Abstract
The efficient production of human neocortical neurons from human embryonic stem cells (hESC) is the primary requirement for studying early stages of human cortical development. We used hESC to obtain radial glial cells (hESC-RG) and then compared them with RG cells isolated from human fetal forebrain. Fate of hESC-RG cells critically depends on intrinsic and extrinsic factors. The expression of Pax6 (intrinsic factor) has a similar neurogenic effect on hESC-RG differentiation as reported for human fetal RG cells. Factors from the microenvironment also play a significant role in determining hESC-RG cell fate. In contrast to control cultures, wherein hESC-RG generate mainly astroglia and far fewer neurons, in co-cultures with human fetal forebrain cells, the reverse was found to be true. This neurogenic effect was partly due to soluble factors from human fetal brain cultures. The detected shift towards neurogenesis has significance for developing future efficient neuro-differentiation protocols. Importantly, we established that hESC-RG cells are similar in many respects to human fetal RG cells, including their proliferative capacity, neurogenic potential, and ability to generate various cortical neuronal sub-types. Unlike fetal RG cells, the hESC-RG cells are readily available and can be standardized, features that have considerable practical advantages in research and clinics.
Keywords: radial glia cells, multipotent progenitors, neurogenetic effect, human fetal co-culture
1.0 INTRODUCTION
Human embryonic stem cells (hESC) provide an ideal system for understanding brain development under both healthy and diseased conditions. They represent a potentially unlimited source of transplantable cells for the therapy of neurodegenerative disorders, and provide an alternative to drug screening and toxicology assays. Numerous studies have investigated the neurogenic potential of embryonic stem cells and demonstrated that they could be the source of a wide variety of differentiated cells including motoneurons (Li et al., 2005), dopaminergic and serotoninergic neurons (Belinsky et al., 2011; Yan et al., 2005), glutaminergic and GABAergic neurons (Li et al., 2009), peripheral neurons (Mizuseki et al., 2003), astrocytes and oligodendrocytes (Brustle et al., 1999), and radial glial (RG) cells (Nat et al., 2007).
Radial glial cells are formed from neuroepithelial cells in the early stages of the of the nervous system development. Along with neuroepithelial markers such as the intermediate filament protein nestin (Hartfuss et. al., 2001; Pollard et al., 2006) and stem cell markers, such as transcription factors Sox2 (Suh et al., 2007) and Pax6 (Bibel et al., 2004; Götz et al., 1998), they also express astroglia markers such as astrocyte-specific glutamate aspartate transporter (GLAST), brain lipid-binding protein (BLBP), vimentin, RC2, and glial fibrillary acidic protein (GFAP) (Campbell et al., 2002; Noctor et. al., 2001). It is the astroglial features that distinguish RG cells from neuroepithelial cells. A surface marker Lex1/CD15, an extracellular matrix-associated carbohydrate, is expressed both in mouse embryonic stem cells (Kim and Morshead, 2003; Capela and Temple, 2006) and human fetal radial glia (Mo et al., 2007). In the present study we identified RG cells in vivo and in vitro by astroglial progenitor/stem markers combined with their typical bipolar shape.
RG cells have been generated from a variety of different sources of embryonic and adult brains, and embryonic stem cells (Conti et al., 2005; Liour et al., 2003; Bibel et al., 2004; Malatesta et al., 2000). In addition to isolating RG cells from human fetal tissue (Mo et al., 2007), it has recently been shown that RG cells can be generated from hESC (Nat et al., 2007). We have used the abbreviation hESC-RG to refer to radial glia cells generated in this manner.
Originally, RG cells were demonstrated to be important in guiding radial migration of neurons (Bentivoglio et al., 1999; Rakic et al., 2003). However, it has been well-documented recently that RG cells are also multipotent progenitor/stem cells, and that they account for the majority of neurogenesis in the developing and postnatal rodent brain (Malatesta et al., 2000; Noctor et al., 2001; Miyata et al., 2001; Götz et al., 2005). In the human brain RG cells express GFAP in early stages of the emerging cerebral cortex (Zecevic, 2004; Howard et al., 2006), in contrast to rodents where this happens much later in corticogenesis. Human RG cells serve as multipotent neural progenitors generating both neurons and glial cells (Mo et al., 2007; Mo and Zecevic, 2008, 2009; Hansen et al., 2010). Transcription factor Pax6 (Pair Box 6) plays a significant role in neurogenetic capabilities of human fetal radial glia cells (Mo and Zecevic, 2008).
The objective of the present investigation was to compare RG cells in the human fetal forebrain (Mo et al., 2007, Mo and Zecevic, 2008) with hESC-RG cells with the idea that these cells can become an unlimited source of neurons available for research.
Our findings suggest that hESC-RG share many antigen characteristics, proliferative capacity, and differentiation pattern with human fetal RG cells and thus are suitable for further research on human brain development.
2.0 MATERIAL AND METHODS
2.1 Human ESC culture
Human ES cell line H9 (Stem Cell Core, UCONN) and H9 stably transfected with EGFP (enhanced green fluorescent protein) under a constitutively active CAG promoter, a gift from Dr. Cai, University of Connecticut Health Center, passages 30–45, were passaged weekly on a feeder layer of irradiated mouse embryonic fibroblasts (MEFs) as previously described (Zhang et al., 2001). The culture medium consisted of Dulbecco`s modified Eagle`s medium (DMEM)/F12 (GIBCO-BRL) with 20% knockout serum replacement, 1 mM glutamine, 1% nonessential amino acid (all from GIBCO-BRL), 0.1 mM β-mercaptoethanol, and 4 ng/ml bFGF2 (basic fibroblast growth factor; PeproTech, Lake Placid, NY).
The colonies were differentiated into neural cells using a protocol previously described by Nat et al., 2007. For immunostaining, flow cytometry and magnetic-activated cell separation, the floating aggregates were treated with Accutase™ (Chemicon) in 37° C incubator for ~ 10 min with to obtain single cells.
2.2 Co-Culture experiments
The hESC-RG expressing EGFP (3×103 cells/well) were plated over mixed cell cultures (1×105 cells/1.7 cm2 well of a 4-well chamber slide, BD Falcon) from the human fetal forebrain (17 to 20 gestational weeks-gw) containing both telencephalon and diencephalon, obtained from the Brain Bank repositories. Human tissue has been collected following rules of appropriate institutions, with written consent, from unidentifiable subjects. The cultures were maintained and processed as previously described (Howard et al. 2006, Zecevic et al., 2005, Mo et al., 2007). To study the effects of secreted factors from human fetal cells, conditioned medium (CM) was collected every 2 days from these primary cultures, filtered through a 0.22-μm membrane and stored at −20° C. This media was added in the same way as commercially available media was added to control cultures.
2.3 Immunostaining
Cell cultures were fixed with 4% paraformaldehyde and immunostaining was performed as previously described (Mo and Zecevic, 2008). Primary antibodies against the following proteins were diluted in the blocking solution and applied overnight: vimentin 1:200, GABA 1:300, MAP2 1:200 (Sigma, Sain Louis, MI), GFAP 1:2000, BrdU (Bromo-deoxyuridine) 1:100 (Dako, Denmark), calretinin 1:500 (Millipore, Temecula, CA), doublecortin 1:200 (Santa Cruz, CA), β-III-tubulin 1:2000, Pax6 1:1000 (Covance, CA), Ki67 1:25 (Beckman Coulter, France), phosphorylated vimentin 4A4 1:500 (MBL), tyrosine hydroxylase 1;1000 (Pel-Freez Biological, Rogers, Arkansas), SMI32 1:1000 (Sternberger Monoclonals Inc, Baltimore, Maryland), SV2 1:500 (Developmental Studies Hybridoma Bank, IA), PDGFRα 1:500 (Pharmingen, San Diego, CA), GFP 1:500 and Foxg1 1:1000 (Abcam, Cambridge, MA) and LeX/CD15 1:100 (Thermo Fisher Scientific, Temecula, CA), Tbr1 1:500 (Chemicon, Billerica, MA), Tbr2 1:500 (Gift from Dr Hevner, University of Washington, WA) NKx2.1 1:250 (Epitomics, Burlingame, CA).
Primary antibodies were followed by secondary antibodies (Jackson ImmunoResearch Laboratories, PA) for 1 hour and a short incubation in the nuclear stain bisbenzamide (Sigma). Coverslips were viewed with the Axioskop microscope (Zeiss, Germany).
2.4 Flow Cytometric Analysis
Flow cytometric analysis was performed by standard staining procedures on BD-FACS Aria (BD Biosciences, San Jose, CA) using APC Mouse Anti-Human CD15 (BD Biosciences). Dead cells were excluded from our analyses by their ability to incorporate propidium iodide (PI) and background fluorescence by using unlabeled cells and isotype controls. Data were analyzed using FlowJo Software (Tree Star, Inc., Ashland, OR).
2.5 Magnetic-activated cell sorting
To enrich RG cells for our in vitro studies, we applied a method based on the expression of LeX carbohydrate epitope (fucose N-acetyl lactosamine, an extracellular matrix-associated carbohydrate also known as LewisX, SSEA-1 or CD15) on the cell surface (Mo et al., 2007). We first generated dorsal forebrain progenitor cells from hESCs and then isolated a LeX+ population of hESC-RG cells using a magnetic cell separation system with LeX (CD15+) isolation kit (MACS, Miltenyi Biotec, Germany) according to manufacturer`s instructions. Selected cells were plated onto poly-L-ornithine-laminin-coated 12 mm coverslips (Carolina Biological Supply, Burlington, NC) and cultured in either proliferation medium (DMEM/F12/B27 supplemented with 20 ng/ml FGF2) or differentiation medium (DMEM/F12/B27 supplemented with 10 ng/ml brain derived neurotrophic factor-BDNF; PeproTech), as indicated. Subsequently, the cells were immuno-labeled with cell-type specific antibodies, as described above.
2.6 Pax6 loss-of-function experiments
Knockdown of Pax6 was achieved as previously described for fetal RG cells (Mo and Zecevic, 2008). hESC-RG cells were electroporated with 5 μg of either interference Pax6 siRNA or scrambled RNA (control) using Amaxa system (Nucleofection, Lonza, Gaithersburg, MD) according to manufacturer`s instructions.
2.7 Proliferation assay
A thymidine analog, bromodeoxyuridine (BrdU, 20 μM, Sigma, St Louis, MO) that incorporates into DNA of dividing cells was added to the cell cultures in proliferation medium for the last 6 hours before fixing and immunostaining as previously described (Mo et al., 2007).
2.8 Cell viability analysis
Ethidium homodimer-1 (EthD-1 Live/Dead Viability/Cytotoxicity kit; Invitrogen) was used in co-culture experiments to quantify cell death as the ratio of EthD-1+ cells to the total cell count.
2.9 Patch clamp
Whole-cell patch recordings were performed in the second week of neurodifferentiation (days 7, 8, and 9) and in the fourth week (days 21, 22, and 23). Patch clamp equipment, and physiological solutions were described previously (Belinsky et al. 2011). Before patching, the GFP-positive cells were identified by fluorescence; standard Olympus GFP filter cube. In one set of experiments, rhodamine-dextran 3000 (50 μM) was added to the intracellular solution. In voltage-clamp configuration, cells were given a series of voltage steps (duration, 50 ms) from −90 to +30 mV from a holding potential of −70 mV. In current-clamp configuration, after determining the actual resting membrane potential, all cells were first clamped at −60 mV using negative direct current (range 5 – 15 pA) and then a series of current steps from −20 to +120 pA was applied to test action potential (AP) firing pattern. Electrical traces were analyzed using Clampfit 9.2 (Molecular Devices). Data are expressed as means ± SEMs.
2.10 Quantitative Real-Time PCR analysis
Total RNA was extracted from cells using TRIZOL® reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Approximately 1 μg of RNA was used in the reverse transcription reaction using M-MuLV reverse transcriptase with random hexamers (Fermentas, Vilnus, Lithuania) according to the manufacturer’s instructions. Real-time PCR was performed in a Realplex2 Mastercycler (Eppendorf, Hamburg, Germany) using 96-well reaction plates (Eppendorf, Hamburg, Germany). The reactions were prepared according to the standard protocol for one-step QuantiTect SYBR Green RT-PCR (Applied Biosystems, Cheshire, UK). The sequences 5′→3′ of the forward (F) and reverse (R) primers were as follows:
GAPDH: (F) ACCACCATGGAGAAGGC/(R) GGCATGGACTGTGGTCATGA
Sox1: (F) CAATGCGGGGAGGAGAAGTC/(R) CTCTGGACCAAACTGTGGCG
Pax6:(F) AACAGACACAGCCCTCACAAACA/(R) CGGGAACTTGAACTGGAACTGAC
Otx2: (F) CCACAGCAGAATGGAGGTCA/(R) CTGGGTGGAAAGAGAAGCTG
Pax7: (F) CCAAGATTCTTTGCCGCTAC/(R) CAGGATGCCGTCGATGCTGT
HoxC8: (F) TTTATGGGGCTCAGCAAGAGG/(R) TCCACTTCATCCTTCGGTTCTG
Gsx2: (F) ACTTCGCACCTGCACTCCTC/(R) ACTTCGCACCTGCACTCCTC
Nkx2.1:(F) ATTGCTAGCGCCACCATGTCGATGAGTCCAAAG/(R) TTAGAATTCACCA GGTCCGACCATA.
Olig2: (F) AGTCATCCTCGTCCAGCACC/(R) TCCATGGCGATGTTGAGGT
The thermal cycle conditions were 95 °C for 2 min followed by 40 cycles of 15 sec at 95 °C, 15 sec at 55 °C and 20 sec at 68 °C. All ass ays were performed in triplicates. Averaged cycle of threshold (Ct) values of GAPDH triplicates were subtracted from Ct values of target genes to obtain ΔCt, and then relative gene expression was determined as 2−ΔΔCt. The results were presented relative to the control value, which was arbitrarily set to 1.
2.11 Cell counting and statistical analysis
Cells stained with the nuclear stain bisbenzamide and various cellular markers were visualized with a Zeiss Axiovision fluorescence microscope. Before quantification, 10 predesignated, adjacent optical fields of view were selected in each culture and examined at magnification 40x (one field has a surface area of 0.5 mm2). The percentage of immunolabeled cells of total bisbenzamide+ (BB) or GFP+ cells was calculated. Data are presented as a mean ± SEM. Statistical differences between the groups were evaluated with a Student’s t- test for unpaired data; level of significance was set at p ≤ 0.05.
3.0 RESULTS
3.1 Generation of hESC-radial glia cells
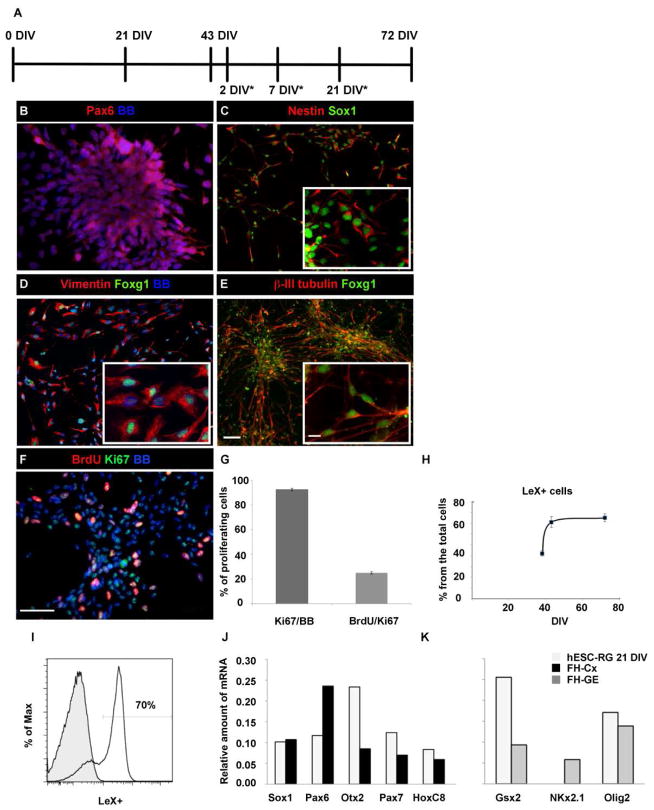
To generate radial glia cells from hESC we used a H9 cell line (passages 30–45) and a protocol from Nat et al., 2007. In our H9 cell cultures, neural tube-like structures became positive for Pax6 at 21 DIV (days in vitro) (Fig. 1A,B). After mechanical isolation and enzymatic dissociation of these neural tube-like structures, immunocytological analysis revealed the homogeneous expression of diagnostic markers of RG cells (Campbell et al., 2002; Noctor et al., 2001; Mo and Zecevic, 2008, 2009), including nestin, Sox1 (Fig. 1C) and vimentin (Fig. 1D) as well as the surface marker LeX.
Figure 1.
Radial glia cells generated from hESC (hESC-RG cells). (A) Experimental design of the time points at which the experiments were performed; DIV43 was a starting point for further experiments that were done at 2DIV*, 7DIV*, 21DIV* after that. (B) Neural tube-like structures labeled with Pax6 at 21 DIV. (C) These cells express neural progenitor markers, Sox1 and Nestin, as well as a forebrain marker Foxg1 in (D) vimentin+ radial glia and in (E) β-III-tubulin+ young neurons. (F) Cell proliferation seen with BrdU and Ki67 at 72DIV. (G) Histogram showing that the majority of cells at 72 DIV were labeled with Ki67 whereas a 25% were both BrdU+ and Ki67. (H) The percentage of LeX+ cells at three time points in culture. (I) Flow cytometry analysis: majority of cells were LeX+ (empty); isotype control antibody was used for background determination (solid). The % of Max is the number of cells in each bin divided by the number of cells in the bin that contains the largest number of cells (FlowJo software). (J,K) Quantitative RT-PCR analysis showing the transcriptional profiling of neural and rosto-caudal markers at 21 DIV. Fetal human cerebral cortex (FH-CX) and ganglionic eminence (FH-GE) were used as a positive control. Insets provide higher magnification of the double-labeled cells. BB- bisbenzamide stained cell nuclei. BrdU/Ki67- cells in phase S calculated as a percentage of BrdU+ from all Ki67+ cells. Ki67/BB- Ki67+ calculated as a percentage from all cells labeled with nuclear stain (BB). Scale bars: 50μm, 10μm (inset).
Immunocytochemical analysis of LeX expression in cultures carried out at 38, 43, and 72 DIV revealed that at 43 DIV the expression of LeX plateaued (Fig. 1H). Hence all subsequent experiments were commenced at 43 DIV (Fig. 1A). Flow cytometric analysis at 43 DIV established that 70±2% of cultured cells were immunopositive for LeX (Fig. 1I). Proliferation analysis with Ki67, a nuclear protein expressed in all phases of the cell cycle, in combination with BrdU, expressed in the S phase of the cell cycle, revealed that the majority of cells (92.5±0.3%) were proliferating. Furthermore, one fourth (25±0.11%) of all Ki67+ cells were in the S phase of the cell cycle as seen by co-labeling with BrdU (Fig 1F,G).
To study whether the cells in our cultures belong to the forebrain, we labeled them with Foxg1, a forebrain transcription factor (Tao et al., 1992). The expression of Foxg1 was detected at 21DIV, 30DIV and is present in 90% of all cells at 43 DIV, consistent with the time line of Foxg1 expression previously described by Li et. al., 2009.
We found that the initially observed expression of Foxg1 in radial glia at 43DIV (Fig. 1D) was maintained after cultures were kept in differentiation medium for additional 7 DIV* (Fig 1A), when cells differentiated in β-III-tubulin+ young neurons (Fig. 1E). Moreover, 90% of these cells were positive for dorsal telencephalic markers such as Pax6 and Tbr2, and only about 1% expressed ventral marker Nkx2.1 (Supplemental Figure 1). The rostro–caudal identity was confirmed by quantitative RT-PCR analysis (qPCR). The Fig 1J,K shows the expression of neuroectodermal marker Sox1, the transcriptional activation of other forebrain markers such as Otx2, but also Pax7, a dorsal marker of mesencephalon and spinal cord and thoracic marker HoxC8. Dorsal-ventral patterning was revealed by a strong expression of dorsal marker Pax6, but also two ventral markers, Gsx2 (lateral ganglionic eminence, LGE marker) and Olig2, whereas Nkx2.1 (marker of the medial GE) was not expressed in hESC-RG. Olig2 expression in these cells is in agreement with its expression in both ventral and dorsal regions of the human fetal forebrain (Jakovcevski and Zecevic, 2005). Our results demonstrate the heterogeneity of hESC-RG cell population, with both ventral and dorsal genes being present.
3.2 Enrichment of LeX+ cells
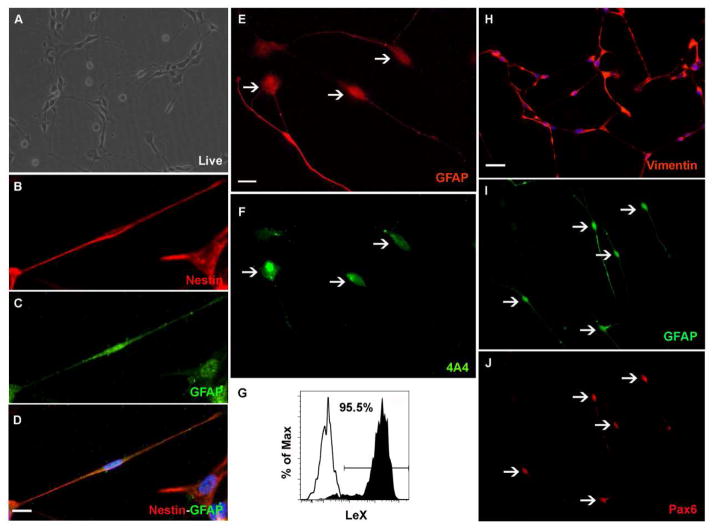
We have previously reported that RG cells can be enriched from human fetal brain tissue on the basis of the surface marker LeX (Mo et al., 2007). Using the same criteria and magnetic cell separation method (MACS), we isolated and characterized RG cells produced during in vitro neural differentiation of hESC (Fig. 2A).
Figure 2.
Characterization of hESC-RG cells isolated by magnetic activated cell separation system (MACS). (A) On adherent conditions the cells have bipolar morphology with long processes. (B–F) Cells co-labeled with nestin (B), GFAP (C), merged image (D) phosphorylated vimentin (4A4 antibody) (E) and GFAP (F). (G)The purity of LeX+ cells after MACS isolation in the stained sample (solid) vs the unstained sample (empty) demonstrates that LeX+ cells represent the majority of purified cells. (H–J) Bipolar cells express radial glia markers: vimentin (H), and co-labeled with GFAP (I) and Pax6 (J) (arrows indicate double-labeled cells. Nuclei were stained with bisbenzamide (Blue). Scale bars: 50μm, 10μm.
After MAC separation, flow cytometry analysis of the LeX+ cell fraction indicated a purity of 95.5% (Fig. 2G). The MACS enriched LeX+ cells were plated under adherent conditions and 24 hours later these cells had bipolar morphology of RG cells (Fig. 2A). Antigen characteristics of these cells confirmed their astroglia (GFAP, vimentin) and stem/progenitor fate (nestin, Pax6) The immunostaining experiments have shown that RG-like cells co-expressed the intermediate filament protein nestin, reported in mitotic radial glia cells (Hockfield et al., 1985; Noctor et al., 2001) and the astroglia marker GFAP (Fig. 2B–D). After 2 DIV* (Fig 1A) in proliferation medium, the cells could be double labeled with a marker of dividing RG cells (phosphorylated vimentin, antibody 4A4) and GFAP (Fig. 2E–F), Pax6 and GFAP (Fig. 2I–J), and vimentin (Fig. 2H). Overall these findings indicate that hESC-RG cells exhibit the same astroglia and stem/progenitor markers as RG cells isolated from human fetal tissue (Zecevic, 2004; Howard et al., 2006; Mo et al., 2007).
3.3 Proliferation and differentiation of hESC-RG cells in vitro
To evaluate the proliferative ability of hESC-RG cells, we added BrdU to cultures after LeX MAC sorting isolation. Immunostaining after 2 DIV* in proliferation medium demonstrated that 14.7% (118/803) of the LeX+ cells incorporated BrdU (Fig. 3A). Within the BrdU+ cells, 9.3% (3/32) were co-labeled with β-III-tubulin and 7.1% (3/42) with DCX (doublecortin), suggesting the presence of neuron-restricted-progenitors.
Figure 3.

Sorted hESC-RG cells are multipotent in vitro. (A) BrdU incorporation after 2 DIV* in proliferation medium. (B–F) In differentiation medium hESC-RG cells generate mainly mature-looking GFAP+ astrocytes (B), neurons labeled with β-III-tubulin (C), DCX+ (D) or MAP2 antibody (E), and rarely PDGFR-α+ oligodendrocyte progenitors (F). (G) Quantification of hESC-RG progeny after 7 DIV* in differentiation medium. Data represent three independent experiments. ***p < 0.0001. Nuclei were stained with bisbenzamide (Blue). Scale bars: 50μm, 10μm.
To assess the differentiation potential of hESC-RG cells, we cultured them in differentiation medium for 7 DIV* (Fig 1A) and applied cell-type specific markers. hESC-RG cells changed their morphology to more mature cell types, and differentiated predominantly (93±1.5%) into GFAP+ cells (Fig. 3B–C), far less (7±0.3%) into neurons labeled by immature neuron markers, βIII-tubulin (Fig. 3C–G) or the microtubule-associated proteins, DCX (Fig. 3D) and MAP2 (Fig. 3E). Few cells were immunolabeled with PDGFRα, a marker for immature oligodendrocyte progenitors (Fig. 3F). These results indicated that hESC-RG cells maintained pluripotency, differentiating mainly into astroglia and less often into neurons or oligodendrocytes, similar to reports on human fetal RG (Mo et al., 2007, Mo and Zecevic, 2008, 2009).
3.4 The role of transcription factor Pax6
Pax6 transcription factor (Pair Box 6) promotes the neurogenic fate of RG cells both in rodents (Götz et al., 1998) and in human fetal cortical cultures (Mo and Zecevic, 2008). Here we study the role of Pax6 in the proliferation and differentiation of hESC-RG by knocking down Pax6 expression.
Progeny of hESC-RG transfected with either interference siRNA Pax6 or control, scrambled RNA (cRNA) were compared. Three days after transfection with siRNA, Pax6 protein was not detected in hESC-RG cells (Supplemental Figure 2), whereas vimentin expression was unaffected (data not shown). Control transfected cells kept their Pax6 expression (Supplemental Figure 2). These results indicate successful knock-down of Pax6 gene in siRNA Pax6 transfected cells that retained their RG identity. Next, we studied the effect of Pax6 on proliferation of hESC-RG as demonstrated with Ki67 labeling. The proliferation rate of cells transfected with siRNA Pax6 was reduced by a factor of 3 (25±10%) compared to control transfected cells (75±13%). This indicates that knocking down Pax6 reduces the proliferation capacity of hESC-RG cells.
We further investigated the role of Pax6 in hESC-RG cell fate determination after culturing transfected cells for 7 DIV* in differentiation medium. The number of Pax6 knock-down cells (15.5%; 11/71) that differentiated into neurons co-labeled with β-III-tubulin was half of that in control transfected cells (34.4%; 21/16). In contrast, the number of GFAP co-labeled cells was 81.4% (22/27) in Pax-6 knockdown cells versus 53.5% (15/28) in control transfected cells. Since transfection efficiency is around 10%, these numbers are not absolute values but rather estimates which illustrate the ratio between neurons and astroglia generated from transfected cells. Overall, these results are similar to the ones reported for human fetal RG (Mo and Zecevic, 2008) and support the idea that Pax6 regulates the proliferation and neurogenic fate of hESC-RG cells in a similar manner.
3.5 The effect of the microenvironment on hESC-RG fate
The effect of the microenvironment on the neurogenic properties of hESC-RG, were examined by co-culturing LeX+ hESC-RG on fetal human brain cells. Fetal brain cultures were made from two different forebrains at mid-gestation (17 and 22 gw) obtained from the Brain Bank repositories. Similar human fetal cultures at mid-gestation were described in more detail in our previous reports (Howard et al. 2006, Zecevic et al., 2005, Mo et al., 2007). After 7DIV two cell types, neurons and astroglia, can be recognized based on immunomarkers in these cultures. GFAP+ cells accounted for 45±4.4% and β-III-tubulin+ neurons for 52±3% of total cells (Supplemental Figure 3).
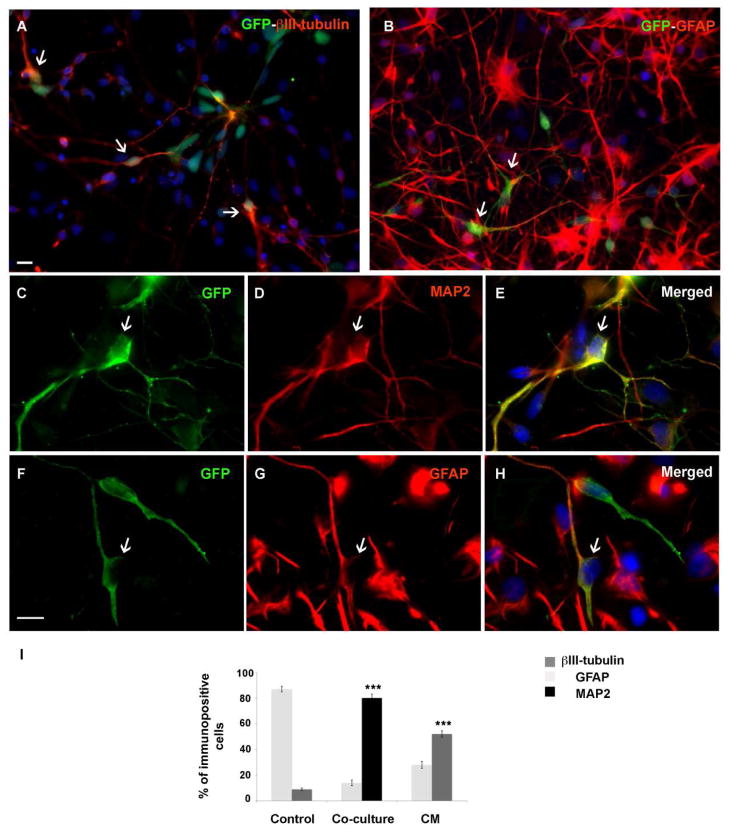
In co-culture system we used stably transfecting hESC with robust levels of EGFP (enhanced green fluorescent protein), which permitted their identification after plating on human fetal cells. Similar to previous experiments, MACS was used to enrich green GFP+ hESC-RG before plating them over either human fetal brain cultures or on poly-L-ornithine-laminin (control cultures).
First, we measured the proliferation rate of GFP+ hESC-RG cells co-cultured with human fetal cells in proliferation medium. After 2 DIV*, the proliferative marker Ki67 was detected in 24% of all GFP+ cells (64 out of 271). To assess differentiation of GFP+ hESC-RG, both control cultures (GFP+ hESC-RG cultured on poly-L-ornithine-laminin) and co-cultures were kept in differentiation medium for 7 DIV*, fixed, and double-labeled with cell-type specific markers, GFAP, β-III-tubulin or MAP2 (Fig. 4). In the co-cultures, the majority of cells differentiated into β-III-tubulin+ (79±2.5%) and MAP2+ (80±3.1%) neurons (Fig. 4). Only 14±2.2% of GFP+ hESC-RG cells differentiated into GFAP+ cells (Fig 4B, F–I). In contrast to this, in control cultures the number of β-III-tubulin+ neurons was 9.0±1% and GFAP+ cells 87.0±2.2% (Fig. 4I) similar to results obtained earlier.
Figure 4.
Co-culture experiments with GFP+ hESC-RG and human fetal brain cells. GFP+ hESC-RG cells differentiated into neurons co-labeled with β-III-tubulin (A) and astroglia cells co-labeled with GFAP (arrows) (B). Higher magnification of GFP+ hESC-RG cells co-labeled with neuronal marker MAP2 (C–E, arrows) or GFAP (F–H, arrows). (I) Quantification shows that more neurons than astroglia are produced from GFP+ hESC-RG cells in the co-culture and treated with conditioned medium (CM). In co-culture, the number of immune-labeled cells is calculated as a percentage of GFP+ cells, whereas in CM and control as a percentage from all cells in the culture. Data represent three independent experiments. ***p < 0.0001. Scale bars: 50μm, 10μm.
Hence, in the presence of the same differentiation medium, the number of neurons derived from GFP+ hESC-RG had increased by a factor of 9 in co-cultures with human fetal forebrain cells, whereas the number of GFAP+ astroglia decreased by a factor of more than 6 in comparison to control cultures kept on poly-L-ornithine-laminin.
The obvious question is whether the above mentioned neurogenic effect is due to cell-cell interaction, agents secreted in the cultures, or both. We thus investigated the differentiation fate of GFP+ hESC-RG cells in conditioned medium (CM) from human fetal cells. After 7 DIV* in CM, GFP+ hESC-RG cells generated significantly more β-III-tubulin+ cells (52±2.5% vs. 9±1%) and fewer GFAP+ cells (28±2.7% vs. 87±2.2%) than those treated with standard differentiation medium (control) (Fig. 4J). Thus, soluble factors from human fetal brain cell cultures are capable of promoting neuronal cell fate of GFP+ hESC-RG cells. However, the neuron/glia ratio in CM alone was 1.67 compared to 5.33 in co-culture conditions (Fig. 4K). Hence, although CM promotes neurogenesis compared to the control medium, the combined effect of cell-cell interaction and soluble factors, was significantly more powerful than the effect of soluble factors alone (Fig. 4K).
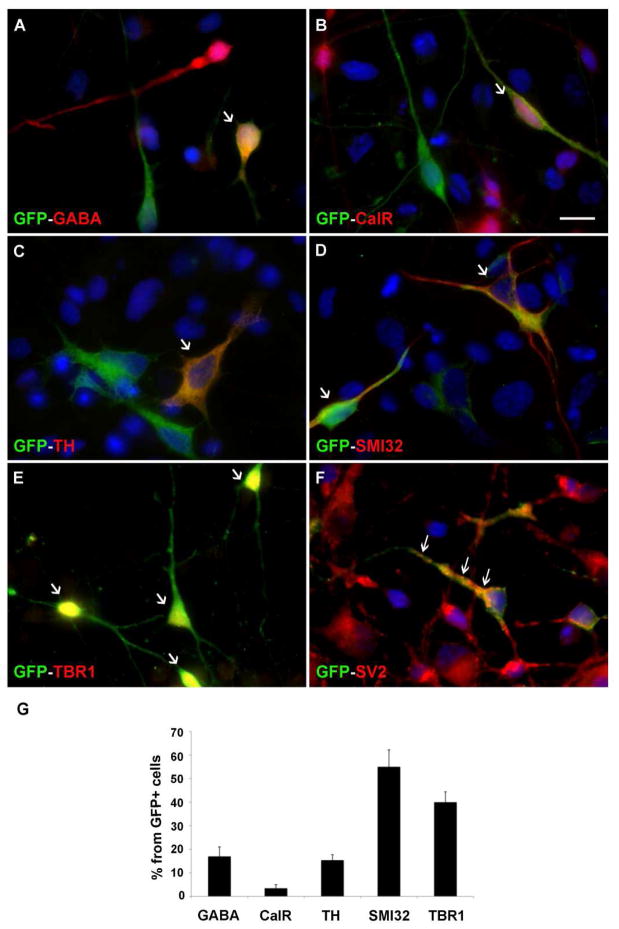
Next, we tested whether specific neuronal sub-types can be generated from green GFP+ hESC-RG in co-cultures. The predominant neuronal cell type generated from GFP+ hESC-RG was pyramidal glutaminergic neurons labeled with SMI-32 (55±7.2%) and Tbr1 (40±4.4%), fewer cells were positive for interneuron markers GABA (17±4%) and calretinin (3.5±1.5%), and TH+ (tyrosine hydroxylase) dopaminergic neurons (15.4±2.3%) (Fig. 5). By 21 DIV* (Fig 1A), the beginning of synaptogenesis was indicated by the expression of the synaptic vesicle protein 2 (SV2) in some presynaptic terminals (Fig. 5F).
Figure 5.
Differentiation of various neuronal cell types from GFP+ hESC-RG in co-cultures after 7 DIV*. Co-expression of GFP and the interneuronal markers, GABA (A), calretinin-CalR (B), dopaminergic marker tyrosine hydroxylase (TH) (C), pyramidal cell markers SMI-32 (D) and Tbr1 (E). Arrows in A–E point to co-labeled cells. (F) Synaptic vesicle labeled with SV2 antibody at 21 DIV (arrows) (G) Percentages of cell-type specific cells. Scale bar: 10μm
Cell culture viability was validated with the EthD-1kit (Invitrogen). After 7 DIV*, relatively low cell death rate of 7.5±0.84% was observed (Supplemental Figure 4). Moreover, 5.5% (21 out of 380 GFP+ cells) were still proliferating (Ki67+). The remaining cells had exited the cell cycle and differentiated as confirmed by a lack of nestin (immature neural protein) labeling.
3.6 Physiological characterizations
Neuronal fate of GFP-hESC-RG was not based only on immunocytochemistry with class specific markers, but also on their physiological properties. Individual neurons were selected for patching by alternating between fluorescence (Fig. 6A1) and infrared (Fig. 6A2) video microscopy. The cell bodies of GFP+ cells were bright, but their thin neuronal processes were not satisfactorily resolved (Fig. 6A1). Ten neurons were injected with rhodamine via the recording patch pipette revealing elaborate axo-dendritic trees consisting of primary, secondary, and tertiary branches (Fig. 6A3). In some cases, thin axon collaterals were documented branching at 90 degrees (Fig. 6A3, arrows). Patch electrode recordings were made on days 7, 8, 9 (Week-2) and days 21, 22, 23 (Week-4) after the initial seeding on human fetal mix cell culture of GFP-hESC-RG cells isolated at 43 DIV. The average resting membrane potential from day 7/8/9 to 21/22/23 were −21.9, −29.0, −20.0 mV and −25.0 mV, −26.8, −34.9 mV, respectively. The sodium current amplitude almost doubled as GFP+ neurons transitioned from the 2nd to the 4th differentiation week (Fig. 6B1). In response to a depolarizing current, the GFP+ neurons generated 5 types of voltage waveforms (Fig. 6C2, AP firing pattern). Cells with a passive electrical response (unable to generate AP) were only found in the 2nd week of neuro-differentiation (Fig. 6C1, Week-2, white bars). Voltage waveforms representative of mature neurons (Repetitive-2) were only encountered in the 4th week of neuro-differentiation (Week-4). Neurons are the only cell type in the brain endowed with a fast sodium current and ability to generate regenerative spikes. Human postmitotic neurons begin to generate nonlinear voltage waveforms (abortive spikes, Fig. 6C2) when their peak sodium current exceeds 200 pA (Moore et al., 2009). Sodium current greater than 200 pA was found in 8 out of 19 neurons tested in Week-2; and in 18 out of 19 neurons tested in Week-4. The electrophysiological recordings of transmembrane currents (Fig. 6B2) and depolarization-induced voltage waveforms (Fig. 6C2), together with elaborate axo-dendritic trees (Fig. 6A3), clearly indicate that hESC-RG gave rise to a neuronal lineage.
Figure 6.
Physiological properties of cells derived from hESC-RG. A1) GFP+ hESC-RG viewed on the electrophysiology station using green fluorescence channel. Scale,20 μm. A2) Same cells as in A1 viewed using infrared DIC video microscopy. “Patch” marks the contours of a patch electrode used for recordings and injection of red fluorescent dye rhodamine. A3) Only the injected cell is visible in red channel. Arrows indicate axon collateral branch points. B1) Average peak sodium current on six recording days. B2) Representative membrane currents in response to voltage steps from −90 to +30 mV, obtained in the second (Week-2) and fourth week (Week-4) of neurodifferentiation. C1) All cells in the present study (n=38) aligned according to the order of patching, from left to right. Each bar corresponds to one cell. The height of the bar indicates the category of AP firing pattern. C2) Representative voltage waveforms in response to a 1s-long depolarizing current pulse.
Combined results from molecular and physiological measurements (Figs 1–6) demonstrate that the human fetal brain microenvironment was efficient in promoting neurogenic fate over gliogenic fate of the hESC-RG cells.
4.0 DISCUSSION
The main findings of this study are that hESC-derived RG cells share numerous properties with human fetal RG cells. Neurogenetic fate of these cells is promoted by transcription factor Pax6 as well as by the microenvironment of human fetal brain.
This is important since unlike human fetal brain tissue, the hESC-RG cells are easily accessible, can be standardized and applied in research related to human brain development and neural tissue repair.
RG cells can be isolated and expanded from mouse and human-derived ES cells or fetal brain and adult subventricular zone (SVZ) (Gregg and Weiss, 2003; Conti et al., 2005; Glaser and Brustle, 2005; Pollard et al., 2006; Pollard and Conti, 2007). However, RG cells from different species can display different properties (Conti et al., 2005; Hansen et al., 2010). For example, whereas, GFAP is expressed early in the human RG-like cells, it is undetectable in mouse-derived RG-like cells in vitro. Thus, it is necessary to characterize RG cells derived from hESC.
In our earlier work (Mo et al., 2007) the surface marker LeX has been used to enrich human fetal RG cells in vitro. In the present study, we found that the same marker can be successfully used to isolate hESC-RG cells. Moreover, these cells are similar to the fetal cortical RG cells in their antigen characteristics, as well as their proliferation and differentiation potentials. Although not all hESC-RG express the LeX antigen, all LeX+ cells were found to express the RG markers (Fig. 1). This is consistent with the heterogeneity of the antigenic characteristics of RG in rodents (Hartfuss et al., 2001; Malatesta et al., 2003) and human fetal brain (Howard et al., 2006). Their differentiation into Foxg1+ forebrain cells was similar to previous reports that in the absence of known morphogens, hESC differentiate into cells with dorsal telencephalic characteristics (Li et al., 2009).
LeX+-RG cells generated from hESC are mitotically active progenitors, with astroglial and stem cell/progenitor characteristics. They are also multipotent cells that can differentiate into all three neural lineages: astrocytes, neurons and oligodendrocytes. In these aspects, hESC-RG cells are similar to what has been reported for RG cells in the mouse (Abramova et al., 2005; Capela and Temple, 2006; Liour et al., 2006) and in the human fetal brain (Mo et al., 2007, Mo and Zecevic, 2008, 2009).
Several lines of evidence suggest that Pax6 transcription factor promotes the neurogenic fate of RG cells in rodents (Götz et al., 1998). Most of the pyramidal neurons in the developing telencephalon are derived from Pax6 positive RG cells (Malatesta et al., 2003). Forced expression of Pax6 in rodent astrocytes is sufficient to drive neurogenesis in these cells (Heins et al., 2002). Moreover, Pax6 is necessary and sufficient for neuroepithelial differentiation from hESC (Zhang et al., 2010). We now demonstrate that transcription factor Pax6 influences proliferation and the number of neurons generated from hESC- RG cells.
This finding is consistent with our observations in human fetal RG cells (Mo and Zecevic, 2008), indicating that in both cell populations Pax6 affects proliferation as well as neuronal fate.
Numerous studies have reported the successful transplantation of hESC into rodent brains (Kelly et al., 2004; Roy et al., 2006; Yang et al., 2008). However, far less is known about how hESC behave when transplanted into the human brain (Lindvall and Kokaia, 2009). This knowledge, however, is very important for the development of future therapies.
In the present study we created a co-culture system in an attempt to mimic the cellular interactions that may occur upon transplantation of hESC-RG cells in the human fetal forebrain. Our experiments demonstrated that the microenvironment of the human fetal forebrain (17–22 gw) promotes neurogenesis in hESC-RG cells when compared to the commonly-used substrate, poly-L-ornithine-laminin (control). In the presence of the same differentiation medium, the number of neurons derived from hESC-RG cells was nine fold higher in co-cultures compared to the control cultures. At the same time the number of derived astroglia decreased more than six fold compared to control cultures. This is in line with our previous report that RG isolated from mid-gestational human fetal forebrain generate more neurons than glia (Mo et al., 2007).
Neuronal fate of co-cultured hESC-RG was determined by general neuronal markers, such as βIII-tubulin and MAP2. Notably, these cells were functionally evaluated by patch-clamp recordings. Three lines of evidence strongly indicate that cells derived from hESC-RG are committed to the neuronal fate. First, these cells exhibited typical neuronal morphologies (Fig. 6A3). Second, the peak sodium current exceeded 200 pA (Fig. 6B2) (Moore et al., 2009). Third, upon depolarization, the GFP+ cells produced regenerative spikes (abortive AP, full-size AP, and repetitive APs, Fig. 6C2). With each day of in vitro cultivation, the two parameters (sodium current and AP firing pattern) clearly shifted towards values characteristic of more mature neurons (Fig. 6B1C1).
Mature forebrain neurons can be separated between GABA-ergic inhibitory and non-GABA-ergic neuron types solely based on their characteristic action potential firing pattern (Connors and Gutnick, 1990). In immature human neurons (4 weeks in vitro), such separation is considerably less reliable because the sodium and potassium channels are not fully expressed in the plasma membrane (Moore et al., 2009; Belinsky et al., 2011). As a consequence of low channel density the AP amplitudes do not overshoot in repetitive firing (Fig. 6C2, Repetitive-1 and Repetitive-2). We think that voltage waveforms termed “Single AP” and “Repetitive-2” (Fig. 6C2) pertains to young glutamatergic neurons, while a delayed-onset high-frequency AP-firing pattern (Repetitive-1) is more indicative of immature fast-spiking GABA-ergic interneurons (Gupta et al., 2000).
Significantly, various neuronal subtypes were generated from hESC-RG in our co-culture system. More than half of the generated neurons by their molecular markers were projection, pyramidal neurons (SMI-32+ and Tbr1), but other subtypes, such as interneurons (GABA+ and calretinin+) and dopaminergic neurons (TH+) were also derived from hESC-RG under these conditions. Based on the expression of various patterning markers, such as rostral and dorsal forebrain markers, (Foxg1, Otx2, Pax6 and Tbr2), but also caudal and ventral markers (Pax7 and HoxC8) (Fig. 1J,K), the hESC-RG cell population is likely a mixed population of both dorsal and ventral progenitors. This is further confirmed by their differentiation into various neuronal cell types of either dorsal (SMI-32, Tbr1) or ventral (TH+, GABA, calretinin) origin (Fig. 5).
In our hESC-RG cultures, the observed increase in neurogenesis was, at least partially, due to secreted factors, because neurogenic effect in was produced with only the medium conditioned with the human fetal forebrain. The major difference between media collected from control cultures (grown on poly-L-ornithine-laminin) and CM from co-cultures, is the presence of astrocytes and neurons in co-cultures; both cell types can release factors, including growth factors and cytokines, that may have a role in the observed increase in the neurogenic fate of GFP hESC-RG.
This is in accord with previous reports, that astrocytes influence both proliferation and differentiation of embryonic and adult neural stem cells, and specifically promote neurogenic fate of neural stem cells (Roy et al., 2006; Nakayama et al., 2003; Song et al., 2002). Factors that affect neural stem cells range from bone morphogenetic protein (BMPs)-(Li et al., 1998), Wnt signaling (Muroyama et al., 2004; Li et al., 2009), to Sonic Hedgehog (Shh) and ciliary neurotrophic factor (CNTF) (Zhu et al., 1999). Not only astrocytes, but also neurons release factors, such as BMPs, that promote neurogenesis from stem cells (Chang et al., 2003). In addition, inhibition of Notch signaling by the γ-secretase inhibitor increases neurogenesis of cultured hESC (Borghese et al., 2010). One cannot, however, exclude the contribution of the regulatory signals provided by cell extracellular matrix (ECM) interaction during this process. Poly-L-ornithine is a non-ECM positively charged polymer, whereas laminin is a component of the ECM which is expressed in the human cortex during developmental stages (Anlar et al., 2002; Flanagan et al., 2006). Laminin/integrin signaling is very important for ECM-cell interactions regulating the fate of neural progenitors (Flanagan et al., 2006; Tate et al., 2004; Ma et al., 2008). Thus, the effect of the entire ECM on the progenitors need be taken into account, since they can influence each other and consequently change the final outcome.
The finding that co-culturing hESC-RG with the human fetal brain tissue or with conditioned media can accelerate production of specific neuronal subtypes, might have relevance for development of cell-replacement therapies for various neurological disorders from Parkinson’s, Huntington’s to Alzheimer’s disease (Lindvall and Kokaia, 2006).
In Parkinson’s disease, a progressive loss of dopaminergic (DA) neurons occurs and transplantation of fetal derived DA neurons has shown some promise, but the use of human stem cells has yet to be accomplished (Lindvall and Kokaia, 2006, 2009; Politis and Lindvall, 2012). Particularly, a recent study showed that radial glial cells are the neural progenitors of DA neurons in the human ventral midbrain (Hebsgaard et al., 2009).
In summary, we compared fetal human radial glia cells and radial glia cells generated by hESC and concluded that these two cell populations share numerous antigen characteristics as well as proliferative capacity and differentiation pattern.
Transcription factor Pax6 influences their neurogenic capability, whereas, the environment of the human fetal forebrain significantly increases the genesis of several neuronal sub-types from hESC-RG cells. These findings may have practical implications in research related to human brain development and to cell replacement therapies for neurological disorders, where it is necessary to generate a sufficient number of neurons.
Supplementary Material
Highlights.
Radial glia (RG) cells are generated from human embryonic stem cells (hESC-RG).
hESC-RG are similar to human fetal RG cells by antigen, proliferation and differentiation properties and can be used in research of human brain development.
Neurogenic fate of hESC-RG is promoted by Pax6 and in co-culture with human fetal brain cells.
Secreted factors from human fetal cultures also promote neurogenic fate of hESC-RG.
Acknowledgments
Supported by NIH grant NS041489-10A and Connecticut Stem Cell grant 2008-013 to NZ and 09-SCA-UCHC-13 to SDA. Human fetal tissue was obtained from StemExpress, CA. Patch clamp recordings were performed in the Stem Cell Physiology and Chemistry Core at UConn Health Center, supported by the Connecticut Stem Cell Initiative/Connecticut Innovations grant No. 10-SCD-01. We thank UCHC Stem Cell Core and UCHC FACS facility, Drs J-A Ortega and N Radonjic for help with qPCR, Dr Xiu-Jun Li for valuable suggestions on the manuscript, Nicole Glidden and Greg Wark for technical support and editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramova N, Charniga C, Goderie SK, Temple S. Stage-specific changes in gene expression in acutely isolated mouse CNS progenitor cells. Dev Biol. 2005;283 (2):269–281. doi: 10.1016/j.ydbio.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 2.Anlar B, Atilla P, Cakar AN, Kose MF, Beksaç MS, Dagdeviren A, Akçören Z. Expression of adhesion and extracellular matrix molecules in the developing human brain. J Child Neurol. 2002;17 (9):707–13. doi: 10.1177/088307380201700913. [DOI] [PubMed] [Google Scholar]
- 3.Belinsky GS, Moore AR, Short SM, Rich MT, Antic SD. Physiological properties of neurons derived from human embryonic stem cells using a dibutyryl cyclic AMP-based protocol. Stem Cells and Development. 2011;20 (10):1733–1746. doi: 10.1089/scd.2010.0501. [DOI] [PubMed] [Google Scholar]
- 4.Bentivoglio M, Mazzarello P. The history of radial glia. Brain Research Bulletin. 1999;49 (5):305–315. doi: 10.1016/s0361-9230(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 5.Bibel M, Richter J, Schrenk K, Tucker KL, Staiger V, Korte M, Goetz M, Barde YA. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat Neurosci. 2004;7 (9):1003–1009. doi: 10.1038/nn1301. [DOI] [PubMed] [Google Scholar]
- 6.Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brüstle O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28 (5):955–964. doi: 10.1002/stem.408. [DOI] [PubMed] [Google Scholar]
- 7.Brustle O, Jones kN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285(5428):754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 8.Campbell K, Gotz M. Radial glia: Multi-purpose cells for vertebrate brain development. Trends Neurosis. 2002;25 (5):235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- 9.Capela A, Temple S. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Dev Biol. 2006;291 (2):300–313. doi: 10.1016/j.ydbio.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Chang MY, Son H, Lee YS, Lee SH. Neurons and astrocytes secret factors that cause stem cells to differentiate into neurons and astrocytes respectively. Mol Cell Neurosci. 2003;23 (3):414–426. doi: 10.1016/s1044-7431(03)00068-x. [DOI] [PubMed] [Google Scholar]
- 11.Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13 (3):99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- 13.Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res. 2006;83 (5):845–56. doi: 10.1002/jnr.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaser T, Brustle O. Retinoic acid induction of ES-cell-derived neurons: the radial glia connection. Trends Neurosci. 2005;28 (8):397–400. doi: 10.1016/j.tins.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Götz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21(5):1031–44. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- 16.Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell. 2005;6 (10):777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287 (5451):273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 18.Gregg C, Weiss S. Generation of functional radial glial cells by embryonic and adult forebrain neural stem cells. J Neurosci. 2003;23 (37):11587–11601. doi: 10.1523/JNEUROSCI.23-37-11587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464 (7288):554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 20.Hartfuss E, Galli R, Heins N, Götz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229 (1):15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- 21.Hebsgaard JB, Nelander J, Sabelström H, Jönsson ME, Stott S, Parmar M. Dopamine neuron precursors within the developing human mesencephalon show radial glial characteristics. Glia. 2009;57(15):1648–58. doi: 10.1002/glia.20877. [DOI] [PubMed] [Google Scholar]
- 22.Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Götz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5 (4):308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 23.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5 (12):3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard B, Chen Y, Zecevic N. Cortical progenitor cells in the developing human telencephalon. Glia. 2006;53 (1):57–66. doi: 10.1002/glia.20259. [DOI] [PubMed] [Google Scholar]
- 25.Howard BM, Mo Z, Filipovic R, Moore AR, Antic SD, Zecevic AD. Radial glia cells in the developing human brain. Neuroscientist. 2008;14 (5):459–473. doi: 10.1177/1073858407313512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakovcevski I, Zecevic N. Olig transcription factors are expressed in oligdendrocyte and neuronal cells in human fetal CNS. J Neurosci. 2005;25 (44):10064–10073. doi: 10.1523/JNEUROSCI.2324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci. 2004;101 (32):11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M, Morshead CM. Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. J Neurosci. 2003;23 (33):10703–10709. doi: 10.1523/JNEUROSCI.23-33-10703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Cogswell CA, LoTurco JJ. Neuronal differentiation of precursors in the neocortical ventricular zone is triggered by BMP. J Neurosci. 1998;18 (21):8853–8862. doi: 10.1523/JNEUROSCI.18-21-08853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from humanembryonic stem cells, Nat. Biotechnol. 2005;23 (2):215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 31.Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136 (23):4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liour SS, Yu RK. Differentiation of radial glia-like cells from embryonic stem cells. Glia. 2003;42 (2):109–117. doi: 10.1002/glia.10202. [DOI] [PubMed] [Google Scholar]
- 33.Liour SS, Kraemer SA, Dinkins MB, Su CY, Yanagisawa M, Yu RK. Further Characterization of embryonic stem cell-derived radial glial cells. Glia. 2006;53 (1):43–56. doi: 10.1002/glia.20257. [DOI] [PubMed] [Google Scholar]
- 34.Lindvall O, Kokaia Z. Stem Cell for treatment of neurological disorders. Nature. 2006;441 (7097):1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 35.Lindval O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons Parkinson`s disease. Trends Pharmacol Sci. 2009;30 (5):260–267. doi: 10.1016/j.tips.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malatesta P, Hartfuss E, Götz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127 (24):5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- 38.Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Götz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37 (5):751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 39.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2 (11):780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 40.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31 (5):727–74. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 41.Mizuseki K, Sakamoto T, Watanabe K, Muguruma K, Ikeya M, Nishiyama A, Arakawa A, Suemori H, Nakatsuji N, Kawasaki H, Murakami F, Sasai Y. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells, Proc. Natl Acad Sci. 2003;100 (10):5828–5833. doi: 10.1073/pnas.1037282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mo Z, Moore AR, Filipovic R, Ogawa Y, Kazuhiro I, Antic SD, Zecevic N. Human cortical neurons originate from radial glia and neuron-restricted progenitors. J Neurosci. 2007;27 (15):4132– 4145. doi: 10.1523/JNEUROSCI.0111-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mo Z, Zecevic N. Is Pax6 critical for neurogenesis in the human fetal brain? Cereb Cortex. 2008;18 (6):1455–1465. doi: 10.1093/cercor/bhm181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mo Z, Zecevic N. Human fetal radial glia cells generate oligodendrocytes in vitro. Glia. 2009;57 (5):490–498. doi: 10.1002/glia.20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore AR, Filipovic R, Mo Z, Rasband MN, Zecevic N, Antic SD. Electrical Excitability of Early Neurons in the Human Cerebral Cortex during the Second Trimester of Gestation. Cereb Cortex. 2011;19 (8):1795–1805. doi: 10.1093/cercor/bhn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muroyama Y, Kondoh H, Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem Biophys Res Commun. 2004;313 (4):915–921. doi: 10.1016/j.bbrc.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Nat R, Nilbratt M, Narkilahti S, Winblad B, Hovatta O, Nordberg A. Neurogenic neuroepithelial and radial glial cells generated from six human embryonic stem cell lines in serum-free suspension and adherent cultures. Glia. 2007;55 (4):385–399. doi: 10.1002/glia.20463. [DOI] [PubMed] [Google Scholar]
- 48.Nakayama T, Momoki-Soga T, Inoue N. Astrocyte-derived factors instruct differentiation of embryonic stem cells into neurons. Neurosci Res. 2003;46 (2):241–249. doi: 10.1016/s0168-0102(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 49.Noctor SC, Flint AC, Weissman TA, Dammmerman RS, Kriegstein AR. Neurons derived from radial glia cells establish radial units in neocortex. Nature. 2001;409 (6821):714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 50.Politis M, Lindvall O. Clinical application of stem cell therapy in Parkinson’s disease. BMC Med. 2012;10:1. doi: 10.1186/1741-7015-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollard SM, Conti L, Sun Y, Goffredo D, Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex. 2006;16 (Suppl 1):i112–i120. doi: 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- 52.Pollard SM, Conti L. Investigating radial glia in vitro. Prog Neurobiol. 2007;83 (1):53–67. doi: 10.1016/j.pneurobio.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13 (6):541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- 54.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human EScell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12 (11):1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 55.Song H, Stevens CF, Gage FH. Astroglia induceneurogenesis from adult neural stem cells. Nature. 2002;417 (6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 56.Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1 (5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tao W, Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8 (5):957–966. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- 58.Tate MC, García AJ, Keselowsky BG, Schumm MA, Archer DR, LaPlaca MC. Specific beta1 integrins mediate adhesion, migration, and differentiation of neural progenitors derived from embryonic striatum. Mol Cell Neurosci. 2004;27 (1):22–31. doi: 10.1016/j.mcn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23 (6):781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26 (1):55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zecevic N. Specific characteristic of radial glia in the human fetal telencephalon. Glia. 2004;48 (1):27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- 62.Zhang SC, Wernig M, Duncan ID, Brustle O, Thompson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19 (12):1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Huang CT, Chen J, Pankratz MT, Xi J, Li J, Yang Y, Lavaute TM, Li XJ, Ayala M, Bondarenko GI, Du ZW, Jin Y, Golos TG, Zhang SC. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7 (1):90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu G, Mehler MF, Zhao J, Yu Yung S, Kessler JA. Sonic hedgehog and BMP2 exert opposing actions on proliferation and differentiation of embryonic neural progenitor cells. Dev Biol. 1999;215 (1):118–129. doi: 10.1006/dbio.1999.9431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.