Abstract
Background & objectives:
Entomological surveillance of the dengue vectors using pupal productivity as indicators can be helpful in effective management. On this basis, an assessment was made on the relative importance of the larval habitats of Aedes mosquitoes in Kolkata, an endemic zone for dengue in West Bengal, India.
Methods:
Monthly collection of larvae and pupae of Aedes from larval habitats categorized as earthen, plastic and porcelain containers and tyres, was carried out from selected sites. Pupal weight was recorded and degree of sexual dimorphism was calculated. The data on pupal weight, sexual dimorphism and immature density were used for regression analysis.
Results:
The number of positive sites for each type of larval habitats varied with months and mosquito species. Based on mean density per month, the plastic containers were the most productive habitats and the tyres were least productive for both Aedes species. The pupal weight of both Ae. aegypti and Ae. albopictus varied with the relative density and type of larval habitats. Significant differences in pupal productivity, positive sites and the proportion of pupae were observed in the habitats. Species-specific differences in the degree of dimorphism were noted with the females being larger in size than males, irrespective of the habitats.
Interpretation & conclusions:
Pupal productivity of Aedes mosquitoes in Kolkata differed in terms of the type of the larval habitats with the immature density affecting the body size of the adults. This habitat-based study is a pioneer effort considering Kolkata and calls for a management plan for source reduction of these habitats to minimize Aedes mosquitoes and thus potential risk of dengue.
Keywords: Aedes aegypti, Ae. albopictus, Kolkata, larval habitats, vector management
Dengue is an arboviral disease prevalent in the tropical and subtropical regions of the world1 transmitted to human by the bites of vectors Aedes aegypti (Linneaus, 1774) and Aedes albopictus (Skuse, 1893) (Diptera: Culicidae). The incidence of dengue is largely dependent on vector populations and the frequency of contact between the vectors and susceptible human hosts, reflected by a positive correlation of Aedes abundance and prevalence with dengue2,3,4. This supports the necessity of entomological surveillance to assess and predict the abundance of vectors and possibilities of occurrence of dengue5,6,7. Conventionally, assessment and prediction of the vector population levels are made using the productive larval habitats and data on human demography as components of different indices recommended by the WHO6. The information provided by these indices is useful supplements for the management strategies against the dengue vectors, in different parts of the world4,6,7. Estimates of Aedes abundance in different dengue endemic regions of India are available that help vector management and enhance necessary precautionary measures. However, uninterrupted monitoring of the vector population is necessary to make successful assessment of the vector population as well as to note the deviation in the population loads, if any. In view of these and considering unique ecological environment of Kolkata, West Bengal, India, being dengue endemic, an appraisal of the dengue vector abundance based on pupal productivity was made to supplement vector management and to provide a platform for comparison with other areas in India and elsewhere in the globe.
Several methods of entomological surveillance have been proposed with higher resolutions of forecasting abundance of mosquitoes. These include sequential stratified sampling methods incorporating pupal density as one of the predictor variable of abundance. In other methods, immature density, habitat types and location are given priority. However, the adult population and its ability to transmit disease depend on the features of the individual mosquitoes that constitute the population. Biological features such as longevity and reproductive success of individual mosquito define the persistence of the population. Longevity and reproductive success are positively correlated with the adult body weight, which is linked to the pupal weight. Thus, pupal weight can be used as an indicator to infer about the adult longevity and reproductive success and the consequences at the population level. Incorporation of pupal weight in the surveillance in addition to the density will help enhance prediction about the population abundance and its possible variations, with higher precision. This proposition has been tested in Thailand8, where adult wing length was found to vary with the Aedes immature density in the habitats and with months. In the present study, pupal weight was used as an indicator of the prospective adult body size, which as a trait contributes to the fitness of the individual and the population as a whole. Thus, evaluation of the pupal weight of Aedes mosquitoes from different larval habitats will help to correlate the density effect on the pupal weight and indirectly the adult features.
Material & Methods
Monthly sampling of selected sites from Kolkata was carried out between January and December 2008. In each site the Aedes mosquito larval habitats were chosen randomly, based on four broad types, earthen pots, plastic and porcelain containers, and tyres. A total of 75 numbers of each habitat were considered per sampling site per month. The features of the habitats surveyed are presented in Table I. From each of the positive larval habitats, the pupae and larvae were sampled and placed in sample containers (100 ml sample container Tarsons®, India) and brought to the laboratory for counting and recording the number of immature collected. This was followed by recording the pupal weight (wet weight up to nearest 0.1 mg using METTLAR-TOLEDO ® Al-104 balance, India), each pupa was placed in vials individually, and allowed to emerge to adult stage. The sex and species of the adults were identified based on appropriate keys9. The numbers of individuals of Ae. aegypti and Ae. albopictus were considered only for analysis. The data on the pupal weight of males and females were used to construct the degree of sexual dimorphism based on the formula10:
Table I.
Features of the Aedes mosquito larval habitats surveyed
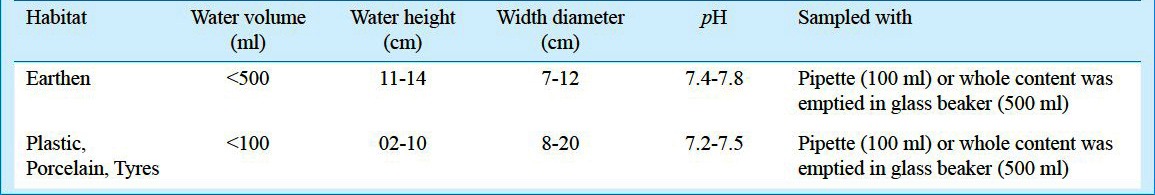
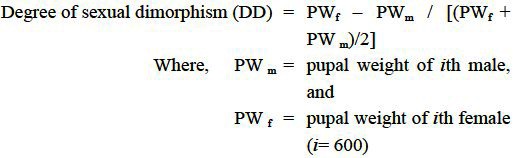
The data on the pupal weight of the females and males were arranged in ascending order in each density class, and for each pair, the DD was calculated using the above formula. To comment on the species specific variation in the abundance of Aedes mosquito, the data on the pupal weight, proportion of positive larval habitats (container index) were subjected to two-way factorial ANOVA analysis, using habitats, species and sex as variables. The data on pupal weight, sexual dimorphism and immature density were considered for regression analysis. The statistical analyses were performed following Zar11 using the SPSS12 and XLSTAT13 software.
Results
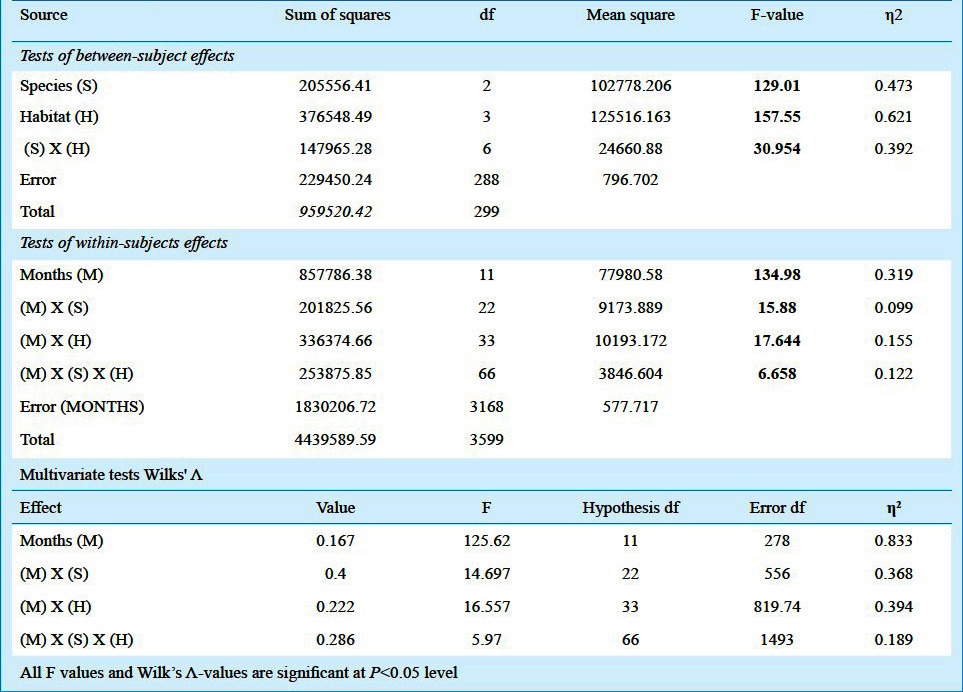
The observations on the Aedes larval habitats revealed that the number of habitats positive for Aedes immature exhibited significant rise and fall with the months and the Aedes species. On an average out of 75 habitats per month, the habitats positive exclusively for Ae. aegypti varied between 0 and 24 (~35%) for earthen, 0 and 23 (~30%) for plastic and porcelain, 0 and 5.5 (~07%) for tyres, depending on the months. The exclusive habitats positive for Ae. albopictus varied between 0 and 23 for earthen (~31%), 0 and 25 for plastic (~35%), 0 and 24 for porcelain (~35%), 0 and 6 for tyres (~10%). The habitats common for both mosquitoes differed between 0 and 19 for earthen (~ 25%), 1 and 14 for plastic (~19%), 0 and 19 for porcelain (~26%), 0 and 6 (~10%) for tyres (Fig. 1). During the months of March no habitat was found to be positive for Ae. aegypti alone, although a few tyre habitats were positive for both Ae. aegypti and Ae. albopictus. The mean number of pupae per positive habitats was observed to vary significantly with the months during the survey period (Fig. 2). During March, no Ae, aegypti or Ae. albopictus was recorded alone in any of the habitats, however, plastic containers recorded both the species in small numbers. The post-monsoon months were found to be most productive in terms of nurturing both the species individually as well as in groups. To carry out ANOVA, the data on pupal productivity were log transformed to ascertain homogeneity of variances among the groups (habitat type). The Levene's test (W) for homogeneity of variance11,12 was applied that yielded a value of 46.103 (df = 3,188, P<0.001), for pupal productivity per habitat indicating the unequal variance among the groups (habitat type) across the months, though their mean values were unequal (indicated by: Welch's robust test of equality of means11,12, which was 30.485; df = 3, 78.54; P < 0.001; F distributed asymptotically). Transformation of the values by log (x+1) yielded homogeneity of variance among the groups Levene's test (W) 2.100; df = 3,188, P=0.102 and Welch's robust test of equality of means was 46.623 (df = 3, 102.63; P<0.001). Similar transformation on the data on the positive habitats did not reduce the heterogeneity of variances among the groups (untransformed data, Levene W=80.465; df=3,188, P<0.001; transformed data, Levene W=113.573; df=3,188, P<0.001) and, therefore, the original values were used for ANOVA. In all of the positive habitats, the pupal productivity varied with the species and habitat types considerably as reflected by the two-way ANOVA. Tukey test revealed significant differences between and among the variables (Table II). Significant correlations between the productivity and the sampling period were observed for both the species irrespective of the habitat types (Table III). The data on pupal productivity was further applied to a 3-way multivariate ANOVA that revealed significant differences in the productivity between the months, habitat types and species concerned (Table IV). The significant differences in the interactions between the month, species and habitat indicate that the pattern of productivity in the habitats varied with the species and months indicating differences in the population dynamics of Ae. aegypti and Ae. albopictus as well as choice of habitats for breeding. The value of Wilk's is indicative of significant variations in the pupal productivity with reference to the habitat type, months and the two species of Aedes.
Fig. 1.
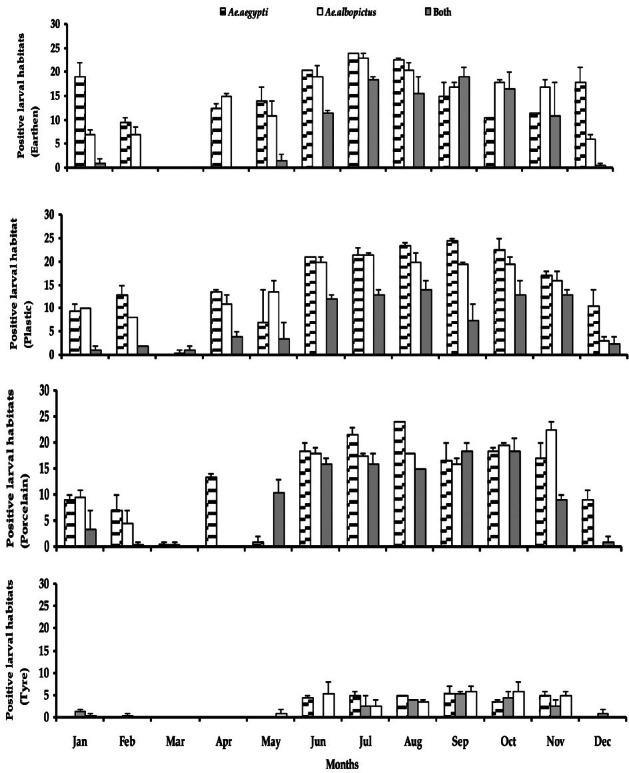
The number (mean ± SE) of habitats positive with only Ae. aegypti, only Ae. albopictus and both during the survey period (n=75 habitats/habitat type/month). This is equivalent to container index (CI).
Fig. 2.
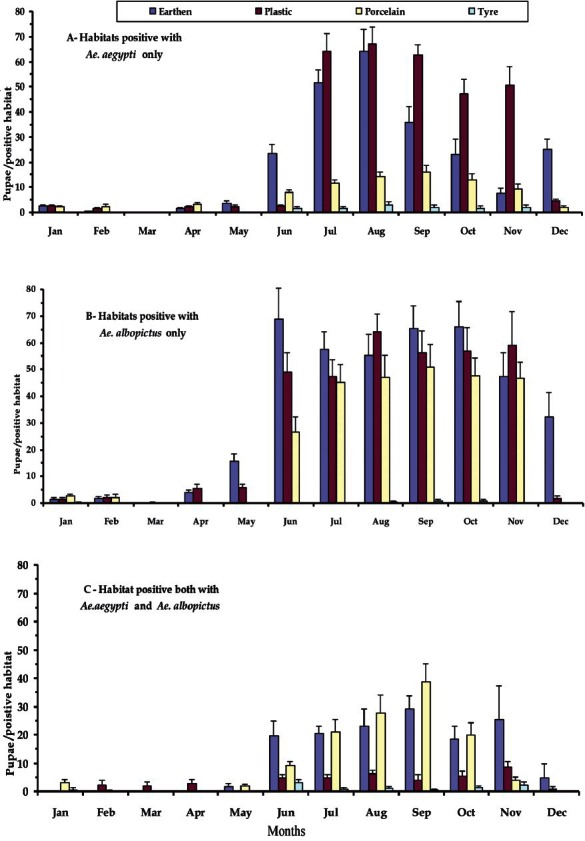
The number (mean ± S.E.) of pupae per positive habitats sampled for only Ae. aegypti, only Ae. albopictus and both, in different months during the survey period.
Table II.
Results of 2-way factorial ANOVA on (a) the variation in positive habitat and (b) the variation in immature Aedes mosquitoes using larval habitats and species as variables.
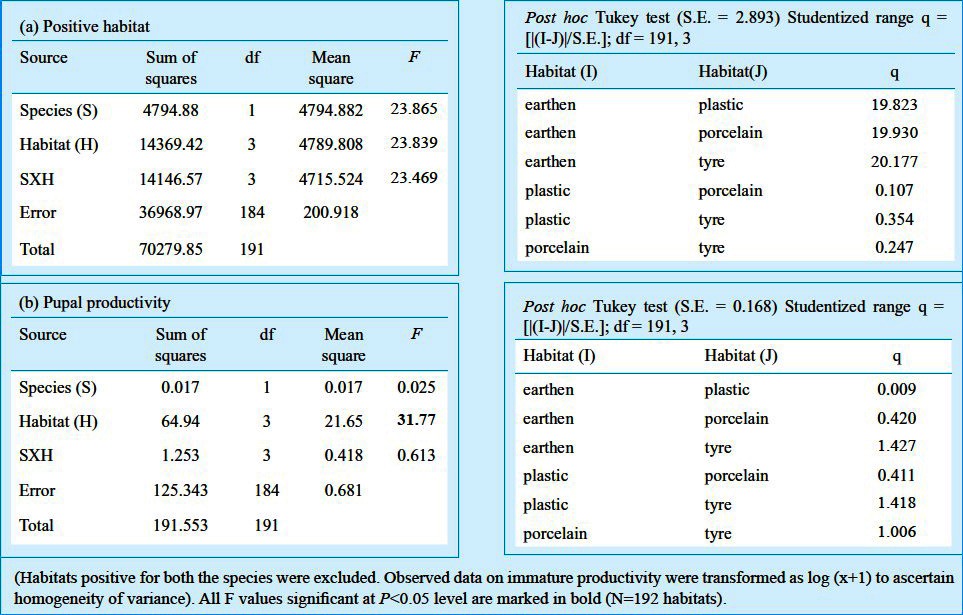
Table III.
Correlations between the months in terms of pupal productivity of Ae. aegypti and Ae. albopictus irrespective of the habitats
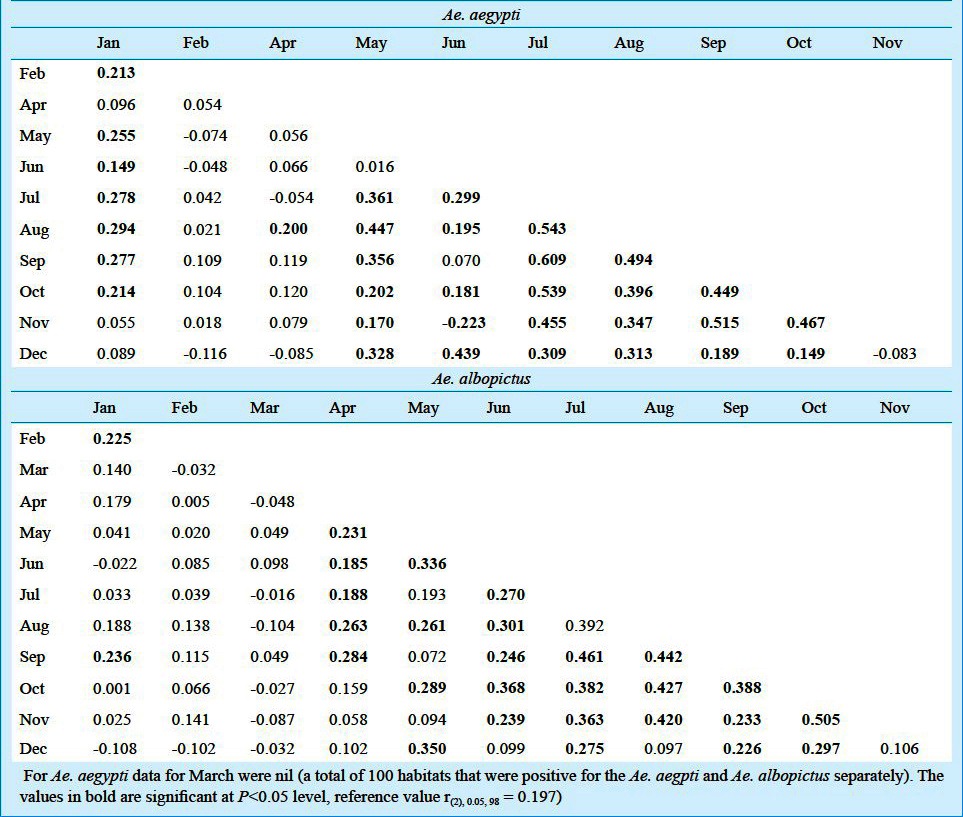
Table IV.
The results of the three-way multivariate ANOVA using the species (Ae. aegypti, Ae. albopictus alone and both), habitats (earthen, plastic, porcelain, tyre) and months as explanatory variables for the observed variations in the pupal density of Aedes mosquitoes in the positive habitats η2 = (1 - Wilk's Λ)
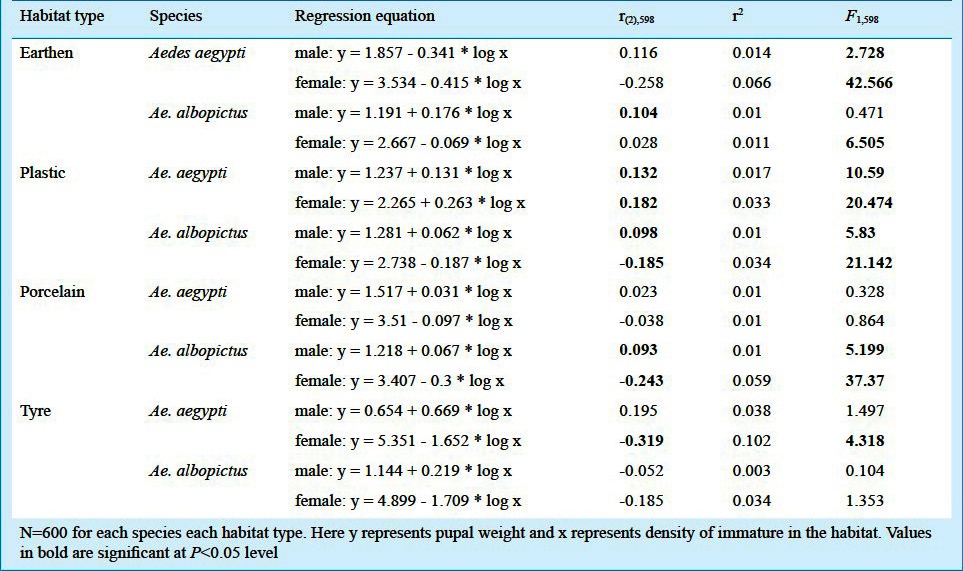
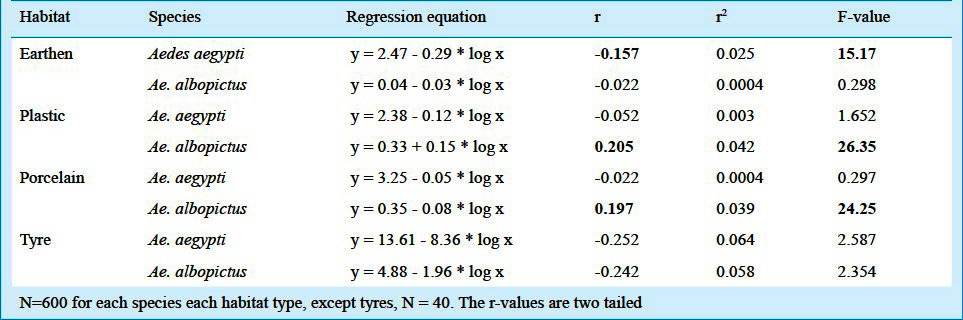
Using the data on the pupal weight as indicator of adult fitness, regression equations revealed pupal weight as a function of density of the habitats (Table V). A significant difference in the relation could be observed with the correlation coefficient values for the density pupal weight relationship varying with the habitat type. In case of both the species, male pupal weight did not vary with the density of the habitats, while for the females in all the habitats, the density affected the pupal weight, i.e. a negative correlation coefficient value was observed. A deviation to this was observed in case of Ae. aegypti from the plastic and Ae. albopictus from the earthen habitats. However, for the males the density effect was seen for the tyre habitats, for Ae. albopictus only.
Table V.
The regression equations showing pupal weight as a function of density (log value) in the different larval habitats surveyed
The degree of dimorphism was prominent for both Ae. aegypti and Ae. albopictus based on the pupal weight. However, a variation in the degree of dimorphism was noted depending on the density of the immature in the concerned habitats. The regression equations and the correlation coefficients suggested that with increasing density the degree of dimorphism decreased (Table VI), except for Ae. albopictus in plastic habitats.
Table VI.
The regression equations of sexual dimorphism as a function of immature density (log value) in the different larval habitats surveyed
Discussion
Entomological surveillance of dengue vectors have been a key aid in understanding the population persistence and dynamics inclusive of relative abundance at a spatiotemporal scale6. The information on these aspects has been useful in predicting the population state of the vector mosquitoes and the possibility of the diseases. Appropriate intervention of the population could be outlined based on the data on the population fluctuations. This is substantiated through the studies in Trinidad4,14, Thailand8, Brazil15,16, Vietnam17,18 and tropical region of Australia19,20. A comprehensive account of the different survey methods and its utility in the vector management suggest a shift from the classical indices for entomological monitoring of the dengue vectors, which has been employed in the studies carried out in recent years. Considering India and Kolkata in particular, such information was fragmentary21, with information on the seasonal prevalence only21,22. In the present study, an attempt was made to emphasize on the importance of the pupal productivity for the entomological monitoring, thereby adding to the possible population and life historical consequences of the vector mosquitoes. The pupal weight was used as an indicator so that the possible fecundity and population growth could be assessed. Our study reveals that the containers with higher density of immature yielded smaller pupae, thereby the prospective adults having lower fitness in terms of longevity and fecundity. This is deduced from the fact that in case of insects and mosquito in particular, the adult life history features are correlated with the pupal weight, which in turn is dependent on the larval development. Both Ae. aegypti and Ae. albopictus exhibited this pattern, for the number of containers surveyed during this year long period. The impact of density effects on the pupal weight could be extended to the sexual dimorphism, which as a biological factor is important in mate choice and successful mating. The changes in the degree of sexual dimorphism with increasing larval density is a possible indication that the vector mosquitoes are able to make a trade-off with the size fecundity relationship such that in situations with lower density they are able to produce larger adults that can live long and serve as continuity and dispersal of the population for the next favourable season. It would be appropriate to judge this proposition through further studies incorporating the life history analysis under variable environmental conditions. The important finding is that higher density of the immature of Ae. aegypti and Ae. albopictus in the larval habitats produces smaller pupae and thus affects the individual fitness of the adults.
The variations in the immature density were prominent with the months, type of containers (larval habitat) and the mosquito species. The trend in the variation was similar for both the species, though the preference of the larval habitats was different. While Ae. albopictus utilized plastic and tyres, Ae. aegypti utilized earthen and porcelain habitats to a higher extent. When both species were found, Ae. albopictus was competitively superior with higher immature density than Ae. aegypti. The competitive effects substantiate the findings of a study23, where the mosquito Ae. albopictus competitively displaced Ae. aegypti under experimental and natural conditions.
In urban areas the landscape pattern may contain barriers for dispersal of Aedes mosquitoes19. Therefore, it is more probable that within restricted areas where habitats are available, the density of the immature will be high, if dispersal is limited. This is supported by the observations of Williams et al24 where water storage in containers facilitated Aedes abundance. However, the differences in pupal productivity in residential and non-residential areas provide further evidence to this proposition. It has been noted that certain species of Aedes like Ae. oceanicus utilizes domestic containers in the similar geographical area25. In the present study, the pupal productivity and its variations in different months followed similar pattern. Theoretically, in rainy season the container habitats had higher chances to be filled with water and thus adult dispersal can be limited26. This further enhances the chance of high density of larvae in the habitats. It has been observed that variation in water storage containers leads to a corresponding variation in pupal productivity17. A positive correlation between the numbers of household water storage containers and abundance of Ae. aegypti immature was found, in rural areas in Vietnam17. Pupae were abundant in large concrete water storage jars or concrete water storage tanks. The relation between the positive containers and the pupal productivity in Kolkata followed a similar pattern. Thus it appears that a common pattern of pupal productivity exists possibly due to the similar socio-economic and geographical settings of the cities in the tropics.
Pupal productivity differed in the larval habitats depending on the size and material of the container type. This was observed in rural areas of American Samoa26. Outdoor, non-potable water storage containers posed significant breeding risk contrast to potable water storage containers. Plastic drums and small plastic containers were the key habitats of Ae. aegypti breeding. Discarded plastic pots were identified as the most productive containers. Similar observations were made from the present study with the plastic containers being more productive than other types. Considering Kolkata and the type and amount of solid waste generated there, it is more probable that the plastic, glass and rubber waste materials generated from household or otherwise augment the availability of possible larval habitats and their utility as oviposition sites by Aedes. This has been observed in urban areas of Dibrugarh and Duliajan, Assam27. Our study reflects that the pupal productivity can be used as an indicator of the abundance of the Aedes mosquitoes, serving as a supplement to assess the possibilities of the dengue in Kolkata. A choice of the larval habitats by Ae. aegypti and Ae. albopictus was observed, though both the species exploited all the container types considered in the study. The individual and population level fitness of the principal vectors of dengue can be evaluated from the densities in the respective container habitats. As an intervention and management strategy, monitoring of the discarded containers and tyres should also be included keeping in view the amount of solid waste generated in the city28,29. This applies to other similar areas of India, so that the possible numbers of larval habitats are checked, thereby reducing the population of Aedes mosquitoes. Further studies should be carried out to note the population dynamics of Aedes in relation to the changing numbers of plastic, porcelain and tyre waste available to substantiate the contribution of solid waste in the maintenance of Aedes population.
Acknowledgment
The authors thank the respective Heads of the Departments of Zoology, University of Calcutta, Kolkata and The University of Burdwan, Burdwan for the facilities provided including DST-FIST. The first author (SB) acknowledges the financial assistance of UGC through SAP-RFSMS fellowship, and CSIR, India, in carrying out the work. The second author (GA) acknowledges the financial assistance of UGC through UGC Research Award.
References
- 1.Gubler DJ. The emergence of epidemic dengue fever and dengue hemorrhagic fever in the Americas: a case of failed public health policy. Rev Panam Saud Publica. 2005;17:221–4. doi: 10.1590/s1020-49892005000400001. [DOI] [PubMed] [Google Scholar]
- 2.Tewari SC, Thenmozhi V, Katholi CR, Manavalan R, Munirathinam A, Gajanana A. Dengue vector prevalence and virus infection in a rural area in south India. Trop Med Int Health. 2004;9:499–507. doi: 10.1111/j.1365-3156.2004.01103.x. [DOI] [PubMed] [Google Scholar]
- 3.Mahadev PVM, Fulmali PV, Mishra AC. A preliminary study of multilevel geographic distribution and prevalence of Aedes aegypti (Diptera: Culicidae) in the state of Goa, India. Indian J Med Res. 2004;120:173–82. [PubMed] [Google Scholar]
- 4.Chadee DD. Dengue cases and Aedes aegypti indices in Trinidad, West Indies. Acta Trop. 2009;112:174–80. doi: 10.1016/j.actatropica.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Dengue status of India in 2006. New Delhi: WHO South East Asian Regional Office; 2006. WHO-SEARO. [Google Scholar]
- 6.Focks DA, Alexander N. Geneva: WHO; 2006. Multicountry study of Aedes aegypti pupal productivity survey methodology: Findings and recommendations. TDR/IRM/DEN/06.1. [Google Scholar]
- 7.Arunachalam N, Tana S, Espino F, Kittayapong P, Abeyewickreme W, Wai KT, et al. Eco-bio-social determinants of dengue vector breeding: a multicountry study in urban and periurban Asia. Bull World Health Organ. 2010;88:173–84. doi: 10.2471/BLT.09.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strickman D, Kittayapong P. Dengue and its vectors in Thailand: calculated transmission risk from total pupal counts of Aedes aegypti and association of wing-length measurements with aspects of the larval habitat. Am J Trop Med Hyg. 2003;68:209–17. [PubMed] [Google Scholar]
- 9.Barraud PJ. V. London, UK: Taylor and Francis; 1964. Fauna of British India, including Ceylon and Burma. Diptera (Family Culicidae: Tribes Megarginini and Culicini) [Google Scholar]
- 10.Sharmila Bharathi N, Prasad NG, Shakarad M, Joshi A. Correlates of sexual dimorphism for dry weight and development time in five species of Drosophila. J Zool Lond. 2004;264:87–95. [Google Scholar]
- 11.Zar JH. 4th ed. New Delhi, India: Pearson Education (Singapore) Pvt. Ltd., (Indian Branch); 1999. Biostatistical analysis. [Google Scholar]
- 12.Kinnear PR, Gray CD. Sussex, UK: Psychology Press; 2000. SPSS for Windows made simple, Release 10. [Google Scholar]
- 13.XLSTAT software, version 9.0. Paris, France: 2009. Addinsoft SARL. Available from: http://www.xlstat.com . [Google Scholar]
- 14.Hemme RR, Thomas CL, Chadee DD, Severson DW. Influence of urban landscapes on population dynamics in a short-distance migrant mosquito: evidence for the dengue vector Aedes aegypti. PLoS Negl Trop Dis. 2010;4:e634. doi: 10.1371/journal.pntd.0000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de-Freitas R, Marques WA, Peres RC, Cunha SP, de Oliveira RL. Variation in Aedes aegypti (Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Mem Inst Oswaldo Cruz. 2007;102:489–96. doi: 10.1590/s0074-02762007005000056. [DOI] [PubMed] [Google Scholar]
- 16.dos Reis CI, Honórioa NA, Codeço CT, Magalhães MDAFM, de-Oliveiraa RL, Barcellos C. Relevance of differentiating between residential and non-residential premises for surveillance and control of Aedes aegypti in Rio de Janeiro, Brazil. Acta Trop. 2010;114:37–43. doi: 10.1016/j.actatropica.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen LAP, Clements ACA, Jeffery JAL, Yen NT, Nam VS, Vaughan G, et al. Abundance and prevalence of Aedes aegypti immatures and relationships with house hold water storage in rural areas in southern Vietnam. Int Health. 2011;3:115–25. doi: 10.1016/j.inhe.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Tsuzuki A, Duoc VT, Riga Y, Yen NT, Takagi M. Effect of peridomestic environments on repeated infestation by preadult Aedes aegypti in urban premises in Nha Trang city, Vietnam. Am J Trop Med Hyg. 2009;81:645–50. doi: 10.4269/ajtmh.2009.08-0175. [DOI] [PubMed] [Google Scholar]
- 19.Tun-Lin W, Kay BH, Barnes A. Understanding productivity, a key to Aedes aegypti surveillance. Am J Trop Med Hyg. 1995;53:595–601. doi: 10.4269/ajtmh.1995.53.595. [DOI] [PubMed] [Google Scholar]
- 20.Tun-lin W, Kay H, Barnes A, Forsyth S. Critical examination of Aedes aegypti indices: Correlations with abundance. Am J Trop Med Hyg. 1996;54:542–7. doi: 10.4269/ajtmh.1996.54.543. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee S, Aditya G, Saha N, Saha GK. An assessment of macroinvertebrate assemblages in mosquito larval habitats - space and diversity relationship. Environ Monit Assess. 2010;168:597–611. doi: 10.1007/s10661-009-1137-9. [DOI] [PubMed] [Google Scholar]
- 22.Pramanik MK, Aditya G, Raut SK. Seasonal prevalence of Aedes aegypti immatures in Kolkata, India. Southeast Asian J Trop Med Public Health. 2007;38:1–6. [PubMed] [Google Scholar]
- 23.Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in south Florida: differences between sites of coexistence and exclusion? Oecologia (Berl) 2004;139:583–93. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams CR, Bader CA, Kearney MR, Ritchie SA, Russell RC. The extinction of dengue through natural vulnerability of its vectors. PLoS Negl Trop Dis. 2010;4:e922. doi: 10.1371/journal.pntd.0000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambdin B, Schmaedick MA, Burkot TR. Utilization of domestic and natural containers by Aedes oceanicus in American Samoa. J Med Entomol. 2008;45:758–62. doi: 10.1603/0022-2585(2008)45[758:uodanc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Burkot TR, Handzell T, Schmaedick MA, Tufa J, Roberts JM, Graves PM. Productivity of natural and artificial containers for Aedes polynesiensis and Aedes aegypti in four American Samoan villages. Med Vet Entomol. 2007;21:22–9. doi: 10.1111/j.1365-2915.2007.00667.x. [DOI] [PubMed] [Google Scholar]
- 27.Dutta P, Khan SA, Khan AM, Sharma CK, Doloi PK, Mahanta J. Solid waste pollution and breeding potential of dengue vectors in an urban and industrial environment of Assam. J Environ Biol. 1999;20:343–5. [Google Scholar]
- 28.Hazra T, Goel S. Solid waste management in Kolkata, India: practices and challenges. Waste Manag. 2009;29:470–8. doi: 10.1016/j.wasman.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhay S, Dutta A, Ray S. Municipal solid waste management in Kolkata, India- a review. Waste Manag. 2009;29:1449–58. doi: 10.1016/j.wasman.2008.08.030. [DOI] [PubMed] [Google Scholar]


