Abstract
Objective
Tight glycemic control (TGC) can potentially reduce morbidity and mortality in the ICU but increases the risk of hypoglycemia. The most effective means to avoid hypoglycemia is to obtain frequent blood glucose (BG) samples, but this increases the burden to nursing staff. The objective of this study was to assess the ability of a real-time continuous glucose monitor (CGM-RT) to reduce hypoglycemia (BG<60 mg/dL [3.3 mmol/L]) during standard care (STD) or TGC effected with a proportional integral derivative (PID) insulin titration algorithm.
Design
CGM-RT profiles obtained from an ongoing prospective randomized trial of TGC were retrospectively analyzed to determine if the continuous glucose measure had prevented instances of hypoglycemia.
Setting
Cardiac ICU.
Patients
Children 3 years of age or less undergoing cardiac surgery were studied.
Interventions
Intravenous insulin infusion and rescue glucose with guided by CGM-RT and the PID algorithm in the TGC arm (N=155; target glucose 80–110 mg/dL [4.4–6.1 mmol/L); CGM-RT in the STD care arm (N=156).
Measurements and Main Results
No reduction in hypoglycemia was observed with CGM-RT alarms set at 60 mg/dL [3.3 mmol/L] (0 of 19 occurrences of BG<60 mg/dL [3.3 mmol/L] detected); 18 of 40 subsequent incidences of hypoglycemia were detected after increasing the alarm threshold to 70 mg/dL [3.9 mmol/L]. In the TGC arm, 8 incidences were reduced in duration and an additional 8 events were prevented with intravenous glucose. In the STD arm, 3 of 9 occurrences of hypoglycemia were detected with the duration reduced in all cases. On average, one to two false hypoglycemia alarms were observed in each patient.
Conclusions
CGM-RT in combination with PID control can reduce hypoglycemia during TGC. CGM-RT can also reduce hypoglycemia during STD. However, false alarms increase the overall nursing workload.
Keywords: tight glycemic control, continuous glucose monitoring, intensive care unit
Introduction
Tight glycemic control (TGC) has been shown to improve outcomes in some (1– 3) but not all studies (4) of tight glycemic control, with several studies having been prematurely halted (5–7) over concerns related to hypoglycemia. Hypoglycemia during insulin therapy has been shown to increase the risk of mortality (8–12), but can potentially be minimized with more frequently monitoring of the blood glucose (BG) level. However, the need for frequent monitoring adds burden to nursing staff (13) and, in the absence of explicit methodology for titrating insulin, may produce varying and ultimately non-reproducible results. Continuous glucose monitoring can potentially reduce the burden associated with frequent blood draws, but questions remain regarding the reliability, and the incorporation of existing monitors in the intensive care unit. Many algorithms exist for titrating insulin rates from intermittent BG measurements (14–17) but none have been explicitly linked to a continuous glucose monitor. In the ambulatory environment, continuous glucose monitoring in real-time (CGM-RT) has been combined with a proportional-integral-derivative (PID) algorithm to effect stable glucose levels in individuals with type 1 diabetes mellitus (18;19). In the pediatric ICU, the algorithm has been linked with intermittent BG values (20).
We are currently conducting a two-center prospective randomized control trial to assess the benefit of TGC in children aged 3 or under undergoing cardiac surgery with cardiopulmonary bypass. The TGC study uses CGM-RT together with the PID titration protocol. The study is not scheduled for completion until 2011 with the primary outcome results to be made available at that time; however, interim data on the performance of CGM-RT device and the PID titration algorithm has been made available for this report evaluating whether the device and algorithm can reduce hypoglycemia during TGC.
Materials and Methods
Study design
Children 3 years of age or less undergoing cardiac surgery with cardiopulmonary bypass were recruited at Children’s Hospital Boston (CHB) and C.S. Mott Children's Hospital (CSMCH) in Michigan in on-going Trial of Euglycemia following Cardiac Surgery study (registered NCT00443599). As of 7/1/2009, 311 subjects were studied with 156 randomized to (standard care) STD and 155 to TGC. Subjects were identified by weekly review of scheduled elective cardiac surgery patients and by daily monitoring of the operating room schedule. Informed consent was obtained from parents. Patients with diabetes mellitus or whose operative plans did not include cardiopulmonary bypass were excluded. Subjects randomized to TGC were monitored for the period in which an arterial line was maintained; for subjects randomized to STD monitoring was discontinued after 72 hours if the patient was clinically stable with no BG <60 mg/dL [3.3 mmol/L] for at least 12 hours. Glucose levels in subjects randomized to STD were managed according to standard practice at each institution, with no explicit protocol requirement for initiating insulin or treating hypoglycemia. In subjects randomized to TGC, glucose levels were managed in accordance to Insulin therapy and glucose rescue algorithm described below. Standard nutritional guidelines were followed at each institution.
All subjects underwent CGM-RT (Guardian Continuous Glucose Monitor Real-Time system, Medtronic-MiniMed, Northridge, CA). CGM-RT sensors were inserted at a 45 degree angle into the patient’s lateral thigh after induction of anesthesia. Each sensor was connected to a wireless transmitter no sooner than 5 minutes after the insertion, and calibration was performed once the patient arrived in the cardiac ICU (time 0 for all analyses). Sensors were replaced every 72 hours or sooner as described in the Insulin therapy and glucose rescue algorithm below. The device was calibrated using arterial BG assessed with bedside meters available at the respective institutions (SureStepFlexx, LifeScan, at CHB and AccuCheck Inform, Roche, at CSMCH) ~3 times per day in STD and 8–18 times per day during TGC, depending on the number of the therapy changes. All values were used for calibration in the STD arm and a subset of values defined by Insulin therapy and glucose rescue algorithm were used in the TGC arm. Arterial BG was also assessed using central laboratory analyzers as dictated by each institution’s standard practice (~6 times per day at CHB using a Bayer RapidLab 860 [Tarrytown, NY];once per day at CSMCH using a Siemens Advia 2400 analyzer [Deerfield, IL]). A VAMP Jr. (Edwards) blood sampling system was introduced to reduce blood loss associated with frequent blood draws. When possible, meter values below 60 mg/dL [3.3 mmol/L] were confirmed by the central laboratory using an independent blood sample; however, if the meter assessment of arterial blood glucose was deemed critically low an exogenous glucose bolus, calculated by the algorithm, was given in advance of performing a second blood draw.
Insulin therapy and glucose rescue algorithm
In the TGC arm, the PID algorithm was used to recommend a variable intravenously (IV) insulin infusion rate. The algorithm has been previously described (20;21) but was modified here to allow SG values to be input more frequently (every 0.5 to 1 hour) than BG values (0.5 to 6 hrs) and for glucose rescue to be given as a bolus rather than an infusion. The algorithm recommended an intravenous insulin infusion rate in proportion to glucose above or below target (P; 0.5 U/kg/hr per gram/dL [9 U/kg/hr per mmol/L] with target = 95 mg/dl [5.3 mmol/L]); the history of glucose above or below target (I; ratio P:I = 300 minutes); and, the rate-of-change of glucose (derivative D; ratio D:P = 30 minutes). If the sum of P, I, and D was negative a recommendation to suspend insulin delivery was made; if glucose fell, or was anticipated by the algorithm to fall, below 60 mg/dL [3.3 mmol/L] a recommendation to suspend insulin delivery and administer IV glucose was made (1.5 g per U/hr below zero). A minimum change in insulin delivery (0.003 U/kg/hr and 20%) was set to prevent frequent minor adjustments requiring blood draws.
Calculations were performed using SG values entered into a spreadsheet at times recommended by the algorithm based on the glucose value and its rate of change; however if a change in therapy was recommended an arterial blood sample was obtained and the recommend insulin delivery rate recalculated using the meter estimate of blood glucose rather than the sensor value. If possible, two consecutive BG values were used to estimate the glucose rate of change; if two consecutive values were not available, consecutive SG values were used. Insulin delivery was suspended during periods in which SG was unavailable for any reason.
During the study period, four additional changes to the PID algorithm were made. 1) When first implemented, a 30% difference between SG and BG used to effect therapy resulted in a request to recalibrate the sensor and any two recalibrations in a three hour period generated a recommendation to replace the sensor. This rule was removed and decisions to replace sensors were made on a case-by-case assessment of sensor performance. 2) The factor used to convert negative requests for insulin delivery to positive recommendations for glucose boluses was increased by a factor of two if a glucose bolus had been delivered at the previous sample time. 3) The CGM-RT hypoglycemic alarm threshold was increased from 60 mg/dL [3.3 mmol/L] to 70 mg/dL [3.9 mmol/L] and a 15 minute forward prediction feature available on the CGM-RT device was enabled. 4) The maximum insulin delivery when glucose was within target range and not changing was decreased from 0.1325 U/kg/hr to 0.03 U/kg/hr. Algorithm changes were made following consultation with the study’s independent Data Safety Monitoring Board (DSMB), appointed and overseen by the National Heart Lung and Blood Institute.
Statistical Analysis
CGM-RT sensor performance was evaluated by calculating the mean-absolute-relative-difference (MARD) between SG and arterial whole BG assessed with bedside meters (MARD = 100•|BG-SG|/BG), and by the regression of SG versus BG. Regression analysis was performed separately for STD and TGC with differences in slope and intercept used to assess any difference in sensor performance in the two study arms. Hypoglycemic alarms were characterized as true-positive (TP) or false-positive (FP), with the analysis performed separately at the 60 [3.3 mmol/L] and 70 mg/dL [3.9 mmol/L] hypoglycemic thresholds. For both thresholds, a TP was defined as an alarm with a confirmed BG less than or equal to 60 mg/dL [3.3 mmol/L], and a FP was defined as an alarm with confirmed BG greater than 60 mg/dL [3.3 mmol/L] (i.e., at the 70 mg/dL [3.9 mmol/L] alarm threshold, an alarm with a confirmed BG between 60 and 70 mg/dL [3.3 and 3.9 mmol/L] was defined as a FP).
To assess whether CGM-RT was effective in reducing hypoglycemia, the number of occurrences in which the CGM alarmed at a glucose value above 60 mg/dL [3.3 mmol/L], but was falling at a rate sufficiently fast for the algorithm to anticipate a value below 60 mg/dL [3.3 mmol/L] within 30 minutes, and which resulted in a glucose rescue bolus in advance of glucose falling below 60 mg/dL [3.3 mmol/L], were counted as “Sensor Enabled Therapy Intervention” (SETI) events. Instances where the device alarmed while glucose was above 60 mg/dL [3.3 mmol/L] but where there was insufficient time to allow staff to prevent the fall in glucose to below 60 mg/dL [3.3 mmol/L], were counted as “Sensor Enabled Therapy Alerts” (SETA). SETI alarms were deemed to have prevented the hypoglycemic event; SETA alarms were deemed to have reduced the duration of the event. SETI and SETA alarms were analyzed separately for the STD and TGC groups.
Average BG profiles were calculated using the central laboratory values obtained each day as dictated by standard practice (CHB) or using bedside meter values (CSMCH). Values were interpolated on a 30 minute interval before averaging. Time-to-target (first value within 80–110 mg/dL [4.4 to 6.1 mmol/L]), time-in-target, and histograms of glucose concentration were calculated from the interpolated curves. Severe hypoglycemia was quantified as the percentage of subjects with any occurrence of BG<40 mg/dL [2.2 mmol/L]. Mild hypoglycemia was quantified as the percentage of subjects with an occurrence of BG greater than or equal to 40 and less than 60 mg/dL [3.3 mmol/L]. Statistical analyses (significance of regression slopes, differences in regression lines, χ2 confidence intervals for percentages, unpaired t-test for other comparisons), were performed using GraphPad Prism version 5.00 (GraphPad Software, San Diego California USA) with P<0.05 considered significant. Data are reported as mean and standard error of the mean (SEM) unless otherwise stated. The study protocol was approved by the CHB and CSMCH institutional review boards.
Results
Subject Enrollment
Consent was obtained from 318 families. Five subjects became ineligible during surgery due to operations performed off bypass, one subject was withdrawn by the surgeon immediately after surgery for severity of illness, and one subject was withdrawn by the investigators prior to surgery based on staff availability. These subjects did not receive any study intervention, including CGM-RT, and did not have data available for analysis. For the remaining 311 subjects, age, weight, severity of illness, and gender were similar between STD and TGC (Table 1). Data reported here exclude a preliminary ten subject pilot phase.
Table 1.
Characteristics of patients undergoing standard care (STD) and tight glycemic control (TGC); data reported as mean ± standard deviation. Severity of illness was assessed using the Risk Adjusted Congenital Heart Surgery (RACHS) score (42) and time spent on Cardio Pulmonary Bypass (CPB)
| STD | TGC | p-value | |
|---|---|---|---|
| Total N | 156 | 155 | |
| Female (N) | 70 | 67 | P=0.82 |
| Age (Mo) | 8.6 ± 9.0 | 8.2 ± 9.2 | P=0.70 |
| Weight (kg) | 6.6 ± 2.9 | 6.2 ± 3.0 | P=0.23 |
| Severity of Illness RACHS Score Minutes on CPB |
2.8 ± 1.2 116 ± 73 |
2.7 ± 1.1 113 ± 62 |
P=0.42 P=0.69 |
Glucose Control
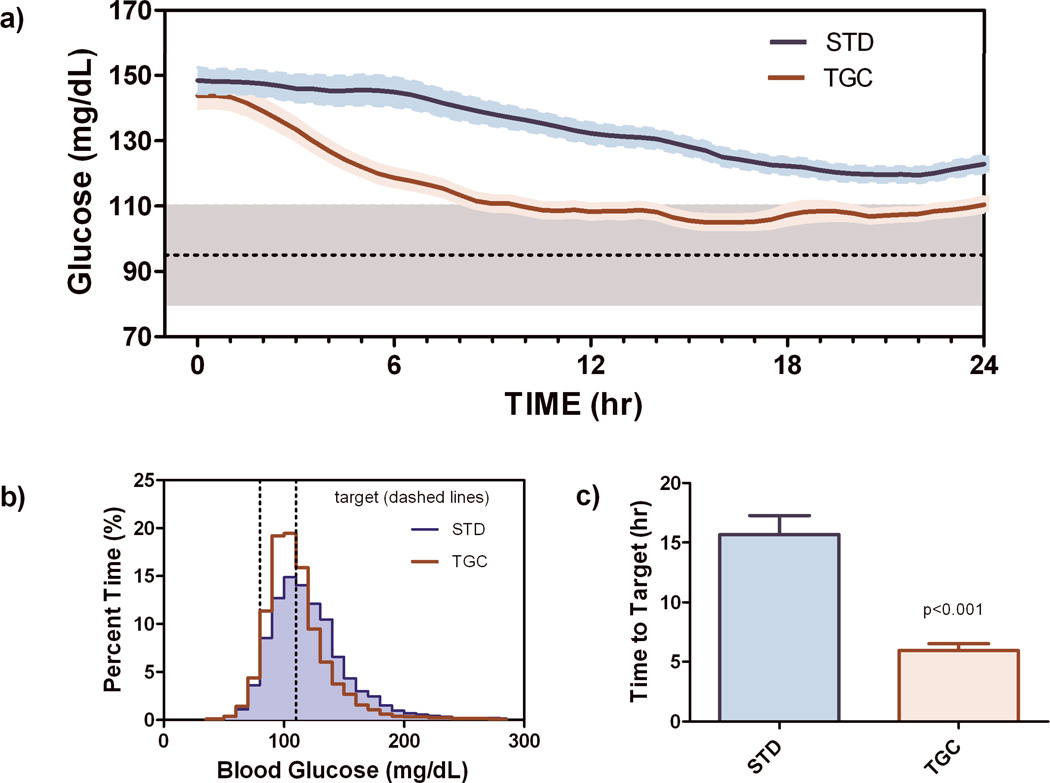
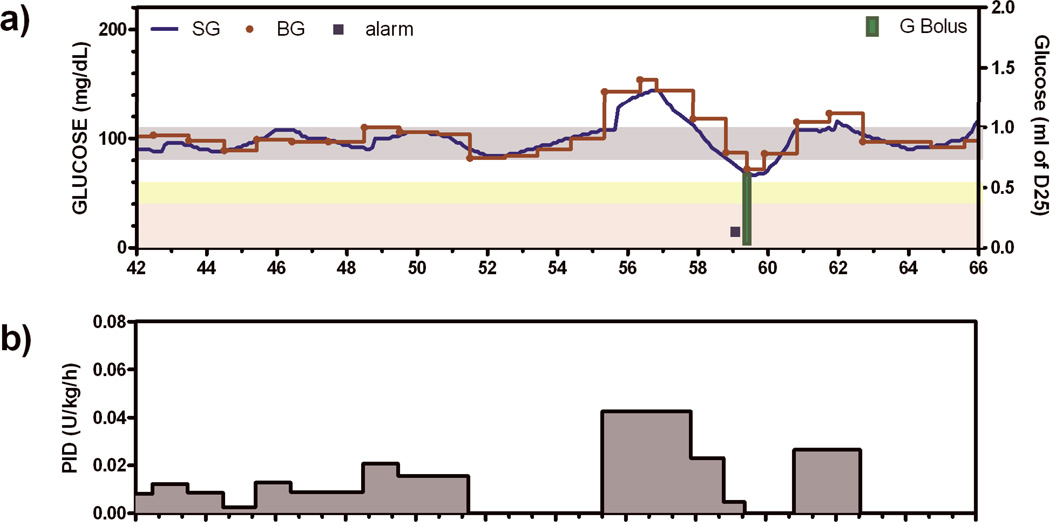
BG was not different in the two treatment groups at time 0 (149 ± 3.92 mg/dl vs. 145 ± 4.11, [8.3 ± 0.2 mmol/L vs. 8.1 ± 0.2 mmol/L] STD versus TGC respectively; p=0.22). Thereafter, patients undergoing TGC achieved target glucose significantly faster (5.96±0.594 vs. 15.0±1.56 hrs; p<0.0001) and were within target range significantly longer (48±1.8% of time vs. 30±2.0%; p<0.0001; Figure 1) than patients undergoing STD. The percent of subjects with severe hypoglycemia during TGC was 3.2% (5 of 155 TGC subjects; 95% Confidence Interval [CI] 1.2 to 7.5%) with one instance of BG < 40 mg/dL [2.2 mmol/L] observed in the STD arm. No subject had more than one hypoglycemic event. In the TGC arm, there were 8 instances of CGM-RT alarming with BG > 60 mg/dL [3.3 mmol/L] but falling at a rate sufficient for the algorithm to recommend a rescue glucose bolus (Table 2; SETI alarms). For these alarms, an example shown in Figure 2 with an alarm at ~59 hours, the SG value was typically confirmed within 20 minutes (23 minutes in Figure 2) and an IV glucose bolus given to prevent any further fall in glucose. In 8 additional instances, the alarm occurred in advance of glucose falling below 60 mg/dL [3.3 mmol/L], but with insufficient time for the BG level to be confirmed and the IV glucose bolus given to prevent the glucose level from falling below 60 mg/dL [3.3 mmol/L] (Table 2; SETA alarms). There were 3 SETA alarms in the STD arm.
Figure 1.
a) Glucose profiles (mean±sem) obtained over the first 24 hours of tight glycemic control (TGC) and standard care (STD). b) Histogram of all glucose levels assessed individually from the start of continuous glucose monitoring, c) Time to target glucose assessed individually.
Table 1.
True positive (TP) and false positive (FP) alarms with alarm thresholds at 60 mg/dL and no forward prediction, and at 70 mg/dL with 15 minute forward prediction. At both alarm settings a TP was defined as an alarm that occurred with a confirmed blood glucose values less than or equal 60 mg/dL and a FP was defined as an alarm with a confirmed blood glucose value greater than or equal to 60 mg/dL. Sensor Enabled Therapy Intervention (SETI) was defined as a sensor alarm that resulted in an exogenous glucose bolus being given in advance of BG falling below 60 mg/dL. Sensor Enabled Therapy Alert (SETA) was defined as a sensor alarm resulting in an exogenous glucose bolus not given in time to prevent BG from falling below 60 mg/dL.
| Subjects (N) | BG<40 mg/dL |
BG 40–60 mg/dL |
TP | FP | % detected |
SETI | SETA |
|---|---|---|---|---|---|---|---|
| Alarm set at 60 mg/dL | |||||||
| All (64) | 3 | 19 | 0 | 9 | 0 | 0 | 0 |
| TGC (30) | 3 | 17 | 0 | 9 | 0 | 0 | 0 |
| STD (34) | 0 | 2 | 0 | 3 | 0 | 0 | 0 |
| Alarm set at 70 mg/dL | |||||||
| All (247) | 3 | 46 | 20 | 334 | 41% | 8 | 11 |
| TGC (125) | 2 | 38 | 18 | 262 | 45% | 8 | 8 |
| STD (122) | 1 | 8 | 3 | 74 | 33% | 0 | 3 |
Figure 2.
a) Example subject in which hypoglycemia was deemed to have been prevented by predictive hypoglycemic alarm (square symbol) and glucose rescue bolus (green bar). Grey bar indicates target glucose, yellow shading indicates mild hypoglycemia, and light red shading indicates severe hypoglycemia b) Insulin delivery recommendations made by the Proportional Integral Derivative (PID) algorithm.
Sensor Performance
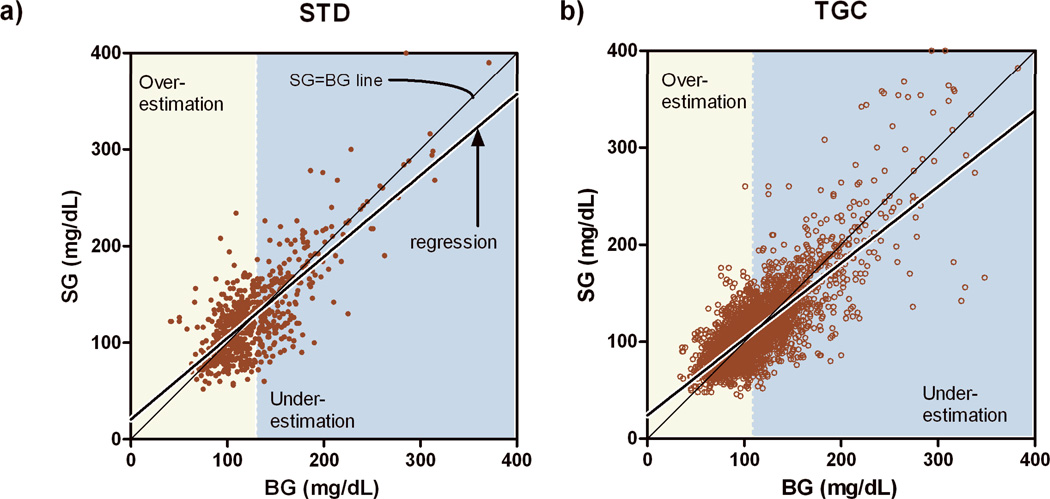
MARD was 16.0%. Regression analysis (Figure 3) indicated slopes less than 1 (0.81 ± 0.027 and 0.78 ± 0.011) and intercepts greater than 0 (24 ± 1.3 mg/dL [1.3± 0.072 mmol/L] and 25 ± 3.6 mg/dL [1.4±0.2 mmol/L ; p<0.05, STD and TGC respectively), with the STD and TGC regression lines different from each other (p<0.05). In STD, the regression line intersected the SG=BG line at BG=132 mg/dL [7.3 mmol/L] (95% CI 130–135 mg/dL [7.22–7.5 mmol/L]) and in TGC the intersection was at 113 mg/dL [6.3 mmol/L] (95% CI 112–114 mg/dL [6.2–6.3 mmol/L]). In each case the intersection point was not different from the average BG value used for calibration (128 mg/dL [7.1 mmol/L] with 95% CI 124–131 mg/dL [6.9–7.3 mmol/L] for STD; 113 mg/dL [6.3 mmol/L] with 95% CI 112–114 mg/dL [6.2–6.3 mmol/L] for TGC). From the regression lines, the expected SG value at the hypoglycemic threshold of 60 mg/dL [3.3 mmol/L] was estimated to be 71 mg/dL [3.9 mmol/L] (95% CI 67 to 75 mg/dL [3.7 to 4.2 mmol/L]) in STD and 72 mg/dL [4.0 mmol/L] (95% CI 70 to 73 mg/dL [3.9–4.1] mmol/L) in TGC. Thus, with the CGM-RT hypoglycemic alarm-threshold set at 60 mg/dL [3.3 mmol/L] (subjects 1–64) 0 of 19 hypoglycemic excursions below 60 mg/dL [3.3 mmol/L] were detected. Increasing the alarm threshold to 70 mg/dL [3.9 mmol/L] and adding a 15 minute forward prediction improved the detection rate to 43% (Table 2) but generated 16–17 false-positive alarms for every true positive (between 1 and 2 false alarms per subject).
Figure 3.
Regression of sensor glucose (SG) versus arterial blood glucose (BG). Bias in regression slope and offset results in overestimation (yellow) of BG at low values and underestimation (blue) at high values of BG. Intersection of SG=BG and regression lines indicates the point at which the sensor is unbiased. Significant differences in STD and TGC regression lines were observed (p<0.05) with SG overestimating BG below 132 mg/dL in the STD group and below 109 mg/dL in the TGC group.
Discussion
Our analysis indicates that the incidence and/or duration of hypoglycemia following cardiac surgery in children aged 3 or less can be reduced by CGM-RT. However, this conclusion is based on retrospective analysis of CGM-RT alarms in an ongoing trial of TGC designed to assess nosocomial infection rate with and without TGC, rather than a prospective study of hypoglycemia during with and without continuous glucose monitoring. With these analyses, it is not possible to ascertain definitively if all patients predicted to become hypoglycemic would have done so had we not administered a glucose bolus. As well, in cases where the alarm occurred near a scheduled blood sample it is difficult to determine if the duration of an individual event was reduced. Nonetheless, the results show that CGM-RT, when combined with an explicit insulin titration algorithm, allows TGC in the 80–110 mg/dL [4.4–6.1 mmol/L] glucose range to be achieved without substantially increasing the risk of hypoglycemia. This requires the CGM-RT alarms be set to the expected sensor value at the hypoglycemic alarm threshold. We estimated that at a hypoglycemic threshold of 60 mg/dL [3.3 mmol/L], the CGM-RT sensor would read ~70 mg/dL [3.9 mmol/L]. Increasing the alarm threshold however, results in a substantial number of false alarms.
False alarms, or any other sensor value falsely indicating the need for a change in therapy, increased nursing workload in this study, as each instance required the nurse to obtain a confirmatory blood glucose sample. While any individual blood sample may only take 2–3 minutes to obtain, repeated alarms interrupt the flow of work and add substantially to the total effort. The increase in workload has to be weighed against the benefit of preventing or reducing hypoglycemia. We estimated ~33% of our subjects benefited by the use of CGM-RT. We arrived at this estimate by examining the hypoglycemic incidence rate with the alarm threshold set at 70 mg/dL (Table 2). At this threshold, we observed 38 instances of mild hypoglycemia and 2 instances of severe hypoglycemia in 125 patients undergoing TGC. We estimated that an additional 8 instances would have occurred had the CGMS-RT system not been used to effect a glucose rescue bolus (SETI alarms), and that the 8 alarms that occurred without sufficient time to prevent the fall in glucose to below 60 mg/dL (SETA alarms) may still have reduced its duration. Thus, 16 of the 48 (33%) total possible hypoglycemic events were prevented or reduced in duration. In the STD arm, we observed 8 instances of mild hypoglycemia and 1 instances of severe hypoglycemia in 122 patients. In 3 of these 9 instances (33%), the duration may have been reduced by the SETA alarms.
The CGM-RT device also failed to detect hypoglycemia in a substantial number of cases (Table 2). The inability of the CGM-RT device to detect hypoglycemia was largely due to a bias in the regression line relating sensor glucose to blood glucose (Figure 3). The bias may be related to an offset in sensor current (23) as this would also explain the observation that the regression lines were different in the STD and TGC groups. That is, bias in regression slope resulting from offset current is a function of the average glucose value used for calibration (24). In this study, the average calibration value was higher in the STD arm than the TGC arm. The observation that the CGM-RT has a different regression bias in the two treatment arms argues that it cannot be used to compare time spent hypo- or hyperglycemic, as these times will be under or overestimated to different degrees.
The use of point-of-care (POC) meters may also have contributed to the bias as well as the overall error. The MARD between the CGM-RT monitor and the point-of-care meter observed here is lower than the value we previously reported in the ICU (16.0% versus 17.6%; (25)), but ambulatory studies have reported still lower values (14% for reference glucose values 121–180 mg/dL (26)). Bias in the regression line has been also been shown in some (19) but not all (18) ambulatory studies. It is difficult to determine what the relative contribution of POC-meter error was to the total MARD. In this study, we introduced a closed blood sampling system designed protect against blood loss. With the closed blood draw system, which we found to improve the consistency of blood draws, we estimate the MARD between BG determinations taken with five minutes of each other to be 6.2% (Children’s Hospital Boston site). We also observed a small, but statistically significant, bias between the POC-meter and laboratory analyzer (regression slope and offset 0.98 and 4.9 mg/dL), but do not believe this small bias in slope (2%) can account for the much larger bias (~20%; Figure 3) observed between the sensor and point of care meter. However, any improvement in the point of care meter accuracy, or decrease in the time required to obtain a glucose value from a central laboratory, can be expected to improve the accuracy of a continuous monitor that uses either value for calibration.
The present study linked the CGM-RT device to a proportional plus integral plus derivative (PID) insulin titration algorithm (20;21). The algorithm required the target glucose value be set to an acceptable range (80–110 mg/dL). The algorithm has an implicit target rate-of-change of glucose ([BG-target]/300 in units of mg/dL per minute; 300 minutes being the ratio P:I). The target range chosen in our study matches the target set in the original Van den Berghe adult trial (2;27) but is higher than the range used by that group in their subsequent pediatric trial (3). Neither the target range, nor how fast it should be achieved is well established at this time. Achieving target more rapidly is generally viewed as a desirable characteristic of insulin titration algorithms (28), but it is unclear if the target needs to be achieved more rapidly than the rate chosen here. Hypoglycemia can intuitively be expected to increase whenever the target is lowered, or the desired rate at which it is to be achieved made faster (21).
The percentage of subjects with severe hypoglycemia (3.2%) in this study was approximately one-half that reported in the NICE-SUGAR study (6%; (4)), and far less than the rates reported in the Volume Substitution and Insulin Therapy in Severe Sepsis study (VISEP (5); 17%) or the Study Comparing the Effects of Two Glucose Control Regimens by Insulin in Intensive Care Unit study (GluControl (6); 9.8%), both of which were stopped over concerns related to hypoglycemia. The study most comparable to that reported here is the pediatric ICU study by Vlasselaers et al. (3) in which 75% of subjects were post cardiac surgery. That study (3) showed an ~ 50% reduction in 30 day mortality but reported a 25% incidence rate of severe hypoglycemia (44% in subjects below one year of age). The study did however target a lower glucose range (50–80 mg/dL in children <1 year of age and 70–100 mg/dL in children >1 year). Thus, while the overall hypoglycemic rate reported here is lower than other TGC trials, the comparisons is confounded by differences in patient populations, target glucose ranges, and the algorithms used to adjust insulin. Prevention of hypoglycemia in our study was due to a combination of CGM-RT and the use of PID algorithm and it is difficult to evaluate the relative contribution of each. A single center RCT study effecting the same titration algorithm with and without CGM observed a reduction in the incidence of hypoglycemia over a 72 hour period, but did not observe an improvement in mean glucose level achieved (29). Still, that study and the results report here support the argument that CGM-RT can reduce the incidence of hypoglycemia.
Conclusions
Use of CGM-RT can prevent some but not all instances of hypoglycemia. The reliability of the hypoglycemia detection can be improved by increasing the alarm threshold but this increases the rate of false alarms, and adds to the nursing workload. Using CGM-RT to improve glucose control in the ICU requires the continuous glucose measurement be combined with an insulin titration algorithm, preferable one that allows for glucose rescue in response to unexpected hypoglycemia. The PID algorithm is well suited to this task, as the target and the rate at which the target is to be achieved, is easily adjusted. The algorithm also provides a systematic means to correct hypoglycemia. Frequent blood glucose values are still required however to protect against sensor error. Still, we reason that until the long-term neurobehavioral consequences of hypoglycemia are better understood, CGM-RT is warranted despite the time required to obtain these samples.
Acknowledgments
Work for the present manuscript was supported by grants from the NIH National Heart, Lung, and Blood Institute (R01HL88448 to MA), National Center for Research Resources (UL1-RR025758 to the Harvard Catalyst Clinical & Translational Science Center) and the National Center for Research Resources (MO1-RR02172 to the CHB General Clinical Research Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: No financial disclosures by any author.
Reference List
- 1.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006 Feb 2;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 3.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van dH I, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009 Feb 14;373(9663):547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 4.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009 Mar 26;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 5.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008 Jan 10;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 6.Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009 Oct;35(10):1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 7.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Ahluwalia JS, Vanhole C, Palmer C, et al. A randomised controlled trial of early insulin therapy in very low birth weight infants, "NIRTURE" (neonatal insulin replacement therapy in Europe) BMC Pediatr. 2007;7:29. doi: 10.1186/1471-2431-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagshaw SM, Egi M, George C, Bellomo R. Early blood glucose control and mortality in critically ill patients in Australia. Crit Care Med. 2009 Feb;37(2):463–470. doi: 10.1097/CCM.0b013e318194b097. [DOI] [PubMed] [Google Scholar]
- 9.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. Jama. 2008 Aug 27;300(8):933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 10.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007 Oct;35(10):2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 11.Chaney MA, Nikolov MP, Blakeman BP, Bakhos M. Attempting to maintain normoglycemia during cardiopulmonary bypass with insulin may initiate postoperative hypoglycemia. Anesth Analg. 1999 Nov;89(5):1091–1095. doi: 10.1213/00000539-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hermanides J, Bosman RJ, Vriesendorp TM, Dotsch R, Rosendaal FR, Zandstra DF, et al. Hypoglycemia is associated with intensive care unit mortality*. Crit Care Med. 2010 Apr 8; doi: 10.1097/CCM.0b013e3181de562c. [DOI] [PubMed] [Google Scholar]
- 13.Vogelzang M, Ligtenberg JJ. Practical aspects of implementing tight glucose control in the ICU. Curr Opin Clin Nutr Metab Care. 2007 Mar;10(2):178–180. doi: 10.1097/MCO.0b013e32801776a3. [DOI] [PubMed] [Google Scholar]
- 14.Wilson M, Weinreb J, Hoo GW. Intensive insulin therapy in critical care: a review of 12 protocols. Diabetes Care. 2007 Apr;30(4):1005–1011. doi: 10.2337/dc06-1964. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg PA, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care. 2004 Feb;27(2):461–467. doi: 10.2337/diacare.27.2.461. [DOI] [PubMed] [Google Scholar]
- 16.Workbook for Improvement: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Society of Hospital Medicine website. 2008 Mar 10; [Google Scholar]
- 17.Meijering S, Corstjens AM, Tulleken JE, Meertens JH, Zijlstra JG, Ligtenberg JJ. Towards a feasible algorithm for tight glycaemic control in critically ill patients: a systematic review of the literature. Crit Care. 2006 Feb;10(1):R19. doi: 10.1186/cc3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006 Dec;55(12):3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 19.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008 May;31(5):934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 20.Wintergerst KA, Deiss D, Buckingham B, Cantwell M, Kache S, Agarwal S, et al. Glucose control in pediatric intensive care unit patients using an insulin-glucose algorithm. Diabetes Technol Ther. 2007 Jun;9(3):211–222. doi: 10.1089/dia.2006.0031. [DOI] [PubMed] [Google Scholar]
- 21.Steil GM, Deiss D, Judy Shih, Buckingham B, Weinzimer SA, Agus MS. ICU Insulin delivery algorithms: Why so many? How to choose? Journal of Diabetes Science and Technology. 2009 Jan 1;3(1):125–140. doi: 10.1177/193229680900300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosiborod M, Inzucchi SE, Goyal A, Krumholz HM, Masoudi FA, Xiao L, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. Jama. 2009 Apr 15;301(15):1556–1564. doi: 10.1001/jama.2009.496. [DOI] [PubMed] [Google Scholar]
- 23.Youssef JE, Castle JR, Engle JM, Massoud RG, Ward WK. Continuous Glucose Monitoring in Subjects with Type 1 Diabetes: Improvement in Accuracy by Correcting for Background Current. Diabetes Technol Ther. 2010 Sep 30; doi: 10.1089/dia.2010.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebrin K, Sheppard NF, Steil GM. Use of Subcutaneous Interstitial Fluid Glucose to Estimate Blood Glucose: Revisiting Delay and Sensor Offset. J Diabetes Sci Technol. 2010;4(5):1087–1098. doi: 10.1177/193229681000400507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piper HG, Alexander JL, Shukla A, Pigula F, Costello JM, Laussen PC, et al. Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics. 2006 Sep;118(3):1176–1184. doi: 10.1542/peds.2006-0347. [DOI] [PubMed] [Google Scholar]
- 26.Tansey MJ, Beck RW, Buckingham BA, Mauras N, Fiallo-Scharer R, Xing D, et al. Accuracy of the modified Continuous Glucose Monitoring System (CGMS) sensor in an outpatient setting: results from a diabetes research in children network (DirecNet) study. Diabetes Technol Ther. 2005 Feb;7(1):109–114. doi: 10.1089/dia.2005.7.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, et al. Clinical review: Intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. 2009 Sep;94(9):3163–3170. doi: 10.1210/jc.2009-0663. [DOI] [PubMed] [Google Scholar]
- 28.Blaha J, Kopecky P, Matias M, Hovorka R, Kunstyr J, Kotulak T, et al. Comparison of three protocols for tight glycemic control in cardiac surgery patients. Diabetes Care. 2009 May;32(5):757–761. doi: 10.2337/dc08-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holzinger U, Warszawska J, Kitzberger R, Wewalka M, Miehsler W, Herkner H, et al. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care. 2010 Mar;33(3):467–472. doi: 10.2337/dc09-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]