Abstract
Objective
To examine whether a racial difference exists in self-reported recommendations for colorectal cancer screening from a health care provider, and whether this difference has changed over time.
Method
Secondary analysis of the 2002, 2004, 2006, and 2008 Maryland Cancer Surveys, cross-sectional population-based random-digit-dial surveys on cancer screening. Participants were 11,368 white and 2,495 black Maryland residents age 50 years.
Results
For each race, recommendations for colonoscopy/sigmoidoscopy increased over time (67%-83% for whites, 57%-74% for blacks; p<0.001 for both), but the race difference remained approximately 10% at each survey. Among respondents without a colonoscopy in the last 10 years (n=5,081), recommendations for fecal occult blood test (FOBT) in the past year decreased over time for whites (37%-24%, p<0.001) and for blacks (36–28%, p=0.05), with no difference by race in any year. In multivariable analysis, the effect of race on the odds of reporting a provider recommendation did not vary significantly across time for either test (p=0.80 for colonoscopy/sigmoidoscopy, p=0.24 for FOBT for effect modification by year).
Conclusion
Whites were more likely than blacks to report ever receiving a provider recommendation for colonoscopy/sigmoidoscopy. Although the proportion of patients receiving recommendations for colonoscopy/sigmoidoscopy increased over time, the gap between races remained unchanged.
Keywords: colorectal cancer, screening, racial disparity, prevention, patient-health care provider interaction
Introduction
Colorectal cancer (CRC) remains the second leading cause of cancer deaths in the United States (Edwards, et al., 2010), despite the availability of screening tests that are effective at preventing and treating CRC (American Cancer Society (ACS), 2008). For average-risk adults age 50 years or older, national CRC prevention guidelines recommend: colonoscopy every 10 years, sigmoidoscopy every 5 years, and/or high-sensitivity fecal occult blood test (FOBT) every year (US Preventive Services Task Force, 2008; Smith et al., 2010; McFarland, et al., 2008). Increasing the proportion of adults age 50 to 75 years receiving CRC screening has been recognized as an important public health objective in Healthy People 2010 and Healthy People 2020 (Anonymous 2010a; Anonymous 2010b).
Lack of a physician recommendation is a primary barrier to CRC screening (Wee, et al., 2005; Guerra, et al., 2007; Klabunde, et al., 2006), and studies have demonstrated a strong association between health care providers’ recommendations and CRC screening (Beydoun and Beydoun, 2008; Sarfaty and Wender, 2007). Increasing the proportion of adults receiving counseling from their providers about CRC screening has been recognized as a developmental objective in Healthy People 2020 (Anonymous, 2010b).
Blacks are known to have a lower prevalence of screening than whites (ACS, 2008; Seeff, et al., 2004; Schenck, et al., 2006; Cooper and Koroukian, 2004) which is an important factor contributing to higher rates of CRC incidence and mortality among blacks compared to whites (ACS, 2008). Age-adjusted incidence rates (per 100,000) of CRC during 2003–2007 were 47.4 for whites and 58.9 for blacks, while mortality rates (per 100,000) during the same period were 17.1 for whites and 24.7 for blacks (Altekruse, et al., 2010). It is therefore of particular interest to examine whether the self-reported prevalence of receiving recommendations for CRC screening varies by patient race. If blacks are less likely to self-report recommendations, this would suggest that interventions to improve CRC screening of blacks may be better aimed at physicians, rather than to the community. Prior studies of the association between race and self-reported screening recommendations have differed, with one study finding racial differences in physician recommendations for FOBT or colonoscopy/endoscopy (Klabunde, et al., 2006) and other studies finding no significant difference for recommendations for any CRC screening tests (Wee, et al., 2005; Burgess, et al., 2010; Shokar, et al., 2006). These studies have also found a strong relationship between the racial gap in recommendations and the gap in up-to-date CRC screening (Wee, et al., 2004; Schenck, et al., 2006; Burgess, et al., 2010), and in a population with no racial difference in recommendations, blacks were significantly more likely than whites to receive CRC screening (Dolan, et al., 2005).
It is also unclear whether the relationship between race and screening recommendations has changed due to interventions to increase the prevalence of CRC screening. Since 2001, local health departments in Maryland have educated providers on the importance of recommending CRC screening to their patients. Thus, this study examines whether a racial difference in self-reported CRC screening recommendations from providers exists in a sample of Maryland adults, and whether this difference changed from 2002 through 2008.
Methods
Study Design
This secondary analysis used data from the 2002, 2004, 2006, and 2008 Maryland Cancer Survey, a set of cross-sectional, population-based, random-digit-dial, computer-assisted land line telephone interview surveys that used list-assisted stratified sampling by geography to oversample rural residences (Steinberger, et al., 2002; Steinberger, et al., 2005; Poppell, et al., 2007; Poppell, et al., 2009). Survey respondents were non-institutionalized Maryland residents age 40 years or older. In 2002, 2004, and 2008, eligible respondents were English-speakers, whereas respondents in 2006 were English- or Spanish-speaking. The Maryland Cancer Surveys were approved by the Institutional Review Boards of the Maryland Department of Health and Mental Hygiene and the University of Maryland, Baltimore.
Participants
A total of 309,535 telephone numbers were screened or called (84,172 in 2002, 66,950 in 2004, 61,273 in 2006, and 97,140 in 2008), resulting in 20,306 completed interviews (5,071 in 2002, 5,007 in 2004, 5,187 in 2006, and 5,041 in 2008). The Council of American Survey Research Organizations (CASRO) response rate for the survey, defined as Completed Interviews/(Known Eligible + Presumed Eligible), was 38.4% in 2002, 38.3% in 2004, 29.7% in 2006, and 40% in 2008.
This analysis was further limited to respondents age 50 years or older, based on national CRC screening guidelines (US Preventive Services Task Force, 2008; McFarland, et al., 2008; Smith, et al., 2003). Respondents who did not report their race (n=109), those who reported a race other than white or black (n=451), and those missing data about CRC screening recommendations (n=347) were excluded, leaving a sample size of 13,863.
Measurements
Respondent race was self-reported as white or black/African American. Hispanic respondents were included in their racial group. Using questions from the National Health Interview Study (Anonymous, 2010e), provider recommendations for CRC screening were determined by describing each test and asking: “Has a doctor or other health professional ever recommended that you have a sigmoidoscopy or colonoscopy?” and “In the past 12 months, has a doctor or other health professional recommended that you have a home blood stool test?” (i.e., FOBT). Respondents who had had a colonoscopy/sigmoidoscopy were asked which test was most recent and how long it had been since that test. These questions were used to determine whether respondents were up-to-date with colonoscopy (defined as colonoscopy within preceding 10 years based on ACS guidelines).
Based on significance in prior studies (Wee, et al., 2005; Klabunde, et al., 2006), covariates chosen from the survey for examination included: gender, age, marital status, education, body mass index (BMI), health care coverage (e.g., health insurance, HMOs, Medicare, Medical Assistance), time since last routine check-up, and family history of CRC (i.e., whether a first degree relative had ever been diagnosed with colon cancer). Employment status and geographic stratum (rural/urban residence, based on the phone number’s location) were also examined.
Statistical Analysis
Because guidelines only recommend annual FOBT for patients who are not up-to-date with colonoscopy, the analysis of recommendations for FOBT in the past year was performed in the subset of respondents who were not up-to-date on colonoscopy; similar results were found when analysis of recommendations for FOBT included all respondents. Analysis of ever receiving a recommendation for colonoscopy/sigmoidoscopy included all respondents.
Logistic regression models were run between respondent race and recommendation for colonoscopy/sigmoidoscopy to determine estimates of odds ratios (OR) and 95% confidence intervals (CI), bivariable for unadjusted estimates and multivariable for adjusted. Similar logistic regression models were run between respondent race and recommendation for FOBT in the past year, among respondents not up-to-date with colonoscopy. Covariates were chosen a priori based on their associations with CRC screening recommendation or race variables; thus, all covariates were included in adjusted models, as was survey year (as a categorical variable).
To examine whether the racial difference in recommendations for colonoscopy/sigmoidoscopy changed over time, the significance of an interaction term between race and survey year was examined in a logistic regression model that included main effects for race and survey year, and in a logistic regression model that included all covariates. Survey year was modeled as a continuous variable. Similar logistic regression models were run to examine whether the association between race and recommendations for FOBT in the past year changed over time, among respondents who were not up-to-date on colonoscopy. Data were analyzed using SAS 9.1 (SAS Institute Inc., Cary, NC). Results were weighted to the Maryland population by race, age, and gender.
Results
The sample included 2,495 black respondents (24%) and 11,368 white respondents (Table 1). All covariates differed significantly (p<0.01) by race. Blacks were less likely than whites to be male (42% vs. 46%, respectively) and to be older. Blacks were also less likely than whites to have health care coverage (92% vs. 97%), and to report a family history of CRC (10% vs. 13%). Blacks were less likely than whites to have ever had a colonoscopy/sigmoidoscopy (62% vs. 69%, p<0.001), to have ever had an FOBT (53% vs. 57%, p=0.001), and to be up-to-date with colonoscopy (53% vs. 58%, p<0.001).
Table 1.
Characteristics by Race of Survey Sample of Marylanders Age 50 Years or Older, Maryland Cancer Survey 2002, 2004, 2006, and 2008
| Respondent Characteristic | Total (n=13,863) | Blacks (n=2,495) | Whites (n=11,368) | pc |
|---|---|---|---|---|
| na (%)b | na (%)b | na (%)b | ||
| Overall | 13,863 (100.0) | 2,495 (24.0) | 11,368 (76.0) | |
| Survey year | 0.10 | |||
| 2002 | 3,232 (22.9) | 577 (21.5) | 2,655 (23.3) | |
| 2004 | 3,380 (24.5) | 657 (23.7) | 2,723 (24.7) | |
| 2006 | 3,585 (25.7) | 551 (26.0) | 3,034 (25.6) | |
| 2008 | 3,666 (26.9) | 710 (28.8) | 2,956 (26.3) | |
| Demographic Characteristics | ||||
| Male | 4,854 (44.8) | 725 (42.2) | 4,129 (45.6) | 0.01 |
| Age | <0.001 | |||
| 50–64 years | 7,549 (59.3) | 1,550 (65.7) | 5,999 (57.3) | |
| 65–74 years | 3,506 (23.0) | 610 (22.3) | 2,896 (23.2) | |
| ≥75 years | 2,808 (17.7) | 335 (12.0) | 2,473 (19.4) | |
| Rural | 5,303 (22.6) | 419 (9.8) | 4,884 (26.6) | <0.001 |
| Marital Status | <0.001 | |||
| Married | 7,635 (65.0) | 980 (50.7) | 6,655 (69.5) | |
| Widowed | 2,963 (14.8) | 551 (16.4) | 2,412 (14.3) | |
| Otherd | 3,210 (20.1) | 952 (32.9) | 2,258 (16.1) | |
| Education | <0.001 | |||
| Less than high school graduation | 1,351 (9.7) | 426 (16.7) | 925 (7.5) | |
| High school graduation/GED | 4,099 (27.8) | 791 (30.5) | 3,338 (26.9) | |
| Some college | 3,127 (22.3) | 584 (24.3) | 2,543 (21.6) | |
| College graduatione | 5,229 (40.2) | 706 (28.5) | 4,523 (43.9) | |
| Employment status | <0.001 | |||
| Employedf | 5,567 (43.7) | 989 (41.9) | 4,578 (44.2) | |
| Retired | 6,619 (44.2) | 1,111 (42.3) | 5,508 (44.8) | |
| Otherg | 1,627 (12.1) | 383 (15.8) | 1,244 (11.0) | |
| Body Mass Indexh | <0.001 | |||
| Normal weight (<25.0) | 4,530 (32.1) | 496 (21.0) | 4,034 (35.6) | |
| Overweight (25.0-<30.0) | 5,030 (38.9) | 901 (38.6) | 4,129 (38.9) | |
| Obese (≥30.0) | 3,779 (29.1) | 1,004 (40.4) | 2,775 (25.5) | |
| Health Care Characteristics | ||||
| Has health care coveragei | 13,303 (95.9) | 2,300 (92.1) | 11,003 (97.1) | <0.001 |
| Time since last routine check-up | <0.001 | |||
| < 1 year | 12,018 (87.3) | 2,259 (91.0) | 9,759 (86.1) | |
| 1 – <5 years | 1,279 (9.6) | 162 (6.5) | 1,117 (10.6) | |
| ≥5 years | 405 (3.1) | 48 (2.6) | 357 (3.2) | |
| Family history of CRCj | 1,748 (12.3) | 271 (10.4) | 1,477 (12.9) | 0.004 |
| Colorectal Cancer Screening History Characteristics | ||||
| Ever had colonoscopy or sigmoidoscopy | 9,307 (67.3) | 1,561 (62.4) | 7,746 (68.8) | <0.001 |
| Ever had fecal occult blood test | 5,969 (56.2) | 1,343 (53.0) | 6,478 (57.3) | 0.001 |
| Up-to-date on colonoscopyk | 7,641 (56.8) | 1,296 (53.1) | 6,345 (58.0) | <0.001 |
| Reported provider recommendation for colonoscopy/sigmoidoscopy | 10,016 (72.4) | 1,635 (65.4) | 8,381 (74.6) | <0.001 |
| Reported provider recommendation for fecal occult blood test in past 12 months | ||||
| Among all respondents | 4,371 (32.0) | 821 (32.7) | 3,550 (31.8) | 0.51 |
| Among respondents up-to-date on colonoscopy | 6,345 (77.7) | 2,016 (77.4) | 4,329 (77.9) | 0.70 |
GED: general educational development tests; CRC: colorectal cancer
Analysis performed accounting for stratification by geography, except results for geographic stratum.
Unweighted sample size; sample size for some variables may not add to column total due to missing responses.
Weighted to the Maryland population; percentages for some variables may not add to 100 due to rounding.
p-value derived from Chi-square test on categories of all variables.
Includes respondents who were divorced, separated, never married, or a partner of an unmarried couple
Includes respondents with masters, doctoral or advanced professional degrees
Includes respondents employed for wages and self employed
Includes students, homemakers, and respondents unable to work, or out of work
Body Mass Index is calculated as weight in kilograms/(height in meters)2
Health care coverage includes health insurance, prepaid plans such as HMOs, or government plans such as Medicare or Medical Assistance
Family history of CRC is defined as a reported history of a diagnosis of colon cancer in a first degree relative (parent, sibling or child)
Up-to-date colonoscopy is colonoscopy within the preceding 10 years (up-to-date according to American Cancer Society guidelines)
Overall, whites were significantly more likely than blacks to report provider recommendations for colonoscopy/sigmoidoscopy (75% vs. 65%, p<0.001). Among respondents reporting a recommendation for colonoscopy/sigmoidoscopy, the proportion of blacks and whites who were up-to-date with colonoscopy were both 73%. The proportion of whites reporting a recommendation for colonoscopy/sigmoidoscopy increased from 67% in 2002 to 83% in 2008 (p<0.001; Figure 1). Similarly, the proportion of blacks reporting a recommendation for colonoscopy/sigmoidoscopy increased from 57% in 2002 to 74% in 2008 (p<0.001). For each race, there was an increase of ~4% between the 2002, 2004, and 2006 surveys, and a ~7% increase by the 2008 survey. The percentage difference between races was significant for each survey (~10%, p<0.001 for each), and this difference did not change over time (p=0.55). The association between race and recommendations for colonoscopy/sigmoidoscopy remained significant after adjustment for demographic and health care characteristics (OR 1.43, 95% CI 1.26–1.63; Table 2). Although the adjusted model also showed a significant increase in the odds of reporting recommendations for colonoscopy/sigmoidoscopy between 2002 and 2008, the effect of race did not change significantly over time (p=0.80 for interaction term). Other significant predictors of reporting recommendations for colonoscopy/sigmoidoscopy included urban residence, being married, increasing education, health care coverage, more recent routine check-up, and family history of CRC.
Figure 1.
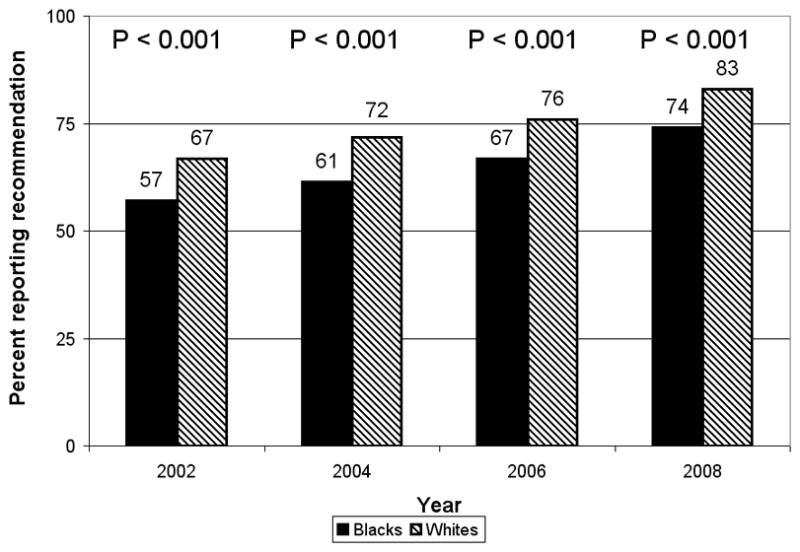
Percentage reporting ever receiving a health care provider recommendation for colonoscopy or sigmoidoscopy, by survey year and race, Maryland Cancer Survey 2002, 2004, 2006, and 2008. p-values derived from Chi-square test on comparison between races for each survey year. p-value for interaction between race and survey year in unadjusted model of reporting recommendations for colonoscopy/sigmoidoscopy was 0.55.
Table 2.
Odds Ratios and 95% Confidence Intervals for Health Care Provider Recommendations for Colorectal Cancer Screening Tests, from Maryland Cancer Survey 2002, 2004, 2006, and 2008 and Weighted to Represent Maryland Population
| Respondent Characteristic | Recommendation for Colonoscopy/Sigmoidoscopy | Recommendation for FOBT in Past Year | ||
|---|---|---|---|---|
| Unadjusteda | Adjustedb | Unadjustedc | Adjustedd | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Race | ||||
| Black | Reference | Reference | Reference | Reference |
| White | 1.55 (1.39, 1.73) | 1.43 (1.26, 1.63) | 0.94 (0.80, 1.11) | 0.90 (0.75, 1.10) |
| Survey year | ||||
| 2002 | Reference | Reference | Reference | Reference |
| 2004 | 1.24 (1.10, 1.40) | 1.24 (1.09, 1.41) | 0.89 (0.76, 1.05) | 0.86 (0.72, 1.03) |
| 2006 | 1.54 (1.36, 1.74) | 1.58 (1.38, 1.81) | 0.56 (0.46, 0.67) | 0.58 (0.47, 0.70) |
| 2008 | 2.27 (1.98, 2.60) | 2.37 (2.05, 2.74) | 0.59 (0.48, 0.73) | 0.60 (0.48, 0.74) |
| Gender | ||||
| Male | Reference | Reference | Reference | Reference |
| Female | 0.94 (0.86, 1.03) | 0.99 (0.89, 1.10) | 1.07 (0.93, 1.23) | 1.02 (0.88, 1.19) |
| Age | ||||
| 50–64 years | Reference | Reference | Reference | Reference |
| 65–74 years | 1.55 (1.39, 1.74) | 1.38 (1.20, 1.59) | 1.20 (1.02, 1.41) | 0.93 (0.76, 1.14) |
| ≥75 years | 0.95 (0.85, 1.06) | 0.81 (0.69, 0.95) | 0.95 (0.80, 1.14) | 0.72 (0.57, 0.93) |
| Geographic stratum | ||||
| Rural | Reference | Reference | Reference | Reference |
| Urban | 1.15 (1.06, 1.26) | 1.16 (1.04, 1.28) | 1.06 (0.93, 1.21) | 0.96 (0.83, 1.12) |
| Marital Status | ||||
| Married | Reference | Reference | Reference | Reference |
| Widowed | 0.66 (0.59, 0.73) | 0.72 (0.63, 0.83) | 0.85 (0.72, 1.00) | 0.88 (0.72, 1.08) |
| Othere | 0.61 (0.55, 0.69) | 0.73 (0.64, 0.83) | 0.76 (0.65, 0.90) | 0.86 (0.72, 1.03) |
| Education | ||||
| Less than high school graduation | Reference | Reference | Reference | Reference |
| High school graduation/GED | 1.80 (1.54, 2.10) | 1.61 (1.35, 1.92) | 1.25 (0.99, 1.59) | 1.23 (0.95, 1.60) |
| Some college | 2.46 (2.08, 2.90) | 2.23 (1.85, 2.69) | 1.36 (1.06, 1.74) | 1.46 (1.11, 1.91) |
| College graduationf | 3.49 (2.99, 4.08) | 3.00 (2.50, 3.61) | 1.58 (1.25, 1.98) | 1.81 (1.39, 2.35) |
| Employment status | ||||
| Employedg | Reference | Reference | Reference | Reference |
| Retired | 1.21 (1.10, 1.33) | 1.33 (1.16, 1.51) | 1.16 (1.01, 1.34) | 1.26 (1.04, 1.54) |
| Otherh | 0.67 (0.58, 0.78) | 0.99 (0.83, 1.17) | 0.73 (0.59, 0.91) | 0.91 (0.71, 1.15) |
| Body Mass Indexi | ||||
| Normal weight (<25.0) | Reference | Reference | Reference | Reference |
| Overweight (25.0-<30.0) | 0.99 (0.89, 1.10) | 0.95 (0.85, 1.07) | 1.07 (0.92, 1.26) | 1.10 (0.93, 1.30) |
| Obese (≥30.0) | 1.01 (0.90, 1.13) | 1.10 (0.97, 1.25) | 1.20 (1.01, 1.42) | 1.23 (1.03, 1.48) |
| Health care coveragej | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 4.74 (3.79, 5.91) | 2.66 (2.05, 3.44) | 2.31 (1.64, 3.24) | 1.47 (1.01, 2.15) |
| Time since last routine check-up | ||||
| < 1 year | Reference | Reference | Reference | Reference |
| 1 – <5 years | 0.56 (0.48, 0.64) | 0.52 (0.44, 0.61) | 0.29 (0.23, 0.38) | 0.28 (0.21, 0.36) |
| ≥5 years | 0.14 (0.11, 0.18) | 0.16 (0.12, 0.21) | 0.15 (0.09, 0.25) | 0.16 (0.10, 0.26) |
| Family history of CRCk | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 1.71 (1.46, 2.00) | 1.75 (1.46, 2.10) | 1.16 (0.91, 1.47) | 1.19 (0.92, 1.56) |
FOBT: fecal occult blood test, OR: odds ratio, CI: confidence interval, GED: general educational development tests, CRC: colorectal cancer
Analysis performed accounting for stratification by geography, except results for unadjusted analysis of geographic stratum, and weighted to the Maryland population.
Due to missing data, n for unadjusted models ranges from 13,339 to 13,863.
Adjusted for all listed variables; n = 13,038
Among the subset of respondents not up-to-date with colonoscopy (within the preceding 10 years). Due to missing data, n for unadjusted models ranges from 5,539 to 5,801.
Among the subset of respondents not up-to-date with colonoscopy (within the preceding 10 years) and adjusted for all listed variables; n = 5,387
Includes respondents who were divorced, separated, never married, or a partner of an unmarried couple
Includes respondents with masters, doctoral or advanced professional degrees
Includes respondents employed for wages and self employed
Includes students, homemakers, and respondents unable to work, or out of work
Body Mass Index is calculated as weight in kilograms/(height in meters)2
Health care coverage includes health insurance, prepaid plans such as HMOs, or government plans such as Medicare or Medical Assistance
Family history of CRC is defined as a reported history of a diagnosis of colon cancer in a first degree relative (parent, sibling or child)
A similar analysis examining reported recommendations for FOBT in the past year was performed among respondents not up-to-date with colonoscopy (n=5,801). The proportions of whites and blacks who reported recommendations for FOBT were similar at each survey year, and in all years combined (Figure 2). These proportions declined over time for both races. For whites, the proportion reporting recommendations for FOBT decreased significantly, from 37% in 2002 to 24% in 2008 (p<0.001); for blacks, the proportion reporting recommendations for FOBT decreased from 36% in 2002 to 28% in 2008, a marginally significant decrease (p=0.05). The racial difference in reporting recommendations for FOBT was not significant at any survey (p>0.30 for each), or in all years combined (p=0.24, OR 0.90, 95% CI 0.75–1.10 for adjusted model). Significant predictors of reporting recommendations for FOBT in the past year included college education, obese BMI, health care coverage, and more recent routine check-up.
Figure 2.
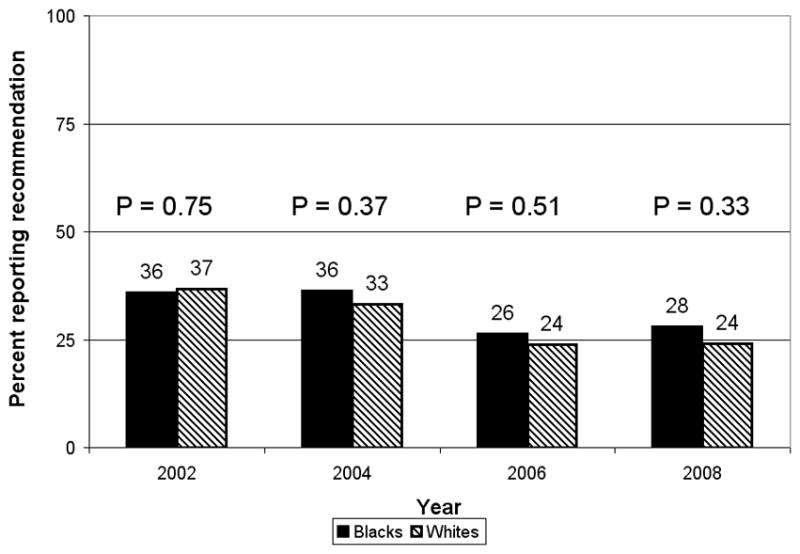
Percentage reporting receiving a health care provider recommendation for fecal occult blood test in the past year, by survey year and race, among respondents not up-to-date on colonoscopy, Maryland Cancer Survey 2002, 2004, 2006, and 2008. p-values derived from Chi-square test on comparison between races for each survey year. p-value for interaction between race and survey year in unadjusted model of reporting recommendations for fecal occult blood test in past year was 0.31.
Discussion
In this large study of non-institutionalized Maryland residents age 50 years or older, blacks were significantly less likely than whites to report ever receiving provider recommendations for colonoscopy/sigmoidoscopy, but there was no significant racial difference in reporting recommendations for FOBT in the past year. Prior studies have differed in their results, with Klabunde, et al. (2006) finding whites more likely than blacks to report physician recommendations for both colonoscopy/sigmoidoscopy and FOBT, whereas other studies found no difference between black and white respondents in reporting counseling about CRC screening tests (Wee, et al., 2005; Burgess, et al., 2010; Shokar, et al., 2006).
Results from these studies and the current study may have differed due to the populations studied. Although Wee et al. (2005) used results from a nationally generalizable survey of US households, the other studies were performed in more selective populations (i.e., Medicare consumers in North and South Carolina (Klabunde, et al., 2006), patients from 24 VA medical facilities (Burgess, et al., 2010), and patients from a primary care clinic (Shokar, et al., 2006)). The racial disparity in recommendations for colonoscopy/sigmoidoscopy in the current study could also result from differences in health care access, which are recognized to contribute to health care disparities (Smedley, et al., 2003). However, health care access may not fully explain this disparity, as results adjusted for health care access variables (health care coverage and time since last check-up) were almost identical to unadjusted results. Differences in provider performance or patient behavior may also explain the observed racial disparity. For example, patients who visit providers mainly for acute illness are unlikely to have preventive care discussed. Thus, if this pattern is more common among blacks, a racial disparity in screening recommendations would result.
The racial difference in reporting recommendations for colonoscopy/sigmoidoscopy persisted at ~10% at all four surveys. This disparity did not change significantly over time even when the analysis was adjusted for several patient characteristics known to be associated with recommendations. It is interesting to note that each race had a consistent rate of improvement over the six years, indicating that the programs and policy changes implemented during this period were equally effective in increasing recommendations for colonoscopy/sigmoidoscopy in both races. Although it is encouraging that there was no racial disparity in reporting recommendations for FOBT in the past year at any survey, it is discouraging that there was no improvement in the disparity in colonoscopy/sigmoidoscopy recommendations. Racial disparities in CRC screening behaviors have been recognized for many years (Breen, et al., 2001), and increased awareness of this difference by physicians could help reduce such disparities if considered when making recommendations (Sarfaty and Wender, 2007). With only 72% of respondents reporting ever receiving a recommendation for colonoscopy/sigmoidoscopy, providers should be promoting CRC screening more widely for all age-eligible patients. Given the persistence of the racial disparity in recommendations for colonoscopy, however, it is critical for providers to be particularly sensitive to the barriers to CRC screening among their black patients. In particular, the racial disparity in up-to-date colonoscopy may be associated with the disparity in provider recommendations. Several effective interventions have been developed that aim to increase CRC screening among blacks (Friedman and Borum, 2007; Khankari, et al., 2007; Ward, et al., 2008; Powe, et al., 2010), and one intervention that included physician health literacy training and priming patients with educational materials has been shown to improve the rates of recommendations to black patients (Khankari, et al., 2007). Thus, there is hope that the racial disparity in provider recommendations, and in turn the disparity in CRC screening, can be narrowed or eliminated by 2015.
Study Limitations and Strengths
Interpretation of the results from this study is limited based on the use of self-report data. There was no validation of recommendations with medical chart review, so self-report of having received a recommendation may be inaccurate. Patients who were screened may be more likely to recall a recommendation. Studies have shown a higher sensitivity and lower specificity for self-report of CRC screening tests by whites compared to blacks (Rauscher, et al., 2008), suggesting that the racial disparity in the current study may be reflect reporting differences, but no studies have compared racial differences in the accuracy of self-report of recommendations for screening. Also, the study sample was limited to non-institutionalized residents using land-line phone numbers; this population may have changed over time. Finally, although the analysis accounted for several likely confounders, residual bias may exist from unmeasured factors including differences in the healthcare system (e.g., numbers and types of providers available), psychosocial factors (e.g., perceived CRC risk, health seeking behavior), and health factors (e.g., health status, chronic disease status). Future research should explore these variables, particularly as they suggest interventions to address the gap in screening.
The Maryland Cancer Surveys are population-based samples, providing generalizability to the Maryland population. Results from this study may not be generalizable beyond Maryland, because its median income is higher than other states’ (Anonymous, 2010d) and it mandates insurance coverage of CRC screening including colonoscopy (Anonymous, 2010c). This study has a large sample size which provides substantial power to test the associations of interest. Finally, the methods of the Maryland Cancer Surveys remained almost identical with respect to the variables in this analysis, permitting appropriate comparisons for time trends.
Conclusions
This study found that blacks were significantly less likely than whites to report receiving provider recommendations for colonoscopy/sigmoidoscopy on each of the Maryland Cancer Surveys, which may contribute to racial differences in CRC screening rates, and thus to the disparity in CRC incidence and mortality. The reasons for this racial difference should be explored further to design interventions eliminating this disparity. Although prevalence of recommendations increased in both blacks and whites, the disparity was unchanged between 2002 and 2008. Providers should be encouraged to recommend colonoscopy/sigmoidoscopy to all eligible patients, especially blacks, which will increase overall screening and may help eliminate the disparity in CRC screening and outcomes.
Highlights.
We examine self-reported colorectal cancer screening recommendations by race.
Blacks and whites reported increasing recommendations for colonoscopy over time.
Blacks reported 10% fewer recommendations than whites in 2002, 2004, 2006, and 2008.
There was no difference by race for recommendations for fecal occult blood tests.
The effect of race on the odds of reporting recommendations did not vary over time.
Acknowledgments
We gratefully acknowledge Dr. Mary-Claire Roghmann for guidance in the design of the study of data from the 2004 Maryland Cancer Survey, and Drs. Patricia Langenberg and Min Zhan for guidance in data analysis.
The first author’s work on this study was supported by training grants T32 AG000262 and F30 AG034008 from the National Institute on Aging, Bethesda, MD. The Maryland Cancer Survey is supported by the Cigarette Restitution Fund Program in Maryland. The results from an analysis of data from the 2004 Maryland Cancer Survey were presented at the 2008 Annual Scientific Meeting of the American Geriatrics Society on May 2, 2008 and at the Internal Medicine 2009 Meeting on April 25, 2009. The results from an analysis of data from the Maryland Cancer Surveys in 2002, 2004, and 2006 were presented at the 137th Annual Meeting of the American Public Health Association on November 10, 2009.
Role of the Funding Source:
The funding sources had no involvement in the conduct of the research or preparation of the article.
Abbreviations
- ACS
American Cancer Society
- BMI
body mass index
- CASRO
Council of American Survey Research Organizations
- CI
confidence interval
- CRC
colorectal cancer
- FOBT
fecal occult blood test
- GED
general educational development tests
- OR
odds ratio
Footnotes
Conflict of Interest Statement:
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fatmatta M. Kuyateh, Email: fkuyateh@gmail.com.
Diane M. Dwyer, Email: ddwyer@dhmh.state.md.us.
Carmela Groves, Email: cgroves@dhmh.state.md.us.
Eileen K. Steinberger, Email: esteinberger@dhmh.state.md.us.
References
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER cancer statistics review, 1975–2007. National Cancer Institute; Bethesda, MD: 2010. [Accessed January 25 2011]. Available at http://seer.cancer.gov/csr/1975_2007/results_merged/sect_06_colon_rectum.pdf. [Google Scholar]
- American Cancer Society (ACS) Colorectal cancer facts & figures 2008–2010. ACS; Atlanta, GA: 2008. [Accessed December 15, 2010]. Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/f861708finalforwebp.df.pdf. [Google Scholar]
- Anonymous. Healthy People 2010: Cancer. US Department of Health and Human Services; Washington, DC: 2010a. [Accessed December, 7, 2010]. Available at http://www.healthypeople.gov/2010/Document/HTML/Volume1/03Cancer.htm. [Google Scholar]
- Anonymous. Healthy People 2020 Summary of Objectives: Cancer. US Department of Health and Human Services; Washington, DC: 2010b. [Accessed December 7, 2010]. Available at http://healthypeople.gov/2020/topicsobjectives2020/pdfs/Cancer.pdf. [Google Scholar]
- Anonymous. Colorectal Cancer Screening Laws by State 2010. [Accessed December, 10, 2010];National Conference of State Legislatures. 2010c Available at http://www.ncsl.org/default.aspx?tabid=14328.
- Anonymous. [Accessed December 10, 2010];Maryland QuickFacts from the US Census Bureau. 2010d Available at http://quickfacts.census.gov/qfd/states/24000.html.
- Anonymous. National Health Interview Survey: Questionnaires, Datasets, and Related Documentation 1997. Present Centers for Disease Control and Prevention/National Center for Health Statistics; Atlanta, GA: 2010e. [Accessed December 15, 2010]. Available at http://www.cdc.gov.proxy-hs.researchport.umd.edu/nchs/nhis/quest_data_related_1997_forward.htm. [Google Scholar]
- Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19:339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93:1704–1713. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- Burgess DJ, van Ryn M, Grill J, Noorbaloochi S, Griffin JM, Ricards J, et al. Presence and correlates of racial disparities in adherence to colorectal cancer screening guidelines. [Accessed December 15, 2010];J Gen Intern Med. 2010 doi: 10.1007/s11606-010-1575-7. Available at http://www.springerlink.com.proxy-hs.researchport.umd.edu/content/y0403n76424521p6/ [DOI] [PMC free article] [PubMed]
- Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. 2004;100:418–424. doi: 10.1002/cncr.20014. [DOI] [PubMed] [Google Scholar]
- Dolan NC, Ferreira MR, Fitzgibbon ML, Davis TC, Rademaker AW, Liu D, et al. Colorectal cancer screening among African-American and white male veterans. Am J Prev Med. 2005;28:479–482. doi: 10.1016/j.amepre.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Borum ML. Colorectal cancer screening of African Americans by internal medicine resident physicians can be improved with focused educational efforts. J Natl Med Assoc. 2007;99:1010–1012. [PMC free article] [PubMed] [Google Scholar]
- Guerra CE, Schwartz JS, Armstrong K, Brown JS, Halbert CH, Shea JA. Barriers of and facilitators to physician recommendation of colorectal cancer screening. J Gen Intern Med. 2007;22:1681–1688. doi: 10.1007/s11606-007-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khankari K, Eder M, Osborn CY, Makoul G, Clayman M, Skripkauskas S, et al. Improving colorectal cancer screening among the medically underserved: a pilot study within a federally qualified health center. J Gen Intern Med. 2007;22:1410–1414. doi: 10.1007/s11606-007-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30:313–319. doi: 10.1016/j.amepre.2005.11.006. [DOI] [PubMed] [Google Scholar]
- McFarland EG, Levin B, Lieberman DA, Pickhardt PJ, Johnson CD, Glick SN, et al. Revised colorectal screening guidelines: joint effort of the American Cancer Society, U.S. Multisociety Task Force on Colorectal Cancer, and American College of Radiology. Radiology. 2008;248:717–720. doi: 10.1148/radiol.2483080842. [DOI] [PubMed] [Google Scholar]
- Poppell CF, Hopkins A, Zhan M, Steinberger EK, Groves C, Gugel D, et al. Maryland Cancer Survey, 2008: A population-based statewide survey on cancer screening and behavioral risk factors. Maryland Department of Health and Mental Hygiene; Baltimore, MD: 2009. [Accessed December 2, 2010]. Available at http://fha.maryland.gov/pdf/cancer/2008_MCS_report.pdf. [Google Scholar]
- Poppell CF, Shebl F, Hopkins A, Zhan M, Steinberger EK, Groves C, et al. Maryland Cancer Survey, 2006: A population-based statewide survey on cancer screening and behavioral risk factors. Maryland Department of Health and Mental Hygiene; Baltimore, MD: 2007. [Accessed December 2, 2010]. Available at http://fha.maryland.gov/pdf/cancer/MCS2006.pdf. [Google Scholar]
- Powe BD, Faulkenberry R, Harmond L. A review of intervention studies that seek to increase colorectal cancer screening among African-Americans. Am J Health Promot. 2010;25:92–99. doi: 10.4278/ajhp.080826-LIT-162. [DOI] [PubMed] [Google Scholar]
- Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- Sarfaty M, Wender R. How to increase colorectal cancer screening rates in practice. CA Cancer J Clin. 2007;57:354–366. doi: 10.3322/CA.57.6.354. [DOI] [PubMed] [Google Scholar]
- Schenck AP, Klabunde CN, Davis WW. Racial differences in colorectal cancer test use by Medicare consumers. Am J Prev Med. 2006;30:320–326. doi: 10.1016/j.amepre.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- Shokar NK, Carlson CA, Shokar GS. Physician and patient influences on the rate of colorectal cancer screening in a primary care clinic. J Cancer Educ. 2006;21:84–88. doi: 10.1207/s15430154jce2102_9. [DOI] [PubMed] [Google Scholar]
- Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. The National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60:99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- Steinberger E, Churchill J, Hopkins A, et al. Maryland Cancer Survey, 2004: A population based statewide survey on cancer screening and behavioral risk factors. Maryland Department of Health and Mental Hygiene; Baltimore, MD: 2005. [Accessed December 2 2010]. Available at http://www.fha.state.md.us/cancer/pdf/MCS2004.pdf. [Google Scholar]
- Steinberger EK, Israel E, Hopkins A, Zahn M, Uman J, Glover M, et al. Maryland cancer survey, 2002: A population-based statewide survey on cancer screening and behavioral risk factors. Maryland Department of Health and Mental Hygiene; Baltimore, MD: 2002. [Accessed December 2, 2010]. Available at http://fha.maryland.gov/pdf/cancer/MCS_Report_2002-V3.pdf. [Google Scholar]
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- Ward SH, Lin K, Meyer B, Bass SB, Parameswaran L, Gordon TF, et al. Increasing colorectal cancer screening among African Americans, linking risk perception to interventions targeting patients, communities and clinicians. J Natl Med Assoc. 2008;100:748–758. doi: 10.1016/s0027-9684(15)31356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41:23–29. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]


