Abstract
Systemic lupus erythematosus (SLE) highlights the dangers of dysregulated B cells and the importance of initiating and maintaining tolerance. In addition to central deletion, receptor editing, peripheral deletion, receptor revision, anergy, and indifference, we have described a new mechanism of B cell tolerance wherein dendritic cells (DCs) and macrophages (MΦs) regulate autoreactive B cells during innate immune responses. In part, DCs and MΦs repress autoreactive B cells by releasing IL-6 and soluble CD40L (sCD40L). This mechanism is selective in that IL-6 and sCD40L do not affect Ig secretion by naïve cells during innate immune responses, allowing immunity in the absence of autoimmunity. In lupus-prone mice, DCs and MΦs are defective in secretion of IL-6 and sCD40L and cannot effectively repress autoantibody secretion suggesting that defects in DC/MΦ-mediated tolerance may contribute to the autoimmune phenotype. Further, these studies suggest that reconstituting DCs and MΦs in SLE patients might restore regulation of autoreactive B cells and provide an alternative to immunosuppressive therapies.
Keywords: Systemic lupus erythematosus, B cell tolerance, Autoimmunity, Dendritic cell, Macrophage, Smith antigen
Learning tolerance: a B cell’s story
A diverse B cell repertoire is critical in combating pathogens, but inherent in generating diversity is the threat of autoimmunity. In the bone marrow, central tolerance mechanisms such as deletion or receptor editing remove high-affinity autoreactive B cells before they exit to the periphery [1–10]. Those that escape are subject to receptor revision [8, 11–14], peripheral deletion [15, 16], or a shortened lifespan because they fail to enter B cell follicles [17–22]. In rare cases, autoreactive B cells are fully functional but indifferent to their specific antigen [23–25]. Finally, many low-affinity autoreactive B cells are maintained in an unresponsive state known as anergy. Anergic B cells do not receive sufficient activation signals to differentiate into plasma cells or secrete immunoglobulin (Ig) in response to antigenic or mitogenic stimulation [26–29]. Their proliferative responses to B cell receptor (BCR) or toll-like receptor (TLR) signaling as well as their lifespans vary in different models [18, 30–35]. Some anergic B cells transduce BCR-derived signals [30, 32, 36–40], while others exhibit desensitized BCRs [30, 33, 41]. Quiescence is dependent on chronic exposure to self-antigen and occupancy of the BCR [42, 43]. Furthermore, ERK activation is critical in sustaining anergy during polyclonal stimulation by TLR ligands [27, 28]. Recently, it was demonstrated that anergy can be mediated by DCs and MΦs. In the presence of TLR ligands, DCs and MΦs release IL-6 and sCD40L that selectively represses Ig secretion from autoreactive B cells [44, 45]. In the absence of DCs/MΦs and their soluble factors, autoreactive B cells regain the ability to secrete Ig, indicating that this mechanism of tolerance is reversible. These data suggest that autoreactive B cells must be maintained in close proximity to DCs/MΦs during innate immune responses to prevent autoimmunity.
The affinity and avidity of the antigen-BCR interaction determines whether developing B cells will be deleted, edited, anergized, or ignored [46]. Self-reactive B cells that survive these developmental checkpoints tend to bind self-antigens with low affinity and they remain anergic in the absence of costimulation by cognate T cells. Many of the early transgenic (Tg) models expressed BCRs with high affinities. However, concerns were raised that these models may not adequately reflect how bona fide low-affinity self-antigens are regulated [1, 26]. To study the tolerance mechanisms regulating low-affinity B cells, new Ig Tg models were generated that expressed BCRs specific for double-stranded (ds) DNA, single-stranded (ss) DNA, rheumatoid factor (RF), insulin, and Smith antigen (Sm).
A short history of Sm
Sm antigens are conserved proteins that are indispensable in RNA splicing. In 1966, Sm was identified as a unique autoantigen, the first non-histone target of autoantibodies in systemic lupus erythematosus (SLE) patients [47]. Named for 15-year-old Stephanie Smith, antibodies to Sm became one of the diagnostic criteria for SLE [48], detectable in 5–30% of SLE patients [48, 49]. Later studies correlated anti-Sm titers with kidney disease [50–53].
The autoantibodies isolated from patient sera proved invaluable in the initial studies of the Sm proteins [54]. The small nuclear RNA (snRNA U1, U2, U4, or U5) in each small nuclear ribonucleic particle (snRNP) is predicted to thread through the center of a heptameric ring of Sm proteins (E, F, G, D1, D2, D3, and B/B’) [55]. Three of the Sm proteins (D1, B, and D3) have long, positively charged tails that contact the pre-mRNA in the 5′ splice site region [56]. This interaction stabilizes the commitment complex formed when U1 snRNP binds the pre-mRNA substrate [57]. The remaining snRNPs, U2 and U4/U5/U6 (the triple snRNP) bind to pre-mRNA, rearrange to form the splicesome, and remove introns from the transcript [58].
Self-antigens composed of protein and DNA or RNA can co-ligate the BCR with TLRs, potentially overcoming tolerance mechanisms and activating autoreactive B cells. For example, concomitant ligation of the BCR and TLR9 by chromatin:IgG immune complexes (ICs) activates RF-specific B cells in the absence of T cell help or overt TLR stimulation [38, 59, 60]. Signaling through both the BCR and TLR9 is necessary to activate NF-κB in these B cells [37]. Recently, RNA-IgG ICs were shown to activate RF-specific B cells via TLR7 [39]. TLR7 binds ssRNA, a viral antigen that acts as a sensor of infection as well as a component of the U1 snRNP splicing complex, a known autoantigen in SLE [61, 62]. Aberrantly high expression of TLR7, or an increased burden of ICs and/or apoptotic cells, aggravates disease in lupus-prone mice. For instance, the gene duplication of TLR7 in Yaa mice results in hyperactive B cells, exacerbation of disease in lupus-prone models, and shifts autoantibody specificities to RNA [63, 64]. Reducing TLR7 gene expression ameliorates disease and increases survival [63]. Hence, the combination of self-proteins and TLR ligands within ICs and on the surface of apoptotic cells can mistakenly activate autoreactive B cells and result in autoimmunity.
Arresting and silencing Sm-specific B cells
Sm-specific autoantibodies are a hallmark of both human and murine lupus. To identify the mechanisms that regulate Sm-specific B cells, the 2–12H Tg mice were generated [35, 65]. In this model, an Ig heavy chain, 2–12H, was identified from an Sm-specific hybridoma derived from an MRL/lpr mouse. The 2–12H chain pairs with a variety of light chains, giving rise to B cells specific for Sm and/or ss-DNA. B cells from the 2–12H model express BCRs of multiple affinities that develop and are regulated on a non-autoimmune background.
Tolerance to Sm is dependent on several cell types. B cells are the most obvious suspects in SLE since disease pathology is mediated by autoantibodies. In vivo, Sm-specific B cells are regulated since 2–12H Tg mice have low titers of anti-Sm antibodies [35, 66]. However, ex vivo non-subsetted 2–12H B cells (uncontaminated by DCs and MΦs) are activated by TLR stimulation (LPS, CpG, dsRNA) in vitro but their Ig secretion is lower than that of C57BL/6 controls [35, 67]. The follicular (FO) B cell subset is repressed by DCs and MΦs secreting IL-6 and sCD40L, while secretion by the MZ B cell subset is partially repressed, but only by MΦs and sCD40L [45]. Some MZ B cells and peritoneal B-1 cells ignore endogenous levels of Sm, but an increase in the number of apoptotic cells can activate peritoneal and MZ B cells [66, 68, 69]. Sm-specific B cells arrested at the pre-plasma cell stage, interrupting plasma cell differentiation and preventing Ig secretion [32].
Restricting the light chain that pairs with 2–12H allowed for the analysis of Sm-specific B cells of moderate and low affinity [32, 70]. The 2–12H/Vκ4 Tg mouse was generated to examine regulation of higher affinity anti-Sm responses [70]. B cells from this mouse are distributed among splenic transitional, FO, and MZ subsets, as well as the peritoneal B-1 subset [70]. 2–12H/Vκ4 B cells are anergic and all subsets are hyporesponsive to LPS in vitro. Additionally, MZ B cells exhibit a block in BCR signaling [70]. LPS-stimulated 2–12H/Vκ4 B cells are repressed by IL-6 and sCD40L (unpublished data). To study low-affinity anti-Sm responses, the 2–12H/Vκ8 Tg mouse was created [32]. In this model, only transitional and FO B cells are present and these cells are regulated by anergy [32]. As in the previous anti-Sm models, 2–12H/Vκ8 B cells are susceptible to IL-6- and sCD40L-mediated repression [44, 45].
T cells are implicated in SLE and Sm-specific T cells are present in the repertoires of both normal and autoimmune mice [71]. Sm-specific T cells in 2–12H Tg mice are anergic and do not proliferate in response to B cells presenting Sm [71]. Anergic T cells are also unable to upregulate CD40L and provide costimulation to their cognate B cells [72]. Anti-Sm B cells do not secrete Ig in vivo [35, 65], perhaps because they are deprived of T cell costimulation. However, in autoimmune situations, autoreactive T cells induce class-switching and somatic hypermutation of anti-Sm B cells, resulting in high levels of pathogenic high-affinity IgG autoantibodies [73, 74]. Paradoxically, anti-Sm B cells are required to tolerize Sm-specific T cells from C57BL/6 mice, but they activate Sm-specific T cells from MRL/lpr mice [71, 75, 76]. This indicates that although T cells are necessary for the development of autoantibodies and disease, they are also regulated by autoreactive B cells in normal individuals.
DCs and MΦs regulate innate and adaptive immune responses by tolerizing or activating T and B cells. The continued ingestion and presentation of self-antigens or the acute presentation of foreign antigen by DCs/MΦs either tolerizes or activates T cells to drive adaptive immune response. The activation of DCs during innate immune responses induces the secretion of IL-6 that promotes immunity by releasing CD4+ T-helper cells from their inhibitory functions [77]. This promotes polyclonal activation of naive B cells, the production of neutralizing antibody and the clearance of the invading pathogen.
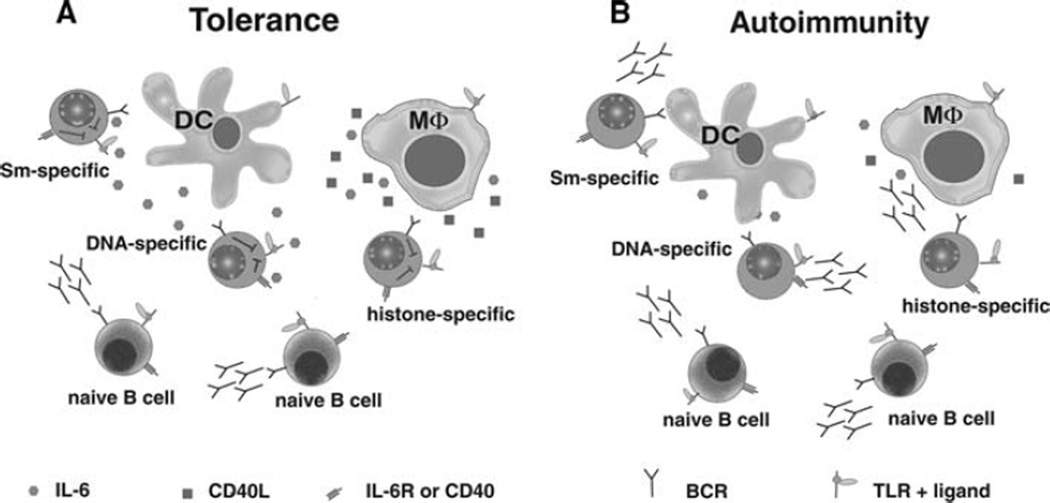
In addition to regulating T cells, DCs and MΦs affect the fate of B cells. They activate naïve B cells by secreting type I interferon, IL-6, and B lymphocyte stimulator (BLyS) [78–80]. They also repress Ig secretion by B cells that have been chronically exposed to antigen. Our laboratory showed that DCs and MΦs regulate autoantibody production, in part through their secretion of IL-6 and sCD40L [44, 45]. Repression is selective in that naïve B cells (not chronically exposed to antigen) are unaffected by the presence of IL-6 and sCD40L while Ig secretion by autoreactive B cells is repressed (Fig. 1a). Coupled with the data showing that IL-6 de-represses regulatory T cells, a mechanism emerges explaining how the pleiotropic affects of IL-6 produced by DCs and MΦs simultaneously promotes immunity and represses autoimmunity during innate immune responses. Signal transduction through many cell surface receptors influences neighboring receptors.
Fig. 1.
DCs and MΦs repress antibody secretion from autoreactive B cells via IL-6 and sCD40L. (a) In non-autoimmune mice, B cell tolerance is maintained during innate immune responses by DCs and MΦs. Although TLR stimulation promotes Ig secretion by B cells, it simultaneously induces DCs and MΦs to secrete IL-6 and sCD40L. These soluble factors repress Ig secretion from autoreactive B cells while allowing innate stimuli to produce a polyclonal response by naïve B cells. (b) In lupus-prone mice, DCs and MΦs are defective in the production of IL-6 and sCD40L, allowing both autoreactive and naïve B cells to produce Ig in response to TLR stimulation
For example, crosstalk between the IFN-αβ and IL-6 receptor (IL-6R) signaling pathways augment transcription factor binding and gene expression in mouse embryonic fibroblasts (MEFs) [81]. Similarly, stimulation of B cells with sCD40L, IL-4, and LPS reprograms the BCR signaling pathway, enhancing ERK activation and bypassing the requirement for phosphatidylinositol 3-kinase (PI3-K) [82–86]. The findings that only B cells chronically exposed to self-antigen are susceptible to repression by IL-6 and sCD40L suggests that chronic BCR-derived signals “reprogram” the outcome of IL-6R and CD40 signal transduction. On a molecular level, the ability of IL-6 and sCD40L to repress Ig secretion reflects diminished BLIMP-1 and XBP-1 mRNA and protein levels. These data indicate that regulation occurs upstream of transcriptional activation. In support of this, pharmacologically inhibiting MEK restores LPS-induced Ig secretion. This suggests that the ability of IL-6/sCD40L to repress TLR4-induced Ig secretion is MEK/ERK-dependent (unpublished data).
Susceptibility of B cells to IL-6/sCD40L requires that B cells be chronically exposed to antigen, consistent with a central role for the BCR in tolerance. High-affinity neo-self-antigens direct a unique tolerance scheme compared to low-affinity self-antigens. Anergic B cells from high-affinity models are characterized by elevated phospho-ERK [42, 87]. In addition, the binding of high-affinity antigen to the BCR and constitutive MEK/ERK activation is sufficient to repress TLR4 and TLR9-induced Ig secretion [27, 28]. In the low-affinity Sm model (2–12H/Vκ8) basal phospho-ERK levels are comparable to those in the HEL model. However, unlike the HEL model, the binding of soluble SmD or snRNPs coupled with elevated phospho-ERK levels does not repress TLR-induced Ig secretion (unpublished data). This indicates that ERK is only part of the “BCR-derived” signals that regulate innate immune responses in vivo. Although B cells expressing high-affinity receptors can be repressed by IL-6 and sCD40L, antigen stimulation is sufficient. In contrast, B cells expressing low-affinity receptors for bona fide self-antigens (Sm and ssDNA) do not achieve sufficient BCR-derived signals to influence TLR4-induced Ig secretion and depend on additional signals from IL-6/sCD40L (unpublished data).
The finding that DCs secrete IL-6 while MΦs secrete IL-6 and sCD40L suggests that the anatomic location within the secondary lymphoid organs might dictate how autoreactive B cells are regulated. Marginal zone B cells are solely repressed by sCD40L while FO B cells are repressed by IL-6 and sCD40L [45]. This specificity may result from the anatomic location, since different subsets of DCs and MΦs localize to specific regions of the spleen. For example, B cells are retained in the marginal zone by MΦs [88] and they are regulated by MΦ-derived sCD40L. However, upon activation and differentiation into pre-plasma cells, they may become susceptible to repression by IL-6 secreted by DCs within the periarteriolar lymphoid sheath (PALS). Thus, depending on the anatomic location of the autoreactive B cell, DCs and/or MΦs can repress autoantibody production during innate immune responses.
Failed tolerance: Sm-specific autoantibodies in a murine model of SLE
MRL/lpr mice are a well-characterized murine model of SLE, with adult-onset disease mediated by autoantibody deposition and tissue destruction. The prevalence of anti-Sm autoantibodies in human SLE patients and MRL/lpr mice is approximately 25% [89, 90]. BCR Tg models have been bred onto the MRL background, allowing B cells of known specificities to be followed throughout development. Developmental arrest, FO exclusion, and receptor editing are defective in MRL/lpr mice [91, 92]. Expression of the 2–12H transgene in MRL/lpr mice increases the prevalence of the anti-Sm response, accelerates disease, and leads to higher serum anti-Sm levels [65]. Sm-specific B cells from 2–12H mice are arrested at the pre-plasma cell stage, while B cells from the 2–12H/MRL/lpr mice bypass this checkpoint and become activated [93].
The presence of class-switched autoantibodies in MRL/lpr mice suggests a breakdown in tolerance within the adaptive immune response. In MRL/lpr mice where somatic hypermutation and isotype switch recombination are blocked (AID−/−), lupus-like symptoms such as glomerulonephritis, proteinuria, and immune complex deposition are ameliorated [94]. Early studies showed a critical role for T cells in disease because thymectomized MRL/lpr mice failed to develop lupus-like disease [95]. Subsequently, it was shown that defects in central deletion and the number and function of T-regulatory cells allow CD4+ T-helper cells to activate autoreactive B cells, induce terminal differentiation and autoantibody production [96–101].
Dysregulation of the innate immune system is apparent in MRL/lpr mice [39]. ICs containing RNA or chromatin stimulate RF-specific B cells through TLR7 and TLR9 to secrete anti-Sm and anti-chromatin [38, 39]. Consistent with a role for TLRs, autoantibody responses were reduced in MyD88−/−/MRL/lpr mice [39] and disease was ameliorated [102]. TLR7−/−/MRL/lpr mice exhibit reduced autoantibody titer and gene duplication of TLR7 shifts autoantibody specificities toward RNA, exacerbating disease [63, 64]. This reveals TLR7 as a key receptor in promoting autoimmunity when tolerance is overcome [63, 103]. Like TLR7−/−/MRL/lpr mice, TLR9−/−/MRL/lpr mice exhibited lower titers of anti-DNA autoantibodies. However unlike TLR7−/−/MRL/lpr mice, they remain plagued by accelerated kidney disease and increased mortality [103]. These data suggest that TLR9 induces anti-DNA responses but also has an anti-inflammatory effect, possibly through its induction of regulatory T cells [104, 105]. The function of regulatory T cells in TLR9−/−/MRL/lpr mice is impaired, potentially allowing autoreactive cells to exacerbate disease [106]. These data indicate that innate immune responses have tremendous and opposing effects on autoantibody production and disease in MRL/lpr mice.
Defects in DC/MΦ-mediated tolerance are evident in lupus-prone mice. Our studies indicate that secretion of IL-6 and sCD40L by DCs/MΦs, as well as reprograming of IL-6R and CD40 in autoreactive B cells, promote tolerance during innate immune responses. This implies that defects in either the secretion of IL-6/sCD40L or the selective response of autoreactive B cells to these factors regulate autoantibody production. Our studies indicate that DCs and MΦs from MRL/lpr mice are unable to repress autoreactive B cells (Fig. 1b). Defects in DC/MΦ-mediated repression are coincident with diminished secretion of IL-6 and sCD40L and failure to sustain IL-6 mRNA production and activation of the IκB/NFκB pathways [107]. Similarly, we found that IL-6 and sCD40L fail to repress B cells from lupus-prone mice (unpublished data). The finding that lupus-prone mice harbor defects in DCs/MΦs and B cells suggests the influence of an environmental stimulus.
Apoptotic cells: dangerous in death
Apoptotic cells pose a unique challenge to the immune system since they are a rich source of nuclear antigens, including DNA, ICs, nucleosomes, histones, snRNPs and snRNAs, Ro, La, and Sm. These self-antigens can be modified by phosphorylation and/or alternate protein cleavage, revealing novel epitopes that were not available during the induction of B and T cell tolerance [108–111]. During cell death, self-antigens are distributed in large and small blebs on the cell surface and act as targets for autoreactive B cells in a membrane-bound, multimeric form [112–114]. The display of self-antigens on apoptotic cells is necessary to develop autoantibodies. For instance, autoantibodies to cytoplasmic but not nuclear antigens develop when nuclear fragmentation is blocked in apoptotic cells [115]. Therefore, it is imperative that scavenger cells such as MΦs and DCs quickly clear apoptotic cells and their associated self-antigens to minimize inflammatory and autoimmune responses.
Apoptotic cells are bound and cleared by a variety of receptors on DCs and MΦs. Ingestion of apoptotic cells reduces the secretion of proinflammatory cytokines by DCs and MΦs, inhibiting their maturation and controlling the activation of T cells and autoimmune responses to apoptotic cells [116–121]. Decreased clearance of apoptotic cells and defects in phagocytosis by MΦs in SLE patients and lupus-prone mice may lead to autoimmunity [69, 108, 122–124]. The increase in apoptotic cells and antigens could dysregulate DCs and MΦs, triggering a chronic anti-inflammatory response that downregulates the production of cytokines important for B cell tolerance [45, 107].
If apoptotic cells fail to be cleared efficiently, they may become necrotic and expel self-antigens and TLR ligands that are opsonized by autoantibodies, forming ICs [125, 126]. Like apoptotic cells, ICs present self-antigens in a multimeric form and enhance phagocytosis by DCs and MΦs [127, 128]. While apoptotic cells stimulate anti-inflammatory responses, ICs bind to Fc receptors (FcRs) and elicit inflammatory responses from MΦs and DCs [128]. Individual FcRs have distinct activating and inhibitory functions to ensure balance in a normal immune system. Activating FcRs include FcγRI and FcγRIII, which phagocytose ICs and provoke inflammatory responses from DCs and MΦs [129–131]. These receptors are necessary and sufficient for glomerulonephritis in (NZB/NZW) F1 mice [132–134]. Co-ligation of TLR9 and FcγRIII by chromatin:IC induces more DC activation than ligation of FcγRIII alone, indicating that TLRs synergize with FcR signaling [135]. In contrast to the activating FcRs, FcγRIIB is an inhibitory FcR with various functions depending on cell type. It modulates MΦ phagocytosis, DC maturation, BCR signaling, IgG secretion, and the expansion of autoreactive IgG-producing B cells [134, 136–145]. Autoantibodies and glomerulonephritis develop in certain strains of mice deficient in FcγRIIB, affirming its role in preventing autoreactive B cell activation [146, 147]. Additionally, antigen internalized by DCs through FcγRIIB, but not FcγRI or FcγRIII, is presented in its native form and activates antigen-specific B cells [148]. ICs and FcRs have important roles in the immune system, but their dysregulation can result in autoantibody secretion and nephritis.
Clearance of apoptotic cells and their associated autoantigens is crucial in preventing activation of B cells, DCs, MΦs, and T cells. When mice are immunized with apoptotic cells or exhibit a defect in apoptotic cell clearance, Sm-specific antibodies are detectable in the serum and MZ and B-1 B cells are inappropriately activated [66, 68, 69]. This suggests that recognition of autoantigens on apoptotic cells can drive B cell terminal differentiation [66, 68]. Impaired clearance of apoptotic cells in Faslpr [69] and merkd [149, 150] mice prevents BCR-mediated reprograming of IL-6R and CD40 (unpublished data). These data suggest that exposure of autoreactive B cells to apoptotic cells or ICs can impact DC/MΦ-mediated tolerance. In summary, apoptotic cells and the self-antigens they display can play both autoimmune and anti-inflammatory roles. Imbalances in apoptotic cell clearance or cellular responses result in inflammatory responses and autoimmune disease.
Selective repression: new treatments for SLE?
Systemic lupus erythematosus is primarily a B cell-mediated autoimmune disease, with symptoms arising from autoantibody deposition and inflammation in target organs, such as the kidneys, skin, and brain. Until recently, treatments for SLE were dependent on immunosuppression, which depresses immune function and causes dangerous side effects such as opportunistic infections [151]. Therapies that target specific cell types or biologic processes are now being developed, with the hopes that they will be more powerful and have less harmful side effects. For instance, chimeric anti-CD20 (rituximab), depletes peripheral B cells and reduces the severity of SLE symptoms in many patients [152]. However, a subset of patients is resistant to rituximab treatment [151]. Additionally, rituximab recently failed a late-stage study when its efficacy in achieving a clinical response was no greater than a placebo [153]. In murine studies of human CD20, B cells in autoimmune-prone strains were refractory to depletion by rituximab [154], compared to non-autoimmune-prone strains. Other biologic agents being studied or developed target complement activation, B cell-T cell interactions, cytokines, TLRs, interferon, or direct removal of antibodies from circulation. However, developing therapies to target each lupus-related autoantigen would be cumber-some and slow. A more efficient approach would be a therapy that selectively targeted autoreactive B cells through a common trait to restore tolerance. In previous studies, we determined that DC- and MΦ-mediated repression via IL-6 and sCD40L is effective on B cells of multiple specificities. These data suggest that any B cell chronically exposed to antigen would be susceptible to DC/MΦ-mediated tolerance. We are currently determining whether the lack of these soluble factors promotes autoimmunity in normal mice during innate immune responses. Preliminary data indicate that Sm-specific B cells adoptively transferred into chimeric mice lacking IL-6 and sCD40L become activated and secrete autoantibodies (unpublished data). This is consistent with our model indicating that DCs/MΦs and their secreted products regulate autoreactive B cells during innate immune responses. Future experiments will examine if tolerance can be restored in lupus-prone mice reconstituted with a mix of autoimmune and non-autoimmune hematopoietic stem cells. If DC/MΦ-mediated tolerance is found to be defective in SLE patients, future therapies could target their in vivo activation or introduce normal DCs/MΦs to reinstate B cell tolerance of newly emerging B cells following B cell depletion therapy. One approach would be non-myeloablative bone marrow transplant (BMT) or nonmyeloablative hematopoietic stem cell transplant (HSCT) to promote mixed chimerism. This may reinstate tolerance by providing a pool of DCs/MΦs that repress autoreactive B cells. Such an approach has shown promise in controlling B cell-mediated autoimmune disease in humans and mouse models [155–160], resulting in remission of rheumatoid arthritis in a human patient [158] and reduction in lupus-like disease in mice [155, 157].
Summary
The autoreactive B cells that incite multi-organ damage in SLE patients become dysregulated long before clinical symptoms appear. Autoantigens and TLR ligands released from apoptotic cells can overcome the tolerance of autoreactive B cells. Autoreactive T cells provide costimulation and promote the secretion of high-affinity, class-switched autoantibodies. These autoantibodies can bind nuclear self-antigens, form ICs, and provoke inflammatory responses from DCs and MΦs. Fortunately, DCs and MΦs specifically repress autoreactive B cells during TLR stimulation by secreting IL-6 and sCD40L. Restoring normal DC and MΦ function in SLE patients might repress autoreactive B cells and re-establish balance among the complex components of the immune system.
Acknowledgments
We would like to thank the current and past members of the Vilen laboratory for their invaluable contributions to this work. We would also like to thank Dr. Stephen Clarke and his laboratory for the many insightful discussions our joint group meeting has generated. This work was supported by NIH/NIAID ROI AI053266 and the Lupus Research Institute.
References
- 1.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 2.Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp Med. 1996;184:1685–1697. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 4.Hippen KL, Schram BR, Tze LE, Pape KA, Jenkins MK, Behrens TW. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. J Immunol. 2005;175:909–916. doi: 10.4049/jimmunol.175.2.909. [DOI] [PubMed] [Google Scholar]
- 5.Retter MW, Nemazee D. Receptor editing occurs frequently during normal B cell development. J Exp Med. 1998;188:1231–1238. doi: 10.1084/jem.188.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casellas R, Shih TA, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, et al. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 7.Ait-Azzouzene D, Verkoczy L, Peters J, Gavin A, Skog P, Vela JL, et al. An immunoglobulin C kappa-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. J Exp Med. 2005;201:817–828. doi: 10.1084/jem.20041854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813–1818. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 12.Papavasiliou F, Casellas R, Suh H, Qin XF, Besmer E, Pelanda R, et al. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997;278:298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Zheng B, Schatz DG, Spanopoulou E, Kelsoe G. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B cells. Science. 1996;274:2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 14.Hikida M, Mori M, Takai T, Tomochika K, Hamatani K, Ohmori H. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–2094. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- 15.Russell DM, Dembic Z, Morahan G, Miller JFAP, Burki K, Nemazee D. Peripheral deletion of selfreactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kench JA, Russell DM, Nemazee D. Efficient peripheral clonal elimination of B lymphocytes in MRL/lpr mice bearing autoantibody transgenes. J Exp Med. 1998;188:909–917. doi: 10.1084/jem.188.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 18.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt KN, Cyster JG. Follicular exclusion and rapid elimination of hen egg lysozyme autoantigen-binding B cells are dependent on competitor B cells, but not on T cells. J Immunol. 1999;162:284–291. [PubMed] [Google Scholar]
- 20.Ekland EH, Forster R, Lipp M, Cyster JG. Requirements for follicular exclusion and competitive elimination of autoantigen-binding B cells. J Immunol. 2004;172:4700–4708. doi: 10.4049/jimmunol.172.8.4700. [DOI] [PubMed] [Google Scholar]
- 21.Paul E, Nelde A, Verschoor A, Carroll MC. Follicular exclusion of autoreactive B cells requires Fc{gamma}RIIb. Int Immunol. 2007;19:365–373. doi: 10.1093/intimm/dxm002. [DOI] [PubMed] [Google Scholar]
- 22.Mandik-Nayak L, Seo S, Eaton-Bassiri A, Allman D, Hardy RR, Erikson J. Functional consequences of the developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J Immunol. 2000;164:1161–1168. doi: 10.4049/jimmunol.164.3.1161. [DOI] [PubMed] [Google Scholar]
- 23.Aplin BD, Keech CL, de Kauwe AL, Gordon TP, Cavill D, McCluskey J. Tolerance through Indifference: autoreactive B cells to the nuclear antigen La show no evidence of tolerance in a transgenic model. J Immunol. 2003;171:5890–5900. doi: 10.4049/jimmunol.171.11.5890. [DOI] [PubMed] [Google Scholar]
- 24.Koenig-Marrony S, Soulas P, Julien S, Knapp A-M, Garaud J-C, Martin T, et al. Natural autoreactive B cells in transgenic mice reproduce an apparent paradox to the clonal tolerance theory. J Immunol. 2001;166:1463–1470. doi: 10.4049/jimmunol.166.3.1463. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Manser T. Antinuclear antigen B cells that down-regulate surface B cell receptor during development to mature, follicular phenotype do not display features of anergy in vitro. J Immunol. 2005;174:4505–4515. doi: 10.4049/jimmunol.174.8.4505. [DOI] [PubMed] [Google Scholar]
- 26.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 27.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 28.Rui L, Healy JI, Blasioli J, Goodnow CC. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4-driven plasma cell differentiation. J Immunol. 2006;177:5337–5346. doi: 10.4049/jimmunol.177.8.5337. [DOI] [PubMed] [Google Scholar]
- 29.Nossal GJ, Pike BL. Clonal anergy: persistence in tolerant mice of antigen-binding B lymphocytes incapable of responding to antigen or mitogen. Proc Natl Acad Sci U S A. 1980;77:1602–1606. doi: 10.1073/pnas.77.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noorchashm H, Bui A, Li H-L, Eaton A, Mandik-Nayak L, Sokol C, et al. Characterization of anergic anti-DNA B cells: B cell anergy is a T cell-independent and potentially reversible process. Int Immunol. 1999;11:765–776. doi: 10.1093/intimm/11.5.765. [DOI] [PubMed] [Google Scholar]
- 31.Acevedo-Suarez CA, Hulbert C, Woodward EJ, Thomas JW. Uncoupling of anergy from developmental arrest in anti-insulin B cells supports the development of autoimmune diabetes. J Immunol. 2005;174:827–833. doi: 10.4049/jimmunol.174.2.827. [DOI] [PubMed] [Google Scholar]
- 32.Borrero M, Clarke SH. Low-affinity anti-Smith antigen B cells are regulated by anergy as opposed to developmental arrest or differentiation to B-1. J Immunol. 2002;168:13–21. doi: 10.4049/jimmunol.168.1.13. [DOI] [PubMed] [Google Scholar]
- 33.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 34.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, et al. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Santulli-Marotto S, Retter MW, Gee R, Mamula MJ, Clarke SH, Autoreactive B. Cell regulation: peripheral induction of developmental arrest by lupus-associated autoantigens. Immunity. 1998;8:209–219. doi: 10.1016/s1074-7613(00)80473-2. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen KA, Mandik L, Bui A, Kavaler J, Norvell A, Monroe JG, et al. Characterization of anti-single-stranded DNA B cells in a non-autoimmune background. J Immunol. 1997;159:2633–2644. [PubMed] [Google Scholar]
- 37.Busconi L, Bauer JW, Tumang JR, Laws A, Perkins-Mesires K, Tabor AS, et al. Functional outcome of B cell activation by chromatin immune complex engagement of the B cell receptor and TLR9. J Immunol. 2007;179:7397–7405. doi: 10.4049/jimmunol.179.11.7397. [DOI] [PubMed] [Google Scholar]
- 38.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 39.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acevedo-Suarez CA, Kilkenny DM, Reich MB, Thomas JW. Impaired intracellular calcium mobilization and NFATc1 availability in tolerant anti-insulin B cells. J Immunol. 2006;177:2234–2241. doi: 10.4049/jimmunol.177.4.2234. [DOI] [PubMed] [Google Scholar]
- 41.Cooke MP, Heath AW, Shokat KM, Zeng Y, Finkelman FD, Linsley PS, et al. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 43.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 44.Kilmon MA, Rutan JA, Clarke SH, Vilen BJ. Low-affinity, Smith antigen-specific B cells are tolerized by dendritic cells and macrophages. J Immunol. 2005;175:37–41. doi: 10.4049/jimmunol.175.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilmon MA, Wagner NJ, Garland AL, Lin L, Aviszus K, Wysocki LJ, et al. Macrophages prevent the differentiation of autoreactive B cells by secreting CD40 ligand and interleukin-6. Blood. 2007;110:1595–1602. doi: 10.1182/blood-2006-12-061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kouskoff V, Famiglietti S, Lacaud G, Lang P, Rider JE, Kay BK, et al. Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. J Exp Med. 1998;188:1453–1464. doi: 10.1084/jem.188.8.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan EM, Kunkel HG. Pillars article characteristics of a soluble nuclear antigen precipitating with sera of patients with systemic lupus erythematosus. J Immunol. 1966;96:464–471. J Immunol. 2006;176: 1297–304. [PubMed] [Google Scholar]
- 48.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 49.Clotet B, Guardia J, Pigrau C, Lience E, Murcia C, Pujol R, et al. Incidence and clinical significance of anti-ENA antibodies in systemic lupus erythematosus. Estimation by counterimmunoelectrophoresis. Scand J Rheumatol. 1984;13:15–20. doi: 10.3109/03009748409102662. [DOI] [PubMed] [Google Scholar]
- 50.Martinez-Cordero E, Martinez-Miranda E, Negrete-Garcia MC, Padilla A, Aguilar Leon DE. Anti-dsDNA and Sm autoantibodies in systemic lupus erythematosus. Clin Rheumatol. 1992;11:341–345. doi: 10.1007/BF02207190. [DOI] [PubMed] [Google Scholar]
- 51.Gripenberg M, Teppo AM, Friman C. Antibodies to Sm and SS-A demonstrated by enzyme immunoassay. Correlation to clinical manifestations and disease activity in patients with systemic lupus erythematosus. Rheumatol Int. 1991;11:209–213. doi: 10.1007/BF00332564. [DOI] [PubMed] [Google Scholar]
- 52.Barada FA, Jr, Andrews BS, Davis JSt, Taylor RP. Antibodies to Sm in patients with systemic lupus erythematosus. Correlation of Sm antibody titers with disease activity and other laboratory parameters. Arthritis Rheum. 1981;24:1236–1244. doi: 10.1002/art.1780241003. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Longo FJ, Gonzalez Fernandez CM, Rodriguez Mahou M, Grau Simo R, Monteagudo Saez I, Meno Garcia AC, et al. Clinical expression of systemic lupus erythematosus with anti-U1-RNP and anti-Sm antibodies. Rev Clin Esp. 1997;197:329–335. [PubMed] [Google Scholar]
- 54.Lerner MR, Steitz JA. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979;76:5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stark H, Dube P, Luhrmann R, Kastner B. Arrangement of RNA and proteins in the spliceosomal U1 small nuclear ribonucleoprotein particle. Nature. 2001;409:539–542. doi: 10.1038/35054102. [DOI] [PubMed] [Google Scholar]
- 56.Zhang D, Abovich N, Rosbash M. A biochemical function for the Sm complex. Mol Cell. 2001;7:319–329. doi: 10.1016/s1097-2765(01)00180-0. [DOI] [PubMed] [Google Scholar]
- 57.Seraphin B, Rosbash M. Identification of functional U1 snRNA-pre-mRNA complexes committed to spliceosome assembly and splicing. Cell. 1989;59:349–358. doi: 10.1016/0092-8674(89)90296-1. [DOI] [PubMed] [Google Scholar]
- 58.Staley JP, Guthrie C. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell. 1998;92:315–326. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- 59.Marshak-Rothstein A, Busconi L, Lau CM, Tabor AS, Leadbetter EA, Akira S, et al. Comparison of CpG s-ODNs, chromatin immune complexes, and dsDNA fragment immune complexes in the TLR9-dependent activation of rheumatoid factor B cells. J Endotoxin Res. 2004;10:247–251. doi: 10.1179/096805104225005850. [DOI] [PubMed] [Google Scholar]
- 60.Rifkin IR, Leadbetter EA, Beaudette BC, Kiani C, Monestier M, Shlomchik MJ, et al. Immune complexes present in the sera of autoimmune mice activate rheumatoid factor B cells. J Immunol. 2000;165:1626–1633. doi: 10.4049/jimmunol.165.3.1626. [DOI] [PubMed] [Google Scholar]
- 61.Eric L, Greidinger RWH. The appearance of U1 RNP antibody specificities in sequential autoimmune human antisera follows a characteristic order that implicates the U1–70 kd and Bprime/B proteins as predominant U1 RNP immunogens. Arthritis Rheum. 2001;44:368–375. doi: 10.1002/1529-0131(200102)44:2<368::AID-ANR55>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 62.Crozat K, Beutler B. TLR7: A new sensor of viral infection. Proc Natl Acad Sci U S A. 2004;101:6835–6836. doi: 10.1073/pnas.0401347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 65.Santulli-Marotto S, Qian Y, Ferguson S, Clarke SH, Anti-Sm B. Cell differentiation in Ig transgenic MRL/Mp-lpr/lpr mice: altered differentiation and an accelerated response. J Immunol. 2001;166:5292–5299. doi: 10.4049/jimmunol.166.8.5292. [DOI] [PubMed] [Google Scholar]
- 66.Qian Y, Santiago C, Borrero M, Tedder TF, Clarke SH. Lupus-specific antiribonucleoprotein B cell tolerance in nonautoimmune mice is maintained by differentiation to B-1 and governed by B cell receptor signaling thresholds. J Immunol. 2001;166:2412–2419. doi: 10.4049/jimmunol.166.4.2412. [DOI] [PubMed] [Google Scholar]
- 67.Chuanlin Ding LW, Al-Ghaw H, Marroquin J, Mamula M, Yan J. Toll-like receptor engagement stimulates anti-snRNP autoreactive B cells for activation. Eur J Immunol. 2006;36:2013–2024. doi: 10.1002/eji.200635850. [DOI] [PubMed] [Google Scholar]
- 68.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172:625–635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 69.Qian Y, Conway KL, Lu X, Seitz HM, Matsushima GK, Clarke SH. Autoreactive MZ and B-1 B-cell activation by Faslpr is coincident with an increased frequency of apoptotic lymphocytes and a defect in macrophage clearance. Blood. 2006;108:974–982. doi: 10.1182/blood-2005-12-006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diz R, McCray SK, Clarke SH. BCR affinity and B cell subset identity integrate to define the effectiveness, affinity threshold, and mechanism of anergy. J Immunol. 2008;181 doi: 10.4049/jimmunol.181.6.3834. in press. http://www.jimmunol.org/future/181.6.shtml. [DOI] [PubMed] [Google Scholar]
- 71.Yan J, Mamula MJ. B and T cell tolerance and autoimmunity in autoantibody transgenic mice. Int Immunol. 2002;14:963–971. doi: 10.1093/intimm/dxf064. [DOI] [PubMed] [Google Scholar]
- 72.Bowen F, Haluskey J, Quill H. Altered CD40 ligand induction in tolerant T lymphocytes. Eur J Immunol. 1995;25:2830–2834. doi: 10.1002/eji.1830251018. [DOI] [PubMed] [Google Scholar]
- 73.Craft J, Peng S, Fujii T, Okada M, Fatenejad S. Autoreactive T cells in murine lupus: origins and roles in autoantibody production. Immunol Res. 1999;19:245–257. doi: 10.1007/BF02786492. [DOI] [PubMed] [Google Scholar]
- 74.Peng SL, Fatenejad S, Craft J. Induction of nonpathologic, humoral autoimmunity in lupus-prone mice by a class II-restricted, transgenic alpha beta T cell. Separation of autoantigen-specific and -nonspecific help. J Immunol. 1996;157:5225–5230. [PubMed] [Google Scholar]
- 75.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- 76.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 77.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 78.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 79.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 80.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell-dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 81.Mitani Y, Takaoka A, Kim S, Kato Y, Yokochi T, Tanaka N, et al. Cross talk of the interferon-alpha/beta signalling complex with gp130 for effective interleukin-6 signalling. Genes Cells. 2001;6:631–640. doi: 10.1046/j.1365-2443.2001.00448.x. [DOI] [PubMed] [Google Scholar]
- 82.Dye JR, Palvanov A, Guo B, Rothstein TL. B cell receptor cross-talk: exposure to lipopolysaccharide induces an alternate pathway for B cell receptor-induced ERK phosphorylation and NF-{kappa}B activation. J Immunol. 2007;179:229–235. doi: 10.4049/jimmunol.179.1.229. [DOI] [PubMed] [Google Scholar]
- 83.Guo B, Rothstein TL. B Cell Receptor (BCR) cross-talk: IL-4 creates an alternate pathway for BCR-induced ERK activation that is phosphatidylinositol 3-kinase independent. J Immunol. 2005;174:5375–5381. doi: 10.4049/jimmunol.174.9.5375. [DOI] [PubMed] [Google Scholar]
- 84.Guo B, Blair D, Chiles TC, Lowell CA, Rothstein TL. Cutting edge: B Cell Receptor (BCR) cross-talk: the IL-4-induced alternate pathway for BCR signaling operates in parallel with the classical pathway, is sensitive to Rottlerin, and depends on Lyn. J Immunol. 2007;178:4726–4730. doi: 10.4049/jimmunol.178.8.4726. [DOI] [PubMed] [Google Scholar]
- 85.Mizuno T, Rothstein TL. B Cell Receptor (BCR) cross-talk: CD40 engagement creates an alternate pathway for BCR signaling that activates I kappa B kinase/I kappa B alpha/NF-kappa B without the need for PI3 K and phospholipase C gamma. J Immunol. 2005;174:6062–6070. doi: 10.4049/jimmunol.174.10.6062. [DOI] [PubMed] [Google Scholar]
- 86.Mizuno T, Rothstein TL. B Cell Receptor (BCR) cross-talk: CD40 engagement enhances BCR-induced ERK activation. J Immunol. 2005;174:3369–3376. doi: 10.4049/jimmunol.174.6.3369. [DOI] [PubMed] [Google Scholar]
- 87.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 88.Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andrews B, Eisenberg R, Theofilopoulos A, Izui S, Wilson C, McConahey P, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 91.Lamoureux JL, Watson LC, Cherrier M, Skog P, Nemazee D, Feeney AJ. Reduced receptor editing in lupus-prone MRL/lpr mice. J Exp Med. 2007;204:2853–2864. doi: 10.1084/jem.20071268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mandik-Nayak L, Seo S-j, Sokol C, Potts KM, Bui A, Erikson J. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J Exp Med. 1999;189:1799–1814. doi: 10.1084/jem.189.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Culton DA, O’Conner BP, Conway KL, Diz R, Rutan J, Vilen BJ, et al. Early preplasma cells define a tolerance checkpoint for autoreactive B cells. J Immunol. 2006;176:790–802. doi: 10.4049/jimmunol.176.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang C, Foley J, Clayton N, Kissling G, Jokinen M, Herbert R, et al. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J Immunol. 2007;178:7422–7431. doi: 10.4049/jimmunol.178.11.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hang L, Theofilopoulos AN, Balderas RS, Francis SJ, Dixon FJ. The effect of thymectomy on lupus-prone mice. J Immunol. 1984;132:1809–1813. [PubMed] [Google Scholar]
- 96.Peng SL, Madaio MP, Hughes DP, Crispe IN, Owen MJ, Wen L, et al. Murine lupus in the absence of alpha beta T cells. J Immunol. 1996;156:4041–4049. [PubMed] [Google Scholar]
- 97.Jevnikar AM, Grusby MJ, Glimcher LH. Prevention of nephritis in major histocompatibility complex class II-deficient MRL-lpr mice. J Exp Med. 1994;179:1137–1143. doi: 10.1084/jem.179.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koh DR, Ho A, Rahemtulla A, Fung-Leung WP, Griesser H, Mak TW. Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells. Eur J Immunol. 1995;25:2558–2562. doi: 10.1002/eji.1830250923. [DOI] [PubMed] [Google Scholar]
- 99.Mudd PA, Teague BN, Farris AD. Regulatory T cells and systemic lupus erythematosus. Scand J Immunol. 2006;64:211–218. doi: 10.1111/j.1365-3083.2006.01808.x. [DOI] [PubMed] [Google Scholar]
- 100.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+ CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 101.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, et al. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 102.Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T, et al. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 103.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 104.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+ CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 105.Obermeier F, Strauch UG, Dunger N, Grunwald N, Rath HC, Herfarth H, et al. In vivo CpG DNA/toll-like receptor 9 interaction induces regulatory properties in CD4+ CD62L+ T cells which prevent intestinal inflammation in the SCID transfer model of colitis. Gut. 2005;54:1428–1436. doi: 10.1136/gut.2004.046946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–342. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- 107.Gilbert MR, Carnathan DG, Cogswell PC, Lin L, Baldwin AS, Jr, Vilen BJ. Dendritic cells from lupus-prone mice are defective in repressing immunoglobulin secretion. J Immunol. 2007;178:4803–4810. doi: 10.4049/jimmunol.178.8.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, et al. SLE—a disease of clearance deficiency? Rheumatology. 2005;44:1101–1107. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 109.Casiano CA, Martin SJ, Green DR, Tan EM. Selective cleavage of nuclear autoantigens during CD95 (Fas/APO-1)-mediated T cell apoptosis. J Exp Med. 1996;184:765–770. doi: 10.1084/jem.184.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Utz PJ, Hottelet M, Le TM, Kim SJ, Geiger ME, van Venrooij WJ, et al. The 72-kDa component of signal recognition particle is cleaved during apoptosis. J Biol Chem. 1998;273:35362–35370. doi: 10.1074/jbc.273.52.35362. [DOI] [PubMed] [Google Scholar]
- 111.Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843–854. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol. 2002;169:159–166. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- 113.Casciola-Rosen L, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–6700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 115.Frisoni L, McPhie L, Kang S-A, Monestier M, Madaio M, Satoh M, et al. Lack of chromatin and nuclear fragmentation in vivo impairs the production of lupus anti-nuclear antibodies. J Immunol. 2007;179:7959–7966. doi: 10.4049/jimmunol.179.11.7959. [DOI] [PubMed] [Google Scholar]
- 116.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 119.Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 120.Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, et al. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109:653–660. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wallet MA, Sen P, Flores RR, Wang Y, Yi Z, Huang Y, et al. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205:219–232. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Licht R, Dieker JWC, Jacobs CWM, Tax WJM, Berden JHM. Decreased phagocytosis of apoptotic cells in diseased SLE mice. J Autoimmun. 2004;22:139–145. doi: 10.1016/j.jaut.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 123.Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 124.Martin Herrmann REV, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Kalden, impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 125.Ren Y, Savill J. Apoptosis: the importance of being eaten. Cell Death Differ. 1998;5:563–568. doi: 10.1038/sj.cdd.4400407. [DOI] [PubMed] [Google Scholar]
- 126.Májai G, Petrovski G, Fésüs L. Inflammation and the apopto-phagocytic system. Immunol Lett. 2006;104:94–101. doi: 10.1016/j.imlet.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 127.Frisoni L, Mcphie L, Colonna L, Sriram U, Monestier M, Gallucci S, et al. Nuclear autoantigen translocation and autoantibody opsonization lead to increased dendritic cell phagocytosis and presentation of nuclear antigens: a novel pathogenic pathway for autoimmunity? J Immunol. 2005;175:2692–2701. doi: 10.4049/jimmunol.175.4.2692. [DOI] [PubMed] [Google Scholar]
- 128.Manfredi AA, Rovere P, Galati G, Heltai S, Bozzolo E, Soldini S, et al. Apoptotic cell clearance in systemic lupus erythematosus: I. Opsonization by antiphospholipid antibodies. Arthritis Rheum. 1998;41:205–214. doi: 10.1002/1529-0131(199802)41:2<205::AID-ART4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 129.Gerber JS, Mosser DM. Stimulatory and inhibitory signals originating from the macrophage Fc[gamma] receptors. Microbes Infect. 2001;3:131–139. doi: 10.1016/s1286-4579(00)01360-5. [DOI] [PubMed] [Google Scholar]
- 130.Barnes N, Gavin AL, Tan PS, Mottram P, Koentgen F, Hogarth PM. Fc[gamma]RI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 131.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, et al. Fc gamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bergtold A, Gavhane A, D’Agati V, Madaio M, Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J Immunol. 2006;177:7287–7295. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- 133.Clynes R, Calvani N, Croker BP, Richards HB. Modulation of the immune response in pristane-induced lupus by expression of activation and inhibitory Fc receptors. Clin Exp Immunol. 2005;141:230–237. doi: 10.1111/j.1365-2249.2005.02847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 135.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Daeron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 137.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 138.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 139.Coggeshall KM. Positive and negative signaling in B lymphocytes. Curr Top Microbiol Immunol. 2000;245:213–260. doi: 10.1007/978-3-642-57066-7_7. [DOI] [PubMed] [Google Scholar]
- 140.Brauweiler A, Tamir I, Marschner S, Helgason CD, Cambier JC. Partially distinct molecular mechanisms mediate inhibitory FcgammaRIIB signaling in resting and activated B cells. J Immunol. 2001;167:204–211. doi: 10.4049/jimmunol.167.1.204. [DOI] [PubMed] [Google Scholar]
- 141.Bolland S, Ravetch JV. Inhibitory pathways triggered by ITIM-containing receptors. Adv Immunol. 1999;72:149–177. doi: 10.1016/s0065-2776(08)60019-x. [DOI] [PubMed] [Google Scholar]
- 142.Clatworthy MR, Smith KGC. Fc{gamma}RIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med. 2004;199:717–723. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Desai DD, Harbers SO, Flores M, Colonna L, Downie MP, Bergtold A, et al. Fc{gamma} receptor IIB on dendritic cells enforces peripheral tolerance by inhibiting effector T cell responses. J Immunol. 2007;178:6217–6226. doi: 10.4049/jimmunol.178.10.6217. [DOI] [PubMed] [Google Scholar]
- 144.Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fc gamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 145.Brownlie RJ, Lawlor KE, Niederer HA, Cutler AJ, Xiang Z, Clatworthy MR, et al. Distinct cell-specific control of autoimmunity and infection by Fc{gamma}RIIb. J Exp Med. 2008;205:883–895. doi: 10.1084/jem.20072565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 147.McGaha TL, Karlsson MCI, Ravetch JV. Fc{gamma}RIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl-MpJ mice. J Immunol. 2008;180:5670–5679. doi: 10.4049/jimmunol.180.8.5670. [DOI] [PubMed] [Google Scholar]
- 148.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 149.Camenisch TD, Koller BH. Earp2, HS, matsushima, GK a novel receptor tyrosine kinase, Mer, inhibits TNF-{alpha} production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- 150.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sfikakis PP, Boletis JN, Tsokos GC. Rituximab anti-B-cell therapy in systemic lupus erythematosus: pointing to the future. Curr Opin Rheumatol. 2005;17:550–557. doi: 10.1097/01.bor.0000172798.26249.fc. [DOI] [PubMed] [Google Scholar]
- 152.Leandro MJ, Edwards JE, Cambridge G, Ehrenstein MR, Isenberg DA. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 2002;46:2673–2677. doi: 10.1002/art.10541. [DOI] [PubMed] [Google Scholar]
- 153.The New York Times. New York: 2008. Reuters, Biotech drug fails critical lupus study. [Google Scholar]
- 154.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 155.Jones OY, Steele A, Jones JM, Marikar Y, Chang Y, Feliz A, et al. Nonmyeloablative bone marrow transplantation of BXSB lupus mice using fully matched allogeneic donor cells from green fluorescent protein transgenic mice. J Immunol. 2004;172:5415–5419. doi: 10.4049/jimmunol.172.9.5415. [DOI] [PubMed] [Google Scholar]
- 156.Burt RK, Slavin S, Burns WH, Marmont AM. Induction of tolerance in autoimmune diseases by hematopoietic stem cell transplantation: getting closer to a cure? Blood. 2002;99:768–784. doi: 10.1182/blood.v99.3.768. [DOI] [PubMed] [Google Scholar]
- 157.Wang B, Yamamoto Y, El-Badri NS, Good RA. Effective treatment of autoimmune disease and progressive renal disease by mixed bone-marrow transplantation that establishes a stable mixed chimerism in BXSB recipient mice. Proc Natl Acad Sci U S A. 1999;96:3012–3016. doi: 10.1073/pnas.96.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Burt RK, Oyama Y, Verda L, Quigley K, Brush M, Yaung K, et al. Induction of remission of severe and refractory rheumatoid arthritis by allogeneic mixed chimerism. Arthritis Rheum. 2004;50:2466–2470. doi: 10.1002/art.20451. [DOI] [PubMed] [Google Scholar]
- 159.Flierman R, Witteveen HJ, van der Voort EIH, Huizinga TWJ, de Vries RRP, Fibbe WE, et al. Control of systemic B cell-mediated autoimmune disease by nonmyeloablative conditioning and major histocompatibility complex-mismatched allogeneic bone marrow transplantation. Blood. 2005;105:2991–2994. doi: 10.1182/blood-2004-09-3715. [DOI] [PubMed] [Google Scholar]
- 160.Burt RK. From animals to clinic: nonmyeloablative conditioning and allogeneic bone marrow transplantation in autoimmune disease. Blood. 2005;105:2623–2624. [Google Scholar]