Abstract
The molecular interaction between adenosine A2A and dopamine D2 receptors (A2ARs and D2Rs, respectively) within an oligomeric complex has been postulated to play a pivotal role in the adenosine-dopamine interplay in the central nervous system, in both normal and pathological conditions (e.g. Parkinson’s disease). While the effects of A2AR challenge on D2R functioning have been largely studied, the reverse condition is still unexplored, a fact that might have impact in therapeutics. Here, we aimed to examine in a real-time mode the D2R-mediated allosteric modulation of A2AR binding when an A2AR/D2R oligomer is established. Thus, we synthesized fluorescent A2AR agonists and evaluated, by means of a flow cytometry homogeneous no-wash assay and a real-time fluorescence resonance energy transfer (FRET)-based approach, the effects on A2AR binding of distinct antiparkinsonian drugs in current clinical use (i.e. pramipexole, rotigotine and apomorphine). Our results provided evidence for the existence of a differential D2R-mediated negative allosteric modulation on A2AR agonist binding that was oligomer-formation dependent, and with apomorphine being the best antiparkinsonian drug attenuating A2AR agonist binding. Overall, the here-developed methods were found valid to prospect the ability of drugs acting on D2Rs to modulate A2AR binding, thus featuring as possible helpful tools for the preliminary selection of D2R-like candidate drugs in the management of Parkinson’s disease.
Keywords: A2AR, D2R, Parkinson’s disease, receptor-receptor allosterism, antiparkinsonian drugs
1. Introduction
Parkinson’s disease (PD) is a progressive systemic neurodegenerative disorder associated with, but not exclusively, the loss of pigmented dopaminergic neurons located at the substantia nigra pars compacta (Lang and Lozano, 1998). Interestingly, the control of this circuitry both in normal and pathological conditions is not only mediated by dopamine receptors, but also by other G protein-coupled receptors (GPCRs), for instance adenosine receptors, which may functionally and molecularly interact with the dopaminergic system (Ferre et al., 2004). Indeed, an interaction between adenosine A2A and dopamine D2 receptors (A2ARs and D2Rs, respectively) has been demonstrated in heterologous expression systems and there is accumulated evidence for the coexistence of reciprocal antagonistic interactions between A2ARs and D2Rs in the same neurons, the GABAergic enkephalinergic neurons (Ferre et al., 2008). Thus, since drugs acting on A2ARs would modulate dopaminergic neurotransmission they have been proposed as therapeutic tools in PD, and in fact, A2AR antagonists have been recently introduced in the symptomatic treatment of the former pathology (for review see (Xu et al., 2005,Morelli et al., 2009,Schwarzschild et al., 2006)). Hence, it has been suggested that the modulation of a feasible A2AR/D2R complex by means of simultaneous D2R activation and A2AR inhibition would represent a valuable target for PD pharmacotherapy. Accordingly, in the present work we aimed to analyze the reciprocal A2AR-D2R antagonistic interaction, concretely the possible D2R-mediated allosteric modulation of A2AR agonist binding. Thus, by means of a real-time fluorescence resonance energy transfer (FRET)- and a flow cytometry-assays, we evaluated the effects of different antiparkinsonian drugs (i.e. pramipexole, rotigotine and apomorphine), in order to provide further information regarding D2R-based drugs before being selected for PD treatment.
2. Materials and Methods
2.1. Materials
The primary antibodies used were: rabbit anti-A2AR polyclonal antibody (Ciruela et al., 2004), rabbit anti-D2R polyclonal antibody (Millipore, Temecula, CA, USA), and mouse anti-A2AR monoclonal antibody (clone 7F6-G5-A2; Millipore). The secondary antibodies were: horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Pierce Biotechnology, Rockford, IL, USA) and Cy3-conjugated donkey anti-mouse IgG antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The ligands used were: adenosine, pramipexole, apomorphine from Sigma-Aldrich (St. Louis, MO, USA); CGS21680, ZM241,385, rotigotine from Tocris Bioscience (Ellisville, MI, USA). Adenosine deaminase was purchased from Roche Diagnostics (GmbH, Mannheim, Germany) and zardaverine from Calbiochem (San Diego, CA, USA).
2.2. Synthesis of the A2AR fluorescent agonists
The APECAlexa488 (MRS5206) and the APECAlexa532 (MRS5424) are functional agonists at the adenosine A2AR, in which the fluorescent dyes Alexa488 and 532, respectively, are covalently attached to the A2AR agonist 2-[[2-[4-[2-(2-aminoethyl)-aminocarbonyl] ethyl]phenyl]ethylamino]-5′-N-ethyl-carboxamidoadenosine (APEC). MRS5424, and similarly MRS5206, were synthesized as previously described (Fernandez-Duenas et al., 2012).
2.3. Plasmid constructs
To perform FRET experiments with the MRS5424 fluorescent agonist, a CFP-tagged A2AR (A2ARCFP) and an AGT-tagged D2R (D2RAGT) were cloned, as previously described (Fernandez-Duenas et al., 2012).
2.4. Cell culture, transfection and membrane preparation
Human embryonic kidney (HEK)-293 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich) supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 100 U/mL streptomycin, 100 mg/mL penicillin and 5% (v / v) fetal bovine serum at 37° C and in an atmosphere of 5% CO2. HEK-293 cells growing in 25 cm2 flasks or six-well plates containing 18 mm coverslips were used for western blot and fluorescence imaging, respectively, were transiently transfected with the cDNA encoding the specified proteins using TransFectin™ Lipid Reagent (Bio-Rad Laboratories, Hercules, CA, USA). Membrane suspensions from transfected HEK cells were obtained as described previously (Fernandez-Duenas et al., 2012).
2.5. Gel electrophoresis, immunoblotting and immunocytochemistry
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) was performed using 7.5 % polyacrylamide gels. Proteins were transferred to PVDF membranes using a semi-dry transfer system, immunoblotted with the indicated antibodies and then bands detected using a chemiluminescent detection kit (Pierce). Immunocytochemistry experiments were performed as previously described (Ciruela et al., 2006).
2.6. [3H]-cAMP assay
The accumulation of cAMP was measured with a [3H]-cAMP assay system (GE Healthcare, Piscataway, NJ, USA). In brief, transiently transfected HEK-293 cells were grown O/N in serum-free DMEM containing adenosine deaminase (0.5 U/mL), preincubated with 50 µM of the phosphodiesterase inhibitor zardaverine for 10 min and then stimulated with adenosine (10 µM) for 15 min at 37° C. Then, we performed the assay protocol, adding the labelled [3H]-cAMP, as described in the manufacturer's manual. The radioactivity was determined in scintillation vials using a Packard 1600 TRI-CARB scintillation counter (PerkinElmer, Waltham, MA, USA).
2.7. Microscopic FRET measurements
Single-cell real-time FRET experiments with the MRS5424 ligand, in cells expressing the A2ARCFP construct and the D2RAGT stained by means of the SNAP-Surface 647 ligand (New England BioLabs, Ipswich, MA, USA), were performed as previously described (Fernandez-Duenas et al., 2012).
2.8. Flow cytometry analysis
Transiently transfected HEK-293 cells were detached with accutase (Sigma-Aldrich) and resuspended in Hank’s balanced salt solution containing 1 g/l glucose (HBSS). Then, cells were incubated with the proper ligands in silicon-coated reaction vessels (3×105 cells/50 µl) during 60 minutes at room temperature and used directly for flow cytometric measurements. For each binding condition tested 104 cells were analyzed using a FACSCalibur™ flow cytometer (Becton Dickinson, Heidelberg, Germany) with excitation at 488 nm and emission at 510 nm. Samples were maintained in the dark during the analysis to avoid photobleaching. Fluorescence intensities were obtained in the FL-1 channel in log mode. Data were collected using Cell Quest Pro software (BD, Franklin Lakes, NJ) and analyzed by GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
2.9. Statistics
The number of samples (n) in each experimental condition is indicated in figure legends. Statistical analysis was performed by one-way ANOVA followed by Dunnett`s multiple comparison post-hoc test. Statistical significance is indicated for each experiment.
3. Results and discussion
In the next years, the search for new drugs specifically targeting the A2AR/D2R complex may represent a novel and therapeutically superior approach for the management of PD. In such way, drugs intended to overcome the tonic A2AR-mediated inhibition of D2R signalling promise to be of great value. Accordingly, in the present work we aimed to study the D2R-mediated allosteric modulation of A2AR agonist binding within the A2AR/D2R oligomer framework. Hence, we developed a FRET- and a flow cytometry-based agonist binding assays devoted to study the adenosine-dopamine receptor-receptor interaction.
3.1. FRET determinations of D2R-mediated modulation of A2AR binding
We recently described a single-cell real-time FRET-based A2AR agonist binding procedure, in which it was possible to ascertain the negative allosteric modulatory effect of a D2R agonist (i.e. quinpirole) on A2AR agonist binding (Fernandez-Duenas et al., 2012). Accordingly, here we aimed to use the same experimental approach to analyze the effects of several clinically used antiparkinsonian drugs (i.e. pramipexole, rotigotine and apomorphine) on A2AR agonist binding (Figure 1A). First, doubly A2ARCFP and D2RAGT transfected cells were visualized at 480 nm (CFP) and 688 nm (SNAP; see methods). Noteworthy, the A2ARCFP construct was, without losing its functionality, mostly targeted to the plasma membrane (see Supplementary Figure 1), where it co-distributed with the D2RAGT (Fernandez-Duenas et al., 2012). Then, we proceeded to analyze the effects of the antiparkinsonian agents on MRS5424 binding. Since it has been previously demonstrated that in real-time FRET determinations there is not receptor reserve and furthermore the measurements are done under continuous superfusion of the ligand (i.e. non-equilibrium conditions) (Ziegler et al., 2011), high saturating concentrations of the antiparkinsonian D2R agonists (pramipexole: 120 µM, rotigotine: 1 µM and apomorphine: 6 µM) (Millan et al., 2002,Scheller et al., 2009) were used to displace the MRS5424 (2 µM) bound to A2ARs. Interestingly, while MRS5424 binding was unaffected by any of the D2R agonists in cells expressing only the A2ARCFP (data not shown), upon D2RAGT co-expression and challenge a significant lower FRET signal was observed (Figure 1B). A close view of the real-time FRET profiles allowed us to discern that apomorphine (58±6%, p < 0.001) was the most efficacious negative allosteric modulator (NAM) of A2AR agonist binding, when compared to rotigotine (29±5%, p < 0.001) or pramipexole (38±5%, p < 0.001) (Figure 1C). Overall, these results indicated that all D2R agonists tested were NAMs of A2AR agonist binding, a phenomenon that would be dependent on A2AR/D2R oligomerization. Indeed, we recently demonstrated that the formation of the A2AR/D2R oligomer was mandatory for the observed receptor-receptor interaction (Fernandez-Duenas et al., 2012).
Figure 1. Real-time FRET determinations of antiparkinsonian D2R agonist-mediated modulation of A2AR agonist binding.
(A) Chemical structures of the investigated antiparkinsonian D2R agonists (pramipexole, rotigotine and apomorphine). (B) Time-resolved changes in FRET signals engaged by MRS5424 (2 µM) in cells transfected with A2ARCFP and D2RAGT in the absence (black trace) or presence (red trace) of pramipexole (120 µM), rotigotine (1 µM) and apomorphine (6 µM) were recorded. Traces are representative of four separate experiments with similar qualitative and quantitative results. (C) The FRET increase (1/F480) was fitted by a simple monoexponential curve and the magnitude of the FRET signal (A, see experimental procedures) calculated for each experimental condition. Data represents the average ± S.E.M. values of four independent experiments. Asterisk indicates data significantly different from the control condition (i.e. in the absence of antiparkinsonian D2R agonist): *p < 0.01 by ANOVA with Dunnett`s multiple comparison post-hoc test.
3.2. Flow cytometric measurements of D2R-agonists effects on A2AR binding
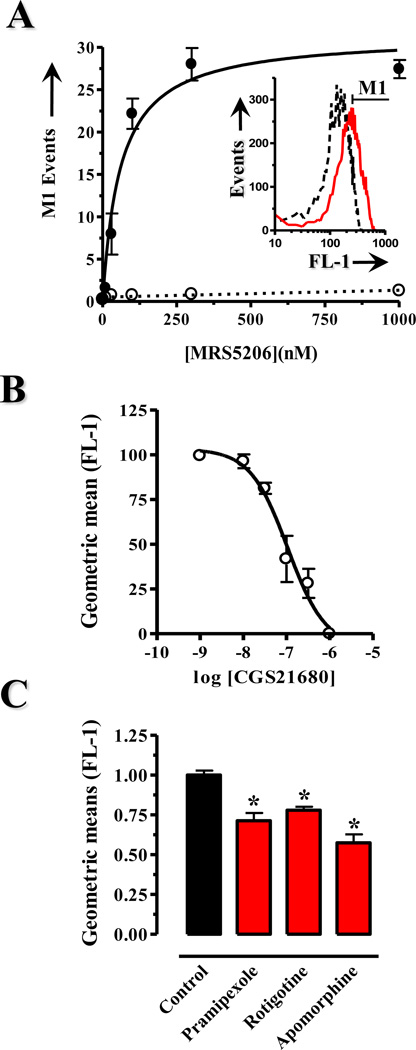
One of the main advantages of FRET-based approaches consists of providing high spatiotemporal resolution of the agonist-receptor interaction. On the contrary, this kind of tool does not permit the collection of a large number of events, therefore not suitable for high throughput screening of drugs. Accordingly, in order to validate our previous results by a different experimental approach and also to generate an easy, fast and reliable way for monitoring A2AR-D2R interactions we aimed to develop a flow cytometry homogeneous no-wash assay. First, a new selective A2AR fluorescent agonist, a pure isomeric APECAlexa488 named MRS5206, was synthesized according to our flow cytometer needs. First, it was tested to be bound selectively to the A2AR by live-cell detection under a fluorescence microscope (see Supplementary Figure 2). And thereafter, we attempted to quantify the occurrence of MRS5206 binding to A2ARs by means of flow cytometry. To this end, A2AR expressing cells were initially incubated with MRS5206 and then separated by flow cytometry with the aid of both forward and sideward scatter signals. Interestingly, while MRS5206 did not bind to mock transfected cells (data not shown), a concentration-dependent binding isotherm for A2AR expressing cells was found when positive cells or events (Figure 2A, inset histogram) were plotted against MRS5206 concentration (Figure 2A). A close analysis of these results revealed that the maximal MRS5206 binding capacity identified ~33% of positive cells, and this was achieved at low nanomolar concentrations of MRS5206. This value may depend on the existing interrelation between cell surface receptor density and transfection efficiency achieved in our experiments. On the other hand, MRS5206 binding was completely blocked by ZM241,385 (1 µM) at any MRS5206 concentration tested, thus demonstrating the specificity of the fluorescent compound (Figure 2A). Also, we evaluated the affinity of MRS5206 by competitive ligand binding experiments. Thus, a fixed dose of MRS5206 (100 nM) was displaced with CGS21680, another A2AR selective agonist, in a concentration-dependent manner (Figure 2B). The dissociation constant of CGS21680 (106±42 nM) was similar to that described in the literature by means of radioligand experiments (Ciruela et al., 2006). Interestingly, these results were consistent with previous observations of fluorescent ligand binding for other GPCRs using flow cytometry under equilibrium conditions (Schneider et al., 2006). Thus, we finally carried out flow cytometry binding experiments using the antiparkinsonian drugs. Importantly, while in cells transfected only with the A2AR-containing plasmid the D2R agonists did not alter MRS5206 binding to A2ARs (data not shown), in A2AR-D2R co-expressing cells all the antiparkinsonian agents tested produced a significant reduction of MRS5206 (100 nM) binding (Figure 2C), with apomorphine again achieving the largest binding inhibition (42±7%, p < 0.01) in comparison to rotigotine (22±3%, p < 0.01) and pramipexole (29±7%, p < 0.01) (Figure 2C).
Figure 2. Flow cytometric determinations of antiparkinsonian D2R agonist-mediated modulation of A2AR agonist binding.
(A) The flow cytometric equilibrium binding assay. HEK-293 cells transiently transfected with A2AR were incubated with increasing concentrations of MRS5206 and analyzed by flow cytometry. In the inset panel a representative histogram of MRS5206 (100 nM) total binding (black line) and nonspecific binding (red line, in the presence of 1 µM of ZM241,385) is shown. The specific fluorescence detection (FL-1) for each MRS5206 concentration tested was determined by M1 cursor (see histogram). Thus, specific events within the M1 region were plotted against MRS5206 concentration. (B) Competition between MRS5206 (100 nM) and increasing concentrations of CGS21680, another specific A2AR agonist. The geometric fluorescence mean within the M1 region events was plotted against each CGS21680 concentration tested. (C) Quantification of antiparkinsonian D2R agonists effects on MRS5206 binding. HEK-293 cells transiently transfected with A2AR and D2R were incubated with 100 nM of MRS5206 in the absence or presence of quinpirole (100 µM), pramipexole (120 µM), rotigotine (1 µM) and apomorphine (6 µM) and analyzed by flow cytometry. The obtained geometric fluorescence mean within the M1 region events for each equilibrium binding condition was plotted. Data represents the average ± S.E.M. values of three independent experiments. Asterisks indicate data significantly different from the control condition (i.e. in the absence of antiparkinsonian D2R agonist): *p < 0.05 and **p < 0.01 by ANOVA with Dunnett`s multiple comparison post-hoc test.
4. Conclusion
The two different experimental approaches presented here permitted us to evaluate the degree of D2R-mediated allosteric modulation of A2AR agonist binding within the A2AR/D2R oligomer framework. Hence, it could be concluded that both our FRET- and flow cytometry-based agonist binding assays would be valuable for determining the D2R agonist NAM activity of different antiparkinsonian agents over A2AR functioning. Needless to say, the relevance of this information for the design of effective D2R-based antiparkinsonian therapies is intrinsically limited, since the experiments were performed in a heterologous system. Thus, it is important to keep in mind that any new emerging D2R-based drug with A2AR NAM activity should be first tested in PD animal models before attempting some clinical extrapolation. And on the other hand, it would also be important to correlate the degree of the discovered properties of apomorphine, rotigotine, and pramipexole with their effectiveness as antiparkinsonian drugs. Overall, the present methods are mainly circumscribed to the mechanistic elucidation of the receptor-receptor interaction occurring between A2ARs and D2Rs, but they may also be viewed as possible complementary tools on the development of D2R-like drugs targeted to the management of Parkinson’s disease.
Supplementary Material
Acknowledgements
This work was supported by grants SAF2011-24779 and Consolider-Ingenio CSD2008-00005 from “Ministerio de Economía y Competitividad” (MINECO) and ICREA Academia-2010 from the Catalan Institution for Research and Advanced Studies (ICREA) to FC. Also, VF-D, MG-S, FN and FC belong to the “Neuropharmacology and Pain” accredited research group (Generalitat de Catalunya, 2009 SGR 232). Support to KAJ, AD, and TSK from the NIDDK Intramural Research Program of the National Institutes of Health, Bethesda, MD, USA is acknowledged. We thank Esther Castaño, Eva Julià, and Benjamín Torrejón, from the Scientific and Technical Services (SCT)-Bellvitge Campus of the University of Barcelona for the technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare that they have not conflict of interest. In addition, we want to state that the experimental work performed within this project was approved by the University of Barcelona.
References
- Ciruela F, Burgueno J, Casado V, Canals M, Marcellino D, Goldberg SR, Bader M, Fuxe K, Agnati LF, Lluis C, Franco R, Ferre S, Woods AS. Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope-epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal.Chem. 2004;76:5354–5363. doi: 10.1021/ac049295f. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J.Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duenas V, Gomez-Soler M, Jacobson KA, Kumar ST, Fuxe K, Borroto-Escuela DO, Ciruela F. Molecular determinants of A(2A) R-D(2) R allosterism: role of the intracellular loop 3 of the D(2) R. J.Neurochem. 2012;123:373–384. doi: 10.1111/j.1471-4159.2012.07956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casado V, Hillion J, Torvinen M, Fanelli F, Benedetti Pd P, Goldberg SR, Bouvier M, Fuxe K, Agnati LF, Lluis C, Franco R, Woods A. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat.Disord. 2004;10:265–271. doi: 10.1016/j.parkreldis.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Ferre S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr.Pharm.Des. 2008;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N.Engl.J.Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J.Pharmacol.Exp.Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Morelli M, Carta AR, Jenner P. Adenosine A2A receptors and Parkinson's disease. Handb.Exp.Pharmacol. 2009;(193):589–615. doi: 10.1007/978-3-540-89615-9_18. [DOI] [PubMed] [Google Scholar]
- Scheller D, Ullmer C, Berkels R, Gwarek M, Lubbert H. The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson's disease. Naunyn Schmiedebergs Arch.Pharmacol. 2009;379:73–86. doi: 10.1007/s00210-008-0341-4. [DOI] [PubMed] [Google Scholar]
- Schneider E, Mayer M, Ziemek R, Li L, Hutzler C, Bernhardt G, Buschauer A. A simple and powerful flow cytometric method for the simultaneous determination of multiple parameters at G protein-coupled receptor subtypes. Chembiochem. 2006;7:1400–1409. doi: 10.1002/cbic.200600163. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson's disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Xu K, Bastia E, Schwarzschild M. Therapeutic potential of adenosine A(2A) receptor antagonists in Parkinson's disease. Pharmacol.Ther. 2005;105:267–310. doi: 10.1016/j.pharmthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Ziegler N, Batz J, Zabel U, Lohse MJ, Hoffmann C. FRET-based sensors for the human M1-, M3-, and M5-acetylcholine receptors. Bioorg.Med.Chem. 2011;19:1048–1054. doi: 10.1016/j.bmc.2010.07.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.