Abstract
We describe a sampling method using glass capillaries for quantitative analysis of trace analytes in small volumes of complex mixtures (~1 μL) using ambient ionization mass spectrometry. The internal surface of a sampling glass capillary was coated with internal standard then used to draw liquid sample and so transfer both the analyte and internal standard in a single fixed volume onto a substrate for analysis. The internal standard was automatically mixed into the sample during this process and the volumes of the internal standard solution and sample are both fixed by the capillary volume. Precision in quantitation is insensitive to variations in length of the capillary, making the preparation of the sampling capillary simple and providing a robust sampling protocol. Significant improvements in quantitation accuracy were obtained for analysis of 1 μL samples using various ambient ionization methods.
Quantitative analysis of complex mixtures is one of the most important applications of mass spectrometry (MS). Representative methods include high-performance liquid chromatography-mass spectrometry (HPLC-MS) and gas chromatography-mass spectrometry (GC-MS), which have been developed over several decades and are widely applied for drug discovery, proteomics, environmental monitoring, food safety, and forensics applications.1 A general procedure for quantitative analysis using a modern MS systems typically starts from sample preparation with the analytes being extracted, purified, preconcentrated, and chromatographically separated before being analyzed by mass spectrometry. Although external calibration can be applied, the use of internal standards (IS) provides the best quantitative results.2 While these standard methods have seen continuous improvement and widening applications for in-lab quantitative chemical analysis, ambient ionization mass spectrometry3, 4 has emerged as a powerful means of rapid chemical analysis which requires minimum sample treatment.
Since the introduction of desorption electrospray ionization (DESI)5 and direct analysis in real time (DART),6 a large number of ambient ionization methods have been developed7 and demonstrated as simple and fast approaches for chemical analysis. Ambient ionization MS analysis represents a promising pathway for transferring MS technology to in field measurements and from analytical laboratory technicians to untrained personnel. For onsite clinical and other regulatory applications, quantitation with mandatory accuracy and limit of quantitation (LOQ) needs to be achieved using operational procedures which involve minimum human intervention. Some of the ambient ionization methods have been explored for quantitative analysis, including low temperature plasma (LTP),8 DESI,9, 10 paper spray,11 easy ambient sonic-spray ionization (EASI),12 DART13, extractive electrospray ionization (EESI),14 and sealing surface sampling probe (SSSP).15 As demonstrated in detail recently using paper spray, the introduction of internal standards is effective in improving the analytical performance for quantitation. For therapeutic drug monitoring in blood,16, 17 LOQs better than 1 ng/mL and RSD better than 15% over the entire therapeutic ranges were obtained with blood samples of volumes as low as 0.4 μL.11, 16 The potential in quantitation has been demonstrated clearly. However, it is critical to develop the most appropriate methods for accurately measuring small amounts of samples and introducing the internal standards while avoiding laboratory techniques like pipetting or vortex mixing. Such methods remain to be developed but are important for retaining the simplicity of ambient ionization analysis.
In a previous study, we explored a simple protocol of IS introduction for paper spray by pre-printing the internal standard onto the paper substrate.18 This method was specific to paper spray and the quantitative performance was found to be highly dependent on the method of depositing the internal standard and sample onto the paper substrate. In this work, we describe a new method in which glass capillaries are coated with internal standard and then used to transfer the sample while automatically mixing the internal standard into the sample. This method should be universally useful for direct MS analysis with a wide range of ambient ionization methods. Excellent quantitative performance was achieved with samples of volumes as small as 1 μL. The protocol developed has been tested with paper spray ionization, LTP and DESI for quantitation of pharmaceutical drugs in blood, illicit drugs in urine, and agricultural chemicals in river water.
EXPERIMENTAL SECTION
Glass capillaries (I.D. 0.4 mm; length, 75 mm) were purchased from Drummond Scientific Co. (Broomall, PA). Pipette tips (1000 μL) were purchased from Eppendorf (Hauppauge, NY). Whatman Grade 1 chromatography paper was purchased from GE Healthcare UK Limited (Buckinghamshire, England). Imatinib was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), imatinib-d8 was purchased from EJY Tech (Rockville, MD). Atrazine, atrazine-d5, cocaine, and cocaine-d3 were purchased from Sigma-Aldrich (Milwaukee, WI). Bovine blood (sodium citrate) was purchased from Innovative Research (Novi, MI). River water was collected from the Wabash River (West Lafayette, IN). Urine was collected from a healthy volunteer.
Blood samples were examined with paper spray ionization by using a TSQ Quantum Access Max (Thermo Scientific, San Jose, CA) in the multiple reaction monitoring (MRM) mode. River water and urine samples were examined with LTP and DESI, respectively, by using an Exactive Orbitrap (Thermo Scientific, San Jose, CA) in full scan mode. For paper spray mass spectrometry,19 chromatography paper was cut into a triangle (5 mm in base and 10 mm in height). The paper triangle was held by a metal clip and placed in front of the MS inlet at a distance of 5 mm. A 4.0 kV DC was applied through the metal clip. Spray solvent (35 μL acetonitrile: water, 90:10, v:v) was applied to the blood spot to extract chemicals for MS detection. For the LTP measurement,20 the river water sample was deposited onto a glass slide and allowed to dry. Experimental conditions for LTP included helium gas flow 0.5 L/min, 10 mm distance between sample and LTP probe, 5 mm distance between sample and MS inlet. For DESI measurements,5 the urine was diluted 10 fold with methanol and deposited on a PTFE sheet and allowed to dry. Experimental conditions for DESI included 150 psi nitrogen gas, 4.5 kV spray voltage, methanol/water (50:50, v:v) solvent delivered at a flow rate of 3 μL/min, 5 mm distance between sample and DESI probe, 1 mm distance between sample and MS inlet.
RESULTS AND DISCUSSION
Sampling capillaries were fabricated by cutting a long glass capillary (0.4 mm I.D.) into ~ 8 mm sections followed by a coating procedure developed to immobilize the internal standard onto the inner wall of the capillary sections. As shown in Figure 1a, one end of the capillary is dipped into a bulk solution (methanol in our experiments) containing the internal standard at a known concentration of CIS. The capillary was filled by capillary action so that the total volume of the solution was equal to the capillary volume VC. The capillary was then held vertically in air at 60 °C for 5 min (for characterization of drying time, see supporting information Table S-1) while the solvent dried completely and a solid coating was formed (Figure 1a). The temperature selected for drying was close to the boiling point of methanol (65 °C) but low enough to avoid decomposition of the internal standard or its loss by sublimation. With 100 ng/mL standard solutions of IS, the coating contained about 0.1 ng internal standard (IS) spread over about 10 mm2 of internal surface. In practical use of these capillary samplers, a plastic pipette tip (1000 μL) was used as a capillary holder for ease of handling (Figure 1b). When used to take a sample of the solution being analyzed (drawn up from a glass slide in our experiments) containing an analyte at a concentration of CA, one end of the capillary touched the sample and the capillary was filled with the sample by capillary action. The volume of the sample taken was also equal to the capillary volume VC. The sample was then transferred to a substrate that is suitable for the subsequent analysis using one of the ambient ionization methods. Figure 3c shows the IS containing dried blood spots prepared on chromatography paper using this protocol. Paper triangles were then cut for paper spray mass spectrometry analysis.
Figure 1.
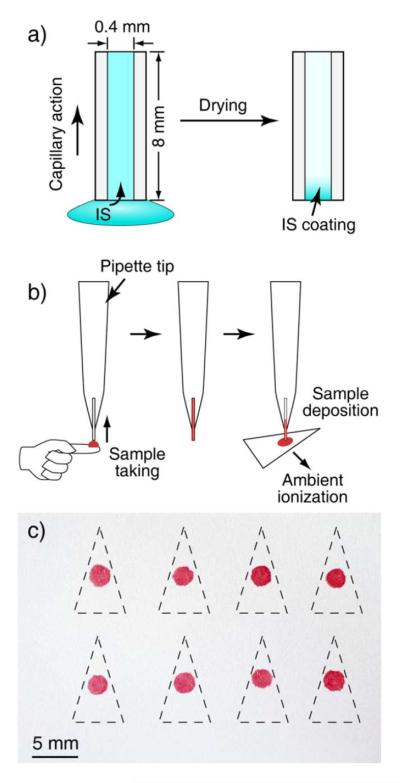
a) A capillary sampler (0.4 mm I.D., about 8 mm long) prepared by filling it with an internal standard (IS) solution through capillary action and drying in air to form an IS coating on its inner surface. b) Use of a capillary sampler with a pipette tip as holder to take the blood sample and to deposit it onto a paper substrate to prepare a dried blood spot containing IS. c) Array of dried blood spots prepared on chromatography paper using capillary samplers. Paper triangles were cut out along the dash lines and used for paper spray ionization. Relative standard deviation (RSD) of the area of the blood spots: was about 8 %, n = 8.
Figure 3.
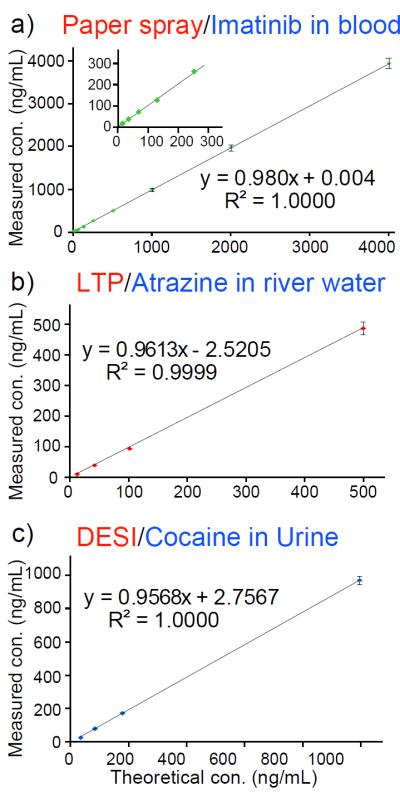
a) Analysis of imatinib in blood (10–4000 ng/mL) using paper spray ionization, capillary sampler coated with imatinib-d8. DC voltage (4.2 kV) was applied to the paper wetted with 35 μL spray solvent (acetonitrile/water, 90:10, v:v). Inset shows the low-concentration range. b) Analysis of atrazine in river water (10–500 ng/mL) using LTP. c) Analysis of cocaine in urine (30–1000 ng/mL) using DESI. TSQ used in MRM mode for paper spray, Exactive Orbitrap used in MS mode for LTP and DESI.
During the process of taking and transferring the sample, the coated internal standard was dissolved and mixed into the sample. In a test of the coating process, haematoxylin solution (blue in color) was used and it was observed that uneven coating could occur due to surface imperfections and gravity. However, this was found to have little impact on the results of the quantitative analysis, presumably due to complete dissolution of the IS coating into the sample. A plastic bulb could also be attached to the pipette to apply a pneumatic pressure and so assist in transferring viscous samples, such as blood, out of the capillary (see supporting information Fig. S-1).
The ratio of the peak intensities for the analyte (IA) and the internal standard (IIS) measured from the MS spectra was used for calibration and calculation of the concentration. As shown with the Equation 1, the ratio of IA/IIS is proportional to the ratio of the total amounts of the analyte and the internal standard dissolved from the coating, which is equal to the ratio of the original concentrations of the analyte in sample and the internal standard in the coating preparation solution. Due to capillary action, the volume of the coating preparation solution consumed and the sample taken both vary with the capillary volume and are always the same. This self-regulating feature of the protocol leads to the important conclusion that the accurate control of the volumes of the sampling capillaries during the capillary fabrication is NOT necessary to achieve good quantitative performance. This should make it extremely easy to adapt the method to mass production of the capillary samplers.
| Equation 1 |
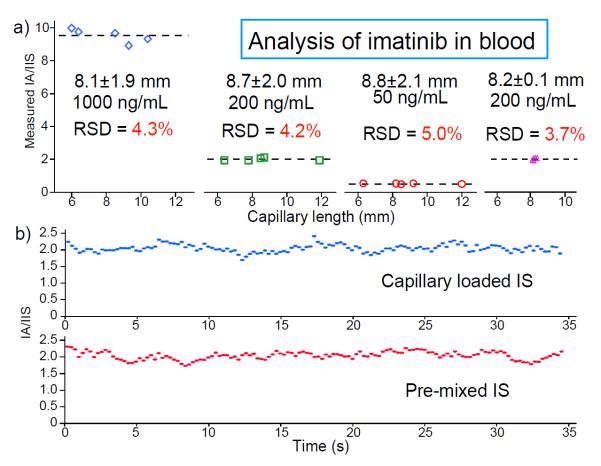
A series of experiments on quantitation of imatinib in blood samples was performed to test this protocol. The capillary samplers with nominal length 8 mm but that varied up to 25% (6–12 mm, corresponding to a range of 0.75–1.5 μL in volume) were coated with IS using 100 ng/mL imatinib-d8 in methanol. These capillary samplers were used to take blood samples containing imatinib at 50, 200 and 1000 ng/mL and to transfer them onto paper to make dried blood spots (DBS). Paper spray MS analysis was then performed using MRM of the fragment ion m/z 394 from imatinib (m/z 494 → m/z 394) and imatinib-d8 (m/z 502 → m/z 394). The ratios of the signals recorded for these two transitions were calculated for each capillary sampler as shown in Figure 2. The variations of the measured ratios are less than 5% (RSD, n=5) for the capillary samplers, which are slightly worse than the 3.7% RSD (n=5) obtained using the capillaries of tightly controlled lengths (Figure 2a right, RSD of 2.4% in capillary length, n=17).
Figure 2.
a) Quantitation of imatinib in blood at 50, 200 and 1000 ng/mL using capillary samplers of the same nominal length but various ranges of lengths. Capillaries were coated with imatinib-d8 (from 100 ng/mL methanol solution) as internal standard. b) Intensity ratios of fragment ions through the reactions of imatinib (m/z 494→ m/z 394, 200 ng/mL) and imatinib-d8 (m/z 502 → m/z 394, 100 ng/mL), paper spray of DBS prepared using an IS coated capillary sampler (top) and pre-mixing the IS using pipetting (bottom).
Comparison in the peak ratio accuracy for quantitation was also made between the protocols using the IS coated capillary samplers and traditional laboratory techniques. A group of dried blood spots on paper were prepared using 1 μL capillary samplers with 100 ng/mL imatinib-d8 introduced into blood samples containing 200 ng/mL imatinib. Another group of dried blood spots on paper were prepared by pipetting 1 μL blood from a bulk sample (1 mL) premixed with imatinib at 200 ng/mL and imatinib-d8 at 100 ng/mL using standard laboratory procedures. Both sets of DBS samples were then analyzed using paper spray. The ratio of the m/z 394 intensities for imatinib and imatinib-d8 as a function of time is shown in Figure 2b for protocols using capillary sampler and the traditional laboratory procedure. The ratio value and variations (RSD of about 5%) observed for the capillary sampler are identical to those for the standard lab procedure. This indicates that good mixing of the internal standard into the blood sample is achieved during sample transfer with the new protocol.
The IS-coated capillary sampler is expected to improve quantitative performance in direct MS analysis of liquid samples and to be useful in conjunction with a wide variety of ambient ionization methods. In this study, we have characterized it for paper spray, low temperature plasma probe and desorption electrospray ionization (Figure 3 and Table S-2). Quantitative analysis of imatinib in blood over the concentration range 10 ng/mL to 4 μg/mL was performed using paper spray analysis of DBS samples. RSDs of 2–4 % (RSD, n ≥ 3) were obtained over the entire range of concentrations (Figure 3a). For analysis of dried sample spots using LTP, river water samples containing atrazine at 10–500 ng/mL were transferred to glass slides using 1 μL capillary samplers coated with IS (100 ng/mL atrazine-d5 in methanol). RSDs (n=5) better than 7.5% were obtained (Figure 3b). For DESI analysis, diluted urine samples containing cocaine at 30 – 1000 ng/mL were deposited onto PTFE substrates using the IS-coated sampler (100 ng/mL cocaine-d3 in methanol), allowed to dry and analyzed by DESI. RSDs (n=5) better than 4% were achieved (Figure 3c).
Adequate quantitative performance was obtained with the 1 μL capillary sampler using all three ambient ionization methods. This method could potentially be used for sampling and IS mixing for laboratory analysis using HPLC-MS, for which larger volumes of sample might be necessary. We tested the sampling protocol using longer capillaries and with volumes up to 8 μL (see Figure S-1 in supporting information). Most liquids could be collected and dispensed with these capillaries, including aqueous solutions, organic solvents, urine, blood and serum. However, the IS coating process and the sampling process were affected by the physical properties of the liquid samples (See Table S-1 and 3). Longer times were required to fill capillaries with samples of higher viscosities, especially for longer capillaries. Sampling with the capillary in a tilted position was found to speed up the loading process. For urine, methanol and serum, sample filling times were about 1 s for an 8 μL capillary (Table S-3 in supporting information). For blood, the sampling time was about 15 s for an 8 μL capillary and less than 0.5 s for a 1 μL capillary, which is fast enough if used for taking finger-stick blood for point-of-care analysis. Dispensing times for different liquids have also been measured. For urine, methanol and serum, all liquids that could be easily dispensed onto paper by capillary action due to the porous nature of paper, the dispensing time was about an order of magnitude longer than the loading time (Table S-3 in supporting information). When dispensing blood, using a bulb on the dispenser holder to put some pressure through the capillary could help significantly and the blood deposition could be completed within one or two seconds.
CONCLUSIONS
The aim of allowing internal standards to be used with ambient ionization mass spectrometry has been achieved without requiring the use of traditional skilled lab procedures. We developed a method for taking liquid samples using IS coated capillary samplers. The effectiveness of this method has been demonstrated for several types of samples and with three ambient ionization methods. Accurate quantitation can be achieved with a starting sample volume at only 1 μL, which is significant for developing POC analysis using least invasive methods to take blood samples. This would also be attractive for preclinical studies, where typically less than 10 μL blood could be taken each time from the tail of a rat. A contribution can also be made to laboratory chemical analysis using HPLC-MS by saving effort, time and reagent in sampling and IS mixing.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Science Foundation (CHE 0847205 and CHE 0848650), National Science Foundation Instrumentation Development for Biological Research (DBI 0852740), National Center for Research Resources (5R21RR031246-02) and the National Institute of General Medical Sciences (8R21GM103454) from the National Institutes of Health.
Footnotes
Supporting Information Fabrication of sampling capillary of different volumes, characterization on the drying coating of internal standard, characterization on the sampling capillary. This supporting material is available.
The authors declare no competing financial interest
REFERENCES
- (1).Hawkridge AM, Muddiman DC. Annu. Rev. Anal. Chem. 2009;2:265–277. doi: 10.1146/annurev.anchem.1.031207.112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Xu RNX, Fan LM, Rieser MJ, El-Shourbagy TA. Journal of Pharmaceutical and Biomedical Analysis. 2007;44:342–355. doi: 10.1016/j.jpba.2007.02.006. [DOI] [PubMed] [Google Scholar]
- (3).Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- (4).Ouyang Z, Zhang XR. Analyst. 2010;135:659–660. doi: 10.1039/c003812c. [DOI] [PubMed] [Google Scholar]
- (5).Takats Z, Wiseman JM, Gologan B, Cooks RG. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- (6).Cody RB, Laramee JA, Durst HD. Anal Chem. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- (7).Harris GA, Galhena AS, Fernandez FM. Anal Chem. 2011;83:4508–4538. doi: 10.1021/ac200918u. [DOI] [PubMed] [Google Scholar]
- (8).Huang GM, Zheng OY, Cooks RG. Chemical Communications. 2009:556–558. doi: 10.1039/b818059h. [DOI] [PubMed] [Google Scholar]
- (9).Manicke NE, Kistler T, Ifa DR, Cooks RG, Ouyang Z. J. Am. Soc. Mass Spectrom. 2009;20:321–325. doi: 10.1016/j.jasms.2008.10.011. [DOI] [PubMed] [Google Scholar]
- (10).Miao ZX, Chen H. J. Am. Soc. Mass Spectrom. 2009;20:10–19. doi: 10.1016/j.jasms.2008.09.023. [DOI] [PubMed] [Google Scholar]
- (11).Wang H, Liu JJ, Cooks RG, Ouyang Z. Angew Chem Int Ed. 2010;49:877–880. doi: 10.1002/anie.200906314. [DOI] [PubMed] [Google Scholar]
- (12).Fernandes AMAP, Tega DU, Jara JLP, Cunha IBS, de Sa GF, Daroda RJ, Eberlin MN, Alberici RM. Energy Fuels. 2012;26:3042–3047. [Google Scholar]
- (13).Perez JJ, Harris GA, Chipuk JE, Brodbelt JS, Green MD, Hampton CY, Fernandez FM. Analyst. 2010;135:712–719. doi: 10.1039/b924533b. [DOI] [PubMed] [Google Scholar]
- (14).Gamez G, Zhu LA, Disko A, Chen HW, Azov V, Chingin K, Kramer G, Zenobi R. Chem. Commun. 2011;47:4884–4886. doi: 10.1039/c1cc10343a. [DOI] [PubMed] [Google Scholar]
- (15).Van Berkel GJ, Kertesz V. Anal. Chem. 2009;81:9146–9152. doi: 10.1021/ac901712b. [DOI] [PubMed] [Google Scholar]
- (16).Zhang ZP, Xu W, Manicke NE, Cooks RG, Ouyang Z. Anal Chem. 2012;84:931–938. doi: 10.1021/ac202058w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Manicke NE, Abu-Rabie P, Spooner N, Ouyang Z, Cooks RG. J. Am. Soc. Mass Spectrom. 2011;22:1501–1507. doi: 10.1007/s13361-011-0177-x. [DOI] [PubMed] [Google Scholar]
- (18).Manicke NE, Yang QA, Wang H, Oradu S, Ouyang Z, Cooks RG. International Journal of Mass Spectrometry. 2011;300:123–129. [Google Scholar]
- (19).Liu JJ, Wang H, Manicke NE, Lin JM, Cooks RG, Ouyang Z. Anal Chem. 2010;82:2463–2471. doi: 10.1021/ac902854g. [DOI] [PubMed] [Google Scholar]
- (20).Harper JD, Charipar NA, Mulligan CC, Zhang XR, Cooks RG, Ouyang Z. Anal Chem. 2008;80:9097–9104. doi: 10.1021/ac801641a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.