Abstract
The discovery of humanin, a novel, mitochondrial-derived peptide, has created a potentially new category of biologically active peptide. As more research unravels the endogenous role of humanin as well as its potential pharmacological use, its role in stress resistance has become clearer. Humanin protects cells from oxidative stress, serum starvation, hypoxia, and other insults in vitro and also improves cardiovascular disease as well as Alzheimer's disease in vivo. In this review, we discuss the emerging role of humanin in stress resistance and its proposed mechanism of action.
Keywords: Apoptosis, Cardiovascular, Diabetes (all), Signal transduction
An overview of mitochondrial function
Mitochondria are generally considered the powerhouse of the cell as the majority of cellular ATP production occurs there. It is within the mitochondria that the Krebs cycle occurs, setting up an electrochemical gradient that will be used to produce ATP (Salway 1994). In addition to its role in energy production and metabolism, mitochondria play a major role in apoptosis, oxidative stress, and calcium sequestration/signaling.
The central role that mitochondria play in apoptosis has been enumerated on several occasions (reviewed in Wang (2001) and Kantari & Walczak (2011)). Mitochondria play a critical role in the intrinsic apoptotic pathway that is triggered by cellular damage of which some cells are dependent on the mitochondria for apoptosis (type 2), while others are not (type 1) (Kantari & Walczak 2011). Furthermore, many nucleusencoded mitochondrial proteins directly activate apoptosis when they are released from the mitochondria into the cytosol or nucleus. In addition to apoptosis, mitochondria are also believed to be the largest generator of free radicals in the cell (reviewed in Balaban et al. (2005)). Free radicals are the major source of oxidative stress within a cell and these free radicals can oxidize and damage proteins, lipids, and DNA. Besides the physiological role that certain free radicals play, increased oxidative stress has been associated with a number of different diseases, although a beneficial effect of transient oxidative stress has been reported as well (Ristow et al. 2009, Ristow & Zarse 2010).
Mitochondria possess an elaborate system for facilitating the transport of Ca2+ in and out of their inner membrane, which plays an important role in cytoplasmic Ca2+ signaling, ATP production, and hormone metabolism. Deregulation of the mitochondrial Ca2+ homeostasis can trigger cell death and disrupt the function of intra-mitochondrial enzymes. Mitochondrial Ca2+ uptake and release differ from other membrane-bound organelles in that Ca2+ uptake is not dependent on ATP and Ca2+ release uses gated channels and Na+ or H+/Ca2+ exchangers (reviewed in Drago et al. (2011)).
Communication from the cell to the mitochondria is well established, but how mitochondria signal to the cell is relatively less understood. In addition to the chemical signaling via Ca2+ and reactive oxygen species (ROS), in simpler organisms like yeast, mitochondria communicate with the cell via the retrograde signaling (RTG) pathway. In animal cells, many of the genes of the RTG pathway do not exist, suggesting that animal mitochondria have an alternative method for signaling the cell. Two recent papers suggest that the mitochondrial unfolded protein response (UPR) may be an alternative way that mitochondria signal to the cell. The first paper used Caenorhabditis elegans to demonstrate that tissue-specific mitochondrial dysfunction lead to a life-increasing, endocrine response and that this increase was dependent on the mitochondrial UPR (Durieux et al. 2011). Another paper showed that mitochondria undergoing temperature stress release degraded protein peptides that trigger mitochondrial UPR (Haynes et al. 2010). Humanin, the topic of this review, is another peptide that possibly acts as a mitochondrial autocrine, paracrine, and endocrine signal.
The discovery of humanin
Approximately 10 years ago, the Nishimoto Lab (Hashimoto et al. 2001b) discovered humanin (MTRNR2) while searching for survival factors in the unaffected brain section of an Alzheimer's patient. Using a cDNA library, they searched for genes that could resist apoptosis induced by a mutant form of amyloid precursor protein (APP V642I), which is a gene that is mutated in one form of familial Alzheimer's disease (AD). They further showed that humanin could protect against other familial AD genes but could not protect against a Huntington's disease model or amyotrophic lateral sclerosis model (Hashimoto et al. 2001a,b). This suggested that humanin conferred specific protection to AD models and did not merely protect cells from all apoptotic events.
Humanin (HN/MTRNR2) has also been cloned as a BAX partner that could suppress apoptosis caused by staurosporine, serum deprivation, or u.v. irradiation (Guo et al. 2003). Using co-immunoprecipitation experiments, humanin specifically bound to BAX, tBID, and BimEL (BCL2L11), but not other BCL2 family proteins such as BCL2 and BCL-B (BCL2L10) (Luciano et al. 2005, Zhai et al. 2005). Furthermore, the anti-apoptotic effect of humanin was specific to BAX-dependent apoptosis, as apoptosis by a BAX-independent stimulus, such as the use of tumor necrosis factor, was not suppressed.
Humanin also interacts with insulin-like growth factor-binding protein 3 (IGFBP3), where it can block IGFBP3-induced apoptosis in glial cell lines but not in neuronal cell lines or primary neurons (Ikonen et al. 2003). Although humanin can block the apoptotic effects of IGFBP3 in glial cells, unexpectedly, IGFBP3 and humanin have a positive, synergistic effect on protecting neurons from amyloid-β-induced apoptosis (Ikonen et al. 2003).
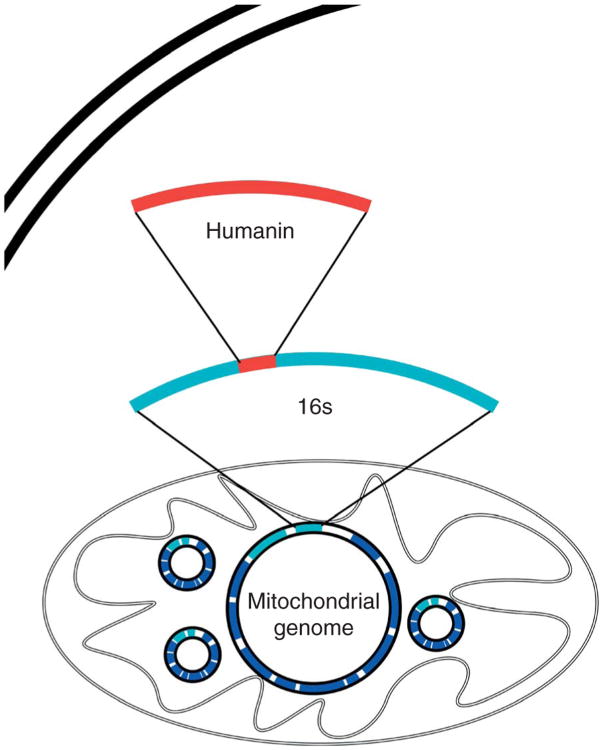
The discovery of humanin is interesting for many reasons. It is a small, secreted, 24 or 21 amino acid peptide, depending on cytoplasmic or mitochondrial translation respectively, with biological activity that does not have a signal peptide for secretion but may act as a signal peptide itself (Yamagishi et al. 2003). More interesting is the fact that it is the first new peptide discovered within the mitochondrial genome since its complete sequencing in 1981 (discussed in further detail below) (Anderson et al. 1981). The mitochondrial genome itself is extremely small and compact (16 569 nucleotides in humans), with no known introns and very few non-coding nucleotides between adjacent genes; traditionally, it has been known to code for only 2 rRNAs, 13 mRNAs, and 22 tRNAs. Humanin is an open reading frame (ORF) found within the 16s rRNA gene (Fig. 1). Like a Russian nesting doll, humanin is a gene within a gene within a genome of an organelle within a cell and may represent the first peptide of a new class of mitochondrial-derived peptides (MDP).
Figure 1.
The humanin open reading frame (ORF). Humanin is encoded by an ORF within the gene for the 16s ribosomal subunit within the mitochondrial genome.
Humanin action in stress
Alzheimer's disease
As mentioned earlier, humanin was discovered during a search for neurosurvival factors in unaffected areas of an AD patient's brain. Thus, it is not surprising that the best-studied aspect of humanin biology is in its beneficial effects in protecting against AD-related stress. These initial studies were first performed in cell culture and then followed by in vivo studies using both pharmacological mimetics of AD as well as mutant APP. The most recent studies have now started using different transgenic models of AD to examine the potential use of humanin to ameliorate AD.
The earliest humanin study showed that it could protect cells from apoptosis induced by amyloid β (Aβ)1–43 and a follow-up study went even further showing that humanin could protect against other mutated genes that cause familial AD such as APP, presenilin 1, and presenilin 2 (Hashimoto et al. 2001a,b). Although other factors such as IGF1 and basic FGF could protect neurons from (Aβ)1–43, only humanin could protect against all the tested AD genes (Hashimoto et al. 2001a).
The first in vivo study to examine the physiological potential of humanin used scopolamine, a muscarinic acetylcholine receptor antagonist that causes memory deficits similar to AD. I.c.v. injections of modified humanin (HNG) ameliorated the short-term memory deficits seen with scopolamine injection (Mamiya 2001). This modification replaced serine 14 for a glycine residue and was shown to increase the neuroprotective effects of humanin by 1000-fold (Hashimoto et al. 2001a,b). Using an alternative anticholinergic drug, 3-quinuclidinyl benzilate, Krejcova et al. (2004) demonstrated that HNG injected i.p. could also help abrogate the memory impairments caused by this drug. This experiment also demonstrated that the positive effects of humanin did not require i.c.v. injections, which would help facilitate the use of humanin as a possible therapeutic drug.
Although the previous studies used a surrogate drug to examine the effects of humanin/HNG on AD-like memory deficits, the first in vivo studies that actually used Aβ also demonstrated that HNG could help prevent the effects of Aβ toxicity on memory defects (Tajima et al. 2005). Injecting Aβ 25–35 into mice (i.c.v.) causes memory deficits within 3 weeks, which was ameliorated by HNG treatment (Tajima et al. 2005). Supporting the memory improvements seen with HNG administration, ex vivo experiments using hippocampal brain slices have also shown that HNG can prevent the inhibition of long-term potentiation caused by Aβ 25–35 applied an hour before recording (Zhang et al. 2009). Examination of acetylcholine-producing cells in the brain by staining for choline acetyltransferase, an enzyme used in the synthesis of acetylcholine, showed that Aβ 25–35 decreased the number of immunoreactive cells and that HNG was able to ameliorate this decrease (Tajima et al. 2005). Further examination of the in vivo effects of humanin/HNG on Aβ toxicity showed that HNG was able to reduce the inflammatory response caused by Aβ 25–35 as demonstrated by a decrease in reactive astrocytes, microglial activation, TNFα levels, and IL6 levels (Miao et al. 2008).
More recent studies have administered HNG to different transgenic models of AD. HNG administered intranasally to 1-year-old mice for 3 months reduced accumulation of Aβ levels in the brain and reduced the memory deficits in the triple transgenic AD model (APPswe, taup310L, PS-1M146V) (Niikura et al. 2011). In mice with preexisting amyloid plaques, HNG administered to double transgenic mice (APPswe, PS-1dE9) reduced the Aβ accumulation and improved the cognitive deficits compared with controls (Zhang et al. 2012). Thus, it seems that humanin may have therapeutic and/or prophylactic promise for treating AD.
Other neurodegenerative diseases
Although AD is the number one cause of dementia in adults, many other neurodegenerative diseases exist. These diseases cause neuronal stress by varying mechanisms but lead to the eventual death of neuronal cells. Because of the anti-apoptotic effects of humanin, several laboratories have examined the use of humanin to prevent neurodegeneration with mixed results.
Polyglutamine toxicity is the underlying cause of a number of different diseases such as spinocerebellar ataxia and Huntington's disease, where mitochondria dysfunction has been noted (reviewed in Takahashi et al. (2010)). The potential use of humanin to prevent polyglutamine toxicity has been investigated with divergent results. In one case, humanin had no protective ability, while in another, humanin partially protected the neuronal cell line (F11 and PC12 cells respectively) (Hashimoto et al. 2001b, Kariya et al. 2005). The many differences between the two studies could explain the equivocal results, but more studies of humanin as a possible treatment for polyglutamine diseases are certainly required.
Prions also cause neuronal stress and cell death and the possibility of humanin helping against this type of stress has also been examined. An initial study demonstrated no protective effect of humanin when using prion peptide 106–126 to induce cell stress and apoptosis (Hashimoto et al. 2001a). A later study confirmed this fact but found that humanin did have a protective effect against prion protein 118–135 (Sponne et al. 2004). Further studies will be required to determine the potential use of humanin in prion diseases.
Oxidative stress
Oxidative stress has been implicated in a multitude of diseases, and because of the success of humanin treatment against Aβ stress, several labs have tested humanin's ability to protect against oxidative stress. Treatment of rat retinal cultures with humanin has shown that it can protect cells from oxidative stress induced by hydrogen peroxide (Yang et al. 2008). The same study also demonstrated that hydrogen peroxide treatment decreases the endogenous protein levels of the rat homolog of humanin, Rattin (Yang et al. 2008). Further examination of humanin's ability to protect against oxidative stress focused on humanin's potential to help against atherosclerosis. In human carotid atherosclerotic plaques, humanin was found to be twice as high in symptomatic patients compared with asymptomatic, suggesting a role for humanin in atherosclerosis (Zacharias et al. 2012). An in vitro study found that humanin was able to protect endothelial cells from oxidative stress induced by oxidized LDL (Bachar et al. 2010). An in vivo follow-up on that observation demonstrated that HNG was able to improve cardiovascular function in Apoe-deficient mice placed on a high-cholesterol diet (Oh et al. 2011). Combining these data suggests that the increased levels of humanin found in atherosclerotic plaques are a protective response to stress and that humanin may be able to protect against oxidative stress.
Ischemia and reperfusion
Ischemic and reperfusion stress contributes to a large number of different physiological injuries such as stroke, myocardial infarction, and sickle cell disease, where it causes a number of different stresses such as oxidative stress, hypoxia, and nutrient deprivation. Accumulating evidence suggests that humanin may be beneficial in attenuating the stresses caused by ischemia and reperfusion. One recent study examined humanin's ability to protect against the cellular effects of the hypoxia mimetic cobalt chloride (CoCl2) (Men et al. 2012). Examining transformed rat ganglion cells, Men et al. (2012) found that humanin could help reduce the number of apoptotic cells caused by this treatment. A lack of growth factors and nutrients is another result of ischemia and serum deprivation increases apoptosis (Kariya et al. 2002). Kariya et al. (2002,2003) as well as Guo et al. (2003) demonstrated that humanin can protect against serum starvation stress as well. Interestingly, they found that humanin increased metabolic activity (measured by MTS assay) and ATP levels of serum-starved cells above that of cells in complete media (Kariya et al. 2003).
In vivo studies have also demonstrated the benefits of humanin treatment. In a stroke model, mice underwent middle cerebral artery occlusion for 75 min. Mice that were either pretreated or posttreated up to 4 h after the occlusion with HNG i.c.v. had up to a 50% reduction in infarct volume size (Xu et al. 2006). Looking at a more clinically applicable method of humanin administration, Xu et al. (2006) also showed that an i.p. injection 2 h following the occlusion could also reduce the infarct size to a similar extent as the i.c.v. injection at 2 h. Similar to the stroke study, humanin can also ameliorate the effects of left coronary occlusion, and both pretreatment with humanin or treatment immediately following the occlusion can confer benefits (Muzumdar et al. 2010). In this study, both infarct size and loss of cardiac function were reduced with humanin treatment (Muzumdar et al. 2010). Thus, growing evidence supports the use of humanin as a cytoprotective agent against ischemia and reperfusion stress.
Humanin and its mechanism of action
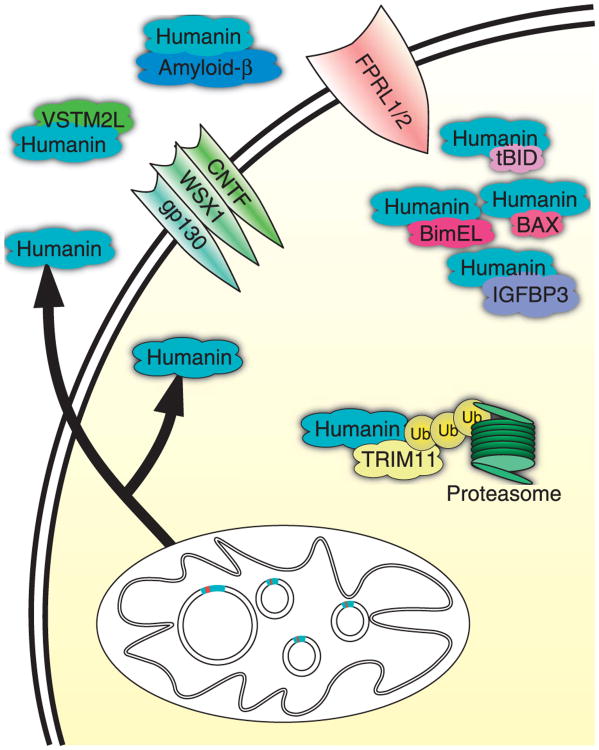
Although all studies show a protective effect of humanin, the mechanisms by which humanin acts is still unclear. Ying et al. (2004) found that both Aβ42 and humanin activate the same receptor, the G protein-coupled formylpeptide-like-1 receptor (FPRL1). Both acted as chemoattractants to monocytes and activated microglia, but humanin prevented the intracellular accumulation of Aβ42 in these cells (Ying et al. 2004). Independently confirming the previous finding, Harada et al. (2004) screened for ligands for orphan G-coupled receptors and found that humanin directly interacts with the FPRL1 and FPRL2 receptors in Chinese hamster ovary (CHO) cells transfected with the corresponding receptor. On the other hand, using a different type of cell, F11 hybrid neuronal cells, Hashimoto et al. (2005) found that the Aβ protection of humanin did not require FPRL1 but did require STAT signaling. Following on that observation, Hashimoto et al. (2009) also found that humanin requires a tripartite cytokine-like receptor complex comprising the CNTF receptor, WSX1, and gp130 (IL6ST) to protect neurons from Aβ. To reconcile these differences, it may be that different cell types employ different receptors to respond to humanin as both FPRL1 and FPRL2 are mainly found on phagocytic cells and not on neurons (reviewed in Le et al. (2002)). Humanin has also been shown to directly interact with Aβ and change the morphology of the Aβ aggregates and this could also be another method by which humanin protects against Aβ (Zou et al. 2003, Maftei et al. 2012). Additionally, HNG administration alone has been shown to increase insulin/IGF signaling in primary mouse neurons as assessed by AKT-1 phosphorylation, although studies in mouse heart has not found the same increase in phosphorylation and instead find an increase in phosphor-AMPK (Xu et al. 2008, Muzumdar et al. 2010). An in vivo experiment has shown that HNG is a potent insulin sensitizer due to its ability to stimulate STAT3 in the hypothalamus (Muzumdar et al. 2009).
While the above pathways demonstrate an extracellular mechanism for humanin action, an intracellular, anti-apoptotic role has also been elucidated. Over several papers, the Reed laboratory has demonstrated that humanin interacts with many proteins in the apoptosis cascade (Guo et al. 2003, Luciano et al. 2005, Zhai et al. 2005). Humanin's anti-apoptotic function has been shown to be due to its interaction with the pro-apoptotic proteins BAX, tBID, and BimEL (Fig. 2). Humanin interacts with each of these proteins and inhibits further downstream signaling. The Reed laboratory further demonstrated that exogenous application of humanin did not prevent apoptosis in these studies and concluded that humanin was likely acting through an intracellular mechanism (Luciano et al. 2005, Zhai et al. 2005). These data seem to contradict data that had previously shown that the anti-apoptotic effects of humanin required extracellular secretion, although the papers used different cell types (Yamagishi et al. 2003).
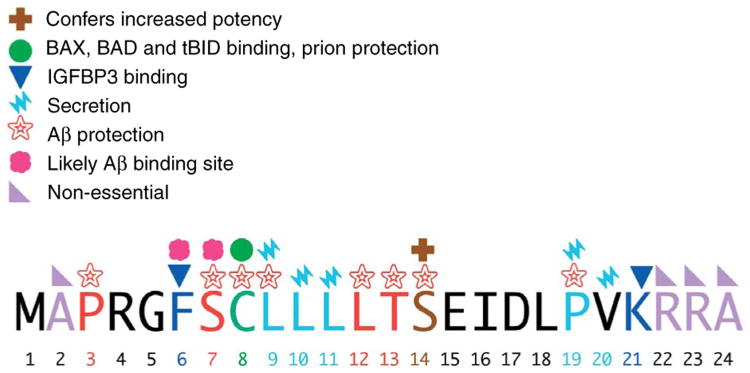
Figure 2.
Function of specific amino acid residues within humanin. Mutational analysis of humanin has allowed for the importance of each amino acid to be elucidated. Important amino acids are marked with their function as noted in the key.
Three other studies have found additional humanin interacting proteins. Using a yeast-2-hybrid system, TRIM11 was discovered (Niikura et al. 2003). TRIM11 likely ubiquinates humanin for degradation and adds another level of regulation to humanin expression (Niikura et al. 2003). In a different yeast-2-hybrid screen, a secreted antagonist of humanin was also found (Rossini et al. 2011). V-set and transmembrane domain containing 2 like (VSTM2L) was discovered to be a secreted protein that inhibits the positive effects of humanin action on neuronal apoptosis and this may be an additional way that the body regulates humanin action (Rossini et al. 2011). Lastly, humanin was found to interact with α-actinin-4, suggesting that humanin may have an unknown role in kidney function (Kigawa et al. 2004). Although there have been many studies examining the mechanism of humanin function, seemingly contradictory results necessitate further investigation.
Few studies have examined the mechanism or conditions by which humanin expression is increased. Humanin levels are known to be increased in endothelial cells of Apoe-deficient mice on a high-cholesterol diet, but the primary cause for this is unknown (Oh et al. 2011). Conversely, humanin levels are known to decrease with age in both rats and humans, and although the primary cause of this increase is unknown as well, it suggests that humanin may play a role in the aging process (Muzumdar et al. 2009, Bachar et al. 2010). More studies are needed to identify possible regulators of humanin transcription and translation.
Structure and function
The exact length of endogenous humanin is still unknown due to the differences in the mitochondrial and cytoplasmic translational machinery. If humanin is translated within the mitochondria, the peptide will be 21 amino acids; on the other hand, if it is translated in the cytoplasm, then the result is a 24 amino acid peptide. Both peptides have been shown to have biological activity (Guo et al. 2003). Because humanin is a relatively short peptide, exhaustive mutational analysis of the importance of each amino acid has been possible (results summarized in Fig. 3). P3, S7, C8, L9, L12, T13, and S14 are important for humanin's protective effects against Aβ toxicity (Hashimoto et al. 2001b, Yamagishi et al. 2003). These residues may be important for the interaction with Aβ as the region between the 5th and 15th amino acid has been shown to interact with Aβ by affinity mass spectrometry (Maftei et al. 2012). Conversely, a mutation substituting glycine for S14 increases the neuroprotective ability of humanin by 1000 times (Hashimoto et al. 2001b). In addition to being important for the neuroprotective effects of humanin, C8 has also been shown to be important for humanin to bind BAX and tBID and also required to protect against prion proteins (Guo et al. 2003, Sponne et al. 2004, Zhai et al. 2005). Some laboratories have shown that humanin secretion is required for it to have its cytoprotective effects, and the amino acid residues L9, L10, L11, P19, and V20 are important for secretion (Yamagishi et al. 2003). In terms of its IGFBP3 binding ability, both F6 and K21 are important for this interaction (Ikonen et al. 2003). Humanin is secreted from cells and can be found in the media of transfected cells (Yamagishi et al. 2003). Although there is no signal peptide to direct the peptide for secretion, humanin itself can act as a signal peptide, and when fused to larger proteins, it can cause the protein to be secreted into the media (Yamagishi et al. 2003).
Figure 3.
Humanin's mechanism of action and interacting partners. Humanin has shown to have both intracellular and extracellular mechanisms of action. Many of its potential effects are mediated by the FPRL receptor as well as the trimeric WSX1/CNTF/gp130 receptor. Intracellularly, it interacts with many different proteins acting as a pro-survival signal.
Humanin-like proteins
In addition to the question of where humanin is translated, another major question that has not yet been fully resolved is whether humanin is translated from a mitochondrially encoded transcript, or if it is a product of a nuclear gene, or both. DNA from the mitochondria is often found dispersed within the nuclear genome, and, in fact, Ramos et al. (2011) found 755 insertion events in the human genome. Of these insertions, ∼75% of them are <500 bp in length and 60% had <80% identity to the mitochondrial sequences (Ramos et al. 2011). With respect to humanin, 13 humanin-like ORFs were found within the nuclear genome, although only ten of them were found to be expressed in tissue samples (Bodzioch et al. 2009). Of these ten ORFs, only one of these codes for a possibly identical protein depending on the polymorphism used (Bodzioch et al. 2009). The rest of the ORFs code for peptides that have amino acid changes in critical residues that may change their function, although this still needs to be carefully tested (see Fig. 4). Thus, the question of the origin of humanin still needs to be clarified.
Figure 4.
Alignment of mitochondrial humanin with possible nuclear homologs. Ten different nuclear-encoded humanin (HN) homologs have been reported with all but one encoding for slightly different proteins than humanin. *Humanin-like protein 8 has a reported polymorphism that would cause it to be identical to mitochondrially encoded humanin.
Future studies
As mentioned earlier, one of the major questions in the field is whether endogenous humanin is derived from the mitochondria, and thus constitutes a novel category of peptides, or if humanin is nuclear in origin. Although the mitochondrial sequence of humanin has been identified independently several times as a cytoprotective peptide, its sequence similarity to other nuclear humanin-like peptides creates ambiguity as to whether it is endogenously expressed or whether its amino acid sequence is merely similar to an endogenously expressed peptide. Additionally, whether humanin is translated in the cytoplasm or mitochondria is also up for debate as both translations have bioactivity (Guo et al. 2003). Examination of mitochondrial RNA has found a high number of poly-adenylation sites within mitochondrial genes, and poly-adenylated mitochondrial sequences have been found in the cytoplasm, providing evidence that cytoplasmic translation may occur (Nakamichi et al. 1998, Mercer et al. 2011). Even if humanin is nuclear in origin, it can still be considered a MDP as the original nucleotide sequence has been transferred from the mitochondrial genome.
From a more practical point of view, it is clear that humanin is a cytoprotective peptide for a number of different stresses. Whether it is Aβ toxicity, serum starvation, hypoxia, oxidative stress, or any of the other stresses previously mentioned, both in vitro and in vivo studies have shown that administration of humanin has a beneficial effect.
The discovery of humanin as a novel, stress-responsive, and cytoprotective peptide derived from a mitochondrial ORF has opened up a new field of research. For decades, the number of genes within the mitochondria has been invariant, but this discovery forces the field to reexamine this assumption. Depending on how one analyzes the genome, there can be anywhere from 100 to over 500 ORFs. The majority are likely non-transcribed ORFs, but even if only a small percentage are real MDPs, there will be many novel discoveries. Because of the mitochondria's central role in stress resistance, these peptides are also likely to play a role in cellular stress resistance and may lead to new pharmacological agents and targets.
Acknowledgments
Funding: This research was supported by a Glenn Foundation Award and National Institute of Health Grants to P C (1R01AG 034430, 1R01GM 090311, 1R01ES 020812, and 1R21DK 089447) and an Ellison Medical Foundation/AFAR fellowship grant to K Y.
Footnotes
Declaration of interest: Dr P C is a consultant and stockholder of CohBar, Inc.
References
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovascular Research. 2010;88:360–366. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bodzioch M, Lapicka-Bodzioch K, Zapala B, Kamysz W, Kiec-Wilk B, Dembinska-Kiec A. Evidence for potential functionality of nuclearly-encoded humanin isoforms. Genomics. 2009;94:247–256. doi: 10.1016/j.ygeno.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Drago I, Pizzo P, Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO Journal. 2011;30:4119–4125. doi: 10.1038/emboj.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- Harada M, Habata Y, Hosoya M, Nishi K, Fujii R, Kobayashi M, Hinuma S. N-formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochemical and Biophysical Research Communications. 2004;324:255–261. doi: 10.1016/j.bbrc.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, Abe Y, Kita Y, Nishimoto I. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults. Journal of Neuroscience. 2001a;21:9235–9245. doi: 10.1523/JNEUROSCI.21-23-09235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Aβ. PNAS. 2001b;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto YY, Suzuki HH, Aiso S, Niikura T, Nishimoto I, Matsuoka MM. Involvement of tyrosine kinases and STAT3 in humanin-mediated neuroprotection. Life Sciences. 2005;77:3092–3104. doi: 10.1016/j.lfs.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Kurita M, Aiso S. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor α/WSX-1/gp130. Molecular Biology of the Cell. 2009;20:2864–2873. doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 C. elegans. Molecular Cell. 2010;37:529–540. doi: 10.1016/j.molcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. PNAS. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochimica et Biophysica Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Kariya S, Takahashi N, Ooba N, Kawahara M, Nakayama H, Ueno S. Humanin inhibits cell death of serum-deprived PC12h cells. Neuroreport. 2002;13:903–907. doi: 10.1097/00001756-200205070-00034. [DOI] [PubMed] [Google Scholar]
- Kariya S, Takahashi N, Hirano M. Humanin improves impaired metabolic activity and prolongs survival of serum-deprived human lymphocytes. Molecular and Cellular Biochemistry. 2003;254:83–89. doi: 10.1023/A:1027372519726. [DOI] [PubMed] [Google Scholar]
- Kariya S, Hirano M, Nagai Y, Furiya Y, Fujikake N, Toda T, Ueno S. Humanin attenuates apoptosis induced by DRPLA proteins with expanded polyglutamine stretches. Journal of Molecular Neuroscience. 2005;25:165–169. doi: 10.1385/JMN:25:2:165. [DOI] [PubMed] [Google Scholar]
- Kigawa A, Wakui H, Maki N, Okuyama S, Masai R, Ohtani H, Komatsuda A, Suzuki D, Toyoda M, Kobayashi R, et al. Interaction of the spectrin-like repeats of α-actinin-4 with humanin peptide. Clinical and Experimental Nephrology. 2004;8:331–338. doi: 10.1007/s10157-004-0322-y. [DOI] [PubMed] [Google Scholar]
- Krejcova G, Patocka J, Slaninova J. Effect of humanin analogues on experimentally induced impairment of spatial memory in rats. Journal of Peptide Science. 2004;10:636–639. doi: 10.1002/psc.569. [DOI] [PubMed] [Google Scholar]
- Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends in Immunology. 2002;23:541–548. doi: 10.1016/S1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- Luciano F, Zhai D, Zhu X, Bailly-Maitre B, Ricci JE, Satterthwait AC, Reed JC. Cytoprotective peptide humanin binds and inhibits proapoptotic Bcl-2/Bax family protein BimEL. Journal of Biological Chemistry. 2005;280:15825–15835. doi: 10.1074/jbc.M413062200. [DOI] [PubMed] [Google Scholar]
- Maftei M, Tian X, Manea M, Exner TE, Schwanzar D, Arnimvon CAF, Przybylski M. Interaction structure of the complex between neuroprotective factor humanin and Alzheimer's β-amyloid peptide revealed by affinity mass spectrometry and molecular modeling. Journal of Peptide Science. 2012;18:373–382. doi: 10.1002/psc.2404. [DOI] [PubMed] [Google Scholar]
- Mamiya T. [Gly14]-humanin improved the learning and memory impairment induced by scopolamine in vivo. British Journal of Pharmacology. 2001;134:1597–1599. doi: 10.1038/sj.bjp.0704429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men J, Zhang X, Yang Y, Gao D. An AD-related neuroprotector rescues transformed rat retinal ganglion cells from CoCl(2)-induced apoptosis. Journal of Molecular Neuroscience. 2012;47:144–149. doi: 10.1007/s12031-011-9701-5. [DOI] [PubMed] [Google Scholar]
- Mercer TR, Neph S, Dinger ME, Crawford J, Smith MA, Shearwood AMJ, Haugen E, Bracken CP, Rackham O, Stamatoyannopoulos JA, et al. The human mitochondrial transcriptome. Cell. 2011;146:645–658. doi: 10.1016/j.cell.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Zhang W, Li Z. S14G-Humanin ameliorates Aβ25-35-induced behavioral deficits by reducing neuroinflammatory responses and apoptosis in mice. Neuropeptides. 2008;42:557–567. doi: 10.1016/j.npep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Muzumdar R, Huffman D, Atzmon G, Buettner C, Cobb L, Fishman S, Budagov T, Cui L, Einstein F, Poduval A, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS ONE. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar RH, Huffman DM, Calvert JW, Jha S, Weinberg Y, Cui L, Nemkal A, Atzmon G, Klein L, Gundewar S, et al. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:1940–1948. doi: 10.1161/ATVBAHA.110.205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamichi N, Rhoads DD, Hayashi JI, Kagawa Y, Matsumura T. Detection, localization, and sequence analyses of mitochondrial regulatory region RNAs in several mammalian species. Journal of Biochemistry. 1998;123:392–398. doi: 10.1093/oxfordjournals.jbchem.a021950. [DOI] [PubMed] [Google Scholar]
- Niikura T, Hashimoto Y, Tajima H, Ishizaka M, Yamagishi Y, Kawasumi M, Nawa M, Terashita K, Aiso S, Nishimoto I. A tripartite motif protein TRIM11 binds and destabilizes humanin, a neuroprotective peptide against Alzheimer's disease-relevant insults. European Journal of Neuroscience. 2003;17:1150–1158. doi: 10.1046/j.1460-9568.2003.02553.x. [DOI] [PubMed] [Google Scholar]
- Niikura T, Sidahmed E, Hirata-Fukae C, Aisen PS, Matsuoka Y. A humanin derivative reduces amyloid β accumulation and ameliorates memory deficit in triple transgenic mice. PLoS ONE. 2011;6:e16259. doi: 10.1371/journal.pone.0016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YK, Bachar AR, Zacharias DG, Kim SG, Wan J, Cobb LJ, Lerman LO, Cohen P, Lerman A. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholestero-lemic ApoE deficient mice. Atherosclerosis. 2011;219:65–73. doi: 10.1016/j.atherosclerosis.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Barbena E, Mateiu L, del Mar Gonzá M, Mairal Q, Lima M, Montiel R, Aluja MP, Santos C. Nuclear insertions of mitochondrial origin: database updating and usefulness in cancer studies. Mitochondrion. 2011;11:946–953. doi: 10.1016/j.mito.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Experimental Gerontology. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kl ting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn C, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. PNAS. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini L, Hashimoto Y, Suzuki H, Kurita M, Gianfriddo M, Scali C, Roncarati R, Franceschini D, Pollio G, Trabalzini L, et al. VSTM2L is a novel secreted antagonist of the neuroprotective peptide humanin. FASEB Journal. 2011;25:1983–2000. doi: 10.1096/fj.10-163535. [DOI] [PubMed] [Google Scholar]
- Salway JG. Metabolism at a Glance. 5. Malden, MA: Blackwell Publishing Ltd; 1994. p. 10. [Google Scholar]
- Sponne I, Fifre A, Koziel V, Kriem B, Oster T, Pillot T. Humanin rescues cortical neurons from prion-peptide-induced apoptosis. Molecular and Cellular Neurosciences. 2004;25:95–102. doi: 10.1016/j.mcn.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Tajima H, Kawasumi M, Chiba T, Yamada M, Yamashita K, Nawa M, Kita Y, Kouyama K, Aiso S, Matsuoka M, et al. A humanin derivative, S14G-HN, prevents amyloid-β-induced memory impairment in mice. Journal of Neuroscience Research. 2005;79:714–723. doi: 10.1002/jnr.20391. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Katada S, Onodera O. Polyglutamine diseases: where does toxicity come from? what is toxicity? where are we going? Journal of Molecular Cell Biology. 2010;2:180–191. doi: 10.1093/jmcb/mjq005. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes and Development. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Xu X, Chua CC, Gao J, Hamdy RC, Chua BHL. Humanin is a novel neuroprotective agent against stroke. Stroke; a Journal of Cerebral Circulation. 2006;37:2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- Xu X, Chua C, Gao J, Chua K, Wang H. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Research. 2008;1227:12–18. doi: 10.1016/j.brainres.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Hashimoto Y, Niikura T, Nishimoto I. Identification of essential amino acids in humanin, a neuroprotective factor against Alzheimer's disease-relevant insults. Peptides. 2003;24:585–595. doi: 10.1016/S0196-9781(03)00106-2. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhang Q, Ge J, Tan Z. Protective effects of tetramethyl-pyrazine on rat retinal cell cultures. Neurochemistry International. 2008;52:1176–1187. doi: 10.1016/j.neuint.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying G, Iribarren P, Zhou Y, Gong W, Zhang N, Yu ZX, Le Y, Cui Y, Wang JM. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. Journal of Immunology. 2004;172:7078–7085. doi: 10.4049/jimmunol.172.11.7078. [DOI] [PubMed] [Google Scholar]
- Zacharias DG, Kim SG, Massat AE, Bachar AR, Oh YK, Herrmann J, Rodriguez-Porcel M, Cohen P, Lerman LO, Lerman A. Humanin, a cytoprotective Peptide, is expressed in carotid artherosclerotic plaques in humans. PLoS ONE. 2012;7:e31065. doi: 10.1371/journal.pone.0031065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. Journal of Biological Chemistry. 2005;280:15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Miao J, Hao J, Li Z, Xu J, Liu R, Cao F, Wang R, Chen J, Li Z. Protective effect of S14G-humanin against β-amyloid induced LTP inhibition in mouse hippocampal slices. Peptides. 2009;30:1197–1202. doi: 10.1016/j.peptides.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang W, Li Z, Hao J, Zhang Z, Liu L, Mao N, Miao J, Zhang L. S14G-humanin improves cognitive deficits and reduces amyloid pathology in the middle-aged APPswe/PS1dE9 mice. Pharmacology, Biochemistry, and Behavior. 2012;100:361–369. doi: 10.1016/j.pbb.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Zou P, Ding Y, Sha Y, Hu B, Nie S. Humanin peptides block calcium influx of rat hippocampal neurons by altering fibrogenesis of Aβ(1–40) Peptides. 2003;24:679–685. doi: 10.1016/S0196-9781(03)00131-1. [DOI] [PubMed] [Google Scholar]