereas Lck- or CD4-cre-mediated deletion prevents normal T-cell development, precluding analysis of its role during T-cell activation.13
In this issue of CDD, Tripathi et al.14 provide compelling evidence that Mcl-1 is critical for promoting an effector T-cell response upon acute viral infection.
Expression analysis on LCMV-specific T cells at the peak of their expansion phase revealed that Mcl-1 levels were increased, whereas Bcl-2 levels dropped when compared with naive T cells.14 In order to demonstrate the relevance of Mcl-1 for the survival of activated T cells, expansion of LCMV-specific T cells was monitored during acute viral infection in mice, where Mcl-1 was deleted by concomitant Cre-expression from the IFN-sensitive Mx1-promoter. Infection led to reduced Mcl-1 levels in virus-specific T cells on day 5, but protein levels were comparable to wildtype on day 8 at the peak of T-cell expansion.14 This indicates that T cells escaping Cre-driven deletion expand to become the dominant virus-specific clones. Notably, despite their reduction in number the pathogen was still cleared effectively. This suggests that the few T cells escaping Cre-deletion can adequately control acute infection. Selection for non-deleting T cells was also seen in Lck–CreMcl-1f/null mice, where some residual mature T cells exist in the periphery.15 A caveat for the interpretation of the Mx-Cre infection experiments is that Mcl-1 is deleted also in numerous other cells, including haematopoietic cells and hepatocytes, which represent an important pool of infected cells that contribute to viral loads and their deletion could impact on viral dynamics.
Despite the normal expression of Mcl-1 in the few effector T cells present on day 8 post infection, the transient reduction of Mcl-1 levels during antigen priming apparently led to a profound loss of LCMV-specific T cells. However, it is not clear if the loss of antigen-specific cells was coincident with T-cell activation, that is, are the virus-specific T cells dying after they undergo TCR activation or is a reduced number of T cells responding because of loss of naive virus-specific precursors prior to TCR activation? The loss of Mcl-1's proposed role in mitochondrial physiology may also lead to limit outgrowth of antigen-specific T-cell clones. To separate these events, the authors generated compound mutant alleles, where either Bim or Bax and Bak were deleted along with Mcl-1, or where Bcl-xL was selectively overexpressed in T cells.
Overexpression of Bcl-xL failed to compensate for Mcl-1 loss in activated CD8+ T cells, indicating a nonredundant role for Mcl-1 in promoting survival in virus-specific CTLs. A general difficulty with the types of analyses performed here is to discriminate defective expansion versus accelerated contraction of effector cells. Circumstantial evidence suggests that Mcl-1 may be more important to mediate survival during expansion. Bim, which competes with Noxa for binding to Mcl-1 upon T-cell activation,5 has been described as the critical factor for T-cell deletion during the contraction phase of the immune response to viral infection,1 but its role in deleting low-affinity clones during T-cell expansion remained untested. To clarify if the loss of LCMV-specific T cells in Mx1Cre-Mcl-1f/f mice was mediated by unrestrained Bim activation upon TCR-ligation, the authors analyzed the antiviral response in Mx1Cre-Mcl-1f/fBimf/f mice. Surprisingly, the concomitant loss of Bim failed to rescue LCMV-specific T cells from the effects of Mcl-1 deficiency. This finding was unexpected, as loss of Bim can rescue T-cell production and increase the number of LCMV-specific T cells in Bcl-2-deficient mice.7 Together these data indicate that Bim is essential in regulating the number of naive T cells that can respond to antigen and limits the numbers of effector T cells during the contraction phase by targeting Bcl-2, but apparently not Mcl-1 during expansion.
Hence, Mcl-1 appears to act in a nonredundant and timed manner with Bcl-2 and Bcl-xL in mediating antigen specific T-cell survival upon infection, but this does not require direct inhibition of Bim, or at least not that of Bim alone. These data pointed towards Noxa or Puma as additional relevant targets.2 To cover all possibilities, the authors decided to delete Mcl-1 and Bax on a Bak-deficient background. Indeed, the concomitant loss of Bax and Bak rescued Mcl-1-deficient LCMV-specific T cells, demonstrating that it is the pro-survival function of Mcl-1 that is of importance here,14 but the identity of the relevant BH3-only pro-apoptotic factor remains unresolved.
Mcl-1 levels were previously reported to rise in parallel to the increased levels of the pro-apoptotic Bcl-2 family member Noxa and Bim in a TCR affinity-dependent manner that co-regulates IL-2R expression.5 The CTL response against LCMV infection is dominated by the accumulation of high-affinity T-cell clones that express TCRs directed against the dominant nucleoprotein NP396 epitope, followed by T cells specific for the glycoprotein GP33 epitope, whereas cells recognizing the subdominant GP276 epitope form a smaller component of the T-cell antiviral response. If Mcl-1 has a role in the prevention of apoptosis of the high-affinity clones, there should be a disproportionate loss of NP396 specific T cells compared with other clones if Mcl-1 is deleted. Insight into this potential affinity related role of Mcl-1 might be gained through the analysis of Mcl-1 haploinsufficient mice or mice that have Mcl-1 temporally deleted in T cells at the time of TCR activation.
In essence, based on the data published here14 and elsewhere,6 we can envision the existence of three distinct apoptotic programs that control T-cell survival during homeostasis, during activation and in effector cells (Figure 1). The size of the naive tolerant T-cell pool appears to be controlled by Mcl-1/Bcl-2 and Bim/Puma crosstalk, downstream of ‘self-peptide' MHC and cytokine receptors. Upon antigen encounter, high-affinity T-cell clones are selected and proliferate exponentially from the naive pool, whereas the low-affinity clones are deleted. This crucial selection step appears regulated by the strength of the TCR-signal that regulates the Noxa/Mcl-1 (and possibly A1) axis and shapes the effector T-cell response during the initial phase of infection.5 Once the pathogen is cleared effector T cells enter the contraction phase that leads to deletion of more than 90% of the effectors and to memory formation. Contraction is initiated by loss of pro-survival cytokines upon antigen clearance that may quickly destabilize Mcl-1 and survival for memory formation is then dictated again mainly by the balance between Bim and Bcl-2. What remains unclear at present, however, is why Bcl-xL is so strongly induced upon TCR ligation and what role the pro-survival Bcl-2 family proteins have during chronic infection, such as those caused by human immunodeficiency virus (HIV) or hepatitis B virus (HBV), that seem to follow different rules of engagement. In the end, when we finally understand all the details it may well come down again to the motto in Alexandre Dumàs novel The Three Musketeers ‘All for one, one for all!'
Acknowledgments
We apologize to scientists whose work could not be cited due to space constraints. Research in our laboratories is funded by the Austrian Science Fund (FWF) and the Australian National Health and Medical Research Council (NHMRC).
References
- Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci USA. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SF, Belz GT, Strasser A. BH3-only protein Puma contributes to death of antigen-specific T cells during shutdown of an immune response to acute viral infection. Proc Natl Acad Sci USA. 2008;105:3035–3040. doi: 10.1073/pnas.0706913105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson F, Kupresanin F, Mount A, Strasser A, Belz GT. Bid and Bim collaborate during induction of T cell death in persistent infection. J Immunol. 2011;186:4059–4066. doi: 10.4049/jimmunol.1001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischner D, Woess C, Ottina E, Villunger A. Bcl-2-regulated cell death signalling in the prevention of autoimmunity. Cell Death Dis. 2010;1:e48. doi: 10.1038/cddis.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensveen FM, van Gisbergen KP, Derks IA, Gerlach C, Schumacher TN, van Lier RA, et al. Apoptosis threshold set by Noxa and Mcl-1 after T cell activation regulates competitive selection of high-affinity clones. Immunity. 2010;32:754–765. doi: 10.1016/j.immuni.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Koenen P, Heinzel S, Carrington EM, Happo L, Alexander WS, Zhang JG, et al. Mutually exclusive regulation of T cell survival by IL-7R and antigen receptor-induced signals. Nat Commun. 2013;4:1735. doi: 10.1038/ncomms2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petschner F, Zimmerman C, Strasser A, Grillot D, Nunez G, Pircher H. Constitutive expression of Bcl-xL or Bcl-2 prevents peptide antigen-induced T cell deletion but does not influence T cell homeostasis after a viral infection. Eur J Immunol. 1998;28:560–569. doi: 10.1002/(SICI)1521-4141(199802)28:02<560::AID-IMMU560>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Wensveen FM, Klarenbeek PL, van Gisbergen KP, Pascutti MF, Derks IA, van Schaik BD, et al. Pro-apoptotic protein Noxa regulates memory T cell population size and protects against lethal immunopathology. J Immunol. 2013;190:1180–1191. doi: 10.4049/jimmunol.1202304. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, He YW. The antiapoptotic protein Bcl-xL is dispensable for the development of effector and memory T lymphocytes. J Immunol. 2005;174:6967–6973. doi: 10.4049/jimmunol.174.11.6967. [DOI] [PubMed] [Google Scholar]
- Ottina E, Grespi F, Tischner D, Soratroi C, Geley S, Ploner A, et al. Targeting antiapoptotic A1/Bfl-1 by in vivo RNAi reveals multiple roles in leukocyte development in mice. Blood. 2012;119:6032–6042. doi: 10.1182/blood-2011-12-399089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perciavalle RM, Opferman JT. Delving deeper: MCL-1's contributions to normal and cancer biology. Trends Cell Biol. 2013;23:22–29. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Koss B, Opferman JT, Hildeman DA. Mcl-1 antagonizes Bax/Bak to promote effector CD4 and CD8 T-cell responses. Cell Death Differ. 2013;20:998–1007. doi: 10.1038/cdd.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–674. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]