Abstract
OBJECTIVE
Cognitive dysfunction and cardiovascular disease are common and debilitating manifestations of systemic lupus erythematosus (SLE). In this study, we evaluated the relationship between cardiovascular events, traditional cardiovascular risk factors, and SLE-specific risk factors as predictors of cognitive dysfunction in a large cohort of participants with SLE.
METHODS
Subjects included 694 participants from the Lupus Outcomes Study (LOS), a longitudinal study of SLE outcomes based on annual telephone survey querying demographic and clinical variables. The Hopkins Verbal Learning Test – Revised (HVLT-R) and the Controlled Oral Word Association Test (COWAT) were administered to assess cognitive function. Multiple logistic regression was used to identify cardiovascular events (myocardial infarction (MI), stroke), traditional cardiovascular risk factors (hypertension, hyperlipidemia, diabetes, obesity, smoking), and SLE-specific risk factors (antiphospholipid antibodies (aPL), disease activity, disease duration) associated with cognitive impairment in year seven of the LOS.
RESULTS
The prevalence of cognitive impairment as measured by verbal memory and verbal fluency metrics was 15%. In adjusted multiple logistic regression analyses, aPL (OR=2.10, 95% CI 1.3-3.41), hypertension (OR=2.06, 95% CI 1.19-3.56), and a history of stroke (OR=2.27, 95% CI 1.16-4.43) were significantly associated with cognitive dysfunction. In additional analyses evaluating the association between these predictors and severity of cognitive impairment, stroke was significantly more prevalent in participants with severe impairment when compared to those with mild or moderate impairment (p=0.036).
CONCLUSIONS
These results suggest that the presence of aPL, hypertension, and stroke are key variables associated with cognitive impairment, which may aid in identification of patients at greatest risk.
Cognitive dysfunction is a common neuropsychiatric manifestation of systemic lupus erythematosus (SLE), with prevalence ranging from 21-81% (1, 2). While existing studies have identified a combination of biologic and socioeconomic factors as potential predictors of cognitive impairment, the etiology of cognitive impairment in SLE remains unclear (3, 4). There is substantial evidence linking cardiovascular disease and Framingham-type risk factors with cognitive dysfunction in the general population. In the Whitehall II Study, for example, coronary heart disease was associated with lower cognitive performance in middle-aged individuals (5). Another study correlated hypertension and diabetes mellitus with cognitive decline in middle-aged adults (6). Similar to the general population, prior SLE studies have also implicated hypertension and diabetes as potential predictors of cognitive dysfunction (3, 4). However, despite the increased burden of premature cardiovascular disease in patients with SLE, the relationship between cardiovascular risk factors and events and cognitive dysfunction has not been fully explored (7-9).
The purpose of this study was to investigate the relationships between cardiovascular events (myocardial infarction (MI), stroke), traditional cardiovascular risk factors (hypertension, hyperlipidemia, diabetes, obesity, smoking), SLE-specific risk factors (antiphospholipid antibodies (aPL), disease activity, disease duration), and cognitive dysfunction in a large cohort of well-characterized individuals with SLE. We aimed to identify the relationship of specific cardiovascular risk factors or events to cognitive dysfunction beyond sociodemographic and disease characteristics.
Subjects and Methods
Subjects
The Lupus Outcomes Study (LOS) is a large cohort of individuals with SLE followed longitudinally through a structured annual telephone interview conducted by trained survey workers. The year-to-year retention rate in the LOS is 92%. Diagnoses of all participants enrolled in the study were confirmed by medical chart review (10). Details regarding subject recruitment have been previously described (11). Briefly, initial subject recruitment occurred between 2002 and 2004, with a second enrollment period beginning in 2006. Participants were recruited through academic medical centers (25%), community rheumatology practices (11%), and various community-based sources including support groups, conferences, and other media (64%). Of 957 total participants enrolled at the time, 694 were included in this study as they had a complete data-set including cognitive function outcomes in year 7, demographics, medical history, depression, disease activity, and at least one available aPL test. The research protocol has been approved by the UCSF Committee on Human Research and all participants gave their informed consent prior to participation.
Data
Data were derived from two sources. The first is the annual structured telephone interviews, containing validated measures pertaining to demographic and socioeconomic characteristics, SLE disease activity and manifestations, medications, general health and comorbidities, depression, employment, health care utilization, and health insurance coverage. The second was medical record reviews to obtain disease duration and laboratory test results.
Measures
Cognitive function
LOS participants were screened for memory impairment by telephone interview using the Hopkins Verbal Learning Test – Revised (HVLT-R), a valid and reliable measure of verbal learning and memory (12, 13). The test consists of a 12-item word list which is presented on 3 successive learning trials and a delayed recall trial, yielding a total recall score and a delayed recall score. The Controlled Oral Word Association Test (COWAT) was employed as a measure of verbal fluency (14, 15). This test consists of 3 trials for which participants generate words beginning with specific letters under timed conditions (15). These measures have been recently validated against a comprehensive cognitive battery and were found to be of sufficient reliability for the detection of cognitive impairment in SLE (16). Age-stratified z-scores were derived for HVLT recall, HVLT delayed recall, and verbal fluency tests. For the purpose of classifying participants and assessing overall cognitive function, we employed a commonly utilized method of data reduction and created a composite z-score index in which z-scores from all three testing domains were averaged (17-19). Based on their composite z-score, participants were considered to have intact cognitive function if they scored better than −1.0 standard deviations below population norms (SD), and cognitive impairment if they scored worse than or equal to −1.0 SD below age-stratified normative values.
Cardiovascular risk, events, and disease-related variables
Cardiovascular events included self-reported MI or stroke in years 1-7 of the LOS. Cardiovascular risk factors included self-reported hypertension, hyperlipidemia, and diabetes in each year. Obesity was defined by current body mass index ≥30, and smoking status was defined as current smoking.
Disease-related factors included disease activity, disease duration, and aPL. Based on medical record reviews, participants were considered to be positive for aPL if they had at least one test result indicating the presence of anticardiolipin antibodies (IgG or IgM), anti-beta2 glycoprotein-1 antibodies (IgG or IgM), or a lupus anticoagulant measured by an abnormal Russell viper venom test at least one point in time. SLE-related disease activity was measured by the Systemic Lupus Activity Questionnaire (SLAQ) score, a validated measure of disease activity in SLE accounting for constitutional, mucocutaneous and musculoskeletal symptoms, and other domains (20, 21). Duration of disease was determined from the date of SLE diagnosis confirmed by medical records.
Covariates
Sociodemographic factors included age, gender, education level (high-school or less, some college, college graduate and post-graduate), and living below poverty (household income below 125% of the Federal Poverty Threshold). The Center for Epidemiological Studies Depression (CES-D) scale, a measure of depressive symptom severity, was used to evaluate depression status. Depression was defined as CES-D score ≥24, as previously described (22, 23).
Analysis
Bivariate comparisons were conducted between impaired and unimpaired individuals on sociodemographic factors, disease activity and duration, depression, aPL, and cardiovascular risk factors and events. Chi-square tests and analysis of variance were used as appropriate to identify differences between groups. We used unadjusted and hierarchical adjusted logistic regression to identify cardiovascular risk factors and events as potential predictors of cognitive dysfunction. The adjusted analyses controlled for sociodemographics (we did not control for age as the cognitive function measures were already age-adjusted) and depression. Variables of interest were evaluated in a hierarchical fashion, with disease-related variables (disease activity, disease duration, and aPL), cardiovascular risk factors (hypertension, hyperlipidemia, diabetes, obesity, current smoking), and cardiovascular events (myocardial infarction, stroke) added successively.
A sensitivity analysis was conducted including a modified SLAQ index excluding symptoms that could overlap with cognitive dysfunction (e.g., concentration problems, depression, reduced energy) and this model yielded highly comparable results (not shown), so the original measure was included in the final model to maintain the psychometric integrity of the SLAQ. Collinearity thresholds were also considered. In additional analyses, we divided the cognitively impaired group into mild-moderate impairment (z-score less than or equal to −1.0 but greater than −2.0) and severe impairment (z-score less than or equal to −2.0). We used chi-square tests to identify differences between the groups for variables identified as significant in the previous adjusted logistic regression.
Results
Six hundred and ninety-four participants were included in this study. Based on the composite cognitive function score, 107 (15%) were classified as having cognitive impairment while 587 (85%) were considered to be unimpaired. Subject characteristics are shown in Table 1. Participants with cognitive impairment were more likely to have a lower level of educational attainment and to be living below the poverty thresholds. Participants with cognitive dysfunction were also more likely to have CES-D scores indicative of depression. Disease duration was similar in both groups, but participants with cognitive impairment had a significantly greater SLAQ index indicative of greater disease activity. With regard to cardiovascular risk factors and events, participants with cognitive dysfunction were more likely to report a history of stroke, diabetes, and hypertension. They were also more likely to be a current smoker, and to have laboratory evidence of aPL positivity. There were no significant differences between groups with regard to MI, hyperlipidemia, and obesity.
Table 1.
Subject Characteristics Stratified by Cognitive Function
| Cognitive function | ||||
|---|---|---|---|---|
| All patients (n=694) |
Unimpaired (n=587) |
Impaired (n=107) |
P | |
| Age, yrs | 50.0±12.9 | 50.3±12.6 | 48.4±14.5 | 0.061 |
| Female, n (%) | 640 (92.2) | 542 (92.3) | 98 (91.6) | 0.791 |
| Education, n (%) | ||||
| high-school or less | 114 (16.4) | 77 (13.1) | 37 (34.6) | 0.000 |
| some college | 282 (40.6) | 230 (39.2) | 52 (48.6) | |
| college graduate | 173 (24.9) | 160 (27.3) | 13 (12.1) | |
| post-graduate (ref.) | 125 (18) | 120 (20.4) | 5 (4.7) | |
| Below poverty, n (%) | 96 (13.8) | 64 (10.9) | 32 (29.9) | 0.000 |
| Depression, n (%) | 129 (18.6) | 79 (13.5) | 50 (46.7) | 0.000 |
| Disease Activity (SLAQ) | 11.4±7.8 | 10.6±7.4 | 15.6±8.7 | 0.025 |
| Disease Duration, yrs | 17.1±8.6 | 17.4±8.6 | 15.7±8.5 | 0.866 |
| Antiphospholipid antibodies, n (%) |
274 (39.5) | 217 (37) | 57 (53.3) | 0.002 |
| Hypertension, n (%) | 401 (57.8) | 325 (55.4) | 76 (71) | 0.003 |
| Hyperlipidemia, n (%) | 276 (39.8) | 233 (39.7) | 43 (40.2) | 0.924 |
| Diabetes, n (%) | 77 (11.1) | 59 (10.1) | 18 (16.8) | 0.040 |
| Obese, n (%) | 189 (27.2) | 153 (26.1) | 36 (33.6) | 0.105 |
| Current smoking, n (%) | 61 (8.8) | 43 (7.3) | 18 (16.8) | 0.001 |
| Myocardial Infarction, n (%) |
46 (6.6) | 37 (6.3) | 9 (8.4) | 0.420 |
| Stroke, n (%) | 71 (10.2) | 50 (8.5) | 21 (19.6) | 0.000 |
All variables are self-reported with the exception of presence of antiphospholipid antibodies and disease duration which were collected from chart review. SLAQ = Systemic Lupus Activity Questionnaire.
Unadjusted and hierarchical adjusted logistic regression analyses predicting cognitive dysfunction are shown in Table 2. In unadjusted models, hypertension (OR=1.98, 95% CI 1.26-3.09), diabetes (OR=1.81, 95% CI 1.02-3.21), current smoking (OR=2.56, 95% CI 1.41-4.63), and stroke (OR=2.62, 95% CI 1.50-4.58) were all associated with cognitive impairment. Disease activity (OR=1.08, 95% CI 1.05-1.11) and aPL (OR=1.94, 95% CI 1.28-2.94) were also associated with cognitive impairment in unadjusted models. Disease duration, hyperlipidemia, obesity, and history of MI were not significantly associated with cognitive impairment.
Table 2.
Hierarchical Logistic Regression Evaluating Cardiovascular Risk Factors and Events as Predictors of Cognitive Dysfunction
| Adjusted Models? | ||||
|---|---|---|---|---|
|
n=694
observations |
Unadjusted Model |
Model 1a | Model 2b | Model 3c |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Disease Activity (SLAQ) |
1.08 (1.05-1.11) | 1.04 (1.01-1.07) | 1.04 (1.00- 1.07) |
1.04 (1.00-1.07) |
| Disease Duration | 0.98 (0.95-1.00) | 0.98 (0.95-1.01) | 0.97 (0.94- 1.00) |
0.97 (0.94-1.00) |
| Antiphospholipid antibodies |
1.94 (1.28-2.94) | 2.14 (1.34-3.41) |
2.24 (1.39-
3.61) |
2.10 (1.3-3.41) |
| Hypertension | 1.98 (1.26-3.09) |
2.08 (1.21-
3.58) |
2.06 (1.19-3.56) | |
| Hyperlipidemia | 1.02 (0.67-1.55) | 0.81 (0.49- 1.35) |
0.74 (0.43-1.26) | |
| Diabetes | 1.81 (1.02-3.21) | 1.35 (0.66- 2.76) |
1.24 (0.6-2.57) | |
| Obese | 1.44 (0.93-2.24) | 0.68 (0.39- 1.17) |
0.71 (0.41-1.24) | |
| Current smoking | 2.56 (1.41-4.63) | 1.47 (0.73- 2.95) |
1.51 (0.74-3.07) | |
| Myocardial Infarction |
1.37 (0.64-2.92) | 0.96 (0.38-2.44) | ||
| Stroke | 2.62 (1.50-4.58) | 2.27 (1.16-4.43) | ||
All models adjusted for gender, education, poverty status, and depression.
Model 1: Disease-related variables.
Model 2: Disease-related variables and cardiovascular risk-factors.
Model 3: Disease-related variables, cardiovascular risk-factors and events.
All variables are self-reported with the exception of presence of antiphospholipid antibodies and disease duration which were collected from chart review.
In a hierarchical logistic regression adjusted for gender, education, poverty status, and depression, three models evaluated potential predictors of cognitive dysfunction. Model one included disease-related variables, model two added general cardiovascular risk factors, and model three added a history of cardiovascular events. In the final model including all potential predictors, aPL (OR=2.10, 95% CI 1.3-3.41), hypertension (OR=2.06, 95% CI 1.19-3.56), and a history of stroke (OR=2.27, 95% CI 1.16-4.43) remained significantly associated with cognitive dysfunction. Disease activity, diabetes, and current smoking were no longer significantly associated with cognitive impairment.
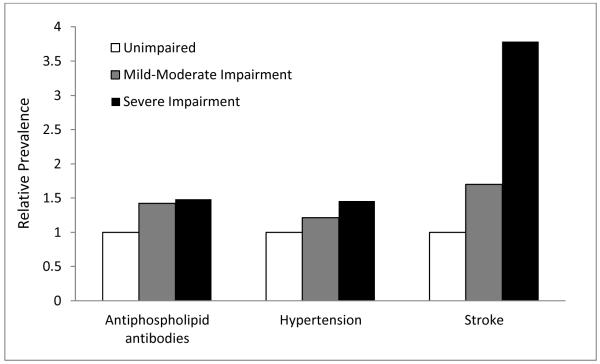
In order to explore the association between significant predictors and severity of cognitive impairment, additional analyses were performed. Characteristics of participants with mild-moderate and severe cognitive impairment are shown in Table 3. While aPL and hypertension were associated with overall cognitive impairment in the previous analysis, subset analysis did not show significant differences between varying levels of cognitive impairment. Stroke, however, was significantly more prevalent in severely impaired participants when compared with mild-moderately impaired participants (p=0.036). Results are graphically displayed in Figure 1, where it can be noted that the relative prevalence of stroke is substantially increased.
Table 3.
Prevalence of risk factors and events in patients with mild-moderate and severe cognitive impairment.
| Mild-Moderate Cognitive Impairment (n=76) |
Severe Cognitive Impairment (n=31) |
P | |
|---|---|---|---|
| Antiphospholipid antibodies, n (%) |
40 (52.6) | 17 (54.8) | 0.836 |
| Hypertension, n (%) |
51 (67.1) | 25 (80.6) | 0.161 |
| Stroke, n (%) | 11 (14.5) | 10 (32.3) | 0.036 |
Mild-Moderate impairment group represents z-scores less than or equal to −1.0 but greater than −2.0, while severe cognitive impairment group represents z-scores less than or equal to −2.0.
Figure 1.
Prevalence of cardiovascular risk factors and events in patients with mild-moderate and severe cognitive impairment relative to unimpaired patients.
Discussion
In this study, we sought to identify cardiovascular risk factors and events that are potentially associated with cognitive dysfunction in SLE. We found that hypertension, stroke, and the presence of aPL were significantly associated with cognitive impairment. In addition, we found that stroke was significantly more prevalent in participants with severe dysfunction when compared to participants with mild-moderate dysfunction.
The relationship between hypertension and cognitive dysfunction in our study was robust and is consistent with at least one prior study in SLE (3). Moreover, hypertension has been well established as a predictor of cognitive dysfunction in the general cardiovascular literature. In a large cohort study of over 10,000 patients, essential hypertension at baseline was associated with cognitive decline over the following six years (6). Furthermore, similar to the cognitive dysfunction seen in our study, the Veterans Affairs Normative Aging Study demonstrated that individuals with uncontrolled hypertension exhibited decrements in recall and verbal fluency with increasing age when compared with normotensive patients (24). While the mechanism linking hypertension with cognitive dysfunction in SLE or non-SLE patients is not entirely understood, studies have correlated hypertension with increased white-matter hyperintensities and brain atrophy, specifically of the prefrontal cortex (25, 26). Although hypertension has been previously reported as a predictor of ischemic stroke in patients with SLE, our data suggest an association that may be in part independent of reported stroke (27, 28).
APL positivity was also significantly associated with cognitive impairment in our adjusted logistic regression. While aPL have been previously found to be associated with cognitive impairment, it remains unclear whether aPL contributes to cognitive decline through a direct pathogenic effect on neurons or through thrombotic or other mechanisms (3, 4, 28-30). APL have a well established association with stroke (28). Stroke is also a well-known cause of cognitive dysfunction in the general population (31, 32). Although an infrequent occurrence, stroke was significantly associated with cognitive dysfunction in our study. Among individuals with documented persistently positive aPL in our cohort, there was also an insignificant trend towards increased frequency of self-reported stroke, which suggests that they may contribute to cognitive dysfunction (data not shown). The association with severe impairment identified in our study suggests that stroke may represent an endpoint on a spectrum of disease pathology that leads to cognitive dysfunction, at which point outcomes are more severe. Given the interrelatedness of stroke with aPL and hypertension, early identification of the latter two may enable clinicians to identify patients at greatest risk for cognitive decline and therapeutically intervene.
It is important to note several limitations in our study when evaluating the contribution of aPL. While our study was designed to evaluate the relationship between Framingham-type cardiovascular risk factors and cognitive dysfunction using self-report data, we controlled for aPL using a single positive test. In doing so, we are unable to fully assess the contribution of specific anti-phospholipid antibodies, titers of antibodies, multiple positive antibodies, or true antiphospholipid antibody syndrome in our cohort. Anti-phospholipid antibody syndrome is most widely defined using the revised Sapporo criteria which includes two positive antibody tests documented at least 12 weeks apart plus the presence of documented vascular thrombosis or pregnancy morbidity (33). In subset analyses evaluating patients who had data from two successive tests and documented clinical evidence of thrombosis or pregnancy morbidity, we were only able to identify 15 patients who met strict criteria for APS, a cohort which was underpowered to evaluate with respect to cognitive function (data not shown).
Another major limitation of our study is the fact that cardiovascular risk factors and events were evaluated via self-report. Although self-report has been deemed a valid and reliable method of data acquisition for some variables (e.g., hypertension), it has been suggested that the validity of self-report for other variables (e.g., stroke) is low and influenced by cognitive function (34, 35). It is certainly possible that self-report was significantly more limited in the patients with cognitive dysfunction, leading to under-reporting of risk factors and perhaps biasing the results of our study toward the null hypothesis. Furthermore, severity, timing, and therapeutic interventions were not queried in association with cardiovascular risk factors and events. Therefore, we do not know the temporal relationship between cognitive dysfunction and stroke, which could be critical to understanding the pathogenesis. There are also limitations to our cognitive dysfunction outcome variable. While these tests capture several “subcortical” cognitive domains observed to be impaired in lupus, they certainly do not capture all possible cognitive deficits (1, 19, 36, 37). As a result, our prevalence rates of cognitive dysfunction were probably reduced in comparison to studies investigating the full breadth of cognitive domains, again potentially biasing our results toward the null. Nevertheless, this is the first study to comprehensively evaluate cardiovascular risk factors and events in association with cognitive dysfunction in a large cohort of patients with SLE.
Cognitive dysfunction is a major cause of morbidity in patients with SLE, but the etiology has not been well established. Cardiovascular disease has emerged as a significant predictor of the development of other aspects of neuropsychiatric SLE, such as depression (38). An understanding of the relationship between cardiovascular risk factors and cognitive dysfunction may allow us to identify patients at greater risk for decline. Importantly, many of these potential risk factors for cognitive dysfunction, including hypertension, are potentially modifiable. Future longitudinal studies are necessary to clarify the causality of these relationships. A better understanding of specific targets for prevention and intervention can serve to minimize the burden of one of the most common neuropsychiatric syndromes occurring in SLE.
Significance and Innovation.
-There is in an increased burden of premature cardiovascular disease as well as a high prevalence of cognitive dysfunction in patients with systemic lupus erythematosus (SLE).
-Despite substantial evidence linking cardiovascular disease with cognitive dysfunction in the general population, the relationship has not been fully explored in patients with SLE.
-Antiphospholipid antibodies, hypertension, and a history of stroke were independently associated with cognitive dysfunction.
-Stroke was significantly more prevalent in participants with severe cognitive impairment.
Acknowledgements
This publication was supported by NIH/NIAMS Grant Number P60AR053308, K08 MH0727240, R01 AR22804, K24 AR02175, The Arthritis Foundation Post-Doctoral Fellowship Award, the Kirkland Scholar Award, and the Rosalind Russell Medical Research Center for Arthritis. The study was performed in part in the General Clinical Research Center, Moffitt Hospital, University of California, San Francisco, California, USA, with funds provided by the National Center for Research Resources, 5 M01 R-00079, US Public Health Service. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57(3):496–500. doi: 10.1212/wnl.57.3.496. [DOI] [PubMed] [Google Scholar]
- 2.Hanly JG, Fisk JD, Sherwood G, Jones E, Jones JV, Eastwood B. Cognitive impairment in patients with systemic lupus erythematosus. J Rheumatol. 1992;19(4):562–7. [PubMed] [Google Scholar]
- 3.Tomietto P, Annese V, D’Agostini S, Venturini P, La Torre G, De Vita S, et al. General and specific factors associated with severity of cognitive impairment in systemic lupus erythematosus. Arthritis Rheum. 2007;57(8):1461–72. doi: 10.1002/art.23098. [DOI] [PubMed] [Google Scholar]
- 4.McLaurin EY, Holliday SL, Williams P, Brey RL. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology. 2005;64(2):297–303. doi: 10.1212/01.WNL.0000149640.78684.EA. [DOI] [PubMed] [Google Scholar]
- 5.Singh-Manoux A, Sabia S, Lajnef M, Ferrie JE, Nabi H, Britton AR, et al. History of coronary heart disease and cognitive performance in midlife: the Whitehall II study. Eur Heart J. 2008;29(17):2100–7. doi: 10.1093/eurheartj/ehn298. PMCID: 2740873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–8. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr., Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145(5):408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 8.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42(2):338–46. doi: 10.1002/1529-0131(199902)42:2<338::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum. 2009;61(10):1396–402. doi: 10.1002/art.24537. PMCID: 2909444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 11.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57(1):56–63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank RM, Byrne GJ. The clinical utility of the Hopkins Verbal Learning Test as a screening test for mild dementia. Int J Geriatr Psychiatry. 2000;15(4):317–24. doi: 10.1002/(sici)1099-1166(200004)15:4<317::aid-gps116>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Brandt J, Benedict RH. Hopkins Verbal Learning Test - Revised. Psychological Assessment Resources, Inc.; 2001. [Google Scholar]
- 14.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–38. [PubMed] [Google Scholar]
- 15.Gladsjo JA, Miller SW, Heaton R. Norms for Letter and Category Fluency: Demographic Corrections for Age, Education, and Ethnicity. Psychological Assessment Resources, Inc.; 1999. [DOI] [PubMed] [Google Scholar]
- 16.Julian LJ, Yazdany J, Trupin L, Criswell LA, Yelin E, Katz PP. Validity of brief screening tools for cognitive impairment in RA and SLE. Arthritis Care Res (Hoboken) 2011 doi: 10.1002/acr.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saczynski JS, Jonsdottir MK, Garcia ME, Jonsson PV, Peila R, Eiriksdottir G, et al. Cognitive impairment: an increasingly important complication of type 2 diabetes: the age, gene/environment susceptibility--Reykjavik study. Am J Epidemiol. 2008;168(10):1132–9. doi: 10.1093/aje/kwn228. PMCID: 2727243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray AM, Pederson SL, Tupper DE, Hochhalter AK, Miller WA, Li Q, et al. Acute variation in cognitive function in hemodialysis patients: a cohort study with repeated measures. Am J Kidney Dis. 2007;50(2):270–8. doi: 10.1053/j.ajkd.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Panopalis P, Julian L, Yazdany J, Gillis JZ, Trupin L, Hersh A, et al. Impact of memory impairment on employment status in persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57(8):1453–60. doi: 10.1002/art.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus. 2003;12(4):280–6. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 21.Yazdany J, Yelin EH, Panopalis P, Trupin L, Julian L, Katz PP. Validation of the systemic lupus erythematosus activity questionnaire in a large observational cohort. Arthritis Rheum. 2008;59(1):136–43. doi: 10.1002/art.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julian LJ, Gregorich SE, Tonner C, Yazdany J, Trupin L, Criswell LA, et al. Using the CES-D to screen for depression in SLE. Arthritis Care Res (Hoboken) 2011 doi: 10.1002/acr.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 24.Brady CB, Spiro A, 3rd, Gaziano JM. Effects of age and hypertension status on cognition: the Veterans Affairs Normative Aging Study. Neuropsychology. 2005;19(6):770–7. doi: 10.1037/0894-4105.19.6.770. [DOI] [PubMed] [Google Scholar]
- 25.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens. 2008;26(8):1636–41. doi: 10.1097/HJH.0b013e3283018333. [DOI] [PubMed] [Google Scholar]
- 26.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117(6):1169–80. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 27.Mikdashi J, Handwerger B, Langenberg P, Miller M, Kittner S. Baseline disease activity, hyperlipidemia, and hypertension are predictive factors for ischemic stroke and stroke severity in systemic lupus erythematosus. Stroke. 2007;38(2):281–5. doi: 10.1161/01.STR.0000254476.05620.14. [DOI] [PubMed] [Google Scholar]
- 28.Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol. 2009;8(11):998–1005. doi: 10.1016/S1474-4422(09)70239-X. [DOI] [PubMed] [Google Scholar]
- 29.Olazaran J, Lopez-Longo J, Cruz I, Bittini A, Carreno L. Cognitive dysfunction in systemic lupus erythematosus: prevalence and correlates. Eur Neurol. 2009;62(1):49–55. doi: 10.1159/000215879. [DOI] [PubMed] [Google Scholar]
- 30.Menon S, Jameson-Shortall E, Newman SP, Hall-Craggs MR, Chinn R, Isenberg DA. A longitudinal study of anticardiolipin antibody levels and cognitive functioning in systemic lupus erythematosus. Arthritis Rheum. 1999;42(4):735–41. doi: 10.1002/1529-0131(199904)42:4<735::AID-ANR17>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57(2):202–7. doi: 10.1136/jnnp.57.2.202. PMCID: 1072451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel MD, Coshall C, Rudd AG, Wolfe CD. Cognitive impairment after stroke: clinical determinants and its associations with long-term stroke outcomes. J Am Geriatr Soc. 2002;50(4):700–6. doi: 10.1046/j.1532-5415.2002.50165.x. [DOI] [PubMed] [Google Scholar]
- 33.Bobba RS, Johnson SR, Davis AM. A review of the sapporo and revised Sapporo criteria for the classification of antiphospholipid syndrome. Where do the revised sapporo criteria add value? J Rheumatol. 2007;34(7):1522–7. [PubMed] [Google Scholar]
- 34.Reitz C, Schupf N, Luchsinger JA, Brickman AM, Manly JJ, Andrews H, et al. Validity of self-reported stroke in elderly African Americans, Caribbean Hispanics, and Whites. Arch Neurol. 2009;66(7):834–40. doi: 10.1001/archneurol.2009.83. PMCID: 2881576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas CM, Burt VL, Gillum RF, Pamuk ER. Validity of self-reported hypertension in the National Health and Nutrition Examination Survey III, 1988-1991. Prev Med. 1997;26(5 Pt 1):678–85. doi: 10.1006/pmed.1997.0190. [DOI] [PubMed] [Google Scholar]
- 36.Monastero R, Bettini P, Del Zotto E, Cottini E, Tincani A, Balestrieri G, et al. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. J Neurol Sci. 2001;184(1):33–9. doi: 10.1016/s0022-510x(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 37.Leritz E, Brandt J, Minor M, Reis-Jensen F, Petri M. “Subcortical” cognitive impairment in patients with systemic lupus erythematosus. J Int Neuropsychol Soc. 2000;6(7):821–5. doi: 10.1017/s1355617700677093. [DOI] [PubMed] [Google Scholar]
- 38.Julian LJ, Tonner C, Yelin E, Yazdany J, Trupin L, Criswell LA, et al. Cardiovascular and disease related predictors of depression in SLE. Arthritis Care Res (Hoboken) 2011 doi: 10.1002/acr.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]