Abstract
The effect of phenethyl isothiocyanate (PEITC), a component of cruciferous vegetables, on the initiation and progression of cancer was investigated in a chemically induced estrogen-dependent breast cancer model. Breast cancer was induced in female Sprague Dawley rats (8 weeks old) by the administration of N-methyl nitrosourea (NMU). Animals were administered 50 or 150 µmol/kg oral PEITC and monitored for tumor appearance for 18 weeks. The PEITC treatment prolonged the tumor-free survival time and decreased the tumor incidence and multiplicity. The time to the first palpable tumor was prolonged from 69 days in the control, to 84 and 88 days in the 50 and 150 µmol/kg PEITC-treated groups. The tumor incidence in the control, 50 µmol/kg, and 150 µmol/kg PEITC-treated groups was 56.6%, 25.0% and 17.2%, while the tumor multiplicity was 1.03, 0.25 and 0.21, respectively. Differences were statistically significant (p < 0.05) from the control, but there were no significant differences between the two dose levels. The intratumoral capillary density decreased from 4.21 ± 0.30 vessels per field in the controls to 2.46 ± 0.25 in the 50 µmol/kg and 2.36 ± 0.23 in the 150 µmol/kg PEITC-treated animals. These studies indicate that supplementation with PEITC prolongs the tumor-free survival, reduces tumor incidence and burden, and is chemoprotective in NMU-induced estrogen-dependent breast cancer in rats. For the first time, it is reported that PEITC has anti-angiogenic effects in a chemically induced breast cancer animal model, representing a potentially significant mechanism contributing to its chemopreventive activity.
Keywords: phenethyl isothiocyanate, estrogen-dependent breast cancer model, anti-angiogenic effects, N-methyl nitrosourea, PEITC, NMU
Introduction
Brassica vegetables of the family Cruciferae (e.g. cabbage, watercress and broccoli) and the genus Raphanus (radishes and daikons) contain isothiocyanates and indoles that have been implicated in the reduction of cancer risk [1]. Phenethyl isothiocyanate (PEITC) is derived from gluconasturtiin, a glucosinolate of PEITC that occurs naturally in cruciferous vegetables [2]. Myrosinase present in cruciferous vegetables, converts gluconasturtiin to PEITC once the vegetable is cut or ingested [3]. Mechanisms of apoptosis induction by PEITC include a reduced expression of BcL-2 and BcL-XL, and an increase in caspase activity through p53 activation [4]. PEITC also down-regulates nicotinamide N-methyltransferase (NNMT) [5], an enzyme known to be a marker for tumor invasiveness and a prognostic tool in cancers such as colorectal, thyroid and renal cancers. In vitro, PEITC is a potent inhibitor of CYP450s that are involved in the metabolism and activation of carcinogens [6]. PEITC, like other isothiocyanates, is a mono-functional inducer of phase II enzymes, i.e. it induces phase II enzymes without inducing phase I enzymes [7]. Mechanisms of isothiocyanate actions have been reviewed elsewhere [8,9].
Phenethyl isothiocyanate has been shown to have a preventive effect in a number of animal cancer models. A dose of 12 µmol PEITC administered 5 days/week reduced the volume of PC-3 prostate cancer xenograft tumors by 57% compared with control animals after 21 days of treatment [10]. A PEITC dose of 5 µmoles three times a week for 28 days reduced the tumor volume by 37%, while doses of 2.5 µmoles of PEITC, combined with 3 µmoles of curcumin, decreased the tumor volume by 77% in PC-3 prostate tumor xenograft mice [11]. A diet containing 3 µmol/g PEITC also inhibited chemically induced lung cancer by 50% in rats [12]. A 94% reduction in oral cancer was observed when 50 mm PEITC was co-applied topically with the carcinogen N-nitrosomethylbenzylamine over 8 to 13 weeks [13]. PEITC has entered clinical trials for lung cancer in smokers [14]. However, the reports concerning the effects of PEITC in mammary gland tumors are conflicting. While one study reported a 15% increase in tumors generated from dimethyl benz(a)anthracene (DMBA), another study reported that PEITC reduced DMBA-induced tumors by 57% after a single dose administered prior to DMBA administration [15,16].
The objective of this study was to investigate the effects of PEITC on the progression of N-methyl nitrosourea (NMU)-induced estrogen-dependent breast cancer in a rat model, and to investigate the mechanisms responsible for its effects. A rat model was chosen since the metabolism of PEITC is similar in rats and humans, but differs between mice and humans [17–19]. To avoid confounding effects such as inhibition of enzymatic formation of the active carcinogen, NMU was used to initiate tumors. N-methyl nitrosourea is activated to its carcinogenic metabolite by non-enzymatic hydrolysis. N-methyl nitrosourea has been used to induce hormone dependent tumors in male and female rats. This model of breast cancer model is well-characterized, widely used and closely mimics breast cancer [20,21]. The tumors formed by NMU are estrogen-receptor positive and regress upon ovariectomy [22]. Gene expression profiling of NMU-induced rat mammary tumors classified the tumors as non-invasive ER + ductal carcinomas that are most similar to ER+ low to intermediate grade human breast cancer [23]. Studies in the NMU animal model have evaluated a number of potential chemopreventive agents including garlic, genistein, 3-cis-retinoic acid, resveratrol and lycopene, among others [21,24–27]. In an interesting study by Su et al. the authors demonstrated that in utero exposure to soy protein isolate can reduce NMU-induced tumor incidence by 20% when the rat offspring are injected with NMU after birth [28]. The model has also been used to evaluate cancer therapeutic agents [29]. Our hypothesis was that PEITC is a chemopreventive agent in estrogen-dependent breast cancer in rats. Two possible mechanisms related to this action were investigated: (a) change in concentrations of estradiol that is crucial in the initiation and progression of breast cancer and (b) tumor invasiveness and anti-angiogenic properties of PEITC, investigated by histological analysis of tumors.
Materials and Methods
Phenethyl isothiocyanate and N-methyl nitrosourea were purchased from Sigma Aldrich (St Louis, MO). The estradiol Coat-a-Count RIA kit was purchased from Siemens Corp. (Los Angeles, CA).
Tumor progression study
Female Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 170–190 g (8 weeks old) were housed in a room with controlled lighting and ambient temperature. The animals were fed with a phytoestrogen-free diet (Tekland 2016S) obtained from Harlan. Animals had free access to food and water throughout the study. Rats were acclimated for 1 week before the start of the study. The research protocol for the study was approved by the Institutional Animal Care and Use Committee at the University at Buffalo.
Starting at week 0, groups of 20–30 pubertal Sprague Dawley female rats received the corn oil vehicle (control) or phenethyl isothiocyanate at doses of 50 µmol/kg or 150 µmol/kg every 2 days by oral gavage. At the end of week 2, mammary gland tumors were induced in the animals by administering a 50 mg/kg dose of N-methyl nitrosourea in saline intraperitoneally. After 12–15 days, a second dose of 50 mg/kg of N-methyl nitrosourea was administered. Animals continued to receive PEITC for 18 weeks until the end of the study. Animals were monitored for new tumors every 2 days and followed until the tumor size reached approximately 5% of their body weight or until 18 weeks. The animals were killed by exsanguination following anesthesia with ketamine: xylazine (90:10 mg/kg), administered intramuscularly. Tumors were weighed and tissues, including liver, mammary gland and uterine horns, were collected and immediately snap frozen in liquid nitrogen. Part of the tumor was fixed in neutral buffered 3.7% formalin and processed for paraffin sectioning and histological evaluation by staining with hematoxylin and eosin (H&E). The estrous stage of the animal at the time of killing was determined by flushing the vagina using 0.9% saline and placing the sample on a glass slide. The cells were stained with 0.4% trypan blue solution and the smears were observed under a light microscope. The criteria used to determine the phase of the estrous cycle have been described previously [30]. Two studies were conducted: one with only one PEITC treatment group, and a second expanded study with two PEITC treatment groups. In the first study, the rats received either 150 µmol/kg PEITC or the vehicle control (n = 10 in each group). In the second study, rats received either 150 µmol/kg PEITC, 50 µmol/kg PEITC or the vehicle control. Terminal blood samples from animals in the second study were collected into heparinized tubes and the plasma was separated by centrifugation of blood at 5000 rpm for 5 min and stored at −80 °C until assayed for estradiol. Estradiol (E2) concentrations were analysed via a Coat-A-Count radioimmunoassay system using 125I-labeled estradiol.
Histological evaluation and functional angiogenesis determination
Blinded evaluation of tumor histology was performed on animals in the second study by using criteria described previously [31]. The intratumoral capillary density was analysed within the tumors by examining (H&E) stained paraffin sections under a 40× objective, using epifluorescen illumination through a Texas red filter. The eosinophilic red blood cells were intensely fluorescent and readily distinguishable from the background staining of extracellular matrix protein, as described previously [31]. The blood vessels were scored as individual incidences of single (in cross section) or multiple (in longitudinal section) red blood cells bounded by an eosinstained and therefore fluorescent basement membrane. In longitudinal capillary sections, individual branches were scored as separate incidences of vessels. When multiple red blood cells were scattered across the extracellular matrix and no limiting basement membrane was detected, this was determined to be a hemorrhage and was not scored as a blood vessel. The vessel density scoring was limited to the interior of the solid epithelial tumors, i.e. blood vessels in the surrounding stroma outside the solid tumor basement membrane were not scored.
Statistical analysis
The statistical analysis of tumor incidence (number of rats with tumors/total number of rats *100%) was performed using a Fisher’s exact test. The tumor-free survival was analysed by the log rank test. The average number of tumors per group (number of tumors per rat in each group) and intra-tumor capillary density were analysed by a Kruskal Wallis test with a Dunn’s multiple comparison post test. Tumor weights were analysed by a one-way ANOVA with Newman Keul’s post-hoc test. Differences in the estrous stage of groups were analysed using a Chi Square test, while circulating estradiol levels were analysed using a one-way ANOVA followed by a Newman Keul’s post test. Data were expressed as mean ± SEM. Statistical differences were significant when the value of p was less than 0.05.
Results
Tumor progression study
Effect of PEITC on breast cancer progression
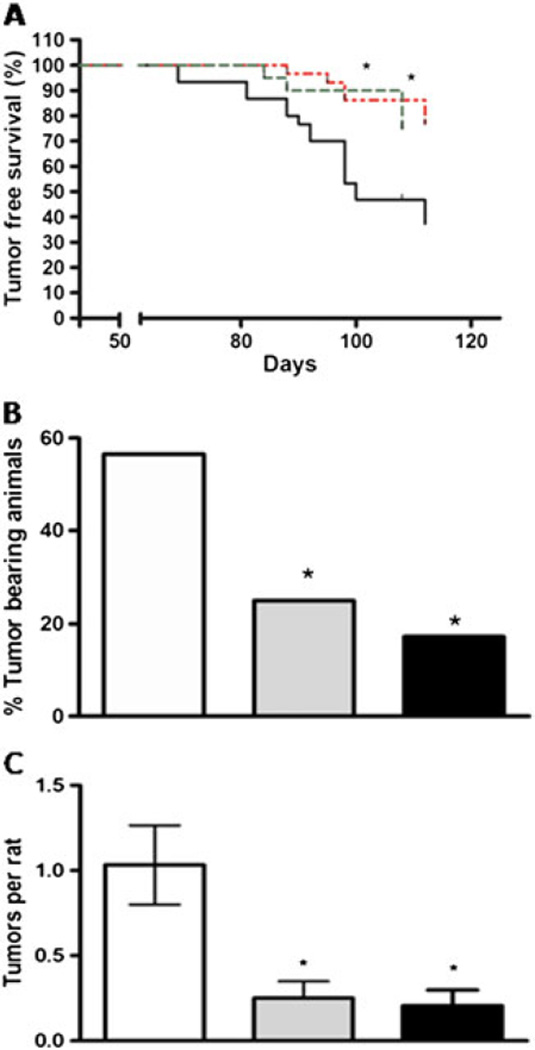
Data from the two studies were combined to measure the effect of PEITC on breast cancer progression. At both doses, the PEITC treatment increased the tumor-free survival time in the rats in this study. The time to the first palpable tumor was prolonged from 69 days in the control, to 84 and 88 days in the 50 and 150 µmol/kg PEITC-treated groups, respectively (Figure 1A). Phenethyl isothiocyanate also reduced the tumor incidence and multiplicity in the treated groups. The tumor incidence rates in the control, 50 µmol/kg, and 150 µmol/kg PEITC groups were 56.6%, 25.0% and 17.2%, while tumor multiplicity (average tumor number) was 1.03, 0.25 and 0.21, respectively (Figures 1B and 1C). The differences in the PEITC-fed rats were statistically significant (p < 0.05) from the control, but there were no significant differences between the two doses of PEITC. A trend was observed suggesting a reduced tumor weight in treated animals, but the results were not statistically significant. There were no differences in the body weights between the groups throughout the study.
Figure 1.
(A) Effect of phenethyl isothiocyanate (PEITC) on tumor-free survival in NMU-treated rats. N-methyl nitrosourea was administered at a dose of 50 mg/kg i.p. at day 0 and between 12 and 15 days. Rats were administered 150 µmol/kg or 50 µmol/kg PEITC in corn oil every 2 days or corn oil alone in the vehicle-treated control group. The black line represents the control group (n = 30), the green dashed line represents the 50 µmol/kg PEITC-treated group (n=20) and the red dotted line represents the 150 µmol/kg PEITC-treated group (n = 29). Curves were determined to be significantly different between the control and treatment groups (Log rank test, p < 0.05). (B) Effect of PEITC on tumor incidence and (C) average number of tumors per group. In panels B and C, the open bar represents the controls, while the gray bar represents the 50 µmol/kg PEITC-treated group, and the black bar represents the 150 µmol/kg PEITC-treated group. *Statistically significant differences from the control group (p < 0.05)
Estrous stage and estradiol levels
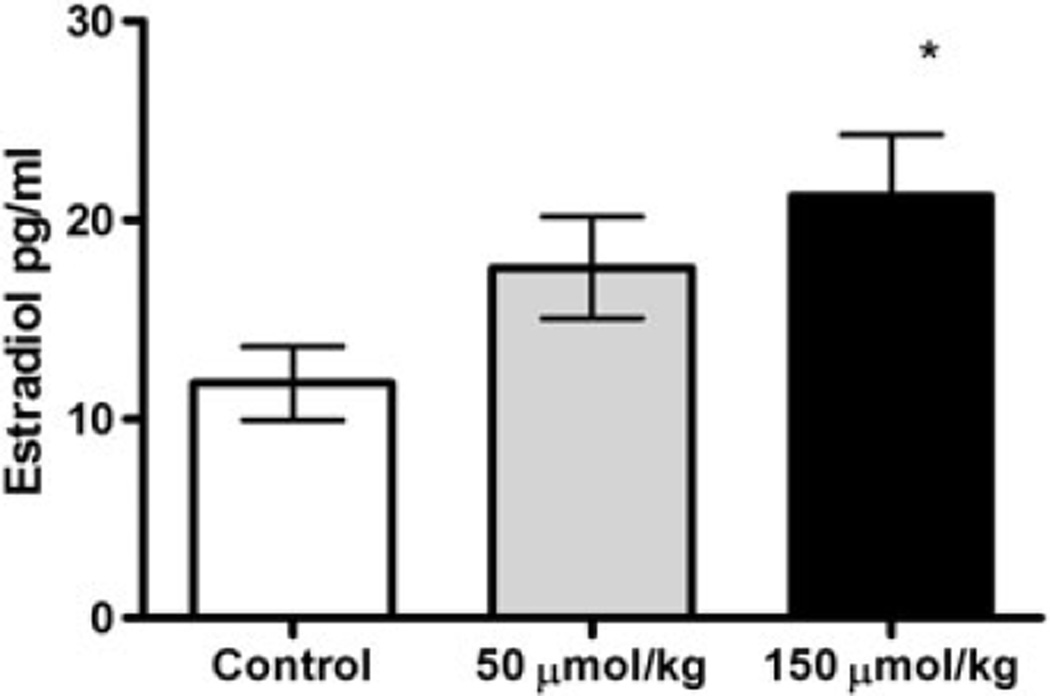
There were no statistical differences in the estrous stages in the rats in the different treatment groups, when determined at the time of killing. Estradiol concentrations were 12 ± 1.8, 18 ± 2.5 and 21 ± 3.0 pg/ml in the control, 50 µmol/kg and 150 µmol/kg PEITC-treatment groups, respectively. The plasma estradiol concentrations were significantly increased for the 150 µmol/kg PEITC-treated group compared with the control group (Figure 2).
Figure 2.
Effect of PEITC on estradiol concentrations. The open bar represents the controls, while the gray bar represents the 50 µmol/kg PEITC-treated group, and the black bar represents the 150 µmol /kg PEITC-treated group. Data are presented ± SEM, n = 12 in each group. *Statistically significant increase compared with control, with p < 0.05
Histological evaluation and functional angiogenesis determination
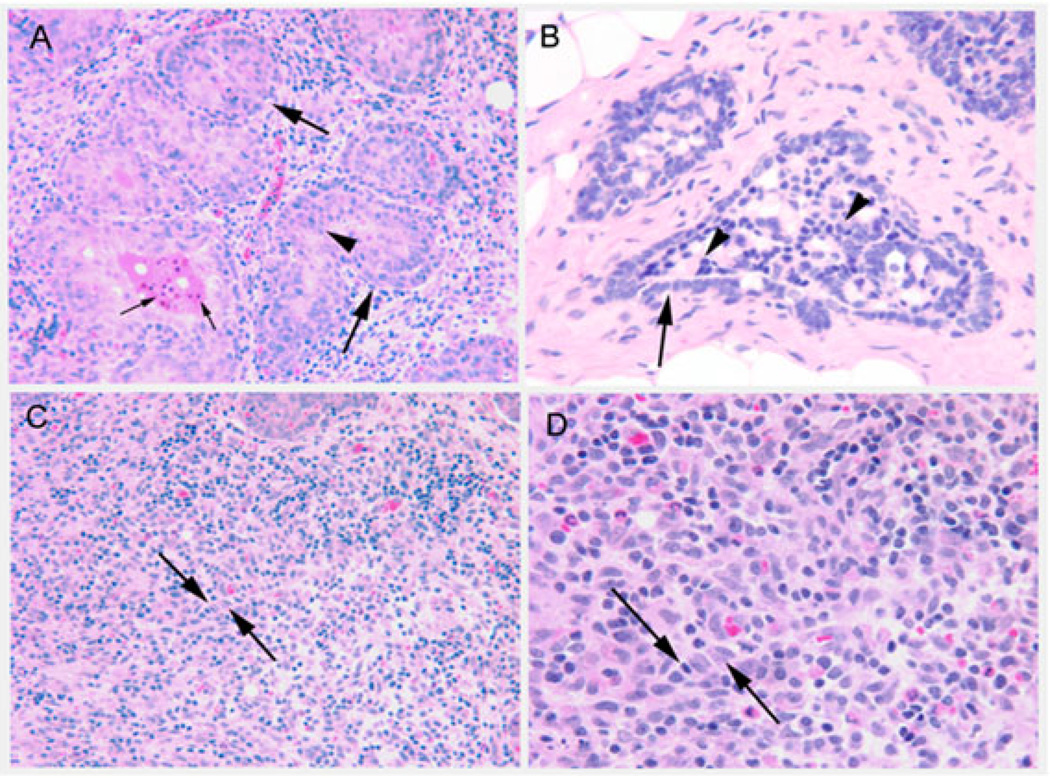
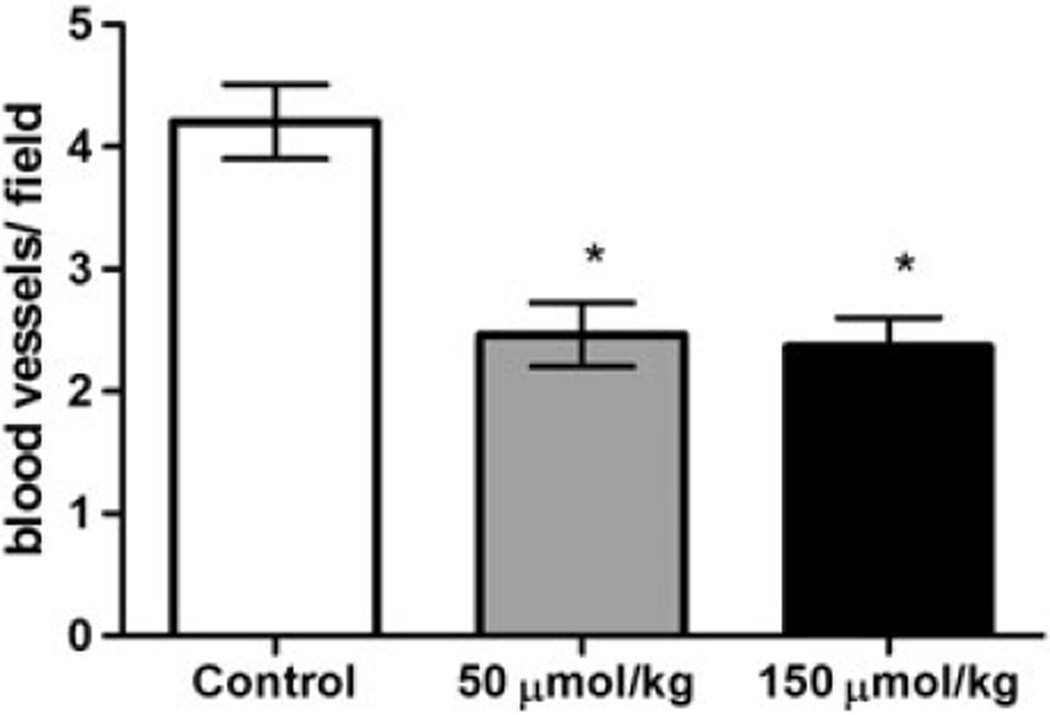
Among the tumors evaluated, 6 of 11 (54.5%) of the control tumors were lobular carcinomas in situ, 2 of 11 (18.1%) were ductal carcinomas in situ, and 2 of 11 were invasive lobular and ductal carcinomas (1 of 11 (9%) each). In the 50 µmol/kg PEITC-treated group, 3 of 5 (60%) tumors were lobular carcinomas, 1 of 5 (20%) was an invasive lobular carcinoma, and 1 of 5 (20%) showed atypical ductal hyperplasia. In the 150 µmol/kg PEITC-treated group, 4 of 4 (100%) tumors were lobular carcinomas in situ. Figure 3 presents images of lobular and ductal carcinomas in situ, as well as invasive carcinomas. The intratumoral capillary density decreased from 4.21 ± 0.30 vessels per field in the controls to 2.46 ± 0.25 vessels per field in the 50 µmol/kg PEITC-treated group and 2.36 ± 0.23 vessels per field in the 150 µmol/kg PEITC-treated group (Figure 4). The intratumoral capillary density in both groups were statistically significantly different from the controls (p < 0.05).
Figure 3.
Images of tumor phenotypes. Panel (A): Lobular carcinoma in situ, showing multilayered, nonpolarized epithelial cells (arrowhead) inside lobular ductules (large arrows). Small arrows indicate apoptotic bodies in the lumen of a lesion. Original magnification with 20× objective. Panel (B): Ductal carcinoma in situ, showing multilayered nonpolarized epithelial cells in a cribriform arrangement (arrowheads) within the lumen of a ductule (arrow). Original magnification, 40× objective. Panel (C): Invasive lobular carcinoma, with epithelial cells arranged in a single file embedded within the interstitial stroma (between arrows). Original magnification, 20× objective. Panel (D) enlargement of panel (C) showing nuclear heterogeneity within the invasive lobular carcinoma lesion (between arrows). Original magnification, 40× objective
Figure 4.
Effect of PEITC on intratumor capillary density. The open bar represents the vehicle controls (n = 9 tumors), the gray bar represents the 50 µmol/kg PEITC-treated group (n=5 tumors), while the black bar represents the 150 µmol/kg PEITC-treated group (n = 3 tumors). The data are presented as mean ± SEM. *Statistically significant differences from controls (p < 0.05) by Kruskal Wallis test followed by Dunn’s multiple comparison test
Discussion
The consumption of vegetables containing isothiocyanates, a class of compounds derived from cruciferous vegetables such as broccoli and kale, has been correlated inversely with the reduction of cancer risk [32]. Our laboratory has previously conducted studies with PEITC and has reported its pharmacokinetic properties, its cytotoxic properties in breast cancer cell lines, and its potential interactions with ATP-dependent binding cassette (ABC) transporters that are overexpressed in many cancer cell lines. Phenethyl isothiocyanate is highly orally bioavailable and a lipophilic agent, which can make it an agent of choice for cancer in organs with high amounts of adipose tissue such as the mammary gland. Our in vitro studies indicated that PEITC may have beneficial protective effects in breast cancer [33]. PEITC, along with other isothiocyanates, was demonstrated to be cytotoxic to MCF7 cells, an estrogen-dependent cancer cell line, with an IC50 of 6.51 ± 0.86 µm. The objective in this study was to demonstrate the effects of PEITC on tumor progression in a carcinogen (NMU)-induced estrogen-dependent breast cancer model in vivo. Past studies have investigated the effects of PEITC in a DMBA-induced mammary gland cancer have yielded conflicting results [15,16]. These studies used PEITC mixed in the diet as a means of delivery of PEITC to the animals. Dietary administration can result in highly variable amounts ingested, in our experience, likely due to the pungent smell or taste of PEITC. Additionally, PEITC may exhibit limited stability when incorporated into diets that are stored and used over a period of time. Whether these potential problems contributed to the variable results from these previous studies is unknown. To ensure the delivery of the intended dose of PEITC, freshly prepared doses of PEITC were administered by oral gavage to the animals.
It was demonstrated previously that at 150 µmol/kg doses in female rats, PEITC can up-regulate the enzyme UDP-glucuronosyltransferase 1a (ugt1a) and down-regulate NNMT, an enzyme shown recently to be an important prognostic marker that may play a role in tumor cell migration and invasiveness [5]. Based on simulations from a pharmacokinetic model published previously [34], this dose yields maximal plasma concentrations of about 100 µm. While the 150 µmol/kg dose is much lower than previously investigated doses of PEITC in mammary cancer, plasma concentrations achieved by this dose are higher than those observed in humans after dietary consumption of watercress [2]. To investigate the effects in the dietary range, a lower dose (50 µmol/kg) was investigated, which would yield a maximal plasma concentration of about 33 µm, which is closer to the maximal plasma concentrations achieved in humans after ingesting 100 g of watercress.
It was observed that chronic PEITC administration at doses of 50 or 150 µmol/kg reduced the incidence of mammary tumors and prolonged tumor-free animal survival. This was accompanied by fewer invasive tumors in the treated groups, a reduction of angiogenesis and an increase in terminal plasma estradiol concentrations. Recent publications have shown that PEITC and other isothiocyanates can exert anti-angiogenic effects, possibly by the modulation of hypoxia inducible factors and NFkB [35,36]. PEITC has been reported to decrease the migration of PC-3 prostate cancer cells [37]. Our histologic evaluations of the formed tumors suggest that the control and low dose PEITC groups demonstrated the presence of invasive tumors, while the 150 µmol/kg group did not show any invasive tumors. A dose of 150 µmol/kg PEITC can down-regulate NNMT [5], which may play a role in this observed reduction in invasiveness. Additionally, the intratumoral capillary density was lower in both the PEITC treatment groups, compared with the control groups. Anti-angiogenic effects of PEITC have been demonstrated previously in vitro in human umbilical vein endothelial cells and ex vivo using a chicken egg chorioallantoic membrane assay by Xiao et al. [37]. Recently, dietary administration of PEITC (100–150 mg/kg body weight/day) was reported to inhibit the expression of tumor platelet/endothelial cell adhesion molecule (PECAM-1/CD31), a marker of angiogenesis, in an androgen-responsive LNCaP human prostate cancer cell xenograft animal model [38]. A PEITC-induced inhibition of the transcription factor hypoxia-inducible factor (HIF), a positive regulator of angiogenesis, has also been reported recently in human breast cancer MCF-7 cells [39]. Importantly, watercress ingestion in humans decreases the phosphorylation of the translation regulator 4E protein -1 (4E-BP1) which can reduce the activity of HIF1α [40]. Our studies show, for the first time, a reduction of angiogenesis and the occurrence of invasive tumors in an in vivo model of chemically induced mammary gland cancer. This and other mechanisms, including the induction of apoptosis, may also be involved in the observed chemopreventive effect of PEITC. Studies examining the effect of low PEITC concentrations on human mammary epithelial cells reported significant increases of the pro-apoptotic genes BAD, BAX, THSB4 and GADD34 [41]. The ERβ expression was also increased and this was associated with increased expression of the tumor suppressor genes cyclin A and Ki67 [41].
While reduced tumor incidence and prolonged tumor-free animal survival were observed, an increase in terminal β-estradiol concentrations was seen in tumor-bearing animals at killing. In the 150 µmol/kg dose group, the terminal mean estradiol concentration was nearly twice the concentration in the controls. The risk of breast cancer has been correlated positively with circulating estradiol concentrations in post-menopausal women [42]. This correlation has been attributed to the capacity of estradiol to (a) interact with the estrogen receptor α, resulting in an increase in cellular proliferation via estrogen-receptor signaling, and (b) to form genotoxic estrogen metabolites [43–45]. The metabolism of estradiol is governed by phase I, as well as phase II enzymes, while the estrogen receptors play a significant role in apoptosis [46]. CYP450s inhibited by isothiocyanates and phase II enzymes, such as UDP-glucuronosyltransferases, and NAD (P)H:quinone oxidoreductase, induced by these isothiocyanates, may play an important role in the conversion of estradiol to its carcinogenic metabolites and its conversion to hydrophilic conjugates, respectively [6,47,48]. Our previous studies reported a 5-fold increase in mRNA expression of the enzyme CYP19 (aromatase), responsible for the conversion of androgens to estrogens, in MCF-7 cells [49], suggesting that increased formation of estradiol may occur as a result of PEITC treatment. However, the importance of the increased plasma estradiol concentrations observed after PEITC treatment is unknown. Isothiocyanates have also been shown to repress estrogen receptor α, which may explain, at least in part, why there was a reduction in the tumor incidence, despite the increased endogenous estradiol concentration [50]. Hormone dependent mammary gland cancers exhibit high expression levels of estrogen receptor α, whose interaction with estradiol plays an important role in enhancing cellular proliferation. The disruption of this receptor may decrease the estradiol interactions with its receptor, contributing to the observed chemopreventive effect of PEITC. A report of PEITC-induced changes in human mammary epithelial cells and human breast cancer MCF-7 cells indicated no change in the mRNA expression of ERα [41]. Further studies are needed to evaluate the potential role of PEITC on ERα expression and function.
Conclusion
In conclusion, dietary isothiocyanates exhibit chemopreventive activities, likely mediated through a number of mechanisms [51]. This investigation represents the first evaluation of PEITC treatment in an NMU-induced breast cancer rat model. In our study, PEITC significantly reduced the incidence of tumors and increased tumor-free survival in an NMU-induced breast cancer rat model. Histological studies also indicated a decrease in invasive mammary tumors, and an anti-angiogenic effect in tumors. Contrary to our expectations, an increase in the circulating estradiol concentrations was observed in PEITC-treated rats. This suggests that the protective effect of chronic administration of PEITC may be related to the upregulation of pro-apoptotic genes and/or the down-regulation of genes promoting proliferation, and anti-angiogenesis, and is not due to a reduction in β-estradiol concentrations.
Acknowledgements
We would like to thank Ms Melanie Felmlee and the Histology Service Laboratory at the UB Department of Pathology and Anatomical Sciences for their assistance.
Financial support was from the National Institutes of Health Grant CA121404 and UB Interdisciplinary Research Development Award. UT was supported in part by a fellowship from Daiichi Sankyo Inc.
Footnotes
Author contributions
All authors participated in the design, interpretation of the studies and/or analysis of the data and all reviewed the manuscript; UA, MEM and PM designed the study, analysed and interpreted the data; UA and YAG conducted the experiments; UA, MEM, YAG and PM all assisted in manuscript preparation.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Zhao BSA, Lee EJ, Poh WT, et al. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prevention. 2001;10:1063–1067. [PubMed] [Google Scholar]
- 2.Ji Y, Morris ME. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Anal Biochem. 2003;323:39–47. doi: 10.1016/j.ab.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Rouzaud G, Young SA, Duncan AJ. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol Biomarkers Prev. 2004;13:125–131. doi: 10.1158/1055-9965.epi-085-3. [DOI] [PubMed] [Google Scholar]
- 4.Huang C, Ma WY, Li J, Hecht SS, Dong Z. Essential role of p53 in phenethyl isothiocyanate-induced apoptosis. Cancer Res. 1998;58:4102–4106. [PubMed] [Google Scholar]
- 5.Telang U, Morris ME. Effect of orally administered phenethyl isothiocyanate on hepatic gene expression in rats. Mol Nutr Food Res. 2010;54:1802–1806. doi: 10.1002/mnfr.200900607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metab Dispos. 2001;29:1110–1113. [PubMed] [Google Scholar]
- 7.Prochaska HJ, Talalay P. Regulatory mechanisms ofmonofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988;48:4776–4782. [PubMed] [Google Scholar]
- 8.Shu L, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- 9.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao D, Lew KL, Zeng Y, et al. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27:2223–2234. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 11.Khor TO, Keum YS, Lin W, et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006;66:613–621. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]
- 12.Morse MA, Wang CX, Stoner GD, et al. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989;49:549–553. [PubMed] [Google Scholar]
- 13.Solt DB, Chang K, Helenowski I, Rademaker AW. Phenethyl isothiocyanate inhibits nitrosamine carcinogenesis in a model for study of oral cancer chemoprevention. Cancer Lett. 2003;202:147–152. doi: 10.1016/j.canlet.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Masonic Cancer Center University of Minnesota, National Cancer Institute Phenethyl Isothiocyanate in Preventing Lung Cancer in Smokers. http://clinicaltrials.gov/ct2/show/NCT00005883.
- 15.Lubet RA, Steele VE, Eto I, Juliana MM, Kelloff GJ, Grubbs CJ. Chemopreventive efficacy of anethole trithione, N-acetyl-l-cysteine, miconazole and phenethylisothiocyanate in the DMBA-induced rat mammary cancermodel. Int J Cancer. 1997;72:95–101. doi: 10.1002/(sici)1097-0215(19970703)72:1<95::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977;58:395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 17.Eklind KI, Morse MA, Chung FL. Distribution and metabolism of the natural anticarcinogen phenethyl isothiocyanate in A/J mice. Carcinogenesis. 1990;11:2033–2036. doi: 10.1093/carcin/11.11.2033. [DOI] [PubMed] [Google Scholar]
- 18.Mennicke WH, Gorler K, Krumbiegel G. Metabolism of some naturally occurring isothiocyanates in the rat. Xenobiotica. 1983;13:203–207. doi: 10.3109/00498258309052256. [DOI] [PubMed] [Google Scholar]
- 19.Adesida A, Edwards LG, Thornalley PJ. Inhibition of human leukaemia 60 cell growth by mercapturic acid metabolites of phenylethyl isothiocyanate. Food Chem Toxicol. 1996;34:385–392. doi: 10.1016/0278-6915(96)00124-x. [DOI] [PubMed] [Google Scholar]
- 20.Cohen LA, Zhao Z, Pittman B, Scimeca JA. Effect of intact and isoflavone-depleted soy protein on NMU-induced rat mammary tumorigenesis. Carcinogenesis. 2000;21:929–935. doi: 10.1093/carcin/21.5.929. [DOI] [PubMed] [Google Scholar]
- 21.Kijkuokool P, Parhar IS, Malaivijitnond S. Genistein enhancesN-nitrosomethylurea-induced ratmammary tumorigenesis. Cancer Lett. 2006;242(1):53–9. doi: 10.1016/j.canlet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Thordarson G, Lee AV, McCarty M, et al. Growth and characterization of N-methyl-N-nitrosourea-induced mammary tumors in intact and ovariectomized rats. Carcinogenesis. 2001;22:2039–2047. doi: 10.1093/carcin/22.12.2039. [DOI] [PubMed] [Google Scholar]
- 23.Chan MM, Lu X, Merchant FM, Iglehart JD, Miron PL. Gene expression profiling ofNMU-induced ratmammary tumors: cross species comparison with human breast cancer. Carcinogenesis. 2005;26:1343–1353. doi: 10.1093/carcin/bgi100. [DOI] [PubMed] [Google Scholar]
- 24.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 25.Nowfar S, Teplitzky SR, Melancon K, et al. Tumor prevention by 9-cis-retinoic acid in the N-nitroso-N-methylurea model of mammary carcinogenesis is potentiated by the pineal hormonemelatonin. Breast Cancer Res Treat. 2002;72:33–43. doi: 10.1023/a:1014912919470. [DOI] [PubMed] [Google Scholar]
- 26.Schaffer EM, Liu JZ, Green J, Dangler CA, Milner JA. Garlic and associated allyl sulfur components inhibit N-methyl-N-nitrosourea induced rat mammary carcinogenesis. Cancer Lett. 1996;102:199–204. doi: 10.1016/0304-3835(96)04160-2. [DOI] [PubMed] [Google Scholar]
- 27.Manni A, Richie JP, Jr, Xu H, et al. Effects of fish oil and Tamoxifen on preneoplastic lesion development and biomarkers of oxidative stress in the early stages of N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. Int J Oncol. 2011 Nov;39:1153–1164. doi: 10.3892/ijo.2011.1133. [DOI] [PubMed] [Google Scholar]
- 28.Su Y, Eason RR, Geng Y, Till SR, Badger TM, Simmen RC. In utero exposure to maternal diets containing soy protein isolate, but not genistein alone, protects young adult rat offspring from NMU-induced mammary tumorigenesis. Carcinogenesis. 2007;28:1046–1051. doi: 10.1093/carcin/bgl240. [DOI] [PubMed] [Google Scholar]
- 29.Moody TW. Thymosin alpha1 as a chemopreventive agent in lung and breast cancer. Ann N Y Acad Sci. 2007;1112:297–304. doi: 10.1196/annals.1415.040. [DOI] [PubMed] [Google Scholar]
- 30.Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 31.Masso-Welch PA, Zangani D, Ip C, et al. Isomers of conjugated linoleic acid differ in their effects on angiogenesis and survival of mouse mammary adipose vasculature. J Nutr. 2004;134:299–307. doi: 10.1093/jn/134.2.299. [DOI] [PubMed] [Google Scholar]
- 32.Ambrosone CBMS, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 33.Tseng E, Kamath A, Morris ME. Effect of organic isothiocyanates on the P-glycoprotein- and MRP1-mediated transport of daunomycin and vinblastine. Pharm Res. 2002;19:1509–1515. doi: 10.1023/a:1020460700877. [DOI] [PubMed] [Google Scholar]
- 34.Ji Y, Kuo Y, Morris M. Pharmacokinetics of dietary phenethyl isothiocyanate in rats. Pharm Res. 2005;22:1658–1666. doi: 10.1007/s11095-005-7097-z. [DOI] [PubMed] [Google Scholar]
- 35.Wang XH, Cavell BE, Syed Alwi SS, Packham G. Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochem Pharmacol. 2009;78:261–272. doi: 10.1016/j.bcp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Boreddy SR, Sahu RP, Srivastava SK. Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-alpha/VEGF/Rho-GTPases: pivotal role of STAT-3. PLoS One. 2011;6:e25799. doi: 10.1371/journal.pone.0025799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 2007;67:2239–2246. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 38.Hudson TS, Perkins SN, Hursting SD, et al. Inhibition of androgen-responsive LNCaP prostate cancer cell tumor xenograft growth by dietary phenethyl isothiocyanate correlates with decreased angiogenesis and inhibition of cell attachment. Int J Oncol. 2012;40:1113–1121. doi: 10.3892/ijo.2012.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavell BE, Syed Alwi SS, Donlevy AM, Proud CG, Packham G. Natural product-derived antitumor compound phenethyl isothiocyanate inhibits mTORC1 activity via TSC2. J Nat Prod. 2012;75:1051–1057. doi: 10.1021/np300049b. [DOI] [PubMed] [Google Scholar]
- 40.Syed Alwi SS, Cavell BE, Telang U, Morris ME, Parry BM, Packham G. In vivo modulation of 4E binding protein 1 (4E-BP1) phosphorylation by watercress: a pilot study. Br J Nutr. 2010;104:1288–1296. doi: 10.1017/S0007114510002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telang U, Brazeau DA, Morris ME. Comparison of the effects of phenethyl isothiocyanate and sulforaphane on gene expression in breast cancer and normal mammary epithelial cells. Exp Biol Med (Maywood) 2009;234:287–295. doi: 10.3181/0808-RM-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas HV, Key TJ, Allen DS, et al. A prospective study of endogenous serum hormone concentrations and breast cancer risk in post-menopausal women on the island of Guernsey. Br J Cancer. 1997;76:401–405. doi: 10.1038/bjc.1997.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 44.Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87:1–25. doi: 10.1016/s0960-0760(03)00390-x. [DOI] [PubMed] [Google Scholar]
- 45.Eissa S, Labib R, Khalifa A, Swelam N, Khalil FE, l-Shenawy AM. Regulators of apoptosis in human breast cancer. Clin Biochem. 1999;32:321–326. doi: 10.1016/s0009-9120(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Basten GP, Bao Y, Williamson G. Sulforaphane and its glutathione conjugate but not sulforaphane nitrile induce UDP-glucuronosyl transferase (UGT1A1) and glutathione transferase (GSTA1) in cultured cells. Carcinogenesis. 2002;23:1399–1404. doi: 10.1093/carcin/23.8.1399. [DOI] [PubMed] [Google Scholar]
- 48.Guo Z, Smith TJ, Wang E, et al. Effects of phenethyl isothiocyanate, a carcinogenesis inhibitor, on xenobiotic-metabolizing enzymes and nitrosamine metabolism in rats. Carcinogenesis. 1992;13:2205–2210. doi: 10.1093/carcin/13.12.2205. [DOI] [PubMed] [Google Scholar]
- 49.Moon YJ, Brazeau DA, Morris ME. Dietary phenethyl isothiocyanate alters gene expression in human breast cancer cells. Evid Based Complement Alternat Med. 2011;2011:462525. doi: 10.1155/2011/462525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang L, Ding L, Wang ZY. Isothiocyanates repress estrogen receptor alpha expression in breast cancer cells. Oncol Rep. 2009;21:185–192. [PMC free article] [PubMed] [Google Scholar]
- 51.Shu L, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]