Abstract
As a critical care community, we have an obligation to provide not only clinical care but also the research that guides initial and subsequent clinical responses during a pandemic. There are many challenges to conducting such research. The first is speed of response. However, given the near inevitability of certain events, for example, viral respiratory illness such as the 2009 pandemic, geographically circumscribed natural disasters, or acts of terror, many study and trial designs should be preplanned and modified quickly when specific events occur. Template case report forms should be available for modification and web entry; centralized research ethics boards and funders should have the opportunity to preview and advise on such research beforehand; and national and international research groups should be prepared to work together on common studies and trials for common challenges. We describe the early international critical care research response to the influenza A 2009 (H1N1) pandemic, including specifics of observational study case report form, registry, and clinical trial design, cooperation of international critical care research organizations, and the early results of these collaborations.
Keywords: critical care, intensive care, registry, H1N1, influenza, pandemic
Clinical research is an essential component of the pandemic response. During the severe acute respiratory syndrome outbreaks in 2003, illness was unexpected, caused by an uncharacterized virus with an uncertain mode of transmission and outcome (1). Only through rapidly performed observational studies were we able to characterize disease course and better-understand predictors of clinical outcome (2, 3). By nature of the rapid response to an unknown disease, treatment options were empirical and there was little opportunity to test these choices with interventional trials using rigorous methodologies (4). In contradistinction, pandemic influenza is well-known and has a frequency that recurs but it has sufficient duration between pandemics to allow a degree of complacency for some, and the illusion of time with which to plan a research response.
During the 1918 pandemic, 50 to 100 million people died (5). After its first description, the 1918 influenza A H1N1 (5) virus underwent at least two major mutations that are believed to have led to its increased pathogenicity (5). Case reports and pathologic studies from 1918 provide rich clinical descriptions; death followed from aggressive secondary bronchopneumonia, influenza-related lung disease, associated cyanosis, and cardiac collapse (6). During the 1918 pandemic, there was unexplained excess influenza mortality in persons 20 to 40 yrs of age, possibly because of limited native immunity and/or a vigorous immune response directed against the virus in healthy young persons (6). In 1918, however, clinical investigation was in its infancy; there was limited opportunity for identification of independent risk factors through rigorous cohort or case-control studies and virtually no opportunity for clinical trials. Today, even without vaccination, the mortality of the 1918 pandemic almost certainly would be reduced because of the availability of intensive care units (ICUs), antibiotics, and antiviral medications, innovations which are the result of much of the clinical research of the mid 20th century. For today’s ICU practitioners, the “cost” will be an increase in critical care admissions and length of stay among patients with severe acute respiratory distress syndrome (ARDS) (7–10).
Planning for the inevitability of sudden events leading to severe illness is essential. However, even the most prescient cannot predict, investigate, or plan for the nuances of the next hurricane, influenza mutation, or act of terror. As a critical care community, we have an obligation to provide not only clinical care but also the research approach to guide the initial and subsequent clinical response during a pandemic.
Challenges to Conducting Research During Pandemic Periods
There are many challenges to conducting research during a pandemic. The first is time. Case report forms must be generated quickly. This can be facilitated by a preexisting template containing core elements modified to the particular scenario. Determining appropriate definitions for those ill (the “cases”) is important, and infectious diseases may require evolving confirmatory laboratory testing. For example, for patients with 2009 influenza A (H1N1), diagnostic testing was not widely available in all clinical settings (the etiology was uncertain and testing was not ubiquitous), and although nasopharyngeal swabs for polymerase chain reaction are associated with excellent sensitivity and specificity when properly performed early after symptom onset, the sensitivity in critically ill patients may not be as good. Failure to include patients with influenza-associated critical illness but with negative nasopharyngeal diagnostic testing results may systematically alter the knowledge base that emerges. Therefore, categorization into confirmed, probable, or possible/suspected allows for greater retrospective precision in defining clinical cases and avoidance of inclusion of patients with non-H1N1 illness. Research questions must be devised and vetted efficiently and decisions must be made about the most important projects. For small or geographically confined outbreaks, when it is possible to collect information on all patients, a cohort study may be ideal. For larger outbreaks, a case series collecting data only for those with illness is more efficient. With the addition of data collection from appropriate patients without illness but with otherwise similar characteristics and opportunity for disease (controls), a case-control study can help to identify patient-based and exposure associations with the disease, which is a critical step for determining risk factors for outcomes.
The second great challenge is availability of personnel. Most are busy with usual duties when an outbreak occurs; however, some must prioritize their time toward a response. Clinicians may be consumed with clinical care. As the outbreak progresses, healthcare workers may become ill or quarantined and taken out of the workforce, placing more demand on those who remain. Research staff, particularly nurses, may be required to return to clinical duties. Research provisions must be made, and some personnel should be devoted to this task.
Third, clinical research must undergo appropriate oversight and research must undergo appropriate ethics approval. In most jurisdictions, urgent public health investigations can occur without delay or need for separate research ethics approval; however, such an outbreak investigation is usually narrow in scope, unable to answer broader questions about the illness, and does not typically encompass interventional trials. In usual times, the process of protocol generation for observational studies and application to a research ethics board may take a minimum of 2 to 3 mos; during most outbreaks, the response ideally should occur in days. This requires an efficient collaboration with research ethics boards and is the subject of another contribution to this Supplement (11). For observational studies recording preexisting data without personal identifying information, we believe there should be no requirement for patient or surrogate-level consent, because this can be associated with authorization bias and invalid results (12). If the research involves collection of patient samples aside from clinical care or involves an interventional design, then there still may be provision for a “no consent” or “deferred consent” model if there is negligible risk and no other reasonable manner with which to perform necessary research (12).
Fourth, this work requires funding. Although clinical investigators, research coordinators, biostatisticians, and administrative staff might be able to devote their time to unfunded outbreak work for short periods (days or weeks), this cannot be sustained for months without other duties suffering. Usual funding cycles occur over a 1-yr period. During a pandemic, funding agencies need to modify their responses, quickly announce funding opportunities, and provide an abbreviated timeline for acceptance of proposals and decisions (weeks or months). Government and health authorities should respond with immediately (days or weeks) available funds for core research endeavors—observational studies and pilot interventions—performed by internal or external groups with the ability to perform this work.
Finally, all the usual challenges in conducting research remain. The clinical case definitions may change with more experience, complicating observational studies. Research hypotheses may not prove fruitful. Multiple groups, even in the same locale, may plan the same study, inefficiently duplicating efforts. Early broad communication (locally, nationally, and internationally) to the research community, ideally using previously established networks, is essential to ensure that the research response is as collaborative and efficient as possible. Some duplication of effort can be desirable, especially when it may identify geographic variation in clinical descriptions, care, or outcomes that are instructive to the response. To facilitate the comparison of findings, similar projects should be undertaken with similar designs and definitions. By definition, research performed during a pandemic must be more flexible and pragmatic than that undertaken in the best of circumstances. As priorities change or new information becomes available, study protocols may require modification.
The Imperative of Early Observational Investigation
This article focuses on observational research activities during pandemic periods, specifically 2009 influenza A (H1N1). Although interventional studies are critical in providing evidence-based prevention and treatment options, other contributions to this Supplement focus on specific clinical trials and the challenges of rapid design and deployment. Observational studies are vitally important for questions that cannot or will not be answered by trials. For example, the identification of risk factors for H1N1 needs to occur through a population-based case-control or cohort study. Furthermore, it is unlikely that there will be equipoise to allow placebo-controlled studies of vaccination or neuraminidase inhibitors, even though their efficacy may be uncertain.
The first steps of any outbreak investigation rely on observational methodologies such as: (1) prepare for field work; (2) establish the existence of an outbreak; (3) verify the diagnosis; (4) define and identify cases; (5) describe and orient the data by time, place, and person; (6) develop hypotheses; (7) evaluate hypotheses; (8) refine hypotheses and perform additional studies; (9) implement control and prevention measures; and (10) communicate findings (13). Once the outbreak is confirmed, the starting point is always defining those with the condition of interest and establishing a case report form.
Considerations for Components of Case Report Forms and Registries
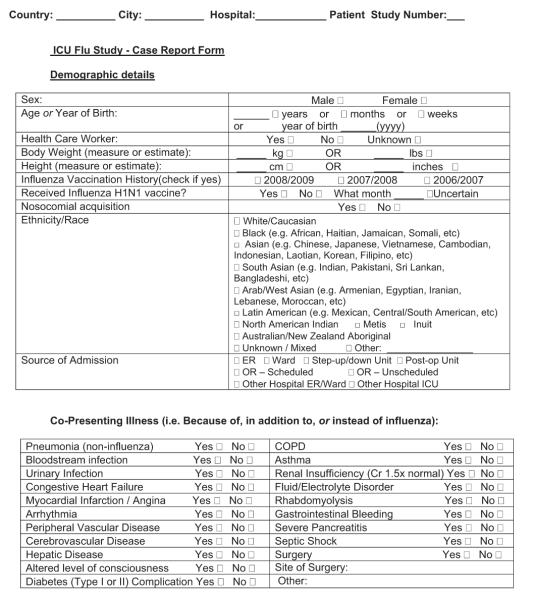
Case report forms for many critical illness-focused outbreaks will contain core elements, including, but not limited to, the following elements. Eligibility criteria that define the patients must be included. For the 2009 H1N1 pandemic, there may be a tendency to include only patients with confirmed H1N1. However, this could prove challenging if diagnostic testing is not 100% sensitive, or if confirmatory testing is not always available or is not be the same in each center (e.g., polymerase chain reaction vs. viral culture vs. enzyme-linked assay). Thus, confirmation may be foregone completely if demand outstrips capacity, making categories of confirmed, probable, and suspected valuable (14). Similarly, although it is possible that H1N1 will comprise the majority of influenza burden in some jurisdictions in 2009 to 2010, it is reasonable to consider including all influenza and subcategorizing when this is known. This will allow comparison of clinical syndromes to other strains and simplify eligibility for study personnel when subtypes are not known. For a critical illness-focused project, there should be some reasonable definition of “critically ill.” Criteria should be defined according to the patient and the illness, not the geographic area of admission (e.g., the ICU). ICU admission criteria are not universal and are often dictated by institutional numbers of beds, which may be exceeded in pandemic periods or may not exist in parts of the developing world. Potential definitions of critical illness include ventilation failure (defined by clinical criteria or receipt of mechanical ventilation), oxygenation failure (defined by clinical criteria and/or receipt of high level of inspired fraction of oxygen), or hypotension (defined by clinical criteria and/or receipt of intravenous vasoactive medications) in any hospital area. If this non-ICU-centric approach is taken, then a mechanism for finding such patients is needed if they do not naturally come under the care of the critical care team. For case series, all and consecutive patients fulfilling the definition must be described to avoid the risk for selective reporting, which often leads to inclusion of the sickest patients—those who more obviously come to clinical attention because of illness severity. This can lead to falsely high mortality estimations in the early stages of pandemics. To facilitate hospital or regional incidence estimates, the case definitions should be harmonized at each level, ideally with international agencies.
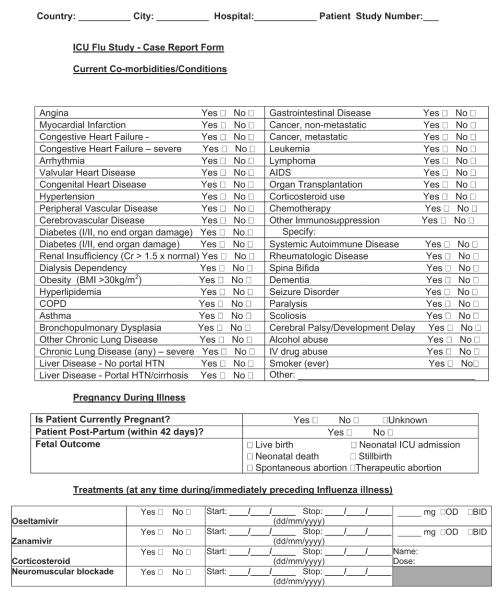
Patient demographics, minimally consisting of age (date of birth may constitute personal health information that is not allowed in certain jurisdictions) and gender, should be collected. Past influenza pandemics have appeared to demonstrate higher mortality in certain racial/ethnic groups. This information should be recorded if possible, adhering to internationally acceptable nomenclatures. Baseline prevention interventions, such as receipt of 2009 H1N1 vaccine, should be considered. Clinical presenting symptoms that may allow differentiation from milder forms of influenza might be sought in addition to comorbid conditions that may prove to be risk factors and can be mapped to various comorbidity indexes (15) or severity of illness scoring systems (16, 17). Because many comorbid conditions differ between adults and children, both should be included for broad applicability (18). Seasonal influenza often presents as an exacerbation of preexisting illness or with secondary bacterial pneumonia, so other presenting illness might also be captured.
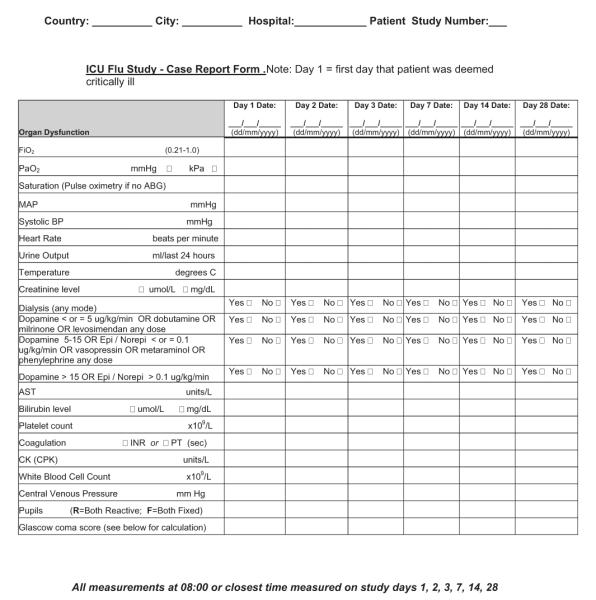
Whenever possible, raw data should be recorded without asking for the application of decisions or categorizations. Such considerations may limit consistency, the ability to apply the data to other purposes, and the power to determine differences when making comparisons (e.g., record the partial pressures of gases instead of a calculated alveolar–arterial difference).
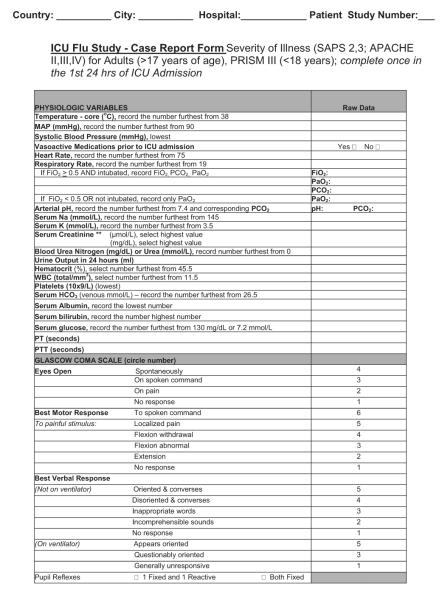
For critical illness-focused studies, it is important to record some measure of illness severity to adjust risk between patients at the onset of critical illness. Common examples include the Acute Physiology and Chronic Health Examination II–IV score and Simplified Acute Physiology III score for adults or the Pediatric Risk of Mortality III score for children (16–18). Despite their differences, these tools contain a surprising overlap of core elements. One flexible approach is to collect physiologic data on the variables necessary for calculation of several (Appendix 1), which then facilitates comparison and risk adjustment among different data sets if one standard cannot be broadly agreed on a priori. Collecting a limited amount of daily data on physiology and organ function, laboratory parameters, treatments, and ventilation may have some benefit, but only those deemed of potential direct relevance to the condition should be captured (e.g., only measures of oxygenation or creatine kinase). If daily data are to be collected on organ function, then variables should be limited to those that can be transformed into standard and validated scoring systems, such as Sequential/Sepsis Organ Failure Assessment (19), Multiple Organ Dysfunction (20), or Pediatric Logistic Organ Dysfunction (21) scores. Especially important is avoiding the temptation to collect data on each day of critical illness, because the burden on the research team can be overwhelming and the information might never be used. Instead, focus should be placed on a period of greatest interest (e.g., first days of admission) and/or limited time points (e.g., weekly) thereafter.
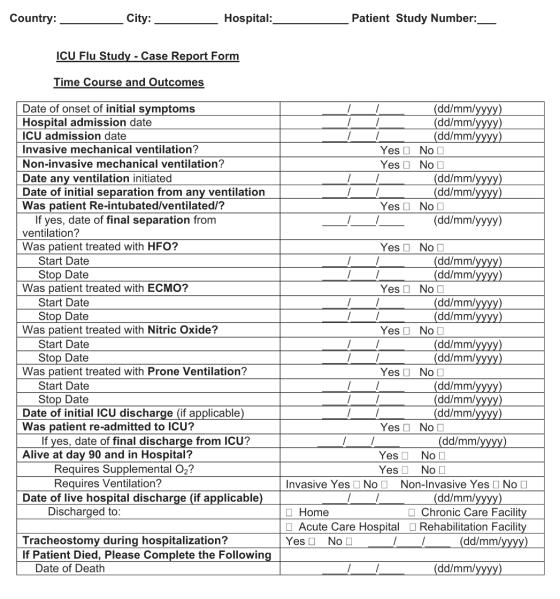
Careful consideration should be given to the level of detail for treatment-based variables, whether to record medication class vs. generic or trade names, dosages, routes, and start/stop/restart/stop dates. We recommend including only those medications believed or postulated to have direct relevance to the course of the illness being studied (e.g., neuraminidase inhibitors, other antiviral medications, corticosteroids, and neuromuscular blockade) and to avoid the temptation to request commonly used medications (e.g., sedatives, analgesics, and antibiotics of varying names, doses, routes, and durations). One alternative is to capture medications as either prescribed or not when timing, dose, and route are of less importance. Other specific therapies are likely relevant, such as the starting and ending of mechanical (invasive and noninvasive) ventilation and rescue therapies (high-frequency oscillatory ventilation, extracorporeal membrane oxygenation, prone ventilation, and nitric oxide).
Outcomes should be appropriate for the illness in question. For H1N1-related conditions characterized by oxygenation failure and prolonged periods of support for relatively young patients, mortality rate at 1 mo (28 days), but also for 60 or 90 days, is necessary for capture of late deaths, in addition to dates of admission and discharge from the hospital and ICU.
Sample patient-based case report forms (Appendix 1) have been disseminated broadly to various jurisdictions. After appropriate assessment by local research ethics boards and jurisdictional data sharing and transfer bodies, these forms have facilitated early reports of critically ill patients for the 2009 H1N1 pandemic. Such forms can be completed quickly in paper format and may be most appropriate in some settings but require transcription into a database for analysis. A more efficient system makes such forms available as a secure Web-based data entry system that can be used wherever an internet connection exists (e.g., http://www.infactglobal.org, http://www.h1n1registry.com and https://www.icnarc.org). Data queries and real-time analyses then can be performed, with efficient reporting to the users and policy makers, in addition to report and manuscript preparation. An electronic, Web-based format also facilitates modification of forms to other purposes, and examples include using the baseline elements of a case report form for translational studies, a subsequent clinical trial that will randomize patients to one treatment or another, or for linking or merging critical care databases with population or other critical care data sets at a later time. Ideally, investigators of unique studies using a common case report form should consider the additional studies or trials beforehand to optimize variable choice, dates of data collection, and merger of data sets.
For the 2009 H1N1 pandemic, detailed critical illness-related data will rarely be reported on all patients. Rather, there should be a balance of minimal reporting at the population level (often performed through public health agencies or preexisting population-based critical care reporting), supplemented by more detailed critical care reporting among an appropriately sized sample of patients, adhering to both statistical and practical principles. Anticipating that centers may be overwhelmed with clinical activity, collection should either begin with or move toward a minimal data set during the outbreak.
The burden of critical illness that a pandemic such as 2009 H1N1 places on ICU and healthcare systems makes it important to attempt to measure this aspect of our response. Ideally, hospitals will be able to track case load, increases in capacity that were necessary or possible, and any reduction in other types of care that occurred. This information is important for future pandemic planning.
Current Global Influenza Case Report Forms and Registries
Since April 2009, a number of critical care groups around the world have collaborated to devise case report forms and plan observational studies that have helped provide the case description of critically ill patients with 2009 H1N1, including baseline patient characteristics, presenting symptoms, organ dysfunction, treatments received, and clinical outcomes, as well as population-based epidemiology of critical illness (8–10, 22, 23). These groups include, but are not limited to, the ARDS Network (ARDS-Net), the Australian and New Zealand Intensive Care (ANZIC) Influenza Investigators, the Canadian Critical Care Trials Group, the European Society of Intensive Care Medicine (ESICM), Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Spanish H1N1 Study Group, and the United Kingdom Intensive Care National Audit and Research Centre (ICNARC) Swine Flu Triage group (Appendix 2). Other national and international critical care societies, government-affiliated agencies, and funders have supported the research undertaken by these groups. This information has been essential in planning for the fall and winter influenza seasons in the southern and northern hemispheres, and in estimating resource requirements for hospitals and healthcare systems. In addition to providing national descriptive studies, the critical care community has come together in a very short period in an attempt to harmonize case report forms, plan for comparative studies, and facilitate enrollment in clinical trials (24), which are now underway.
CONCLUSIONS
The 2009 H1N1 pandemic has provided our specialty with one of this century’s first great challenges, as well as one of our greatest opportunities. The undertaking of research to better the understanding of critical illness and care of acutely ill patients is vital to the care of patients currently affected, those who will become ill during the next surges, and for planning of the inevitable next pandemic, whenever it may occur. Thus far, the critical care community has shown great promise in its ability to work together under difficult circumstances. Our subsequent challenge will be to maintain this spirit, combine our efforts, work together on common projects, and translate this effort and knowledge gained globally.
Acknowledgments
The ARDS and PALISI collaborative registry is funded by the NHLBI contract HHSN268200536179C; The Canadian Institutes of Health Research; The Public Health Agency of Canada; and The UK Medical Research Council.
Dr. Cobb is supported by NIH grant U13GMO83407. Dr. Thompson has received grant support from the NIH and ARDSNetwork.
APPENDIX 1. SAMPLE PATIENT-BASED CASE REPORT FORMS
 |
 |
 |
 |
 |
 |
APPENDIX 2. CURRENT GLOBAL INFLUENZA RESEARCH GROUPS AND CLINICAL REGISTRY INITIATIVES
The Canadian Critical Care Trials Group
The Canadian Critical Care Trials Group is dedicated to the pursuit of excellence in and advancement of critical care research in Canada. The group currently has >200 members in pediatric and adult critical care medicine from all provinces and has representation from both academic and community intensive care units. In April 2009, critical care colleagues in Mexico City faced with the early burden of H1N1-related critical illness contacted Canadian colleagues to collaborate on an observational study. Using experience gained through the SARS outbreak and reporting system, the group constructed a case report form for critically ill patients and then performed a pilot test of the form with clinicians and research coordinators in both countries. Research ethics approval for an observational study was granted on April 30, 2009, and the forms were disseminated in Canada and Mexico and to critical care societies in other regions of the world on May 3 (http://www.ccctg.ca/news_events.php). Data were transmitted by secure research fax to the methods center and preliminary results from the Mexico City experience were presented to the American Thoracic Society on May 19, 2009. The observational study continued in Canada and Mexico through August 2009, and both experiences were published together online on October 12, 2009 (8, 9), in conjunction with presentation of results at the European Society of Intensive Care Medicine’s annual scientific symposium in Vienna. The case report form underwent modification, was transitioned to Web-based data entry by October 2009, and is currently enrolling patients in university and community adult centers and in all pediatric critical care hospitals (in conjunction with the PALISI Network) in Canada.
ANZIC Influenza Initiative
ANZIC Influenza Investigators comprise adult and pediatric intensivists and infectious diseases physicians. The group was formed on May 28, 2009, in response to the spread of the H1N1 2009 influenza virus in North America. The group included many researchers who have been active within the ANZIC Society Clinical Trials Group (ANZICS CTG), and the influenza registry established by these investigators was endorsed by the ANZICS CTG. Later, in the midst of the epidemic, it became apparent that many patients with proven or suspected influenza were being treated with extracorporeal membrane oxygenation (ECMO), prompting the formation of a separate group of researchers, which also included cardiac surgeons and anesthetists, to establish a registry for patients treated with ECMO.
The ANZIC Research Centre (ANZIC RC) at Monash University acted as the methods center for both the influenza and the ECMO registries. The links to the ANZIC RC and the ANZIC CTG have been essential in the conduct of registry activities. At establishment, ANZIC Influenza Investigators established contact with all 187 ICUs and obtained agreement from all to screen and submit data on patients meeting the influenza registry entry criteria (admission to an ICU and evidence of active or recent infection with influenza A). All 15 ICUs that provide ECMO contributed to the ECMO registry. All registry activity was initially unfunded, and data were provided by all sites with no guarantee of reimbursement for data collection costs.
Submission of patient data to the registries was approved by the Health Research Ethics Committee at all participating sites, and a model of waived consent was utilized. The accessibility of this established ANZIC RC infrastructure was critical to initiating effective early data collection, especially because the epidemic was well-established in some regions by the time Web-based data collection became available. The ANZIC Influenza Investigators made a decision to collect a very limited data set, comprising only demographic information, variables related to potential risk factors, method of testing that confirmed influenza A infection, key treatment-related factors, including use of antivirals and glucocorticoids and daily use of an invasive airway, mechanical ventilation, ECMO, and use of vasopressors, and vital status. No physiologic data, including severity of illness or organ failure scores, were collected. Patients with ICU admissions between June 1 and August 31 were followed-up as of September 7. For the influenza registry, complete follow-up of the enrolled cohort was achieved by September 14, a manuscript was submitted for publication 2 days later, and the information was available online on October 8 (10). A manuscript describing patients treated with ECMO was published a few days later (22). Both registries remain active, with new cases being submitted, although the incidence of ICU admission attributable to infection with H1N1 2009 has decreased dramatically.
United States Department of Health and Human Services H1N1 Critical Illness Registries The Acute Respiratory Distress Syndrome Network
The Acute Respiratory Distress Syndrome Network (ARDSNet) is a consortium of academic medical centers and affiliated hospitals across the United States funded by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). The Office of the Assistant Secretary for Preparedness and Response within the United States Department of Health and Human Services and the United States Centers for Disease Control and Prevention have collaborated with NHLBI and ARDSNet to establish an adult registry of critically ill novel influenza H1N1 because it has been successful at conducting multicentered clinical trials and because of its diverse regional representation and established infrastructure. Representatives from the United States Food and Drug Administration and the National Institute of Allergy and Infectious Diseases are also involved with the registry. The goal of the registry is to obtain a better understanding of the burden of disease, severity of illness, clinical course, and resource utilization needed to optimize patient care for H1N1-associated critical illness. The ARDSNet data set will collect information from 12 core academic medical centers and >50 ICUs from 42 hospitals. The PALISI Network joined this novel partnership and is leading the registry efforts regarding the pediatric experience.
The ARDSNet and PALISI database, funded by the NHLBI, will contain information on up to 2250 individuals admitted to the ICU with confirmed or suspected influenza, both novel H1N1 and seasonal influenza. Most patients will be collected prospectively; however, a subset of patients will be accrued retrospectively. The registry will enroll patients admitted to the ICU with confirmed H1N1 or nonsubtypeable influenza A infection after May 1, 2009. Prospective data collection will include patients with both confirmed and suspected influenza, either novel H1N1 or seasonal. Because obtaining complete information on every patient with influenza in designated ICUs is vital for accurate estimation of clinical resource utilization, and to avoid authorization bias attributable to biased exclusion of patients without surrogates, data in the registry will be collected using a waiver of informed consent and a waiver of authorization for both the prospective and retrospective portions. Institutional Review Boards have been receptive to approving the waivers of consent and authorization for these data collections.
No samples or specimens will be collected as part of the registry effort, although substudies may include such collection. Data will be collected from the medical record and transferred electronically to the electronic database, Research Electronic Data Capture (http://www.project-redcap.org), for storage. Many of the data elements have been harmonized via the International Forum for Acute Care Trialists (InFACT) group to simplify combining data with other databases and registries in the future. Detailed demographic and treatment information will be collected. Crude outcomes, including survival status, need for ICU care, dialysis, vasopressors, noninvasive or mechanical ventilation, and rescue ventilation therapies will be collected weekly to have a real-time picture of the clinical course, allowing resource utilization decisions to be modified and adjusted to provide optimal patient care during the pandemic. At ICU discharge, death, or day 28, more in-depth outcomes, such as severity of organ dysfunction and bacterial coinfections or nosocomial infections, will be collected. The development of additional organ failures, such as myositis, myocarditis, or encephalitis/seizure/delirium, also will be collected retrospectively at this time. Treatments specific for influenza during the ICU course, including administration of antiviral medications or immune plasma or immunoglobulin, also will be collected for the ICU course. Survival data will be collected to day 60 on patients who remain hospitalized beyond day 28. Cause of death will be recorded for all deceased patients, and a de-identified autopsy report will be requested.
The Office of the Assistant Secretary for Preparedness and Response is hiring additional analytic and biostatistical support to assist the networks’ clinical coordination center to analyze data rapidly and allow for near-real time reporting to inform government response policy, as well as timely reporting to frontline clinicians. In addition, the United States Critical Illness and Injury Trials Group is working with National Institute of Allergy and Infectious Diseases and the NIH to enroll a broader group of hospitalized patients with H1N1 who are at risk for organ failure.
The PALISI Network
The PALISI Network is a consortium of clinical investigators at >75 ICUs in the United States and Canada (http://pedsccm. org/PALISI_network.php). Starting in January 2009, 36 sites in the PALISI Network enrolled children admitted to pediatric ICU with community-acquired influenza infection in an ongoing Centers for Disease Control and Prevention-funded and NIH-funded study of genetic susceptibility to life-threatening and fatal influenza in children and young adults. ICU cases are screened daily for probable cases of influenza. As soon as possible after admission, parents are approached for consent, and symptomatic children confirmed to have probable influenza infection from preliminary screening tests (rapid testing or direct fluorescent antibody) or confirmed influenza infection from viral culture or polymerase chain reaction-based testing are enrolled. Data collection consists of patient demographics, clinical information including vaccination status, risk factors, medical history, clinical status on day of admission, bacterial and viral test results on presentation, and ICU and hospital course via medical chart abstraction and survey questionnaire. The same computerized database is also used for the Canadian pediatric epidemiologic component of the ICU-Flu study. All 17 pediatric ICUs across Canada are screening daily for patients. A weekly screening tally is sent to the coordinating center to produce a weekly report on influenza infection in Canadian pediatric ICU.
The ESICM H1N1 Registry
At the end of July 2009, the ESICM developed an H1N1 Registry for critically ill patients with novel H1N1 virus. Although this registry focuses on the European Union, all adult and pediatric ICUs are welcome to add their patients (http:// www.h1n1registry.com/). Requests for approval by Helsinki Committees with a waiver of informed consent are to be obtained by each country. The ESICM registry comprises 123 ICUs in 29 countries with registered patients, including Argentina, Belgium, Bolivia, Brazil, Chile, Colombia, Czech Republic, Denmark, Ecuador, France, Greece, Hong Kong, Iceland, India, Iran, Ireland, Israel, Italy, Mauritius, the Netherlands, Norway, Peru, Portugal, Spain, Sweden, Switzerland, United Kingdom, Vietnam, and Yugoslavia. As of November 12, 363 patients have been enrolled.
The common data set encompasses such variables as demographic details, eligibility criteria, chronic comorbidities, severity scores, and admission data, including medications, clinical manifestations, diagnosis, specific findings (including bacterial pneumonia, other life-threatening bacterial or fungal infections, shock, asthma attack or chronic obstructive pulmonary disease exacerbation, acute coronary syndrome, renal failure, altered level of consciousness, rhabdomyolysis, and cardiac function), time course, microbiology, treatments, outcomes, complications, residual organ dysfunction, and clinical variables at baseline, days 1 to 7, and discharge.
To make the ESICM data set compatible with others and to reduce the participant’s burden of data entry, the ESICM Steering Committee decided to have three types of data: (1) minimal data: data in the ESICM data set that are present in the agreed-on InFACT minimal data set; (2) optional data: data in the ESICM data set that are not part of the InFACT minimal data set; and (3) preferred data: data in the InFACT minimal data set that will be added to the ESICM data set. Participants are asked to complete all three data categories if possible. If they cannot, then they should complete the minimal data and preferred data.
The Spanish H1N1 Study Group
The Spanish H1N1 Study Group was created in June 2009 by members of the “Grupo de Trabajo de Enfermedades Infecciosas” (GTEI) from the Spanish Society of Critical Care. GTEI is a research group of >300 active members with 20 yrs of experience in conducting clinical and translational research in severe infections in the ICU in Spain. More than 400 ICU admissions with severe H1N1 infections from 120 ICUs, mainly primary bacterial pneumonia, were registered before vaccination was implemented. A periodical newsletter is providing feedback to members. Update of the pandemic progression by ICU admissions, influenza rate, and deaths is updated each week. Clinical and epidemiologic details from critically ill patients with respiratory failure through July 31 were reported September 11 (23). The case report form is faxed to the coordinating center, where dedicated investigators complete an electronic case report form. Sites of this group represent the core of the IMMUNOFLU project, in which multidisciplinary collaboration by virologists, immunologists, bioinformatics personnel, and other specialists facilitate investigation of translational aspects comparing critically ill with noncritically ill H1N1 patients.
The United Kingdom ICNARC Swine Flu Triage Registry
Since 1996, United Kingdom ICNARC (www.icnarc.org) has coordinated a national outcome audit for adult critical care called the Case Mix Programme. With the support of trained, local data collectors, the Case Mix Programme collects, on a quarterly basis, data for defined specific case mix, outcome, and activity variables on consecutive admissions to adult critical care units in England, Wales, and Northern Ireland (219 units; 85% coverage). After extensive local and central data validation, quarterly comparative reports presenting approximately 20 key potential quality/performance indicators, including risk-adjusted hospital mortality (using the UK Acute Physiology and Chronic Health Evaluation II model, 2009, and the ICNARC model, 2009) are disseminated.
At the start of the H1N1 swine influenza pandemic, participating Case Mix Programme units were asked to submit data for confirmed H1N1 cases for rapid analysis and feedback. In addition, after a commission from the government, ICNARC rapidly (in 6 wks) established the eligibility criteria, data set, and Web portal, and gained ethics and research regulatory approval for approximately 250 acute hospitals in England, Ireland, Northern Ireland, Scotland, and Wales to collect data on confirmed/ suspected H1N1 cases referred and assessed as requiring critical care and on non-H1N1 cases referred and assessed as requiring critical care (under usual nonpandemic circumstances) and not admitted to a critical care unit because of the pandemic. Assessment included critical care and daily critical care data collected on all eligible cases. When possible, attempts have been made to ensure that these data are compatible with other efforts internationally.
Fostering International Collaborations
The InFACT is a recently formed collaborative network of investigator-led research groups (such as those mentioned) that study the optimal care of acutely ill patients from their initial presentation in the community through ICU support to their rehabilitation and integration back into society. InFACT is guided by a steering committee of representatives of the various research groups that have recognized the value of closer international collaboration to advance the study of the care of the acutely ill. One near-term goal of InFACT is to aid in communication and collaboration among groups to enable common reporting of observational studies of H1N1-related critical illness, as well as broader collaboration in interventional and translational studies. One of the planned initiatives for the H1N1 pandemic is to facilitate a common reporting structure of aggregate data among those groups and countries already leading registries. The various member groups have worked collaboratively to ensure, when possible, common definitions and reporting structures that will allow this to occur (24).
Footnotes
The authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;349:709–711. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 2.Fowler RA, Lapinsky SE, Hallett D, et al. Toronto SARS Critical Care Group. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 3.Lew TWK, Kwek T-K, Tai D, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 4.National Advisory Committee on SARS and Public Health [Accessed November 12, 2009];Learning from SARS— Renewal of public health in Canada. A report of the National Advisory Committee on SARS and Public Health. 2003 Oct; http://www.phac-aspc.gc.ca/publicat/sars-sras/naylor/
- 5.Belshe RB. The origins of pandemic influenza— Lessons from the 1918 Virus. N Engl J Med. 2009;353:2209–2211. doi: 10.1056/NEJMp058281. [DOI] [PubMed] [Google Scholar]
- 6.Morens DM, Fauci AS. The 1918 influenza pandemic: Insights for the 21st century. J Infect Dis. 2007;195:1018–1028. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 7.Richard N, Hackme C, Stamm D, et al. Influenza in pediatric intensive cure unit. Arch Pediatr. 2004;11:879–884. doi: 10.1016/j.arcped.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Zarychanski R, Pinto R, et al. Canadian critical care trials group H1N1 collaborative. Critically ill patients with 2009 influenza A (H1N1) in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 10.The ANZIC Influenza Investigators Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–193. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 11.Cook DJ, Burns K, Finfer S, et al. Clinical research ethics for critically ill patients during the H1N1 pandemic. Crit Care Med. 2010;38:e138–e142. doi: 10.1097/CCM.0b013e3181cbaff4. [DOI] [PubMed] [Google Scholar]
- 12.Tu JV, Willison DJ, Silver FL, et al. Impracticability of informed consent in the registry of the Canadian Stroke Network. N Engl J Med. 2004;350:1414–1421. doi: 10.1056/NEJMsa031697. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control [Accessed November 12, 2009];Steps in an outbreak investigation. Available at: www.cdc.gov/excite/classroom/outbreak/steps.htm.
- 14.Public Health Agency of Canada [Accessed November 13, 2009];Case definitions for national surveillance H1N1 flu virus. Available at: http://www.phac-aspc.gc.ca/alert-alerte/h1n1/hp-ps-info definition-eng-.php.
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 17. [Accessed November 13, 2009];Simplified Acute Physiology Score 3: SAPS 3 Project. Available at: http://www.saps3.org.
- 18.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Marshall JC, Cook DJ, Christou N, et al. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dys-function (PELOD) score: Prospective, observational, multicentre study. Lancet. 2003;19:362, 192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 22.The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 23.Rello J, Rodríguez A, Ibañez P, et al. The H1N1 SEMICYUC working group. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1v) in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The InFACT Global H1N1 Collaboration In-FACT: A global critical care research response to H1N1. Lancet. 2010;375:11–13. doi: 10.1016/S0140-6736(09)61792-X. [DOI] [PubMed] [Google Scholar]


