Abstract
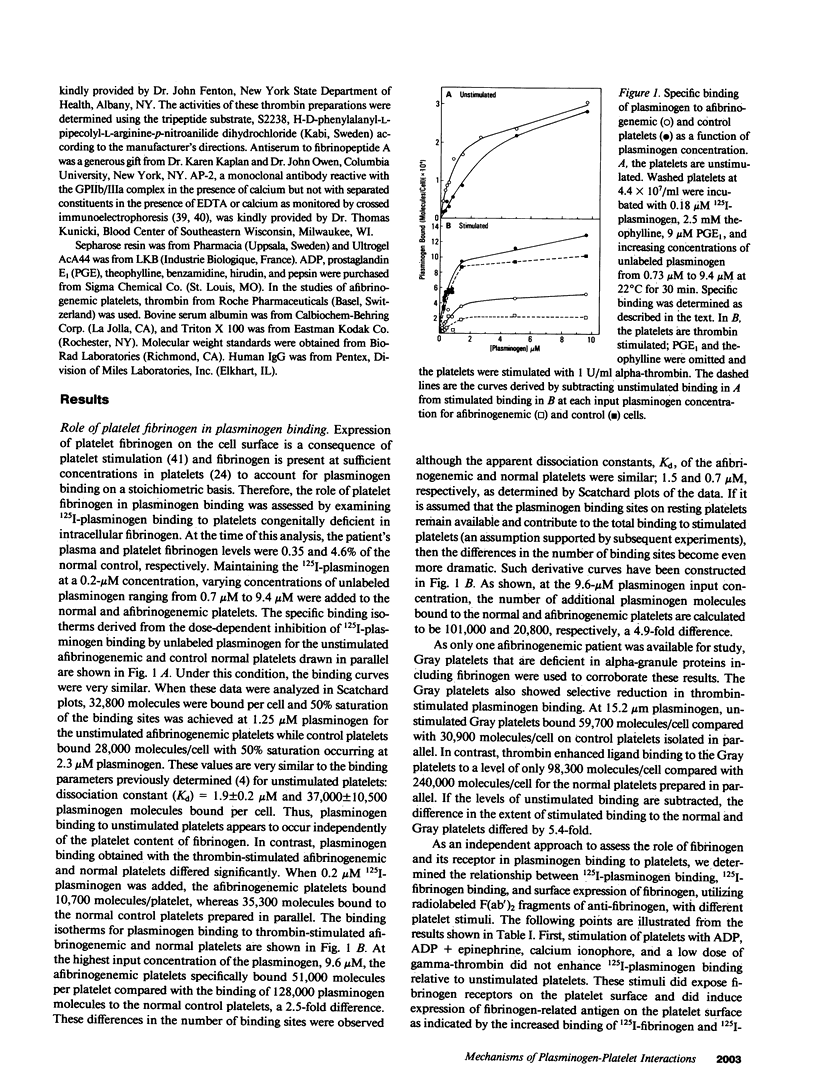
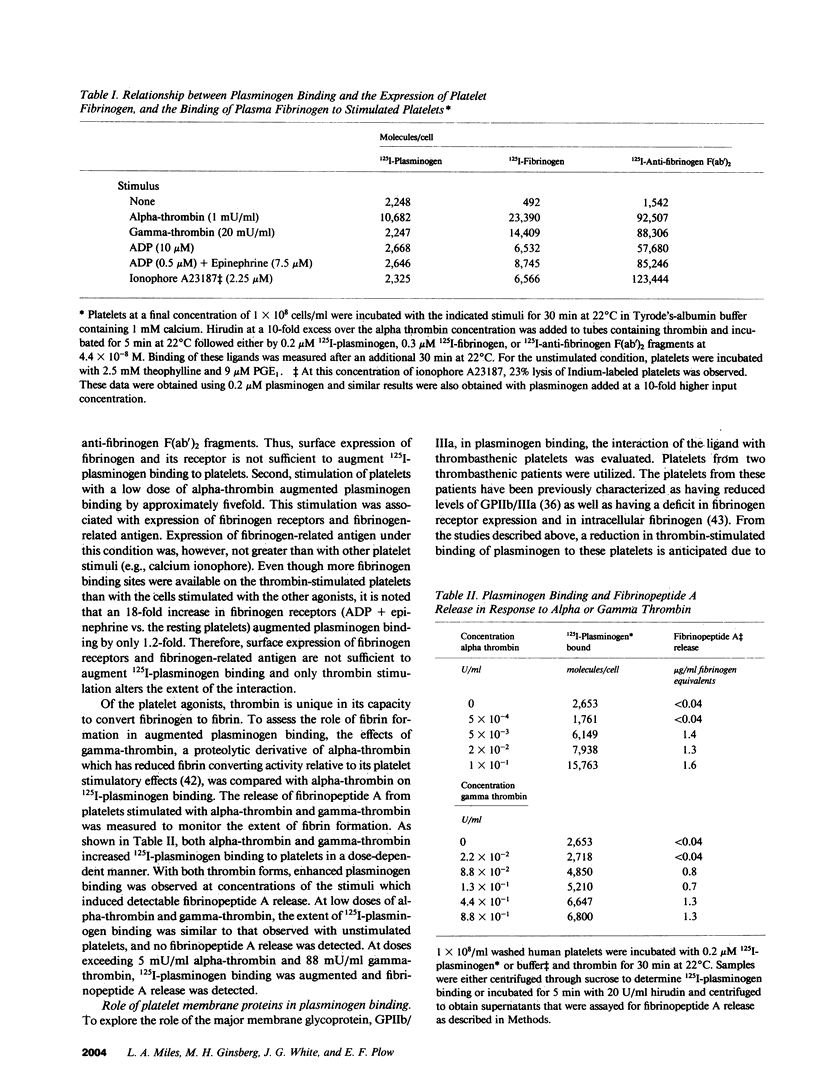
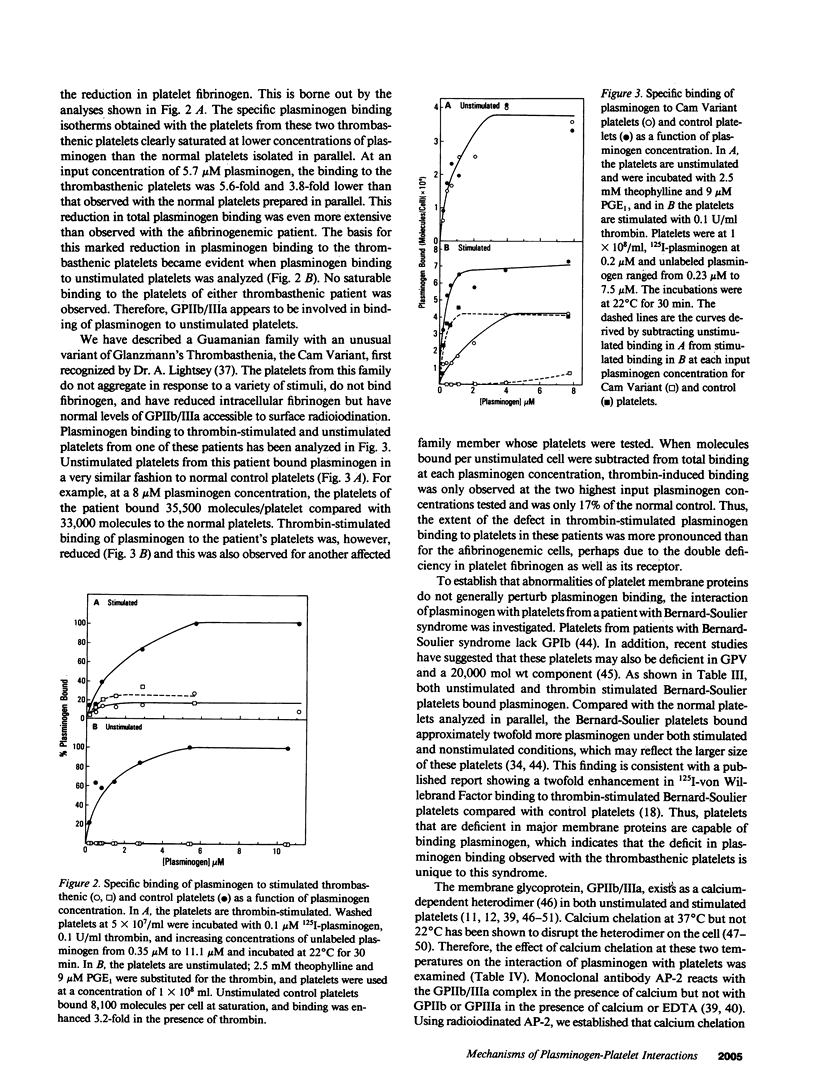
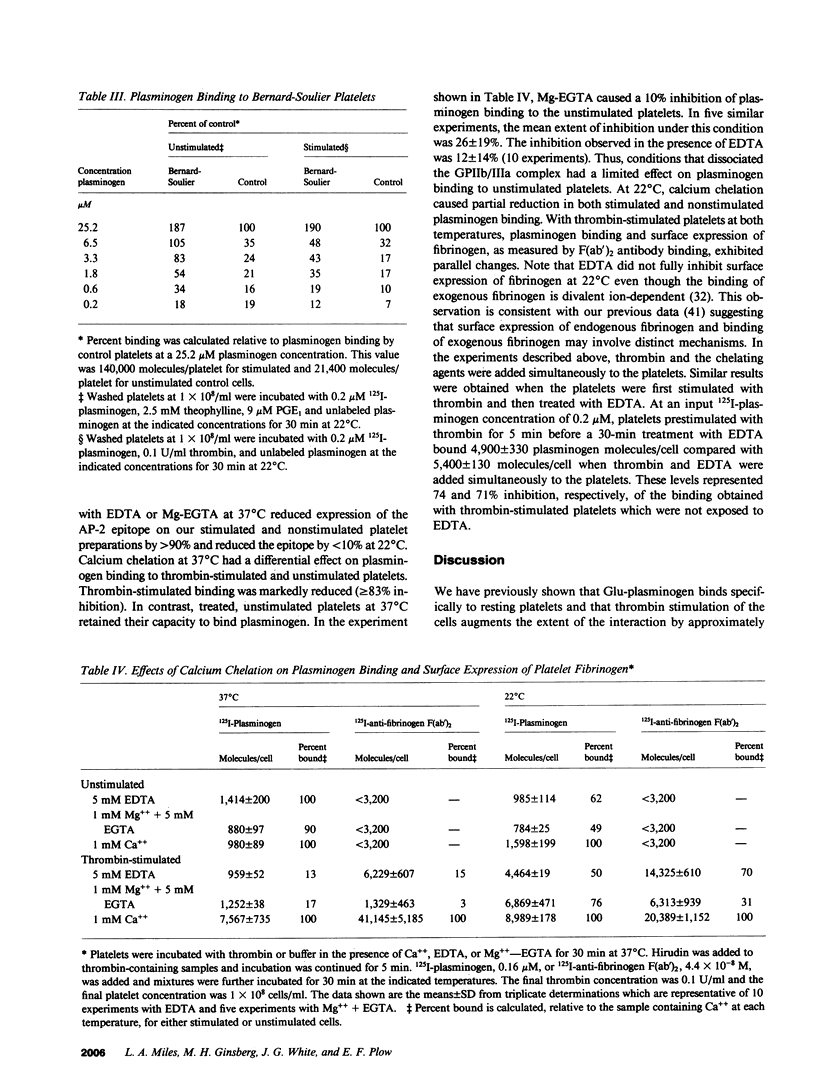
Glu-plasminogen, the native form of plasminogen, interacts in a specific and saturable manner with unstimulated human platelets, and the binding is enhanced fivefold by thrombin stimulation (Miles and Plow, 1985. J. Biol. Chem. 260:4303). This study characterizes the nature of the Glu-plasminogen binding sites by analyzing platelets deficient in selected proteins and functions. Platelets from patients with afibrinogenemia, Gray platelet syndrome, and the Cam Variant of thrombasthenia, a form of thrombasthenia with near normal levels of glycoprotein IIb/IIIa (GPIIb/IIIa), showed minimal augmentation of plasminogen binding to thrombin-stimulated platelets but normal binding to unstimulated platelets. This selective deficiency indicates that two distinct mechanisms are involved in the interaction of plasminogen with platelets. These abnormal platelets share a deficiency in fibrinogen. Surface expression of platelet fibrinogen, however, was not sufficient for enhanced plasminogen binding to stimulated platelets, and experiments with alpha-thrombin and gamma-thrombin indicated that fibrin formation on the platelet surface is necessary for the augmented plasminogen binding. Unstimulated and stimulated thrombasthenic platelets deficient in GPIIb/IIIa bound markedly reduced levels of plasminogen, which suggests a role for GPIIb/IIIa in plasminogen binding to unstimulated platelets. Treatment of platelets to dissociate the heterodimeric complex of GPIIb/IIIa did not significantly perturb plasminogen binding to unstimulated platelets, but the complex may be necessary for thrombin-stimulated plasminogen binding via its interaction with platelet fibrin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman B., Michelson A. D., Loscalzo J., Greenberg J., Handin R. I. Plasmin effect on platelet glycoprotein Ib-von Willebrand factor interactions. Blood. 1985 Jan;65(1):32–40. [PubMed] [Google Scholar]
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt M. C., Gregory C., Chong B. H., Zola H., Castaldi P. A. Additional glycoprotein defects in Bernard-Soulier's syndrome: confirmation of genetic basis by parental analysis. Blood. 1983 Oct;62(4):800–807. [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J., Kunicki T. J., Bennett J. S. Effect of calcium on the stability of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Jul 5;260(13):7875–7881. [PubMed] [Google Scholar]
- Charo I. F., Feinman R. D., Detwiler T. C. Interrelations of platelet aggregation and secretion. J Clin Invest. 1977 Oct;60(4):866–873. doi: 10.1172/JCI108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Schubert D., Schwartz S. A. Amino acid sequence studies on artiodactyl fibrinopeptides. I. Dromedary camel, mule deer, and cape buffalo. Arch Biochem Biophys. 1967 Feb;118(2):456–467. doi: 10.1016/0003-9861(67)90374-8. [DOI] [PubMed] [Google Scholar]
- Fujimura K., Phillips D. R. Calcium cation regulation of glycoprotein IIb-IIIa complex formation in platelet plasma membranes. J Biol Chem. 1983 Sep 10;258(17):10247–10252. [PubMed] [Google Scholar]
- George J. N. Studies on platelet plasma membranes. IV. Quantitative analysis of platelet membrane glycoproteins by (125I)-diazotized diiodosulfanilic acid labeling and SDS-polyacrylamide gel electrophoresis. J Lab Clin Med. 1978 Sep;92(3):430–446. [PubMed] [Google Scholar]
- Gerrard J. M., Phillips D. R., Rao G. H., Plow E. F., Walz D. A., Ross R., Harker L. A., White J. G. Biochemical studies of two patients with the gray platelet syndrome. Selective deficiency of platelet alpha granules. J Clin Invest. 1980 Jul;66(1):102–109. doi: 10.1172/JCI109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Forsyth J., Lightsey A., Chediak J., Plow E. F. Reduced surface expression and binding of fibronectin by thrombin-stimulated thrombasthenic platelets. J Clin Invest. 1983 Mar;71(3):619–624. doi: 10.1172/JCI110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Plow E. F., Forsyth J. Fibronectin expression on the platelet surface occurs in concert with secretion. J Supramol Struct Cell Biochem. 1981;17(1):91–98. doi: 10.1002/jsscb.380170111. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Wencel J. D., White J. G., Plow E. F. Binding of fibronectin to alpha-granule-deficient platelets. J Cell Biol. 1983 Aug;97(2):571–573. doi: 10.1083/jcb.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- Joist J. H. Platelets and fibrinolysis. Thromb Haemost. 1977 Dec 15;38(4):955–962. [PubMed] [Google Scholar]
- Kornecki E., Niewiarowski S., Morinelli T. A., Kloczewiak M. Effects of chymotrypsin and adenosine diphosphate on the exposure of fibrinogen receptors on normal human and Glanzmann's thrombasthenic platelets. J Biol Chem. 1981 Jun 10;256(11):5696–5701. [PubMed] [Google Scholar]
- Kunicki T. J., Newman P. J., Amrani D. L., Mosesson M. W. Human platelet fibrinogen: purification and hemostatic properties. Blood. 1985 Oct;66(4):808–815. [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Leung L. L., Harpel P. C., Nachman R. L., Rabellino E. M. Histidine-rich glycoprotein is present in human platelets and is released following thrombin stimulation. Blood. 1983 Nov;62(5):1016–1021. [PubMed] [Google Scholar]
- Lijnen H. R., Hoylaerts M., Collen D. Isolation and characterization of a human plasma protein with affinity for the lysine binding sites in plasminogen. Role in the regulation of fibrinolysis and identification as histidine-rich glycoprotein. J Biol Chem. 1980 Nov 10;255(21):10214–10222. [PubMed] [Google Scholar]
- Lucas M. A., Fretto L. J., McKee P. A. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1983 Apr 10;258(7):4249–4256. [PubMed] [Google Scholar]
- MORSE E. E., JACKSON D. P., CONLEY C. L. ROLE OF PLATELET FIBRINOGEN IN THE REACTIONS OF PLATELETS TO THROMBIN. J Clin Invest. 1965 May;44:809–816. doi: 10.1172/JCI105193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerie G. A., Edgington T. S., Plow E. F. Interaction of fibrinogen with its platelet receptor as part of a multistep reaction in ADP-induced platelet aggregation. J Biol Chem. 1980 Jan 10;255(1):154–161. [PubMed] [Google Scholar]
- Marguerie G. A., Thomas-Maison N., Larrieu M. J., Plow E. F. The interaction of fibrinogen with human platelets in a plasma milieu. Blood. 1982 Jan;59(1):91–95. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- Miles L. A., Plow E. F. Binding and activation of plasminogen on the platelet surface. J Biol Chem. 1985 Apr 10;260(7):4303–4311. [PubMed] [Google Scholar]
- Moake J. L., Olson J. D., Troll J. H., Tang S. S., Funicella T., Peterson D. M. Binding of radioiodinated human von Willebrand factor to Bernard-Soulier, thrombasthenic and von Willebrand's disease platelets. Thromb Res. 1980 Jul 1;19(1-2):21–27. doi: 10.1016/0049-3848(80)90400-4. [DOI] [PubMed] [Google Scholar]
- Moroi M., Aoki N. Isolation and characterization of alpha2-plasmin inhibitor from human plasma. A novel proteinase inhibitor which inhibits activator-induced clot lysis. J Biol Chem. 1976 Oct 10;251(19):5956–5965. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Dupuis D., Kunicki T. J., Caen J. P. Analysis of the glycoprotein and protein composition of Bernard-Soulier platelets by single and two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Clin Invest. 1981 May;67(5):1431–1440. doi: 10.1172/JCI110172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. M., Wehring B. Isoelectric characteristics and surface radioiodination of normal and thrombasthenic platelet membrane glycoproteins. Thromb Res. 1981 Apr 1;22(1-2):53–65. doi: 10.1016/0049-3848(81)90308-x. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Prasanna H. R. Ca2+-mediated association of glycoprotein G (thrombinsensitive protein, thrombospondin) with human platelets. J Biol Chem. 1980 Dec 25;255(24):11629–11632. [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Plow E. F., Collen D. The presence and release of alpha 2-antiplasmin from human platelets. Blood. 1981 Dec;58(6):1069–1074. [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. Unique immunochemical features and intracellular stability of platelet fibrinogen. Thromb Res. 1975 Nov;7(5):729–742. doi: 10.1016/0049-3848(75)90198-x. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Marguerie G. A., Ginsberg M. H. Fibronectin binding to thrombin-stimulated platelets: evidence for fibrin(ogen) independent and dependent pathways. Blood. 1985 Jul;66(1):26–32. [PubMed] [Google Scholar]
- Plow E. F., Marguerie G. A. Participation of ADP in the binding of fibrinogen to thrombin-stimulated platelets. Blood. 1980 Sep;56(3):553–555. [PubMed] [Google Scholar]
- Robbins K. C., Summaria L. Plasminogen and plasmin. Methods Enzymol. 1976;45:257–273. doi: 10.1016/s0076-6879(76)45025-5. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Brass L. F., Bennett J. S., Pandhi P. Biochemical and functional consequences of dissociation of the platelet membrane glycoprotein IIb-IIIa complex. Blood. 1985 Jul;66(1):92–98. [PubMed] [Google Scholar]
- Silverstein R. L., Leung L. L., Harpel P. C., Nachman R. L. Complex formation of platelet thrombospondin with plasminogen. Modulation of activation by tissue activator. J Clin Invest. 1984 Nov;74(5):1625–1633. doi: 10.1172/JCI111578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P., Wiman B. Characterization of human plasminogen. II. Separation and partial characterization of different molecular forms of human plasminogen. Biochim Biophys Acta. 1972 Jan 26;257(1):122–134. doi: 10.1016/0005-2795(72)90261-9. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Tschopp T. B., Baumgartner H. R., Sussman I. I., Johnson M. M., Egan J. J. Decreased adhesion of giant (Bernard-Soulier) platelets to subendothelium. Further implications on the role of the von Willebrand factor in hemostasis. Am J Med. 1974 Dec;57(6):920–925. doi: 10.1016/0002-9343(74)90170-3. [DOI] [PubMed] [Google Scholar]
- Wiman B., Collen D. Purification and characterization of human antiplasmin, the fast-acting plasmin inhibitor in plasma. Eur J Biochem. 1977 Aug 15;78(1):19–26. doi: 10.1111/j.1432-1033.1977.tb11709.x. [DOI] [PubMed] [Google Scholar]



