Abstract
Globally, tuberculosis (TB) still remains a major public health problem. India is a high TB burden country contributing to 26 per cent of global TB burden. During 1944-1980, TB became treatable and short-course chemotherapy emerged as the standard of care. When TB elimination seemed possible in the early 1980s, global human immunodeficiency virus (HIV) infection/acquired immunodeficiency syndrome (AIDS) pandemic resulted in a resurgence of TB. Widespread occurrence of multidrug-resistant and extensively drug-resistant TB (M/XDR-TB) is threatening to destabilize TB control globally. Atypical clinical presentation still poses a challenge. Disseminated, miliary and cryptic TB are being increasingly recognized. Availability of newer imaging modalities has allowed more efficient localization of lesions and use of image guided procedures has facilitated definitive diagnosis of extrapulmonary TB. Introduction of liquid culture, rapid drug-susceptibility testing (DST), molecular diagnostic methods has helped in rapid detection, speciation and DST profiling of Mycobacterium tuberculosis isolates. While treatment of TB and HIV-TB co-infection has become simpler, efforts are on to shorten the treatment duration. However, drug toxicities and drug-drug interactions still constitute a significant challenge. Recently, there has been better understanding of anti-TB drug-induced hepatotoxicity and its frequent confounding by viral hepatitis, especially, in resource-constrained settings; and immune reconstitution inflammatory syndrome (IRIS) in HIV-TB. Quest for newer biomarkers for predicting a durable cure, relapse, discovery/repurposing of newer anti-TB drugs, development of newer vaccines continues to achieve the goal of eliminating TB altogether by 2050.
Keywords: Clinical manifestations, diagnosis, epidemiology, extensively drug-resistant tuberculosis, human immunodeficiency virus co-infection, multidrug-resistant tuberculosis, treatment, tuberculosis
Introduction
The “captain of all these men of death”, tuberculosis (TB) has been a scourge of the humankind from time immemorial. Till date, no other disease in history matches the sheer magnitude of the misery inflicted by TB on the human race in terms of morbidity and mortality. The social and economic consequences of TB have had a profound effect on human existence. Historically, even though several other diseases like smallpox and plague have killed millions of people, their reign has been relatively short-lived; TB has been ever present. The inexorable march of time has witnessed the changing face of TB: from an incurable disease to the hype and hope of being an eminently curable one. However, even today TB remains as a formidable foe threatening to annihilate the human race. This review attempts to provide an overview of our understanding of TB, availability of rapid diagnostic tests including imaging modalities and anti-TB drugs and to outline the challenges that lie ahead in TB control.
Historical Background
Since ancient times, there have been references to TB or illnesses resembling TB from several parts of the world from many civilizations. The earliest references to TB can be found in the language Samskritam (Sanskrit). In the ancient Indian scriptures, The Vedas, TB was referred to as Yakshma (meaning wasting disease). Description of a TB-like disease has been documented in ancient Chinese and Arabic literature1,2,3. In English literature, the word “consumption” (derived from the Latin word consumer) has also been used to describe TB. The word “tuberculosis” appears to have been derived from the Latin word tubercula (meaning “a small lump”)4,5.
Fracastorius (1443-1553) believed that TB was contagious. Thomas Willis (1621-1675) had documented the clinical presentation of consumption in detail in his treatise Pthisiologica. Richard Morton (1637-1698) had described several pathological appearances of TB2,4,6. John Jacob Manget gave the description of classical miliary TB in 17007. In 1720, Benjamin Marten conjectured that TB could be caused by “certain species of animalcula or wonderfully minute living creatures”. In 1865 Jean Antoine Villemin presented his results suggesting that TB was a contagious disease1,2. However, it was Robert Koch who announced the discovery of the tubercle bacillus during the monthly evening meeting of the Berlin Physiological Society on 24th March 18828. On this day, after thousands of years, Mycobacterium tuberculosis, the organism causing TB finally revealed itself to humans. Commemorating the centenary of this event, since 1982, 24th March is being celebrated as “World TB Day” world over. Wilhelm Conrad Roentgen's discovery of X-rays, facilitated radiographic visualization of changes caused by TB in a living person. Thus, it was in the early years of 20th century that basic concepts related to aetiological agent of TB, consequent pathological changes in humans and detection of the organism became established.
Discovery of streptomycin, para-amino salicylic acid (PAS) and the availability of isoniazid ushered in modern era of effective treatment of TB in the mid-1940s. With the emergence of ‘short-course’ treatment cure for TB has become a reality. In the late 1970s, though TB continued to ravage developing countries like India, there was an optimism in the developed world that TB may cease to be a public health problem1.
The emergence of the human immunodeficiency virus (HIV) infection and the acquired immuno-deficiency syndrome (AIDS) ended this optimism and fuelled the resurgence of TB worldwide. Recognizing the importance of the impact of TB globally, the World Health Organization (WHO) took an unprecedented step and declared TB to be a “global emergency” in April 19938. The late 1990s also witnessed the resurgence of drug-resistant TB (DR-TB) with multidrug-resistant TB (MDR-TB) emerging as a major threat9,10,11. The first decade of the 21st century has been ravaged by extensively drug-resistant TB (XDR-TB)12. Recently, concern has been expressed regarding the occurrence of extremely drug-resistant TB (XXDR-TB)13,14, super XDR-TB15, totally drug-resistant TB (TDR-TB)15,16 from some parts of the world. The report on the occurrence of TDR-TB from India17 has raised concern and consternation18. Over the millennia, TB never respected anyone and had treated the rich and poor alike with equal disdain.
Definitions
Certain key definitions concerning clinically important forms of TB, drug-resistant TB are listed in TablesIA19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 and IB9,10,11,12,13,14,15,16,31,32,33,34,35,36,37 respectively.
Table IA.
TB: key clinical definitions

Table IB.
Drug-resistant TB: key definitions

Epidemiology
Global burden of TB
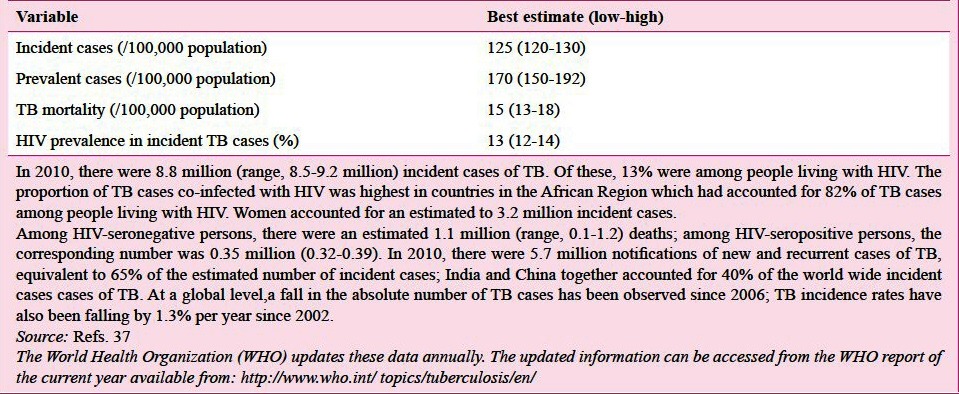
The global burden of TB as described in the 16th global report on TB published by WHO in 201237 is shown in Table IIA; most of the cases occurred in Asia (59%) and Africa (26%).
Table IIA.
Estimates of global burden of TB 2011
Indian scenario
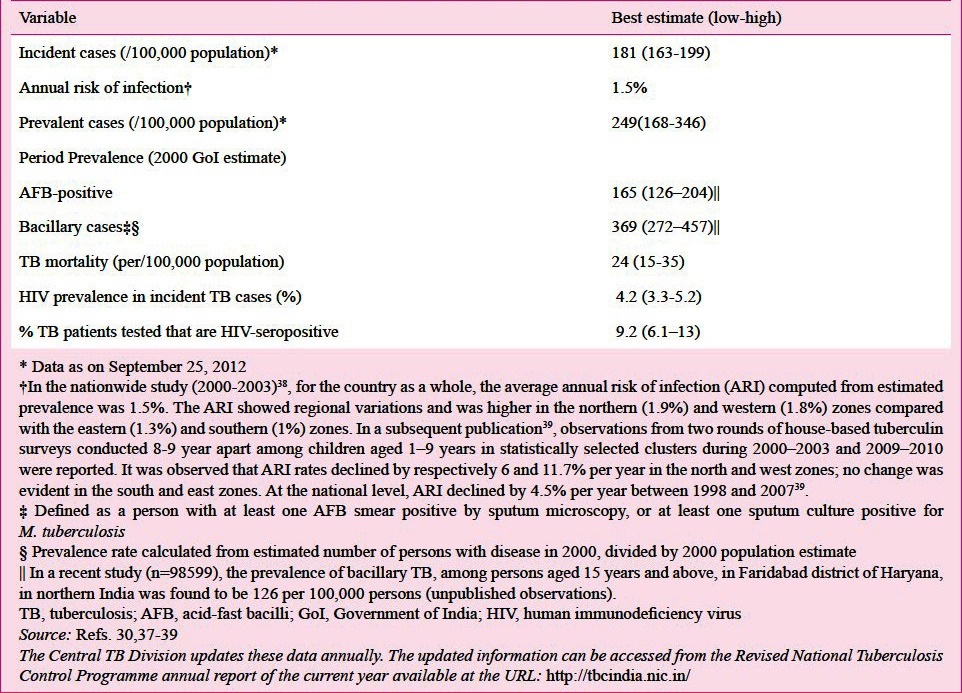
The current estimated TB burden in India is listed in Table IIB30,37,38,39. India has featured among the 22 high TB burden countries; and has accounted for an estimated one quarter (26%) of all TB cases worldwide30
Table IIB.
Provisional estimates of TB burden in India 2010
M/XDR-TB
The results of surveillance data on MDR-TB should be interpreted carefully keeping in mind the fact that globally, less than 4 per cent of new bacteriologically-positive cases and 6 per cent of previously treated cases were tested for MDR-TB in 2011 in accredited laboratories, with particularly low levels of testing in the South-East Asia (where India is located) and Western Pacific regions37. The recent global epidemiological data on M/XDR-TB are shown in Table IIIA, Figs 1A and 1B. XDR-TB has been documented from many parts of the world (Fig. 1C)40,41.
Table IIIA.
Global epidemiology of MDR-TB and XDR-TB

Fig. 1.
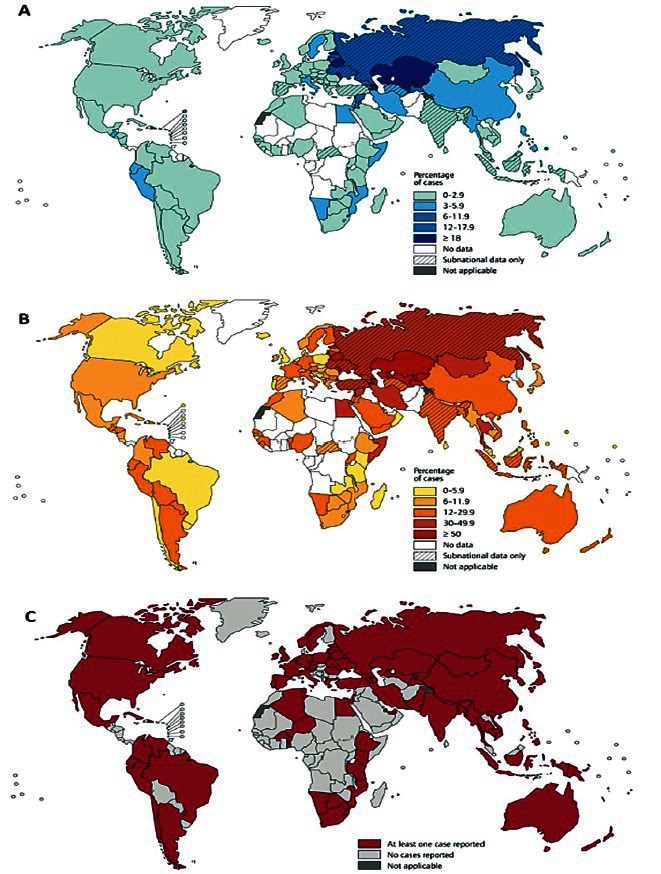
(A). Distribution of percentage of new tuberculosis cases with MDR-TB 1994-2011. (B). Distribution of percentage of previously treated tuberculosis cases with MDR-TB 1994-2011. (C). Countries that had reported at least one XDR-TB case 1994-2011. MDR-TB, multidrug-resistant tuberculosis; XDR-TB, extensively drug-resistant tuberculosis.
Reproduced with permission from World Health Organization (reference 41)
Indian scenario
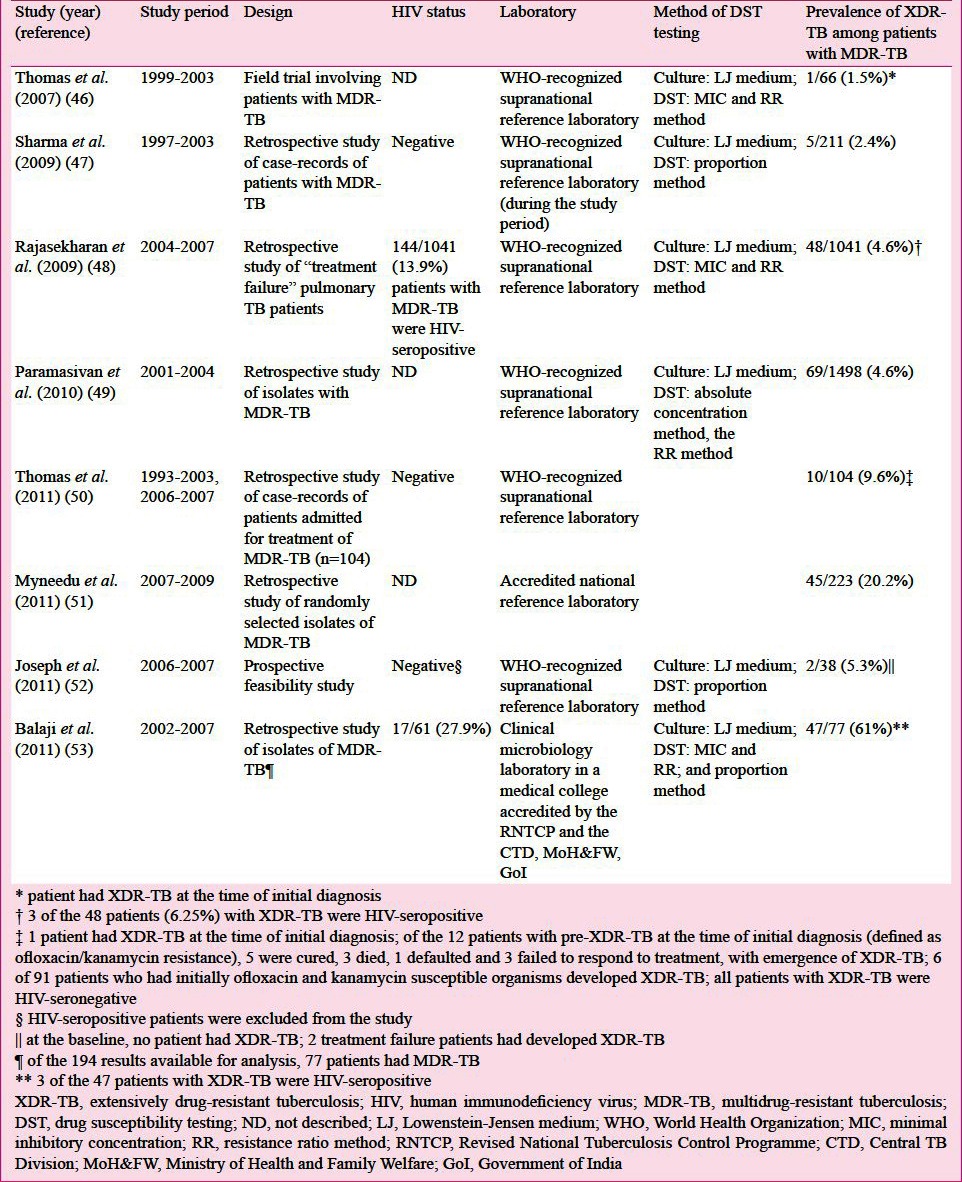
Observations from reliable accredited mycobacteriology laboratories from India suggest that the prevalence of MDR-TB is quite low in new TB cases (<3%) compared with previously treated patients (15-30%)42,43,44,45 (Table IIIB). The prevalence of XDR-TB in studies published from India where drug-susceptibility testing (DST) was carried out in quality-assured, accredited laboratories is shown in Table IIIC46,47,48,49,50,51,52,53.
Table IIIB.
Epidemiology of MDR-TB in India 2011
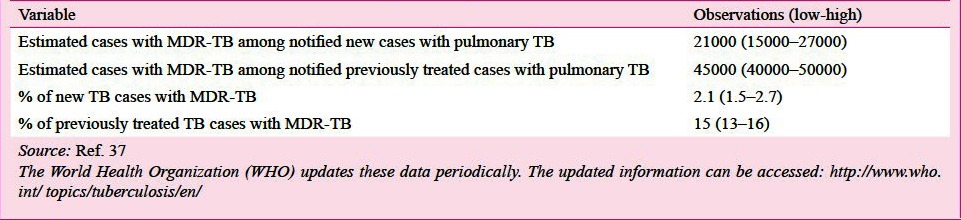
Table IIIC.
Prevalence of XDR-TB in studies published from accredited laboratories for mycobacteriology from India
Risk factors
Conventionally several genetic, social, environmental and biological determinants of health have been intuitively recognized by clinicians as risk factors for TB (Table IV)54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73. Some of these risk factors are discussed below.
Table IV.
Social, environmental and biological determinants of health considered to be risk factors for TB

Genetic factors
Certain key issues should be considered while evaluating genetic susceptibility to TB disease. Susceptibility to TB does not follow a Mendelian pattern and is polygenic and multifactorial. Presence of two different genomes, (of the TB bacillus and the host) and their interaction can have influence on the disease58. Several reports have implicated a long list of genes with risk of developing TB (Table IV)58,59,60,61.
HIV infection
HIV infection and AIDS stand out as the most significant among all the risk-factors for TB and has consistently and significantly altered the incidence rate of TB over the last three decades62,63,64,65,66,67,68,69,70,71,72,73. The impact of HIV/AIDS has been most profound in HIV prevalence sub-Saharan Africa where a dramatic increase in TB notification rates have been documented concurrent with increasing HIV prevalence. Among persons living with HIV (PLWH) TB can develop at any stage of HIV infection and there is a strong evidence suggesting that a declining CD4+ T-lymphocyte count and high viral load are risk factors for disease, while treatment with highly active antiretroviral therapy (HAART) reduces risk62,63,64.
HIV infection and MDR-TB: Even though several institutional outbreaks of MDR-TB among HIV-infected patients drew attention to the problem two decades ago74,75,76,77,78 as per currently available evidence79,80,81, HIV infection per se does not appear to be a risk-factor for MDR-TB.
Diabetes mellitus
The lethal interaction between diabetes mellitus (DM) and TB is being increasingly recognized world over82,83,84. Epidemiological modelling data suggest that in India, 14.8 per cent of all pulmonary TB cases and 20 per cent of sputum smear-positive cases have DM84 suggesting that DM substantially contributes to the burden of TB, especially sputum smear-positive pulmonary TB in India.
Use of immunomodulator biologicals
Use of immunomodulator drugs (biologicals) has been associated with the development of fatal TB in rheumatoid arthritis85,86.
Tobacco smoking
Data from recent systematic reviews on tobacco smoking and TB suggest that tobacco smokers have about three-fold higher risk of TB than non-smokers; even after adjustment for other factors87,88,89,90,91.
Changing clinical presentation of TB
Natural history of TB
The natural history of TB (Fig. 2)23,27,62,92,93,94,95,96 is influenced by several factors, the course being determined by the balance between the host immunity and the virulence of the TB bacillus. This understanding also facilitates identification of areas where interventional strategies can be identified for control of TB.
Fig. 2.

Natural history of Mycobacterium tuberculosis infection and scope for intervention. TB, tuberculosis; BCG, bacille Calmette-Guerin; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; TNF, tumour necrosis factor; LTBI, latent TB infection; SS, sputum smear.
Atypical clinical presentations
Cryptic miliary TB: Miliary TB that was earlier seen primarily as a disease of children, is being increasingly encountered in adults since the 1970s23,27,28,97. Apyrexial presentation with progressive wasting strongly mimicking a metastatic carcinoma (cryptic miliary TB) that has been described especially among older people23,27,28,98,99 often used to be diagnosed only at autopsy. This entity is being increasingly diagnosed during life in young immunosuppressed persons presently. This has been possible by the advances in imaging studies and increasing use of interventional procedures to procure tissue for confirming the diagnosis.
Acute lung injury and acute respiratory distress syndrome: TB as a primary cause of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) is also being reported in the recent years especially in areas where TB is highly endemic100,101,102,103. Increased awareness regarding this entity among intensivists, invasivists and internists, has resulted in a focused search for TB as the treatable cause in patients presenting with ARDS of obscure aetiology.
TB in patients receiving immunomodulator biologicals: Data regarding the clinical presentation of TB in patients receiving immunomodulator biological, such as, anti-tumour necrosis factor (anti-TNF) antibodies infliximab, adalimumab, golimumab and certolizumab pegol; and the soluble TNF receptor etanercept are emerging. The rate of TB was three to four-fold higher in patients receiving infliximab and adalimumab than in those receiving etanercept86.
Presentation as ‘pyrexia of unknown origin’: In areas where the disease is endemic, TB tops the list of aetiological causes of pyrexia of unknown origin (PUO)104,105. Till about two decades ago, clinicians either empirically administered anti-TB treatment or had to resort to invasive surgical procedures such as scalene node biopsy, laparotomy to ascertain the diagnosis. Often, the TB was diagnosed only on post-mortem examination. In patients presenting with PUO, miliary TB27,28, intrathoracic (e.g., paratracheal, mediastinal, hilar) and intraabdominal (e.g., retroperitoneal, porta hepatis) lymph node TB94,106, intestinal, omental and mesenteric, hepatic, splenic TB107, vertebral TB (often with paraspinal cold abscess)108, pelvic ascites109,110 are important occult locations that are identified as the focus of fever by imaging methods facilitating ante-mortem diagnosis. Many times, bone marrow aspirate and biopsy smear, mycobacterial culture and molecular test evidence could be the only discernible cause of TB in these patients62,94.
Sudden cardiac death: Sudden cardiac death due to TB myocarditis, especially in young persons is increasingly being recognized. This condition is often diagnosed at autopsy and extensive TB infiltration of the myocardium with minimal systemic involvement has been described; occult miliary TB has been implicated as the possible cause of myocarditis111,112. Ante-mortem diagnosis of this condition is now possible with echocardiography, and cardiac magnetic resonance imaging (MRI)113,114.
TB among healthcare workers
Health-care workers (HCWs), who are often in close proximity to patients with TB are at an increased risk of developing TB. It has been estimated that in areas of high TB incidence (>100/100,000 population), the stratified pooled estimates for LTBI and TB incidence rate ratios were 8.4 (95% CI 2.7-14.0%) and 3.7 per cent (95% CI 2.9-4.5), respectively; median estimated population-attributable fraction for TB was as high as 0.4% (115). These figures serve as warning bells, especially with regard to HCWs caring for patients with X/MDR-TB116 and highlight the need for institution of preventive measures. Further, it has been shown that institution of basic administrative and engineering controls and personal protection measures can be effective in reducing the annual tuberculin skin test (TST) conversion rates in HCWs117.
Diagnosis
Latent TB infection
Diagnosis of LTBI has been considered important as a tool for assessing the burden of TB for epidemiological purposes. Because LTBI contributes significantly to the pool of active TB cases later on, its recognition is assuming importance in high-risk groups where there is a potential for instituting treatment for this condition92. The tuberculin skin test (TST) and interferon-gamma release assays (IGRAs) have been used as diagnostic tests for the detection of LTBI (Table VA)118,119,120,121.
Table VA.
Diagnostic methods for latent TB infection
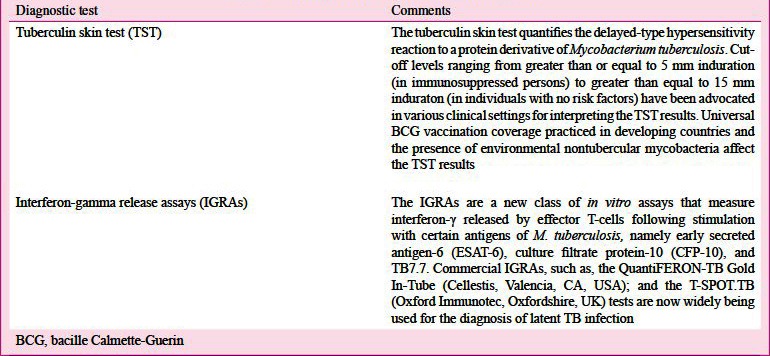
In high burden TB countries, neither IGRAs nor TST have been found to be adequate in accurately identifying persons who will benefit from treatment of LTBI with false positivity rates greater than 50 per cent being reported for both122,123,124,125. In this connection, a recent policy statement issued by the WHO125 and the European Centre for Disease Prevention and Control guidelines124 discourage the use of IGRAs in preference to TST, in areas where TB is highly endemic.
Diagnosis of active TB disease
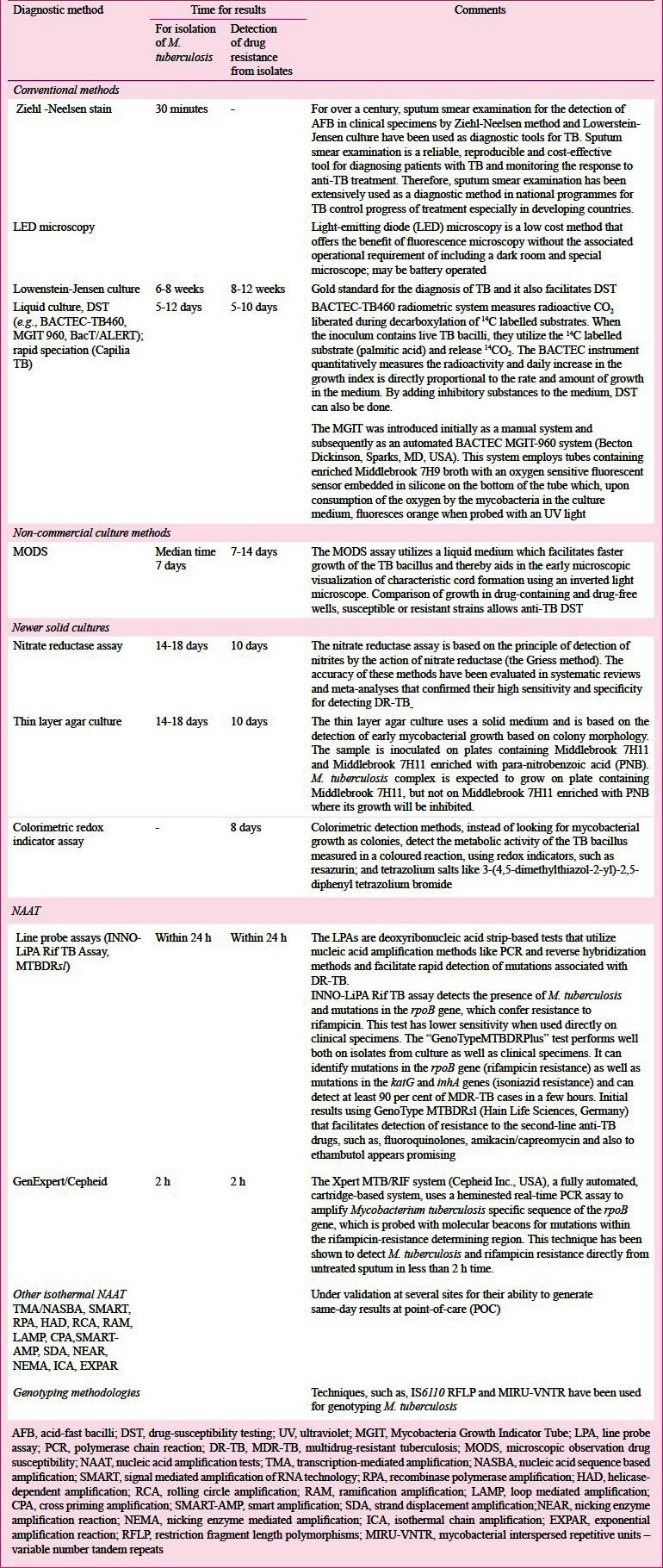
The current diagnostic and genotyping methods for TB are listed in Table VB125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141. Innovations such as use of fluorescent staining, light-emitting diode (LED) based microscopes have helped optimizing the yield of sputum smear examination126. Sputum mycobacterial culture is considered to be the ‘gold standard’ for the diagnosis of TB and it also facilitates DST. However, reliable, periodically accredited facilities for mycobacterial culture and DST are not widely available in TB high burden countries limiting their usefulness. Conventional sputum mycobacterial culture takes 6-8 weeks time and valuable time is lost in establishing the definitive diagnosis. In the 1980s, new semi-automated and automated culture systems based on liquid culture medium became available, such as the BACTEC-TB460 radiometric system (Becton Dickinson, Sparks, MD, USA) and facilitated rapid culture and detection of M. tuberculosis with a turn-around time of about 10 days127,129. For nearly a two decades, this was used for rapid culture and DST. The non-radiometric rapid liquid culture methods like Mycobacteria Growth Indicator Tube (MGIT) and BacT/ALERT (BioMe´rieux) then emerged. In 2007 the WHO endorsed the use of liquid culture assays, DST129 and rapid speciation (strip speciation) tests that detect a TB-specific antigen from positive liquid or solid cultures to confirm the presence of TB bacillus [Capilia TB; Tauns Laboratories Inc., Shizuoka, Japan]130 for faster diagnosis of TB and MDR-TB37.
Table VB.
Some of the current diagnostic, genotypic methods for TB125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141
The nucleic acid amplification based TB diagnostic tests (NAAT) are based on the amplification of short specific sequences of DNA or RNA of M. tuberculosis complex by PCR and the amplified products are then detected by agarose/acrylamide gel electrophoresis, or by various hybridization methods142. Several in-house PCR assays and commercial kits have been used for rapid diagnosis of TB.
Integrated automated NAAT, the GeneXpert (Cepheid Inc., Sunnyvale, CA, USA) platform combines automated sample preparation, real-time PCR amplification, identification of M. tuberculosis and detection of rifampicin resistance in less than 120 minutes142. GeneXpert has the advantage of being simple to use even in field conditions and appears promising technology for rapid diagnosis of TB. However, this test requires uninterrupted electric power supply and is expensive. This test is being evaluated by Revised National Tuberculosis Control Programme (RNTCP) in field conditions.
Promising results from studies carried out in low-resource countries suggest that loop mediated isothermal amplification (LAMP) has the potential to be a candidate as a molecular test for the rapid diagnosis of TB in clinical samples143,144. M. tuberculosis produces volatile organic compound (VOC) metabolites in-vitro, and their presence in the breath has been considered to be apparent biomarkers of infection145,146.
Serodiagnostic Tests
A broad range of serodiagnostic tests with a varying degree of reliability, repeatability and concordance have been used for the diagnosis of TB. Since no commercial serological assay could consistently result in an improved outcome, the WHO recently recommended that commercial serological tests should not be used for the diagnosis of pulmonary and extrapulmonary TB147. This view has been endorsed by the RNTCP of Government of India also148.
Diagnosis of extrapulmonary TB (EPTB)
The yield of conventional AFB smear and mycobacterial culture and some commonly used non-conventional tests in the diagnosis of EPTB is shown in Table VI149,150,151,152,153,154,155,156,157,158,159,160,161,162,163. A focused diagnostic approach, procurement of appropriate body fluid and tissue specimens and subjecting these to a battery of diagnostic tests including culture (and DST where feasible) will enhance the diagnostic yield in EPTB. There is a need to systematically evaluate and establish the utility of recently available newer tests such as GeneXpert to facilitate an early diagnosis and increase the diagnostic yield.
Table VI.
Diagnostic yield of commonly used tests in the diagnosis of certain forms of extrapulmonary TB

Development of accreditation system, quality assured laboratory network expansion
The eventual goal of controlling and probably eliminating TB hinges on rapidly and correctly identifying all TB cases and ensure that all diagnosed patients receive individualized treatment with anti-TB drugs tailored to the DST profile of the isolates obtained164. The ongoing struggle to meet the global target for case detection of diagnosing at least 70 per cent of new smear-positive cases, reflects the yawning gap between the need and the availability of quality assured laboratory infrastructure, especially in developing countries like India. Strengthening the capacity of public-sector laboratory networks, ensuring their accreditation initially and periodically thereafter so that a network of reliable quality assured accredited laboratories is eventually available to take TB control forwards are initial steps in this direction. The Expanding Access to New Diagnostics for TB (EXPAND-TB) project165, a collaboration among WHO, the Global Laboratory Initiative (GLI), Foundation for Innovative New Diagnostics (FIND) and the Global Drug Facility (GDF), and funded by UNITAID and other partners aims to improve capacity to diagnose MDR-TB in upgraded laboratory services in 27 countries.
In India, The RNTCP has also adopted a rigorous procedure for granting accreditation to culture and DST laboratories both in public and private sectors and medical colleges to provide accurate and reliable services for MDR-TB diagnosis and treatment follow-up. By 2015, it is expected that universal access to MDR-TB diagnosis and treatment will be made available for all smear positive TB cases under the RNTCP30.
Imaging Studies
Imaging modalities such as conventional radiography, ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography CT (PET-CT) have been used to localize the disease, assess the extent of organ involvement and evaluate response to treatment. The chest radiograph is the mainstay of imaging pulmonary TB (Fig. 3A–3E). However, the chest radiograph can be normal in HIV-infected patients with late HIV disease with active TB disease and in some patients with miliary TB166,167. Ultrasonography helps in detecting pleural effusion and ascites (which may sometimes be loculated), focal lesions in the liver and spleen, cold abscesses, intra-abdominal lymphadenopathy, involvement of other abdominal organs. High resolution CT (HRCT), thin-section multidetector row CT (MDCT) have been helpful in identifying pulmonary and miliary lesions, even in those in whom the chest radiograph is normal and has facilitated more frequent antemortem diagnosis of ‘cryptic miliary TB’ that was earlier diagnosed only at autopsy. CT of the thorax also allows detection of intrathoracic lymphadenopathy, calcification, pleural, pericardial and vascular lesions (Figs. 3F–3I, 4). CT and MRI of the brain and CT of the abdomen have also been extensively used to study CNS TB and abdominal TB, respectively (Fig. 5). Magnetic resonance spectroscopy (MRS) has been found to be useful in patients with intracranial tuberculomas where a characteristic large lipid peak with reduced n-acetyl aspartate peak can be seen. PET-CT using 18F labelled 2-deoxy-D-glucose (FDG) is helpful in locating, defining the extent of activity of TB at various organ sites (that may sometimes not be clinically discernible) (Fig. 6) especially in patients with disseminated TB and assessing the activity of lesions that might persist following anti-TB treatment on follow-up. 11C-choline PET scans can help differentiate between lung cancer and tuberculoma. The standard uptake value of tuberculoma is low in 11C-choline PET scans168.
Fig. 3.
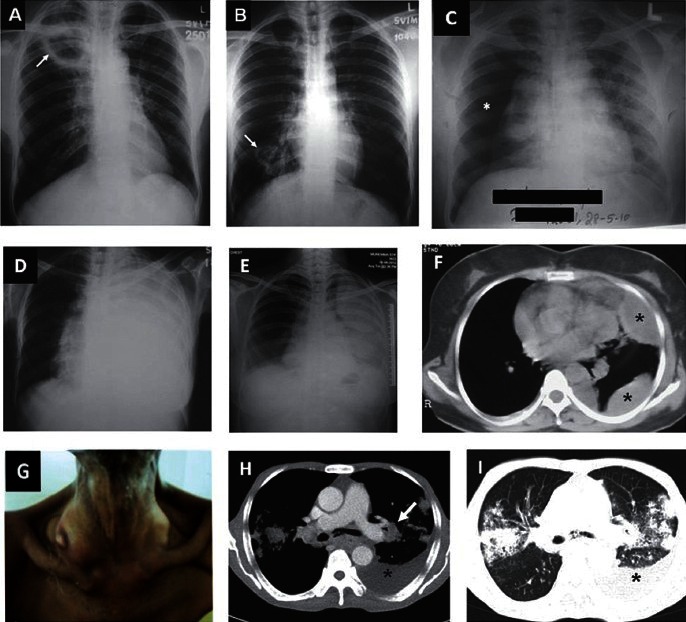
(A) Chest radiograph (postero-anterior view) showing a cavity in the right upper zone (arrow), and (B) lower zone (arrow). (C). Chest radiograph (postero-anterior view) in a patient presenting to the emergency room with severe breathlessness showing right-sided pneumothorax (asterisk). Sputum smear was positive for acid-fast bacilli. (D). Chest radiograph (poster-anterior view) showing left-sided massive pleural effusion. (E). Chest radiograph (poster-anterior view) in another patient showing left-sided loculated pleural effusion. (F). CECT (chest) of the same patient showing left-sided loculated pleural effusion (asterisks). (G) Clinical photograph of a patient with disseminated TB showing right-sided cervical lymphadenopathy with cold abscess. (H) CECT chest of the same patient showing left-sided pleural effusion (asterisk), left hilar lymphadenopathy [arrow (mediastinal window)]; and (I) and bilateral parenchymal infiltrates and left sided pleural effusion (asterisk) (lung window).
Fig. 4.
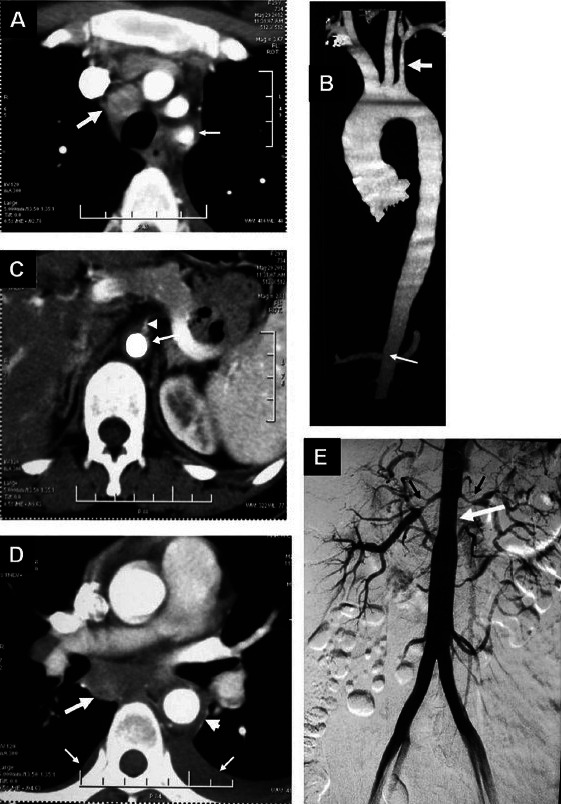
Nonspecific aortoarteritis. (A) Axial contrast enhanced CT showing an enlarged mediastinal lymph node (thick arrow) and diffuse wall thickening of left subclavian artery (Thin arrow) (B). Volume rendered CT angiography image showing diffuse long segment narrowing of left subclavian artery (thick arrow) and abdominal aorta at the renal artery origin level as well as infrarenal segment (thin arrow) (C). Axial contrast enhanced CT showing diffuse wall thickening of abdominal aorta (long arrow) with luminal narrowing of the origin of superior mesenteric artery (arrow head) (D). Axial contrast enhanced CT showing an enlarged subcarinal mediastinal lymph node (thick arrow) and diffuse wall thickening of descending thoracic aorta (arrow head). Bilateral pleural effusions [left more than right (thin arrows)] can also be seen (E). Digital substraction angiography (DSA) showing diffuse long segment narrowing of abdominal aorta at the renal artery origin level and infrarenal segment (white arrow). There is a marked narrowing of the bilateral renal arteries (black arrows)
Fig. 5.
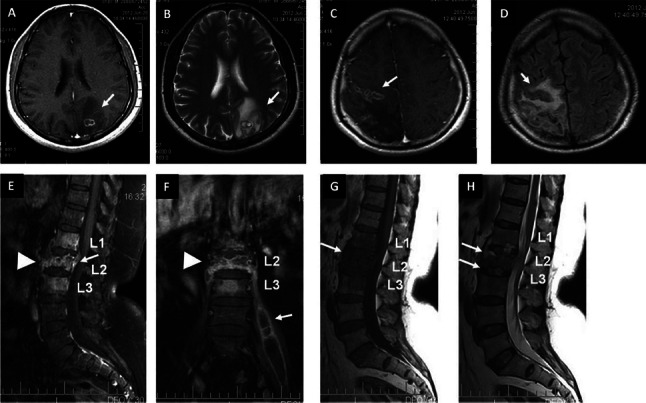
(A) T1-weighted pre-contrast and (B) contrast-enhanced MRI images showing ring enhancing lesion with perileional oedema in the left parietal lobe (arrows). (C) T1-weighted axial MRI image showing conglomerate ring enhancing lesions in the right frontoparietal regions (arrow) (D). FLAIR sequence showing hypointense lesions with perilesional oedema (arrow) (E). T1-weighted contrast enhanced MRI showing collapse of L2 vertebral body with abnormal enhancement in L1-L3 vertebrae, prevertebral regions (arrow-head) and epidural abscess (arrow) at L2 (F). Coronal image of the same patient showing collapse of L2 vertebral body (arrow-head) and left-sided psoas abscess (arrow). (G) Pre-Contrast T1-weighted sagittal MRI showing hypointense signal in L1-L3 vertebral bodies (arrow). T2-weighted sagittal image showing hyperintensities in the end-plates of L1-L2 and L2-L3 vertebral bodies and intervening discs (arrows).
Fig. 6.
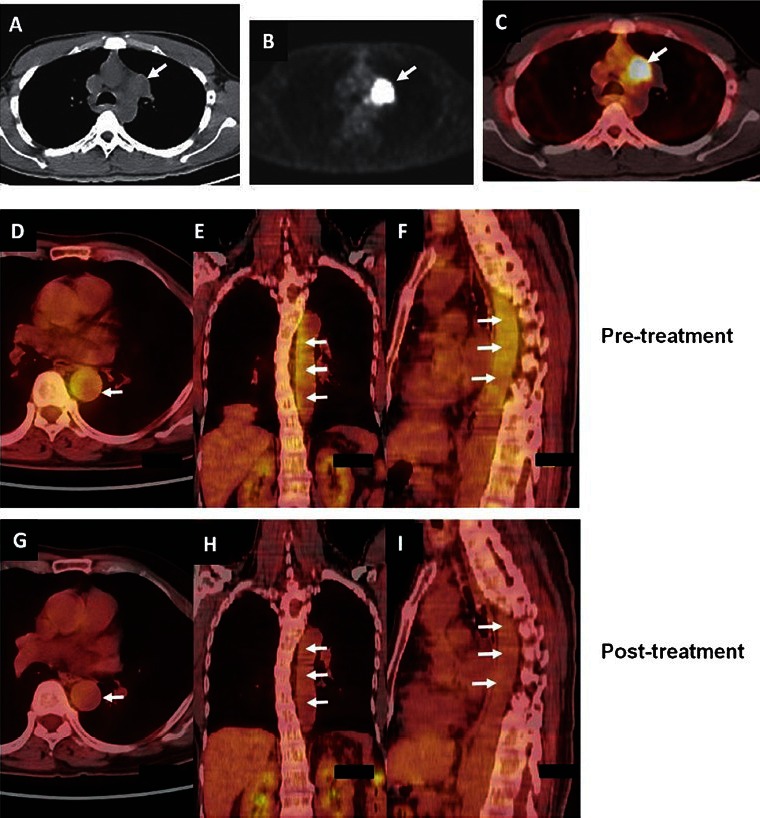
Intrathoracic lymph node TB. (A) CT of the chest (mediastinal window), FDG-PET (B) showing left-sided hilar lymphadenopathy (arrow). (C) PET-CT image of the same patient showing increased uptake in the lesion (arrow). (D,E,F, upper panel). Nonspecific aortoarteritis. PET-CT images showing increased uptake (arrows) at the time of initial presentation. (G,H,I lower panel) The post-treatment images of the same patient shows significant decrease in the uptake suggestive of resolution of lesions with treatment.
Kind courtesy: Dr TC Kalawat, Department of Nuclear Medicine, Sri Venkateswara Institute of Medical Sciences, Tirupati (Figures 6 A-C and Drs. Arun Malhotra, Rakesh Kumar, Department of Nuclear Medicine, All India Institute of Medical Sciences, New Delhi (Figures 6D-I)
In patients presenting with PUO with no focal localizing clue, nuclear medicine techniques using gallium-67 citrate, technetium-99m methylene diphosphonte (Fig. 7), radiolabelled white blood cells, and human immune globulin imaging have been used to identify occult foci of infection especially in the bones. However, these techniques have been relatively non-specific and have not been able to distinguish bacterial mediated infection from non-bacterial inflammation due to other causes169. Recently, the radiopharmaceutical technetium-99m labelled ciprofloxacin (99mTc-CPF) has been developed and shown to localize in high concentrations in bacterial abscesses, and not in areas of sterile inflammation170,171. Though not specific for TB, this technique has been used to localize foci of TB osteomyelitis as well172 and also assess the adequacy of short-course treatment of TB osteomyelitis173.
Fig. 7.
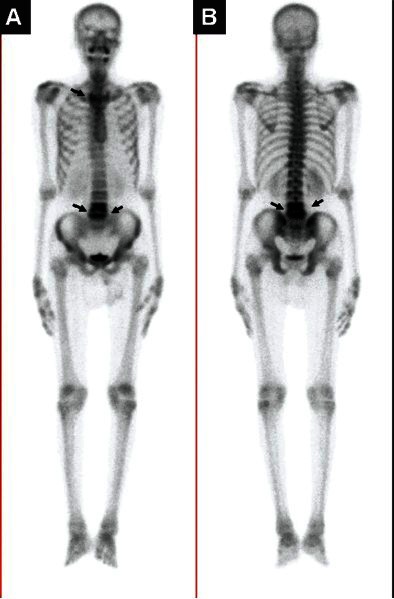
(A) 99mTc-methylene diphosphonte (MDP) whole body anterior and (B) posterior sweep views in a patient presenting with backache and low-grade fever showing diffuse increased radiotracer localization in the body of L4 and 5 vertebrae (arrows) suggestive of spinal TB.
(Kind courtesy: Dr TC Kalawat, Department of Nuclear Medicine, Sri Venkateswara Institute of Medical Sciences, Tirupati)
Procurement of body fluids/tissues for diagnostic testing
Radiographic image guided interventional procedures, biopsy of peripherally accessible lesions (e.g., lymph nodes), endoscopic procedures like laparoscopy, colonoscopy, thoracoscopy, can be used in appropriate settings to procure material for diagnostic testing for TB. Endoscopic interventions, such as, endobronchial ultrasound bronchoscopy (EBUS) and endoscopic oesophageal ultrasound, FNACs, biopsies are useful in situations where patients are sputum smear-negative, are unable to produce sputum, present with intrathoracic (e.g., mediastinal, sub-carinal, hilar) lymphadenopathy to procure tissue. Material thus obtained should be subjected to cytopathological, histopathological, microbiological (including DST) and molecular methods for confirmation of TB diagnosis.
Treatment
Evolution of modern multiple drug treatment
The humankind had to wait for more than 60 years following Robert Koch's momentous announcement of the discovery Mycobacterium tuberculosis for drug(s) that could cure TB to become available (Fig. 8)174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194. The first controlled clinical trial in the history of medicine conducted by the British Medical Research Council (BMRC)179 demonstrated the activity of streptomycin. The BMRC assessed the addition of PAS to streptomycin in a controlled clinical trial181 which showed a lower rate of clinical deterioration, higher rate of culture conversion, and a lower rate of streptomycin resistance in patients receiving streptomycin plus PAS, suggesting that combination treatment with PAS was helpful in preventing the emergence of drug resistance to streptomycin. The first clinical trial with isoniazid was initiated in 1951182. Subsequent studies by BMRC183,184 further assessed the utility of using two of the three drugs, namely, streptomycin, isoniazid and PAS in various combinations to treat TB. A later clinical trial by BMRC185 established the duration of anti-TB treatment that would effectively prevent relapse, to be 18-24 months.
Fig. 8.

A brief history of development of antituberculosis drugs.
Ethambutol, discovered in 1961 got added to the armamentarium of anti-TB drugs186 and soon replaced PAS in the standard regimens. In the 1960s seminal research conducted by Wallace Fox and co-workers at the National Institute for Research in Tuberculosis (NIRT), Chennai [then called as Tuberculosis Chemotherapy Centre, Madras; later renamed as Tuberculosis Research Centre (TRC), Madras in 1978] showed that home or ambulatory treatment was almost as effective as sanatorium treatment “provided the regular use of anti-TB medication was well organized and supervised”, a fact that is often neglected even today187. The classic NIRT studies187,188 also established the efficacy of intermittent administration of anti-TB medications. These fundamental principles have since then remained the pillars on which modern treatment of TB is based.
Subsequently, with the introduction of rifampicin189 based on the data from studies conducted in the 1970s190 the standard treatment duration of anti-TB treatment could be shortened to 9 months. By then the 9-month rifampicin containing regimens replaced the then prevailing standard treatment of 18-months. In 1972, the therapeutic role of pyrazinamide used in a reduced dosage was rediscovered in the studies conducted by East Africa Medical Council and BMRC193. Combining pyrazinamide to rifampicin containing regimens then ensued. The 6-month regimens containing rifampicin and pyrazinamide were as effective as either rifampicin or pyrazinamide containing regimens in the clinical trials by the British Thoracic Society194 heralding the modern 6-month short-course chemotherapy endorsed by the WHO that has become the standard of care worldover. Since then, most countries have been using the WHO endorsed standardized daily or thrice-weekly intermittent treatment regimens in National TB Control Programmes.
National Tuberculosis Programme (NTP)
In 1962, the NTP was started in India, the first time ever a ‘national programme’ was conceived to tackle the menace of TB in the world. Short-course chemotherapy was introduced in the NTP by 1985. However, uninterrupted drug supply and treatment adherence continued to plague the NTP and the programme did not make a significant epidemiological impact on the prevalence of TB in the country195. This led to introspection and a comprehensive joint review of the TB programme in India by members of several organizations including the Government of India, WHO and Swedish International Development Agency (SIDA) in 1992 identified many issues that were affecting the programme performance196.
Revised National Tuberculosis Control Programme
To revamp TB control in India, in 1993, the RNTCP, based on the WHO-recommended DOTS strategy, began operations in five pilot sites. Following pilot testing, large scale expansion of RNTCP in India began in 1997 in a phased manner and by March 24, 2006, the whole country was covered by the RNTCP. The Programme aims at achieving and maintaining a cure rate of at least 85 per cent among new sputum positive (NSP) patients, and to achieve and maintain case detection of at least 70 per cent of the estimated NSP cases in the community196,197.
Treatment of active TB disease: issues concerning dosing frequency and duration of treatment
The current treatment regimens listed in the recent WHO guidelines for national programmes29 and the RNTCP of Government of India30 are shown in Tables VIIA, VIIB and VIIC. The WHO guidelines29 suggest that HIV patients co-infected with TB should be treated with daily regimens. This recommendation has been based on evidence from meta-analyses198,199 that showed that HIV co-infected patients with pulmonary TB were at a higher risk of acquired rifampicin resistance, when failing a three times weekly short-course intermittent regimen. In the meta-analysis199 of treatment of active TB in HIV co-infected patients, data from six randomized trials and 21 cohort studies showed that compared with daily therapy in the initial phase (n=3352 patients from 35 study arms), thrice-weekly therapy (n=211 patients from 5 study arms) was associated with higher rates of treatment failure (adjusted risk ratio, 4.0; 95% CI 1.5-10.4) and relapse (adjusted risk ratio 4.8; 95% CI 1.8-12.8) and a trend toward higher relapse rates if rifamycins were used for only 6 months, compared with 8 months or more, or if antiretroviral therapy was not used. In a study from India200 the outcome of fully intermittent thrice-weekly antituberculosis treatment regimens of 6-month isoniazid, rifampicin, pyrazinamide and ethambutol, followed by 4 months of isoniazid and rifampicin; and a 9-month regimen where the intensive phase was same but continuation phase was 7 months, were assessed in HIV-TB co-infected patients. In the ‘intent-to-treat analysis’, among patients who had a favourable outcome at the end of treatment, bacteriologically confirmed recurrence rate was significantly higher with the 6-month regimen compared with the 9-month regimen.
Table VIIA.
WHO standard treatment regimens for new TB patients

Table VIIB.
WHO standard treatment regimens for previously treated TB patients

Table VIIC.
Categorization and treatment regimens under RNTCP
Daily vs thrice-weekly treatment: Under the RNTCP, in India, 15,852,745 patients have been treated with thrice-weekly intermittent treatment and 2,853,494 lives have been saved201 reflecting the huge success achieved by the RNTCP programme over the last 15 years. While thrice-weekly intermittent treatment seems adequate in HIV-seronegative patients, use of daily therapy is an important issue to contend with in HIV-seropositive persons. The prospect of considering the implementation of a daily treatment regimen and the logistics of direct observation of treatment are being actively considered and a clear-cut government policy on the same is expected to be available soon. However, head-on comparisons of adequately powered, fully daily, partial daily (daily intensive phase and thrice-weekly intermittent continuation phase) and fully thrice-weekly intermittent regimens are not available in the literature and studies of this nature will help in arriving at optimal dosing frequency issue so that policy can be modified.
There is also a lack of consensus regarding the optimal duration of therapy in patients with EPTB, especially bone and joint TB, neurological TB; disseminated and miliary TB. While 6-months of treatment may be adequate in HIV-seronegative new patients with pulmonary TB and focal extrapulmonary TB, individual patients may require 9 to 12 months of treatment when TB meningitis is present given the serious risk of disability and mortality; and 9 months of treatment when bone and joint TB is also present. The efficacy and safety of erstwhile Category III (intermittent thrice-weekly rifampicin, isoniazid and pyrazinamide for 2 months, followed by rifampicin and isoniazid for 4 months) DOTS has been documented in the patients with uncomplicated small (<1500 ml) unilateral pleural effusion202, and peripheral lymph node TB203.
Treatment of HIV-TB co-infection and X/MDR-TB
Treatment of active TB in patients co-infected with HIV requires careful consideration of drug-drug interactions between anti-TB and anti-retroviral drugs (Fig. 9)204,205. Treatment of X/MDR-TB is expensive and time-consuming, and requires special facilities with adequate infrastructure, reliable, access to periodically accredited mycobacterial culture and sensitivity laboratories, medical, nursing and para-medical personnel trained in the management of X/MDR-TB206. In India, the RNTCP started treatment of X/MDR-TB through the DOTS-Plus services in 2007 in a phased manner and at present all Indian states have been covered30.
Fig. 9.

Guidelines on timing of antiretroviral treatment in patients with HIV-TB co-infection
ART, antiretroviral treatment; BHIVA, British HIV Association; EFV, efavirenz; HAART, highly active antiretroviral treatment; HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitors;
Key issues in TB treatment monitoring
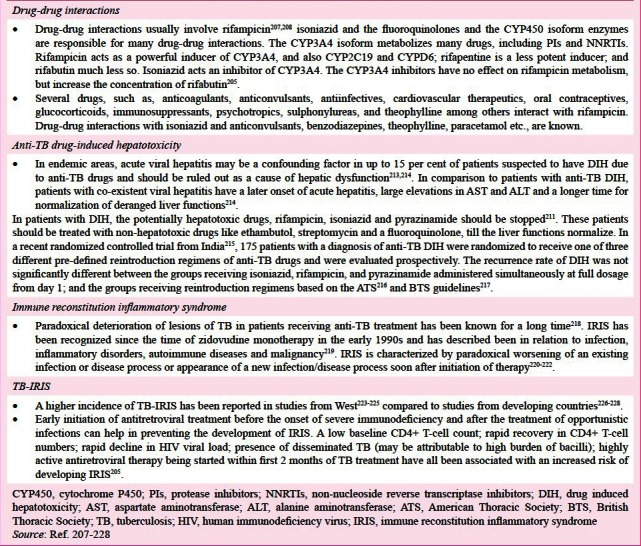
Drug-drug interactions
Clinically significant interactions during anti-TB treatment should be carefully monitored as these may sometimes result in therapeutic failure or drug toxicity207,208. This is particularly important in HIV-co-infected persons, the elderly and those with significant co-morbidities receiving treatment for the same (Box I)207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228.
Box 1.
Key issues in monitoring treatment of TB
Drug induced hepatotoxicity (DIH)
Principles underlying evaluation of patients with DIH are listed in Box I207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228.
Immune reconstitution inflammatory syndrome (IRIS)
Paradoxical deterioration of lesions of TB in patients receiving anti-TB treatment has been known for a long time (218). Key developments in the understanding of IRIS are listed in Box I219,220,221,222,223,224,225,226,227,228. Minor manifestations of IRIS can be managed with non-steroidal anti-inflammatory drugs (NSAIDS). Moderate-to-high dose corticosteroid treatment, sometimes for prolonged periods may be required for the treatment of paradoxical TB-IRIS and may be beneficial in TB-IRIS with CNS manifestations, tracheal compression due to lymphadenopathy, acute kidney injury and acute respiratory distress syndrome (ARDS)218,219.
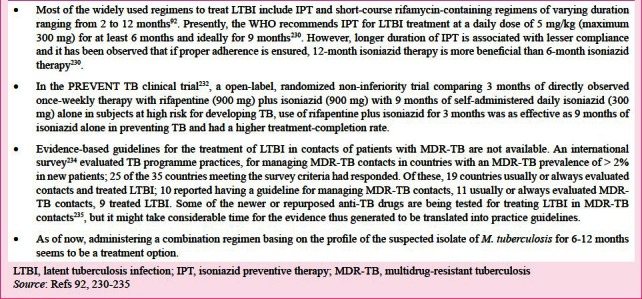
Treatment of LTBI
The WHO has stressed on the importance of interventions like ART provision and the three Is for HIV-TB coinfection, namely intensified TB case finding, infection control, and isoniazid preventive therapy (IPT), as part of prevention, care and treatment services229. The diagnosis and treatment of LTBI have been extensively reviewed recently92. It is necessary to rule out active TB disease before initiating treatment for LTBI. Important issues concerning LTBI and its treatment92,230,231,232,233,234 are listed in Box 2
Box 2.
Latent TB infection: key issues
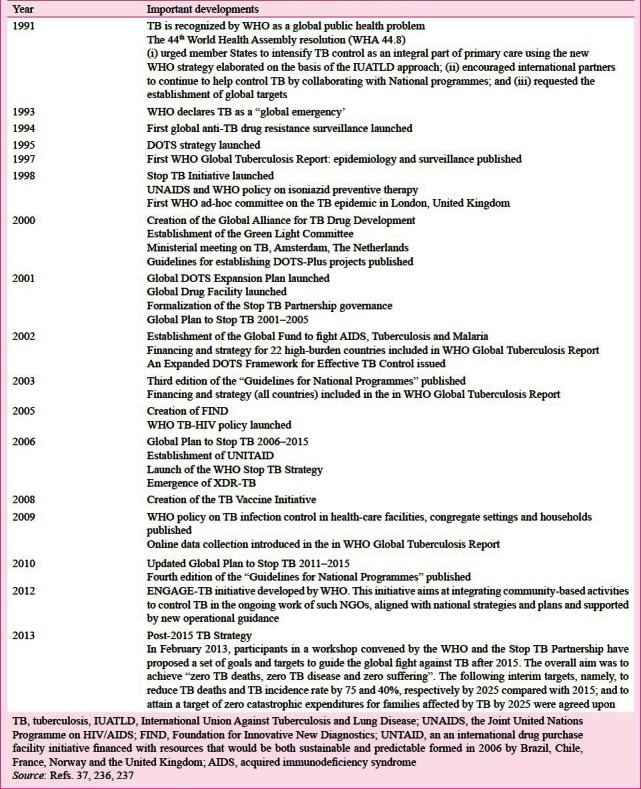
Control of TB
Global measures: India conceived the NTP and set an example to the world in the programmatic approach to TB control in 1962197. Since then several efforts have been undertaken globally to achieve control of TB and are summarized in Box III235,236,237,238. These developments reflect the efforts towards achieving universal access to preventive, diagnostic and treatment services for all forms of TB.
Box 3.
Global efforts at TB control (1991-2013): a journey
As a result of the implementation of RNTCP, prevalence of all forms of TB has been brought down (from 338/100,000 population in 1990 to 249/100,000 population in 2009); TB mortality has also reduced (from >42/100,000 population in 1990 to 23/100,000 population in 2009) in India. The Phase II (2006-2012) of the RNTCP has achieved the set goals, the country is coursing towards achieving “universal access” for control of TB197 and appears to be on track to achieve the TB related United Nations Millenium development Goals (UNMDG). Jointly with National AIDS Control Programme (NACP), RNTCP has developed “National framework of joint TB/HIV collaborative activities” that are being implemented in the country197.
Involvement of Medical Colleges in TB control
For the first time in the global history of TB control, Indian Medical Colleges were involved in the RNTCP. This unique experiment in the history of TB control has resulted in medical colleges providing diagnostic services (Designated Microscopy Centres), treatment (DOT Centres), referral for treatment, recording and reporting data, carrying out advocacy for RNTCP and conducting operational research on the RNTCP238.
Prevention
Even though the declining trends observed in the global burden of TB currently37,239,240,241,242, this trend seems insufficient to achieve the global target of elimination of TB in 2050164. Therefore, the need for other measures including infection control measures, newer or repurposed anti-TB drugs, newer and better vaccines for TB is pressing.
Airborne infection control measures
Recognizing the importance of airborne infection control and in view of the association of TB with HIV and the emergence of X/MDR-TB, guidelines have been issued for implementing the same by the WHO239 and the RNTCP240 as well. However, there is a wide gap between the needs and the actual implementation of these guidelines presently especially in the overcrowded government hospitals in India and other developing countries.
TB Vaccine
Till date the bacille Calmette-Guerin (BCG) vaccine is the only vaccine available for TB241,242,243,244. Though BCG confers consistent protection against severe forms of TB, such as TB meningitis, disseminated and miliary TB in children in areas where TB is endemic its ability to protect against pulmonary TB has been found to be variable245,246. New candidate vaccines for TB are in various stages of development and are listed in Table VIII241,242,247.
Table VIII.
Some of the potential TB vaccines in various stages of development

The summary of important changes in TB during the last 130 years since the time of Robert Koch in 1882 are listed in Table IX.
Table IX.
Summary of important changes in TB

The Future
The search for newer and more efficient biomarkers for predicating a durable (non-relapsing) cure, indication of reactivation risk, prediction of eradication of LTBI; and prediction of vaccine efficacy248,249, discovery of newer anti-TB drugs250,251,252,253 and development of newer additional candidate vaccines254,255,256,257 is required to achieve the WHO and UNMDG goal of halting the incidence, prevalence and death rates associated with TB by 2015 and eliminating the disease altogether by 2050197. Translation of newer innovative diagnostics for TB, such as, use of a ‘hand-held’ nuclear magnetic resonance (NMR) apparatus capable of offering a 30-minute diagnosis of TB258, applications of nanotechnology259,260 to point-of-care diagnostic tests needs to be pursued.
Newer, repurposed drugs in pipeline
Presently, several newer or repurposed drugs are in pipeline in various stages of development as anti-TB drugs252,261. Among the newer drugs, delamanid (OPC67683), bedaquiline (TMC207) and the nitroimidazole-oxazine PA-824 have been found to be active against both drug-sensitive and drug-resistant strains. Bedaquiline (TMC207) has been approved for use by the United States-Food and Drug Administration (US-FDA). Their efficacy and safety have been demonstrated in MDR-TB patients in double-blind, placebo controlled phase II clinical trials262,263,264. The Government of India is planning to regulate the introduction of these newer drugs in a systematic fashion by streamlining the conduct of clinical trials in India. Evidence is available that isoniazid-resistant clinical isolates of M. tuberculosis remain fully susceptible to the drug pyridomycin, a compound produced by Dactylosporangium fulvum with specific bactericidal activity against mycobacteria265. Pyridomycin merits further evaluation as an anti-TB drug.
Initiatives like the Critical Path to New TB Regimens (CPTR) involving several pharmaceutical companies and non-governmental organizations have been attempting to develop the newer drugs concomitantly in combination trials so that the best regimen and the shortest duration of time can be evolved266. Additional vaccine candidates are likely to enter clinical trials in future254,255,256,257.
The last 70 years have witnessed an initial euphoria of emergence of drug treatment of TB that raised hopes and even signalled a likely ‘elimination’ of TB. Inspite of political commitment, global and national programmatic strategy to contain and control TB, eventual elimination of TB in near future appears to be a mirage as of now. The widespread occurrence of X/MDR-TB threatens to take us back to the era of untreatable TB. TB has come a long way, from despair and the status of an incurable malady, through a brief interlude of a curable disease to a scourge that is menacingly threatening the return to dark ages. The fall in the absolute number of TB cases globally observed since 2006 is heartening. With newer and repurposed anti-TB drugs emerging and becoming available for use, the march of the humankind towards the goal of TB elimination, i.e., reducing the annual incidence to less than 1 case/1,000,000 population by 2050267 appears to be on course in the right direction.
References
- 1.Mohan A, Sharma SK. History. In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2009. pp. 7–15. [Google Scholar]
- 2.Rubin SA. Tuberculosis. Captain of all these men of death. Radiol Clin North Am. 1995;33:619–39. [PubMed] [Google Scholar]
- 3.Rosenblatt MB. Pulmonary tuberculosis: evolution of modern therapy. Bull NY Acad Med. 1973;49:163–96. [PMC free article] [PubMed] [Google Scholar]
- 4.Dubos R, Dubos J. Boston: Little, Brown and Company; 1952. The white plague. Tuberculosis, man and society. [Google Scholar]
- 5.Waksman SA. Berkeley and Los Angeles: University of California Press; 1964. The conquest of tuberculosis. [Google Scholar]
- 6.Keers RY. London: Bailliere-Tindall; 1978. Pulmonary tuberculosis - A journey down the centuries. [Google Scholar]
- 7.Sakula A. Robert Koch: centenary of the discovery of the tubercle bacillus, 1882. Thorax. 1982;37:246–51. doi: 10.1136/thx.37.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO Report on the TB epidemic. WHO/TB/94.177. Geneva: World Health Organization; 1994. TB. A global emergency. [Google Scholar]
- 9.Sharma SK, Mohan A. Multidrug-resistant tuberculosis. Indian J Med Res. 2004;120:354–76. [PubMed] [Google Scholar]
- 10.Sharma SK, Mohan A. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest. 2006;130:261–72. doi: 10.1378/chest.130.1.261. [DOI] [PubMed] [Google Scholar]
- 11.Guidelines on Programmatic Management of Drug Resistant TB (PMDT) in India. New Delhi: Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare; 2012. Central TB Division. Revised National Tuberculosis Control Programme. [Google Scholar]
- 12.Extensively drug-resistant tuberculosis (XDR-TB): recommendations for prevention and control. Wkly Epidemiol Rec. 2006;81:430–2. [No authors listed] [PubMed] [Google Scholar]
- 13.Migliori GB, De Iaco G, Besozzi G, Centis R, Cirillo DM. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill. 2007;12:E070517.1. doi: 10.2807/esw.12.20.03194-en. [DOI] [PubMed] [Google Scholar]
- 14.Migliori GB, Loddenkemper R, Blasi F, Raviglione MC. 125 years after Robert Koch's discovery of the tubercle bacillus: the new XDR-TB threat. Is “science” enough to tackle the epidemic? Eur Respir J. 2007;29:423–7. doi: 10.1183/09031936.00001307. [DOI] [PubMed] [Google Scholar]
- 15.Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, et al. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest. 2009;136:420–5. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- 16.Velayati AA, Farnia P, Masjedi MR, Ibrahim TA, Tabarsi P, Haroun RZ, et al. Totally drug-resistant tuberculosis strains: evidence of adaptation at the cellular level. Eur Respir J. 2009;34:1202–3. doi: 10.1183/09031936.00081909. [DOI] [PubMed] [Google Scholar]
- 17.Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. Totally drug-resistant tuberculosis in India. Clin Infect Dis. 2012;54:579–81. doi: 10.1093/cid/cir889. [DOI] [PubMed] [Google Scholar]
- 18.Mudur G. Indian health ministry challenges report of totally drug resistant tuberculosis. BMJ. 2012;344:e702. doi: 10.1136/bmj.e702. [DOI] [PubMed] [Google Scholar]
- 19.Hill AR, Premkumar S, Brustein S, Vaidya K, Powell S, Li PW, et al. Disseminated tuberculosis in the acquired immunodeficiency syndrome era. Am Rev Respir Dis. 1991;144:1164–70. doi: 10.1164/ajrccm/144.5.1164. [DOI] [PubMed] [Google Scholar]
- 20.Sharma SK, Mohan A, Gupta R, Kumar A, Gupta AK, Singhal VK, et al. Clinical presentation of tuberculosis in patients with AIDS: an Indian experience. Indian J Chest Dis Allied Sci. 1997;39:213–20. [PubMed] [Google Scholar]
- 21.Wang JY, Hsueh PR, Wang SK, Jan IS, Lee LN, Liaw YS, et al. Disseminated tuberculosis: a 10-year experience in a medical center. Medicine (Baltimore) 2007;86:39–46. doi: 10.1097/MD.0b013e318030b605. [DOI] [PubMed] [Google Scholar]
- 22.Sharma SK, Mohan A. Miliary tuberculosis. In: Agarwal AK, editor. Clinical medicine update - 2006. New Delhi: Indian Academy of Clinical Medicine; 2006. pp. 353–60. [Google Scholar]
- 23.Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5:415–30. doi: 10.1016/S1473-3099(05)70163-8. [DOI] [PubMed] [Google Scholar]
- 24.Sahn SA, Neff TA. Miliary tuberculosis. Am J Med. 1974;56:494–505. doi: 10.1016/0002-9343(74)90482-3. [DOI] [PubMed] [Google Scholar]
- 25.Sharma SK, Mohan A. Disseminated and miliary tuberculosis. In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2009. pp. 494–518. [Google Scholar]
- 26.Baker SK, Glassroth J. Miliary tuberculosis. In: Rom WN, Garay SM, editors. Tuberculosis. Philadelphia: Lippincott Williams and Wilkins; 2004. pp. 427–44. [Google Scholar]
- 27.Sharma SK, Mohan A. Miliary tuberculosis. In: Schlossberg D, editor. Tuberculosis and non-tuberculous mycobacterial diseases. 6th ed. Washington: ASM Press; 2011. pp. 415–35. [Google Scholar]
- 28.Sharma SK, Mohan A, Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. Indian J Med Res. 2012;135:703–30. [PMC free article] [PubMed] [Google Scholar]
- 29.Treatment of tuberculosis. Guidelines. 4th ed. Geneva: World Health Organization; 2010. World Health Organization. WHO/HTM/ TB/2009.420. [PubMed] [Google Scholar]
- 30.Revised National TB Control Programme. Annual Status Report. New Delhi: Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare; 2012. [accessed on February 28, 2013]. Central TB Division, Government of India. TB India 2012. Available from: http://tbcindia.nic.in/pdfs/TB%20India%202012-%20Annual%20Report.pdf . [Google Scholar]
- 31.Frieden TR, Munsiff SS. The DOTS strategy for controlling the global tuberculosis epidemic. Clin Chest Med. 2005;26:197–205. doi: 10.1016/j.ccm.2005.02.001. v. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee R, Allen J, Westenhouse J, Oh P, Elms W, Desmond E, et al. Extensively drug-resistant tuberculosis in california, 1993-2006. Clin Infect Dis. 2008;47:450–7. doi: 10.1086/590009. [DOI] [PubMed] [Google Scholar]
- 33.Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, et al. Treatment outcomes and survival based on drug resistance patterns in multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:113–9. doi: 10.1164/rccm.200911-1656OC. [DOI] [PubMed] [Google Scholar]
- 34.Migliori GB, Centis R, D’Ambrosio L, Spanevello A, Borroni E, Cirillo DM, et al. Totally drug-resistant and extremely drug-resistant tuberculosis: the same disease? Clin Infect Dis. 2012;54:1379–80. doi: 10.1093/cid/cis128. [DOI] [PubMed] [Google Scholar]
- 35.Zumla A, Abubakar I, Raviglione M, Hoelscher M, Ditiu L, McHugh TD, et al. Drug-resistant tuberculosis--current dilemmas, unanswered questions, challenges, and priority needs. J Infect Dis. 2012;205(Suppl 2):S228–40. doi: 10.1093/infdis/jir858. [DOI] [PubMed] [Google Scholar]
- 36.Geneva, Switzerland: WHO/HQ; [accessed on February 28, 2013]. “Totally Drug-Resistant TB”: a WHO consultation on the diagnostic definition and treatment options 21-22 March 2012. Available from: http://www.who.int/tb/challenges/xdr/Report_Meeting_totallydrugresistantTB_032012.pdf . [Google Scholar]
- 37.Global tuberculosis control: WHO report 2012. WHO/HTM/ TB/2012.6. Geneva: World Health Organization; 2012. [accessed on February 28, 2013]. World Health Organization. Available from: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf . [Google Scholar]
- 38.Chadha VK, Kumar P, Jagannatha PS, Vaidyanathan PS, Unnikrishnan KP. Average annual risk of tuberculous infection in India. Int J Tuberc Lung Dis. 2005;9:116–8. [PubMed] [Google Scholar]
- 39.Chadha VK, Sarin R, Narang P, John KR, Chopra KK, Jitendra R, et al. Trends in the annual risk of tuberculous infection in India. Int J Tuberc Lung Dis. 2013;17:312–9. doi: 10.5588/ijtld.12.0330. [DOI] [PubMed] [Google Scholar]
- 40.Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K, et al. Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007-2010. Bull World Health Organ. 2012;90:111–119D. doi: 10.2471/BLT.11.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Stop TB Department, World Health Organization. Available data on anti-TB drug resistance. [accessed on February 28, 2013]. Available from: http://www.who.int/tb/challenges/mdr/drs_maps.pdf .
- 42.Ramachandran R, Nalini S, Chandrasekar V, Dave PV, Sanghvi AS, Wares F, et al. Surveillance of drug-resistant tuberculosis in the state of Gujarat, India. Int J Tuberc Lung Dis. 2009;13:1154–60. [PubMed] [Google Scholar]
- 43.Paramasivan CN. Antituberculosis drug resistance surveillance. In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2009. pp. 714–33. [Google Scholar]
- 44.Sharma SK, Kumar S, Saha PK, George N, Arora SK, Gupta D, et al. Prevalence of multidrug-resistant tuberculosis among category II pulmonary tuberculosis patients. Indian J Med Res. 2011;133:312–5. [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma SK, Kaushik G, Jha B, George N, Arora SK, Gupta D, et al. Prevalence of multidrug-resistant tuberculosis among newly diagnosed cases of sputum-positive pulmonary tuberculosis. Indian J Med Res. 2011;133:308–11. [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas A, Ramachandran R, Rehaman F, Jaggarajamma K, Santha T, Selvakumar N, et al. Management of multi drug resistance tuberculosis in the field: Tuberculosis Research Centre experience. Indian J Tuberc. 2007;54:117–24. [PubMed] [Google Scholar]
- 47.Sharma SK, George N, Kadhiravan T, Saha PK, Mishra HK, Hanif M. Prevalence of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: a retrospective hospital-based study. Indian J Med Res. 2009;130:392–5. [PubMed] [Google Scholar]
- 48.Rajasekaran S, Chandrasekar C, Mahilmaran A, Kanakaraj K, Karthikeyan DS, Suriakumar J. HIV coinfection among multidrug resistant and extensively drug resistant tuberculosis patients - a trend. J Indian Med Assoc. 2009;107:281. [PubMed] [Google Scholar]
- 49.Paramasivan CN, Rehman F, Wares F, Sundar Mohan N, Sundar S, Devi S, et al. First- and second-line drug resistance patterns among previously treated tuberculosis patients in India. Int J Tuberc Lung Dis. 2010;14:243–6. [PubMed] [Google Scholar]
- 50.Thomas A, Joseph P, Nair D, Rao DV, Rekha VV, Selvakumar N, et al. Extensively drug-resistant tuberculosis: experience at the Tuberculosis Research Centre, Chennai, India. Int J Tuberc Lung Dis. 2011;15:1323–5. doi: 10.5588/ijtld.10.0530. [DOI] [PubMed] [Google Scholar]
- 51.Myneedu VP, Visalakshi P, Verma AK, Behera D, Bhalla M. Prevalence of XDR TB cases - a retrospective study from a tertiary care TB hospital. Indian J Tuberc. 2011;58:54–9. [PubMed] [Google Scholar]
- 52.Joseph P, Desai VB, Mohan NS, Fredrick JS, Ramachandran R, Raman B, et al. Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, India. Indian J Med Res. 2011;133:529–34. [PMC free article] [PubMed] [Google Scholar]
- 53.Balaji V, Daley P, Anand AA, Sudarsanam T, Michael JS, Sahni RD, et al. Risk factors for MDR and XDR-TB in a tertiary referral hospital in India. PLoS One. 2010;5:e9527. doi: 10.1371/journal.pone.0009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain S, Rodrigues C, Mehta A, Udwadia ZF. High prevalence of XDR-TB from a tertiary care hospital in India. Am J Respir Crit Care Med. 2007;175:A510. [Google Scholar]
- 55.Datta BS, Hassan G, Kadri SM, Qureshi W, Kamili MA, Singh H, et al. Multidrug-resistant and extensively drug resistant tuberculosis in Kashmir, India. J Infect Dev Ctries. 2009;4:19–23. doi: 10.3855/jidc.669. [DOI] [PubMed] [Google Scholar]
- 56.Murray M, Oxlade O, Lin HH. Modeling social, environmental and biological determinants of tuberculosis. Int J Tuberc Lung Dis. 2011;15(Suppl 2):S64–70. doi: 10.5588/ijtld.10.0535. [DOI] [PubMed] [Google Scholar]
- 57.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 58.Möller M, de Wit E, Hoal EG. Past, present and future directions in human genetic susceptibility to tuberculosis. FEMS Immunol Med Microbiol. 2010;58:3–26. doi: 10.1111/j.1574-695X.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- 59.Najmi N, Kaur G, Sharma SK, Mehra NK. Human Toll-like receptor 4 polymorphisms TLR4 Asp299Gly and Thr399Ile influence susceptibility and severity of pulmonary tuberculosis in the Asian Indian population. Tissue Antigens. 2010;76:102–9. doi: 10.1111/j.1399-0039.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- 60.Sharma S, Rathored J, Ghosh B, Sharma SK. Genetic polymorphisms in TNF genes and tuberculosis in North Indians. BMC Infect Dis. 2010;10:165. doi: 10.1186/1471-2334-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rathored J, Sharma SK, Singh B, Banavaliker JN, Sreenivas V, Srivastava AK, et al. Risk and outcome of multidrug-resistant tuberculosis: vitamin D receptor polymorphisms and serum 25(OH)D. Int J Tuberc Lung Dis. 2012;16:1522–8. doi: 10.5588/ijtld.12.0122. [DOI] [PubMed] [Google Scholar]
- 62.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis & management. Indian J Med Res. 2005;121:550–67. [PubMed] [Google Scholar]
- 63.Padmapriyadarsini C, Narendran G, Swaminathan S. Diagnosis & treatment of tuberculosis in HIV co-infected patients. Indian J Med Res. 2011;134:850–65. doi: 10.4103/0971-5916.92630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pawlowski A, Jansson M, Sköld M, Rottenberg ME, Källenius G. Tuberculosis and HIV co-infection. PLoS Pathog. 2012;8:e1002464. doi: 10.1371/journal.ppat.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–35. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 66.Daley CL, Small PM, Schecter GF, Schoolnik GK, McAdam RA, Jacobs WR, Jr, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction fragment length polymorphisms. N Engl J Med. 1992;326:231–5. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 67.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis. 2010;10:455–63. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358:1687–93. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 69.Small PM, Shafer RW, Hopewell PC, Singh SP, Murphy MJ, Desmond E, et al. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N Engl J Med. 1993;328:1137–44. doi: 10.1056/NEJM199304223281601. [DOI] [PubMed] [Google Scholar]
- 70.Korenromp EL, Scano F, Williams BG, Dye C, Nunn P. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an analytical review. Clin Infect Dis. 2003;37:101–12. doi: 10.1086/375220. [DOI] [PubMed] [Google Scholar]
- 71.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 72.Narain JP, Raviglione MC, Kochi A. HIV-associated tuberculosis in developing countries: epidemiology and strategies for prevention. Tuber Lung Dis. 1992;73:311–21. doi: 10.1016/0962-8479(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 73.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis. 2003;188:1146–55. doi: 10.1086/378676. [DOI] [PubMed] [Google Scholar]
- 74.Centers for Disease Control (CDC) Transmission of multidrug-resistant tuberculosis among immunocompromised persons in a correctional system- New York, 1991. MMWR Morb Mortal Wkly Rep. 1992;41:507–9. [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention (CDC) Outbreak of multidrug-resistant tuberculosis at a hospital - New York City, 1991. MMWR Morb Mortal Wkly Rep. 1993;42(427):433–4. [PubMed] [Google Scholar]
- 76.Pearson ML, Jereb JA, Frieden TR, Crawford JT, Davis BJ, Dooley SW, et al. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis. A risk to patients and health care workers. Ann Intern Med. 1992;117:191–6. doi: 10.7326/0003-4819-117-3-191. [DOI] [PubMed] [Google Scholar]
- 77.Beck-Sague C, Dooley SW, Hutton MD, Otten J, Breeden A, Crawford JT, et al. Hospital outbreak of multidrug-resistant Mycobacterium tuberculosis infections. Factors in transmission to staff and HIV-infected patients. JAMA. 1992;268:1280–6. doi: 10.1001/jama.1992.03490100078031. [DOI] [PubMed] [Google Scholar]
- 78.Fischl MA, Daikos GL, Uttamchandani RB, Poblete RB, Moreno JN, Reyes RR, et al. Clinical presentation and outcome of patients with HIV infection and tuberculosis caused by multiple-drug-resistant bacilli. Ann Intern Med. 1992;117:184–90. doi: 10.7326/0003-4819-117-3-184. [DOI] [PubMed] [Google Scholar]
- 79.Swaminathan S, Paramasivan CN, Ponnuraja C, Iliayas S, Rajasekaran S, Narayanan PR. Anti-tuberculosis drug resistance in patients with HIV and tuberculosis in South India. Int J Tuberc Lung Dis. 2005;9:896–900. [PubMed] [Google Scholar]
- 80.Pereira M, Tripathy S, Inamdar V, Ramesh K, Bhavsar M, Date A, et al. Drug resistance pattern of Mycobacterium tuberculosis in seropositive and seronegative HIV-TB patients in Pune, India. Indian J Med Res. 2005;121:235–9. [PubMed] [Google Scholar]
- 81.Wells CD, Cegielski P, Nelson LJ, Laserson KF, Holtz TH, Finlay A, et al. HIV infection and multidrug resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 82.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harries AD, Billo N, Kapur A. Links between diabetes mellitus and tuberculosis: should we integrate screening and care? Trans R Soc Trop Med Hyg. 2009;103:1–2. doi: 10.1016/j.trstmh.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010;36:1185–206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 86.Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A. BSR BR Control Centre Consortium, Symmons DP; BSR Biologics Register. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Ann Rheum Dis. 2010;69:522–8. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pai M, Mohan A, Dheda K, Leung CC, Yew WW, Christopher DJ, et al. Lethal interaction: the colliding epidemics of tobacco and tuberculosis. Expert Rev Anti Infect Ther. 2007;5:385–91. doi: 10.1586/14787210.5.3.385. [DOI] [PubMed] [Google Scholar]
- 88.Ferrara G, Murray M, Winthrop K, Centis R, Sotgiu G, Migliori GB, et al. Risk factors associated with pulmonary tuberculosis: smoking, diabetes and anti-TNF alpha drugs. Curr Opin Pulm Med. 2012;18:233–40. doi: 10.1097/MCP.0b013e328351f9d6. [DOI] [PubMed] [Google Scholar]
- 89.Gajalakshmi V, Peto R, Kanaka TS, Jha P. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35000 controls. Lancet. 2003;362:507–15. doi: 10.1016/S0140-6736(03)14109-8. [DOI] [PubMed] [Google Scholar]
- 90.Lin H-H, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4:e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slama K, Chiang CY, Enarson DA, Hassmiller K, Fanning A, Gupta P, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis. 2007;11:1049–61. [PubMed] [Google Scholar]
- 92.Sharma SK, Mohanan S, Sharma A. Relevance of latent TB infection in areas of high TB prevalence. Chest. 2012;142:761–73. doi: 10.1378/chest.12-0142. [DOI] [PubMed] [Google Scholar]
- 93.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–53. [PubMed] [Google Scholar]
- 95.Marais BJ. Childhood tuberculosis: epidemiology and natural history of disease. Indian J Pediatr. 2011;78:321–7. doi: 10.1007/s12098-010-0353-1. [DOI] [PubMed] [Google Scholar]
- 96.Sharma SK, Kadhiravan T, Banga A. A clinical prediction rule to identify patients with tuberculosis at high risk for HIV co-infection. Indian J Med Res. 2009;130:51–7. [PubMed] [Google Scholar]
- 97.Sharma SK, Mohan A, Pande JN, Prasad KL, Gupta AK, Khilnani GC. Clinical profile, laboratory characteristics and outcome in miliary tuberculosis. QJM. 1995;88:29–37. [PubMed] [Google Scholar]
- 98.Proudfoot AT, Akhtar AJ, Douglas AC, Horne NW. Miliary tuberculosis in adults. Br Med J. 1969;2:273–6. doi: 10.1136/bmj.2.5652.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu YL, Chow WH, Humphries MJ, Wong RW, Gabriel M. Cryptic miliary tuberculosis. QJM. 1986;59:421–8. [PubMed] [Google Scholar]
- 100.Deng W, Yu M, Ma H, Hu LA, Chen G, Wang Y, et al. Predictors and outcome of patients with acute respiratory distress syndrome caused by miliary tuberculosis: a retrospective study in Chongqing, China. BMC Infect Dis. 2012;12:121. doi: 10.1186/1471-2334-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee K, Kim JH, Lee JH, Lee WY, Park MS, Kim JY, et al. Acute respiratory distress syndrome caused by miliary tuberculosis: a multicentre survey in South Korea. Int J Tuberc Lung Dis. 2011;15:1099–103. doi: 10.5588/ijtld.10.0557. [DOI] [PubMed] [Google Scholar]
- 102.Sharma SK, Mohan A, Banga A, Saha PK, Guntupalli KK. Predictors of development and outcome in patients with acute respiratory distress syndrome due to tuberculosis. Int J Tuberc Lung Dis. 2006;10:429–35. [PubMed] [Google Scholar]
- 103.Mohan A, Sharma SK, Pande JN. Acute respiratory distress syndrome (ARDS) in miliary tuberculosis: a twelve year experience. Indian J Chest Dis Allied Sci. 1996;38:157–62. [PubMed] [Google Scholar]
- 104.Hayakawa K, Ramasamy B, Chandrasekar PH. Fever of unknown origin: an evidence-based Review. Am J Med Sci. 2012;344:307–16. doi: 10.1097/MAJ.0b013e31824ae504. [DOI] [PubMed] [Google Scholar]
- 105.Bandyopadhyay D, Bandyopadhyay R, Paul R, Roy D. Etiological study of fever of unknown origin in patients admitted to medicine ward of a teaching hospital of eastern India. J Glob Infect Dis. 2011;3:329–33. doi: 10.4103/0974-777X.91052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grossman C, Kushinka J, Brath LK. A 41-year-old woman with AIDS, mediastinal lymphadenopathy, and fever of unknown origin. Am J Med Sci. 2008;336:349–52. doi: 10.1097/MAJ.0b013e318167b0e4. [DOI] [PubMed] [Google Scholar]
- 107.Sharma SK, Smith-Rohrberg D, Tahir M, Mohan A, Seith A. Radiological manifestations of splenic tuberculosis: a 23-patient case series from India. Indian J Med Res. 2007;125:669–78. [PubMed] [Google Scholar]
- 108.Kingma BJ, van den Berg W, Schuurmans MM, Molenaar AH. A patient with back pain and fever. Eur Respir J. 1992;5:1292–5. [PubMed] [Google Scholar]
- 109.Ibrarullah M, Mohan A, Sarkari A, Srinivas M, Mishra A, Sundar TS. Abdominal tuberculosis: diagnosis by laparoscopy and colonoscopy. Trop Gastroenterol. 2002;23:150–3. [PubMed] [Google Scholar]
- 110.Arch-Ferrer JE, Velázquez-Fernández D, Sierra-Madero J, López-Karpovitch X, Angeles-Angeles A, Gamino R, et al. Laparoscopic approach to fever of unknown origin. Surg Endosc. 2003;17:494–7. doi: 10.1007/s00464-002-8589-0. [DOI] [PubMed] [Google Scholar]
- 111.Liu A, Hu Y, Coates A. Sudden cardiac death and tuberculosis - How much do we know? Tuberculosis (Edinb) 2012;92:307–13. doi: 10.1016/j.tube.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 112.Biedrzycki OJ, Baithun SI. TB-related sudden death (TBRSD) due to myocarditis complicating miliary TB: a case report and review of the literature. Am J Forensic Med Pathol. 2006;27:335–6. doi: 10.1097/01.paf.0000233633.16185.32. [DOI] [PubMed] [Google Scholar]
- 113.Breton G, Leclerc S, Longuet P, Leport C, Vildé JL, Laissy JP. Myocardial localisation of tuberculosis: the diagnostic value of cardiac MRI. Presse Med. 2005;34:293–6. doi: 10.1016/s0755-4982(05)83909-0. [DOI] [PubMed] [Google Scholar]
- 114.Bansal M, Mehrotra R, Kasliwal RR. Loss of left ventricular torsion as the predominant mechanism of left ventricular systolic dysfunction in a patient with tubercular cardiomyopathy. Echocardiography. 2012;29:E221–5. doi: 10.1111/j.1540-8175.2012.01768.x. [DOI] [PubMed] [Google Scholar]
- 115.Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17:488–94. doi: 10.3201/eid1703.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jarand J, Shean K, O’Donnell M, Loveday M, Kvasnovsky C, Van der Walt M, et al. Extensively drug-resistant tuberculosis (XDR-TB) among health care workers in South Africa. Trop Med Int Health. 2010;15:1179–84. doi: 10.1111/j.1365-3156.2010.02590.x. [DOI] [PubMed] [Google Scholar]
- 117.Welbel SF, French AL, Bush P, DeGuzman D, Weinstein RA. Protecting health care workers from tuberculosis: a 10-year experience. Am J Infect Control. 2009;37:668–73. doi: 10.1016/j.ajic.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 118.Screening for tuberculosis and tuberculosis infection in high risk populations. Recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19–34. [No authors listed] [PubMed] [Google Scholar]
- 119.Pinto LM, Grenier J, Schumacher SG, Denkinger CM, Steingart KR, Pai M. Immunodiagnosis of tuberculosis: state of the art. Med Princ Pract. 2012;21:4–13. doi: 10.1159/000331583. [DOI] [PubMed] [Google Scholar]
- 120.Denkinger CM, Dheda K, Pai M. Guidelines on interferon-gamma release assays for tuberculosis infection: concordance, discordance or confusion? Clin Microbiol Infect. 2011;17:806–14. doi: 10.1111/j.1469-0691.2011.03555.x. [DOI] [PubMed] [Google Scholar]
- 121.Metcalfe JZ, Everett CK, Steingart KR, Cattamanchi A, Huang L, Hopewell PC, et al. Interferon-γ release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011;204(Suppl 4):S1120–9. doi: 10.1093/infdis/jir410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, et al. Predictive value of interferon- g release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for Its Prevention and Control. NICE clinical guideline 117. London, England: Royal College of Physicians of London; 2011. [accessed on February 28, 2013]. National Collaborating Centre for Chronic Conditions and the Centre for Clinical Practice at NICE. Available from: http://www.nice.org.uk/nicemedia/live/13422/53642/53642.pdf . [Google Scholar]
- 124.Use of interferon-gamma release assays in support of TB diagnosis. Stockholm: European Centre for Disease Prevention and Control; 2011. [accessed on February 28, 2013]. European Centre for Disease Prevention and Control. Available from: http://ecdc.europa.eu/en/publications/publications/1103_gui_igra.pdf . [Google Scholar]
- 125.Use of tuberculosis interferongamma release assays (IGRAs) in Low- and Middle-Income Countries: Policy Statement. WHO/HTM/TB/ 2011.18. Geneva: World Health Organization; 2011. [accessed on February 28, 2013]. World Health Organization. Available from: http://whqlibdoc.who.int/publications/2011/9789241502672_eng.pdf . [PubMed] [Google Scholar]
- 126.Dheda K, Ruhwald M, Theron G, Peter J, Yam WC. Point-of-care diagnosis of tuberculosis: past, present and future. Respirology. 2013;18:217–32. doi: 10.1111/resp.12022. [DOI] [PubMed] [Google Scholar]
- 127.Palomino JC. Current developments and future perspectives for TB diagnostics. Future Microbiol. 2012;7:59–71. doi: 10.2217/fmb.11.133. [DOI] [PubMed] [Google Scholar]
- 128.Maiga M, Abaza A, Bishai WR. Current tuberculosis diagnostic tools & role of urease breath test. Indian J Med Res. 2012;135:731–6. [PMC free article] [PubMed] [Google Scholar]
- 129.Rodrigues C, Shenai S, Sadani M, Sukhadia N, Jani M, Ajbani K, et al. Evaluation of the Bactec MGIT 960 TB system for recovery and identification of Mycobacterium tuberculosis complex in a high through put tertiary care centre. Indian J Med Microbiol. 2009;27:217–21. doi: 10.4103/0255-0857.53203. [DOI] [PubMed] [Google Scholar]
- 130.Shen GH, Chen CH, Hung CH, Wu KM, Lin CF, Sun YW, et al. Combining the Capilia TB assay with smear morphology for the identification of Mycobacterium tuberculosis complex. Int J Tuberc Lung Dis. 2009;13:371–6. [PubMed] [Google Scholar]
- 131.Tortoli E, Marcelli F. Use of the INNO LiPA Rif. TB for detection of Mycobacterium tuberculosis DNA directly in clinical specimens and for simultaneous determination of rifampin susceptibility. Eur J Clin Microbiol Infect Dis. 2007;26:51–5. doi: 10.1007/s10096-006-0240-x. [DOI] [PubMed] [Google Scholar]
- 132.Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2010;48:1683–9. doi: 10.1128/JCM.01947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dorman SE. New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clin Infect Dis. 2010;50(Suppl 3):S173–7. doi: 10.1086/651488. [DOI] [PubMed] [Google Scholar]
- 134.Parsons LM, Somoskövi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24:314–50. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shikama Mde L, Silva RR, Martins MC, Giampaglia CM, Oliveira RS, Silva RF, et al. Rapid detection of resistant tuberculosis by nitrate reductase assay performed in three settings in Brazil. J Antimicrob Chemother. 2009;64:794–6. doi: 10.1093/jac/dkp284. [DOI] [PubMed] [Google Scholar]
- 136.Martin A, Portaels F, Palomino JC. Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;59:175–83. doi: 10.1093/jac/dkl477. [DOI] [PubMed] [Google Scholar]
- 137.Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6:1067–82. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Theron G, Pinto L, Peter J, Mishra HK, Mishra HK, van Zyl-Smit R, et al. The use of an automated quantitative polymerase chain reaction (Xpert MTB/RIF) to predict the sputum smear status of tuberculosis patients. Clin Infect Dis. 2012;54:384–8. doi: 10.1093/cid/cir824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Zyl-Smit RN, Binder A, Meldau R, Mishra H, Semple PL, Theron G, et al. Comparison of quantitative techniques including Xpert MTB/RIF to evaluate mycobacterial burden. PLoS One. 2011;6:e28815. doi: 10.1371/journal.pone.0028815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a metaanalysis. Eur Respir J. 2008;32:1165–74. doi: 10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 141.Martin A, Panaiotov S, Portaels F, Hoffner S, Palomino JC, Angeby K. The nitrate reductase assay for the rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2008;62:56–64. doi: 10.1093/jac/dkn139. [DOI] [PubMed] [Google Scholar]
- 142.Ling DI, Flores LL, Riley LW, Pai M. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression. PLoS One. 2008;3:e1536. doi: 10.1371/journal.pone.0001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neonakis IK, Spandidos DA, Petinaki E. Use of loop-mediated isothermal amplification of DNA for the rapid detection of Mycobacterium tuberculosis in clinical specimens. Eur J Clin Microbiol Infect Dis. 2011;30:937–42. doi: 10.1007/s10096-011-1195-0. [DOI] [PubMed] [Google Scholar]
- 144.Boehme CC, Nabeta P, Henostroza G, Raqib R, Rahim Z, Gerhardt M, et al. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J Clin Microbiol. 2007;45:1936–40. doi: 10.1128/JCM.02352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Phillips M, Basa-Dalay V, Bothamley G, Cataneo RN, Lam PK, Natividad MP, et al. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb) 2010;90:145–51. doi: 10.1016/j.tube.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 146.Phillips M, Basa-Dalay V, Blais J, Bothamley G, Chaturvedi A, Modi KD, et al. Point-of-care breath test for biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb) 2012;92:314–20. doi: 10.1016/j.tube.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 147.Commercial serodiagnostic tests for diagnosis of tuberculosis: policy statement. WHO/HTM/TB/2011.5. Geneva: World Health Organization; 2011. World Health Organization. [PubMed] [Google Scholar]
- 148.Kumar A. Letter of Deputy Director General, Cenral TB Division, Ministry of Health and Family Welfare, Government of India. [accessed on February 28, 2013]. Available from: http://www.tbcindia.nic.in/pdfs/Letter_Serodiagnosis.pdf .
- 149.Seibert AF, Haynes J, Jr, Middleton R, Bass JB., Jr Tuberculous pleural effusion. Twenty-year experience. Chest. 1991;99:883–6. doi: 10.1378/chest.99.4.883. [DOI] [PubMed] [Google Scholar]
- 150.Escudero Bueno C, Garcia Clemente M, Cuesta Castro B, Molinos Martin L, Rodriguez Ramos S, Gonzalez Panizo A, et al. Cytologic and bacteriologic analysis of fluid and pleural biopsy specimens with Cope's needle. Study of 414 patients. Arch Intern Med. 1990;150:1190–4. doi: 10.1001/archinte.150.6.1190. [DOI] [PubMed] [Google Scholar]
- 151.Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131:880–9. doi: 10.1378/chest.06-2063. [DOI] [PubMed] [Google Scholar]
- 152.Sharma SK, Suresh V, Mohan A, Kaur P, Saha P, Kumar A, et al. A prospective study of sensitivity and specificity of adenosine deaminase estimation in the diagnosis of tuberculosis pleural effusion. Indian J Chest Dis Allied Sci. 2001;43:149–55. [PubMed] [Google Scholar]
- 153.Sharma SK, Banga A. Pleural fluid interferon-gamma and adenosine deaminase levels in tuberculosis pleural effusion: a cost-effectiveness analysis. J Clin Lab Anal. 2005;19:40–6. doi: 10.1002/jcla.20054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989–99. [PubMed] [Google Scholar]
- 155.Guirat A, Koubaa M, Mzali R, Abid B, Ellouz S, Affes N, et al. Peritoneal tuberculosis. Clin Res Hepatol Gastroenterol. 2011;35:60–9. doi: 10.1016/j.gcb.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 156.Sharma SK, Tahir M, Mohan A, Smith-Rohrberg D, Mishra HK, Pandey RM. Diagnostic accuracy of ascitic fluid IFN-gamma and adenosine deaminase assays in the diagnosis of tuberculous ascites. J Interferon Cytokine Res. 2006;26:484–8. doi: 10.1089/jir.2006.26.484. [DOI] [PubMed] [Google Scholar]
- 157.Fowler NO. Tuberculous pericarditis. JAMA. 1991;266:99–103. [PubMed] [Google Scholar]
- 158.Reuter H, Burgess L, van Vuuren W, Doubell A. Diagnosing tuberculous pericarditis. QJM. 2006;99:827–39. doi: 10.1093/qjmed/hcl123. [DOI] [PubMed] [Google Scholar]
- 159.Galimi R. Extrapulmonary tuberculosis: tuberculous meningitis new developments. Eur Rev Med Pharmacol Sci. 2011;15:365–86. [PubMed] [Google Scholar]
- 160.Cherian A, Thomas SV. Central nervous system tuberculosis. Afr Health Sci. 2011;11:116–27. [PMC free article] [PubMed] [Google Scholar]
- 161.Murthy JM. Tuberculous meningitis: the challenges. Neurol India. 2010;58:716–22. doi: 10.4103/0028-3886.72178. [DOI] [PubMed] [Google Scholar]
- 162.Tuon FF, Higashino HR, Lopes MI, Litvoc MN, Atomiya AN, Antonangelo L, et al. Adenosine deaminase and tuberculous meningitis - a systematic review with meta-analysis. Scand J Infect Dis. 2010;42:198–207. doi: 10.3109/00365540903428158. [DOI] [PubMed] [Google Scholar]
- 163.Xu HB, Jiang RH, Li L, Sha W, Xiao HP. Diagnostic value of adenosine deaminase in cerebrospinal fluid for tuberculous meningitis: a meta-analysis. Int J Tuberc Lung Dis. 2010;14:1382–7. [PubMed] [Google Scholar]
- 164.Stop TB Partnership and WHO. The global plan to stop TB, 20112015: transforming the fight towards elimination of tuberculosis. Geneva: World Health Organization; 2010. World Health Organization. [Google Scholar]
- 165.World Health Organization. Expanding and accelerating access to diagnostics for patients at risk of multidrugresistant tuberculosis. [accessed on February 28, 2013]. Available from: http://www.who.int/tb/publications/factsheet_expand_tb.pdf .
- 166.McAdams HP, Erasmus J, Winter JA. Radiologic manifestations of pulmonary tuberculosis. Radiol Clin North Am. 1995;33:655–78. [PubMed] [Google Scholar]
- 167.Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol. 2008;191:834–44. doi: 10.2214/AJR.07.3896. [DOI] [PubMed] [Google Scholar]
- 168.Hara T, Kosaka N, Suzuki T, Kudo K, Niino H. Uptake rates of 18F-fluorodeoxyglucose and 11C-choline in lung cancer and pulmonary tuberculosis: a positron emission tomography study. Chest. 2003;124:893–901. doi: 10.1378/chest.124.3.893. [DOI] [PubMed] [Google Scholar]
- 169.Iyengar KP, Vinjamuri S. Role of 99mTc Sulesomab in the diagnosis of prosthetic joint infections. Nucl Med Commun. 2005;26:489–96. doi: 10.1097/00006231-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 170.Singh B, Prasad V, Bhattacharya A, Singh AK, Bhatnagar A, Mittal BR, et al. Diagnosis of mandibular osteomyelitis in probable coexisting tumor recurrence: role of Tc-99m ciprofloxacin imaging. Clin Nucl Med. 2008;33:525–7. doi: 10.1097/RLU.0b013e31817e6de9. [DOI] [PubMed] [Google Scholar]
- 171.Zhang H, Jiang NY, Zhu L. Experimental studies on imaging of infected site with (99m)Tc-labeled ciprofloxacin in mice. Chin Med J (Engl) 2009;122:1907–9. [PubMed] [Google Scholar]
- 172.Sharma R, Tewari KN, Bhatnagar A, Mondal A, Mishra AK, Singh AK, et al. Tc-99m ciprofloxacin scans for detection of tubercular bone infection. Clin Nucl Med. 2007;32:367–70. doi: 10.1097/01.rlu.0000259322.31974.e8. [DOI] [PubMed] [Google Scholar]
- 173.Bhardwaj V, Agrawal M, Suri T, Sural S, Kashyap R, Dhal A. Evaluation of adequacy of short-course chemotherapy for extraspinal osteoarticular tuberculosis using 99mTc ciprofloxacin scan. Int Orthop. 2011;35:1869–74. doi: 10.1007/s00264-010-1162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Donald PR, van Helden PD. Progress in Respiratory Research. Vol. 40. Basel: Karger; 2011. Antituberculosis chemotherapy. [Google Scholar]
- 175.Dubos RJ. Bactericidal effect of an extract of a soil bacillus on Gram positive cocci. Proc Soc Exp Biol Med. 1939;40:311–2. [Google Scholar]
- 176.Feldman WH, Hinshaw HC. Effects of streptomycin on experimental tuberculosis in guinea pigs: a preliminary report. Proc Staff Meet Mayo Clin. 1944;19:593–9. [Google Scholar]
- 177.Feldman W, Hinshaw HC, Mann FC. Streptomcin in experimental tuberculosis. Am Rev Tuberc. 1945;52:269–98. [Google Scholar]
- 178.Hinshaw HC, Feldman WH, Pfuetze KH. Treatment of tuberculosis with streptomycin: a summary of observations on one hundred cases. J Am Med Assoc. 1946;132:778–82. doi: 10.1001/jama.1946.02870480024007. [DOI] [PubMed] [Google Scholar]
- 179.Streptomycin treatment of pulmonary tuberculosis. Br Med J. 1948;2:769–82. [No authors listed] [PMC free article] [PubMed] [Google Scholar]
- 180.Lehmann J. Paraaminosalicylic acid in the treatment of tuberculosis. Lancet. 1946;1:15–6. doi: 10.1016/s0140-6736(46)91185-3. [DOI] [PubMed] [Google Scholar]
- 181.Treatment of pulmonary tuberculosis with streptomycin and para-amino-salicylic acid; a Medical Research Council investigation. Br Med J. 1950;2:1073–85. [PMC free article] [PubMed] [Google Scholar]
- 182.New drugs for tuberculosis. N Engl J Med. 1952;246:797–9. Anonymous. [Google Scholar]
- 183.Isoniazid in the treatment of pulmonary tuberculosis; Second report to the Medical Research Council by their Tuberculosis Chemotherapy Trials Committee. Br Med J. 1953;1:521–36. [No authors listed] [PMC free article] [PubMed] [Google Scholar]
- 184.Medical Research Council Investigation. Isoniazid in combination with streptomycin or with PAS in the treatment of pulmonary tuberculosis. Br Med J. 1953;2:1005–14. [PMC free article] [PubMed] [Google Scholar]
- 185.Long term chemotherapy in the treatment of chronic pulmonary tuberculosis with cavitation: a report to the Medical Research Council by their Tuberculosis Chemotherapy Trials Committee: Tubercle. 1962;43:201–67. [Google Scholar]
- 186.Wilkerson RG, Sheperd RG, Thomas DP, Baughn C. Stereospecificity in a new type of antituberculosis agent. J Am Chem Soc. 1961;83:2212–3. [Google Scholar]
- 187.Fox W. Ambulatory chemotherapy in a developing country: clinical and epidemiological studies. Adv Tuberc Res. 1963;12:28–149. [PubMed] [Google Scholar]
- 188.Fox W. Methods of chemotherapy in developing countries. Bull Int Union Tuberc. 1966;337:249–60. [Google Scholar]
- 189.Sensi P. History of the development of rifampin. Rev Infect Dis. 1983;5(Suppl 3):S402–6. doi: 10.1093/clinids/5.supplement_3.s402. [DOI] [PubMed] [Google Scholar]
- 190.Short-course chemotherapy in pulmonary tuberculosis. A controlled trial by the British Thoracic and Tuberculosis Association. Lancet. 1976;ii:1102–4. [No authors listed] [PubMed] [Google Scholar]
- 191.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- 192.Yeager RL, Munroe WG, Dessau FI. Pyrazinamide (Aldinamide) in the treatment of pulmonary tuberculosis. Am Rev Tuberc. 1952;65:523–46. [PubMed] [Google Scholar]
- 193.Controlled clinical trial of short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1972;1:1079–85. [No authors listed] [PubMed] [Google Scholar]
- 194.A controlled trial of 6 months’ chemotherapy in pulmonary tuberculosis. Final report: results during the 36 months after the end of chemotherapy and beyond. British Thoracic Society. Br J Dis Chest. 1984;78:330–6. [No authors listed] [PubMed] [Google Scholar]
- 195.Chauhan LS. RNTCP: past, present and future of TB control programme in India. J Commun Dis. 2006;38:191–203. [PubMed] [Google Scholar]
- 196.Granich R, Chauhan LS. The Revised National Tuberculosis Control Programme (RNTCP) In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2009. pp. 894–917. [Google Scholar]
- 197.Sachdeva KS, Kumar A, Dewan P, Kumar A, Satyanarayana S. New vision for Revised National Tuberculosis Control Programme (RNTCP): Universal access - “Reaching the un-reached”. Indian J Med Res. 2012;135:690–4. [PMC free article] [PubMed] [Google Scholar]
- 198.Menzies D, Benedetti A, Paydar A, Martin I, Royce S, Pai M, et al. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med. 2009;6:e1000146. doi: 10.1371/journal.pmed.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khan FA, Minion J, Pai M, Royce S, Burman W, Harries AD, et al. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis. 2010;50:1288–99. doi: 10.1086/651686. [DOI] [PubMed] [Google Scholar]
- 200.Swaminathan S, Narendran G, Venkatesan P, Ramachandran R, Sekar L, Iliayas S, et al. Acquired rifampicin resistance in HIV-infected and -uninfected patients with TB treated with a thrice-weekly short-course regimen. Poster presented at Conference on Retroviruses and Opportunistic Infections (CROI), Montreal. 2009. [accessed on December 29, 2012]. Available from: http://www.retroconference.org/2009/Abstracts/35234.htm .
- 201.Tuberculosis Control India. [accessed on February 28, 2013]. Available from: http://tbcindia.nic.in .
- 202.Sharma SK, Solanki R, Mohan A, Jain NK, Chauhan LS Pleural Effusion Study Group. Outcomes of Category III DOTS treatment in immunocompetent patients with tuberculosis pleural effusion. Int J Tuberc Lung Dis. 2012;16:1505–9. doi: 10.5588/ijtld.12.0233. [DOI] [PubMed] [Google Scholar]
- 203.Jindal SK, Aggarwal AN, Gupta D, Ahmed Z, Gupta KB, Janmeja AK, et al. Tuberculous lymphadenopathy - a multicentre operational study of 6-month, thrice weekly, directly observed therapy. Int J Tuberc Lung Dis. 2013;17:234–9. doi: 10.5588/ijtld.12.0333. [DOI] [PubMed] [Google Scholar]
- 204.Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach: 2010 revision. Geneva: World Health Organization; 2010. World Health Organization. [PubMed] [Google Scholar]
- 205.Pozniak AL, Coyne KM, Miller RF, Lipman MC, Freedman AR, Ormerod LP, et al. BHIVA Guidelines Subcommittee. British HIV Association guidelines for the treatment of TB/HIV coinfection 2011. HIV Med. 2011;12:517–24. doi: 10.1111/j.1468-1293.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- 206.Guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Geneva: World Health Organization; 2011. World Health Organization. [PubMed] [Google Scholar]
- 207.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 208.Yew WW. Treatment of tuberculosis. In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2009. pp. 751–75. [Google Scholar]
- 209.Sharma SK, Mohan A. Antituberculosis treatment induced hepatotoxicity: from bench to bed-side. In: Gupta SB, editor. Medicine update. Mumbai: Association of Physicians of India; 2005. pp. 479–84. [Google Scholar]
- 210.Sharma SK. Antituberculosis drugs and hepatotoxicity. Infect Genet Evol. 2004;4:167–70. doi: 10.1016/j.meegid.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 211.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee. An official ATS statement: hepatotoxicity of anti tuberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–52. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 212.Singla R, Sharma SK, Mohan A, Makharia G, Sreenivas V, Jha B, et al. Evaluation of risk factors for antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2010;132:81–6. [PubMed] [Google Scholar]
- 213.Sharma SK, Singla R, Sreenivas V, Kumar S, Jha B, Rathored J, Singh S. Acute viral hepatitis as a confounding factor in patients with antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2009;130:200–1. [PubMed] [Google Scholar]
- 214.Sarda P, Sharma SK, Mohan A, Makharia G, Jayaswal A, Pandey RM, et al. Role of acute viral hepatitis as a confounding factor in antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2009;129:64–7. [PubMed] [Google Scholar]
- 215.Sharma SK, Singla R, Sarda P, Mohan A, Makharia G, Jayaswal A, et al. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis. 2010;50:833–9. doi: 10.1086/650576. [DOI] [PubMed] [Google Scholar]
- 216.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 217.Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations. Thorax. 1998;53:536–48. [No authors listed] [PMC free article] [PubMed] [Google Scholar]
- 218.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. International Network for the Study of HIV-associated IRIS. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sharma SK, Soneja M. HIV & immune reconstitution inflammatory syndrome (IRIS) Indian J Med Res. 2011;134:866–77. doi: 10.4103/0971-5916.92632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sinha S, Sharma SK. Tuberculosis associated immune reconstitution inflammatory syndrome. Indian J Tuberc. 2010;57:177–9. [PubMed] [Google Scholar]
- 221.Tahir M, Sinha S, Sharma SK, Mitsuyasu RT. Immune reconstitution inflammatory syndrome manifesting as disseminated tuberculosis, deep venous thrombosis, encephalopathy and myelopathy. Indian J Chest Dis Allied Sci. 2008;50:363–4. [PubMed] [Google Scholar]
- 222.Tahir M, Sharma SK, Sinha S, Das CJ. Immune reconstitution inflammatory syndrome in a patient with cryptococcal lymphadenitis as the first presentation of acquired immunodeficiency syndrome. J Postgrad Med. 2007;53:250–2. doi: 10.4103/0022-3859.37514. [DOI] [PubMed] [Google Scholar]
- 223.Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 224.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 225.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–61. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 226.Kumarasamy N, Chaguturu S, Mayer KH, Solomon S, Yepthomi HT, Balakrishnan P, et al. Incidence of immune reconstitution syndrome in HIV/tuberculosis-coinfected patients after initiation of generic antiretroviral therapy in India. J Acquir Immune Defic Syndr. 2004;37:1574–6. doi: 10.1097/00126334-200412150-00007. [DOI] [PubMed] [Google Scholar]
- 227.Sharma SK, Dhooria S, Barwad P, Kadhiravan T, Ranjan S, Miglani S, et al. A study of TB-associated immune reconstitution inflammatory syndrome using the consensus case-definition. Indian J Med Res. 2010;131:804–8. [PubMed] [Google Scholar]
- 228.Karmakar S, Sharma SK, Vashishtha R, Sharma A, Ranjan S, Gupta D, et al. Clinical characteristics of tuberculosis associated immune reconstitution inflammatory syndrome in North Indian population of HIV/AIDS patients receiving HAART. Clin Dev Immunol 2011. 2011:239021. doi: 10.1155/2011/239021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.WHO Three I's Meeting: Intensified Case Finding (ICF), Isoniazid Preventive Therapy (IPT) and TB Infection Control (IC) for People Living with HIV. Geneva: World Health Organization; 2008. [accessed on February 28, 2013]. World Health Organization, HIV/AIDS Department. Available from: http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf . [Google Scholar]
- 230.Implementing the WHO Stop TB strategy: A handbook for national tuberculosis control programmes. WHO/HTM/TB/2008.401. Geneva: World Health Organization; 2008. World Health Organization. [PubMed] [Google Scholar]
- 231.Menzies D, Al Jahdali H, Al Otaibi B. Recent developments in treatment of latent tuberculosis infection. Indian J Med Res. 2011;133:257–66. [PMC free article] [PubMed] [Google Scholar]
- 232.Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, et al. TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–66. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 233.Sharma SK, Sharma A, Kadhiravan T, Tharyan P. Isoniazid monotherapy versus other monotherapies or combination chemotherapy for preventing active tuberculosis in HIVnegative persons (Protocol) Cochrane Database Syst Rev. 2009. [Accessed on February 28, 2013]. (1) . Art. No.: CD007545. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007545/pdf .
- 234.Cain KP, Nelson LJ, Cegielski JP. Global policies and practices for managing persons exposed to multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2010;14:269–74. [PubMed] [Google Scholar]
- 235.Lienhardt C, Glaziou P, Uplekar M, Lönnroth K, Getahun H, Raviglione M. Global tuberculosis control: lessons learnt and future prospects. Nat Rev Microbiol. 2012;10:407–16. doi: 10.1038/nrmicro2797. [DOI] [PubMed] [Google Scholar]
- 236.Wares DF. Global tuberculosis control: the future prospects. In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2009. pp. 874–93. [Google Scholar]
- 237.Landmark meeting on post-2015 TB targets held in Geneva. [accessed on February 28, 2013]. Available from: http://www.stoptb.org/news/stories/2013/ns13_012.asp .
- 238.Sharma SK, Mohan A, Chauhan LS, Narain JP, Behera D, Kumar A, et al. for Task Force for Involvement of Medical Colleges in the Revised National Tuberculosis Control Programme, Govt. of India. Contribution of medical colleges to tuberculosis control in India under the Revised National Tuberculosis Control Programme (RNTCP): lessons learnt & challenges ahead. Indian J Med Res. 2013;137:283–94. [PMC free article] [PubMed] [Google Scholar]
- 239.WHO policy on TB infection control in health-care facilities, congregate settings and households. WHO/HTM/TB/2009.419. Geneva: World Health Organization; 2009. World Health Organization. [PubMed] [Google Scholar]
- 240.New Delhi: Directorate General of Health Services, Ministry of Health & Family Welfare; 2010. Directorate General of Health Services, Ministry of Health & Family Welfare. Guidelines on airborne infection control in healthcare and other settings in the context of tuberculosis and other airborne infections April 2010 (Provisional) [Google Scholar]
- 241.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Brennan MJ, Thole J. Tuberculosis vaccines: a strategic blueprint for the next decade. Tuberculosis (Edinb) 2012;92(Suppl 1):S6–S13. doi: 10.1016/S1472-9792(12)70005-7. [DOI] [PubMed] [Google Scholar]
- 243.Trial of BCG vaccines in south India for tuberculosis prevention. Indian J Med Res. 1979;70:349–63. [PubMed] [Google Scholar]
- 244.Fifteen year follow up of trial of BCG vaccines in south India for tuberculosis prevention. Tuberculosis Research Centre (ICMR), Chennai. Indian J Med Res. 1999;110:56–69. [PubMed] [Google Scholar]
- 245.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 246.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 247.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. the MVA85A 020 Trial Study Team. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–8. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010;375:1920–37. doi: 10.1016/S0140-6736(10)60359-5. [DOI] [PubMed] [Google Scholar]
- 249.Wallis RS, Wang C, Doherty TM, Onyebujoh P, Vahedi M, Laang H, et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis. 2010;10:68–9. doi: 10.1016/S1473-3099(10)70003-7. [DOI] [PubMed] [Google Scholar]
- 250.Swindells S. New drugs to treat tuberculosis. F1000 Med Rep. 2012;4:12. doi: 10.3410/M4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zumla A, Hafner R, Lienhardt C, Hoelscher M, Nunn A. Advancing the development of tuberculosis therapy. Nat Rev Drug Discov. 2012;11:171–2. doi: 10.1038/nrd3694. [DOI] [PubMed] [Google Scholar]
- 252.Villemagne B, Crauste C, Flipo M, Baulard AR, Déprez B, Willand N. Tuberculosis: the drug development pipeline at a glance. Eur J Med Chem. 2012;51:1–16. doi: 10.1016/j.ejmech.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 253.Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. The challenge of new drug discovery for tuberculosis. Nature. 2011;469:483–90. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- 254.Kaufmann SH. Fact and fiction in tuberculosis vaccine research: 10 years later. Lancet Infect Dis. 2011;11:633–40. doi: 10.1016/S1473-3099(11)70146-3. [DOI] [PubMed] [Google Scholar]
- 255.McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos Trans R Soc Lond B Biol Sci. 2011;366:2782–9. doi: 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Brennan MJ, Thole J. Tuberculosis vaccines: a strategic blueprint for the next decade. Tuberculosis (Edinb) 2012;92(Suppl 1):S6–13. doi: 10.1016/S1472-9792(12)70005-7. [DOI] [PubMed] [Google Scholar]
- 258.Lee H, Yoon TJ, Weissleder R. Ultrasensitive detection of bacteria using core-shell nanoparticles and an NMR-filter system. Angew Chem Int Ed Engl. 2009;48:5657–60. doi: 10.1002/anie.200901791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Qin D, He X, Wang K, Tan W. Using fluorescent nanoparticles and SYBR Green I based two-color flow cytometry to determine Mycobacterium tuberculosis avoiding false positives. Biosens Bioelectron. 2008;24:626–31. doi: 10.1016/j.bios.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 260.Veigas B, Machado D, Perdigão J, Portugal I, Couto I, Viveiros M, et al. Au-nanoprobes for detection of SNPs associated with antibiotic resistance in Mycobacterium tuberculosis. Nanotechnology. 2010;21:415101. doi: 10.1088/0957-4484/21/41/415101. [DOI] [PubMed] [Google Scholar]
- 261.Working Group on New TB Drugs. Drug pipeline. [accessed on February 28, 2013]. Available from: http://www.newtbdrugs.org/pipeline.php .
- 262.Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366:2151–60. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 263.Diacon AH, Donald PR, Pym A, Grobusch M, Patientia RF, Mahanyele R, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother. 2012;56:3271–6. doi: 10.1128/AAC.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, et al. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob Agents Chemother. 2012;56:3027–31. doi: 10.1128/AAC.06125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hartkoorn RC, Sala C, Neres J, Pojer F, Magnet S, Mukherjee R, et al. Towards a new tuberculosis drug: pyridomycin - nature's isoniazid. EMBO Mol Med. 2012;4:1032–42. doi: 10.1002/emmm.201201689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Spigelman M, Woosley R, Gheuens J. New initiative speeds tuberculosis drug development: novel drug regimens become possible in years, not decades. Int J Tuberc Lung Dis. 2010;14:663–4. [PubMed] [Google Scholar]
- 267.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–86. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]


